Allogeneic NKG2D CAR-T Cell Therapy: A Promising Approach for Treating Solid Tumors
Abstract
1. Introduction
2. CAR-T Therapy as an Example of Adoptive Cell Therapy for Cancer Diseases
3. CAR-T Cell Therapy: Autologous and Allogeneic Approaches
| Characteristics | Autologous CAR-T Cells | Allogeneic CAR-T Cells |
|---|---|---|
| Source of T cells | Patient | Healthy donor |
| Manufacturing process | Characteristics of CAR-T cells vary depending on the patient’s condition and previous therapy; long interval between leukapheresis and infusion of CAR-T cells; logistical difficulties | Possibility of standardizing the characteristics of CAR-T lymphocytes; large-scale industrial process for obtaining large numbers of CAR-T lymphocytes from a single donor; immediate availability of the drug for patient treatment. |
| Persistence in the patient’s body | From a few months to several years | From a few weeks to several months |
| Main disadvantages and side effects | Cytokine release syndrome; neurotoxicity; immune effector cell-associated neurotoxicity syndrome; long-term side effects (B-cell aplasia for anti-CD19 CAR-T cells) | Cytokine release syndrome; “off-target” mutations; graft-versus-host disease; rejection of modified CAR-T cells |
| Main disadvantages and side effects | Cytokine release syndrome; neurotoxicity; immune effector cell-associated neurotoxicity syndrome; long-term side effects (B-cell aplasia for anti-CD19 CAR-T cells) | Cytokine release syndrome; “off-target” mutations; graft-versus-host disease; rejection of modified CAR-T cells |
| Targeted tumor type | Hematological malignancies; solid malignancies | Hematological malignancies; solid malignancies |
| Reinfusion of CAR-T cells | Limited by the number of CAR-T cells | Not limited by number of CAR-T cells; increased risk of alloimmunization with each reinfusion |
| Cost | High cost ($300,000–475,000 per dose) | The ability to manage prices while developing the manufacturing process ($4000–10,000 per dose) |
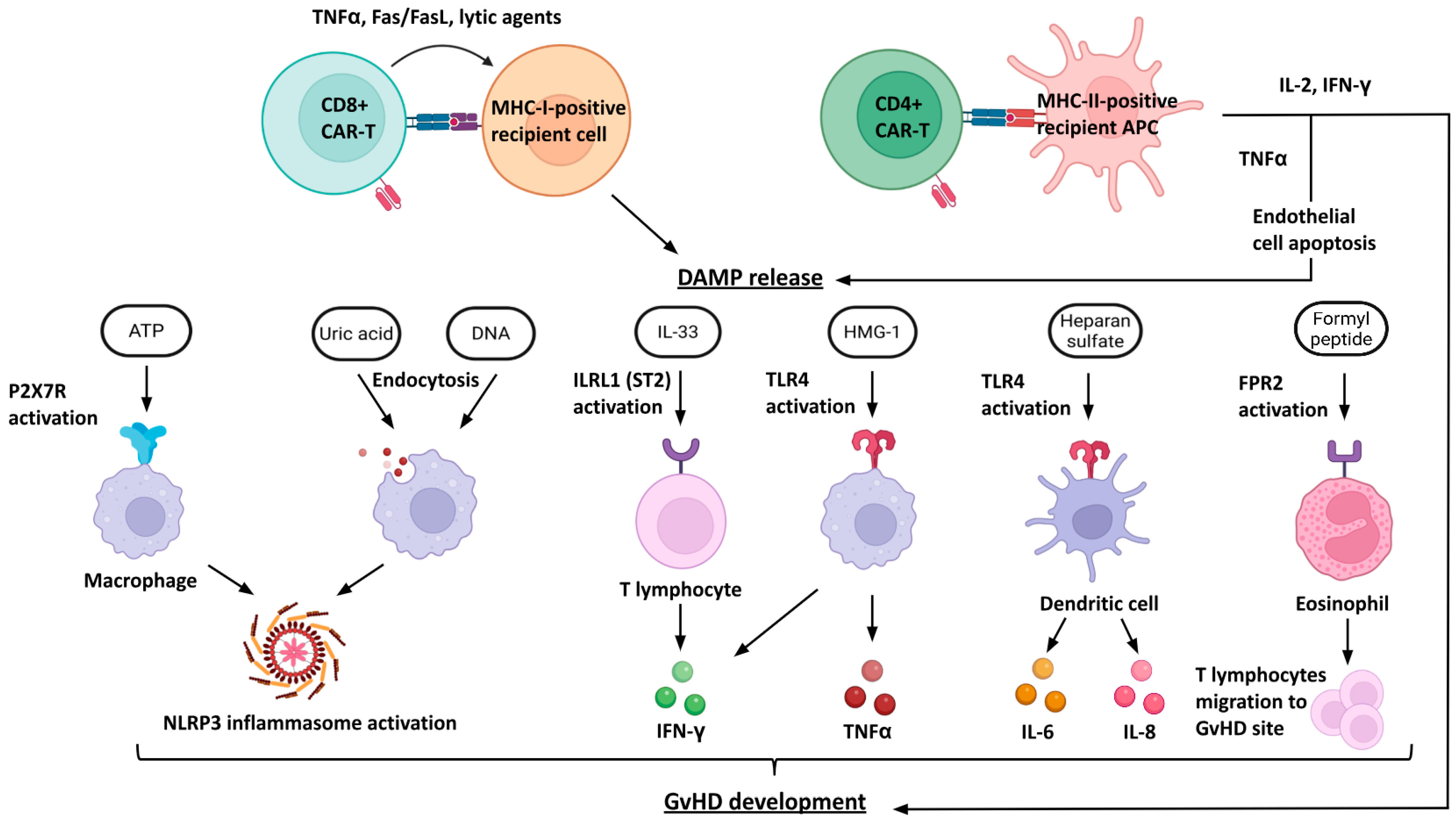
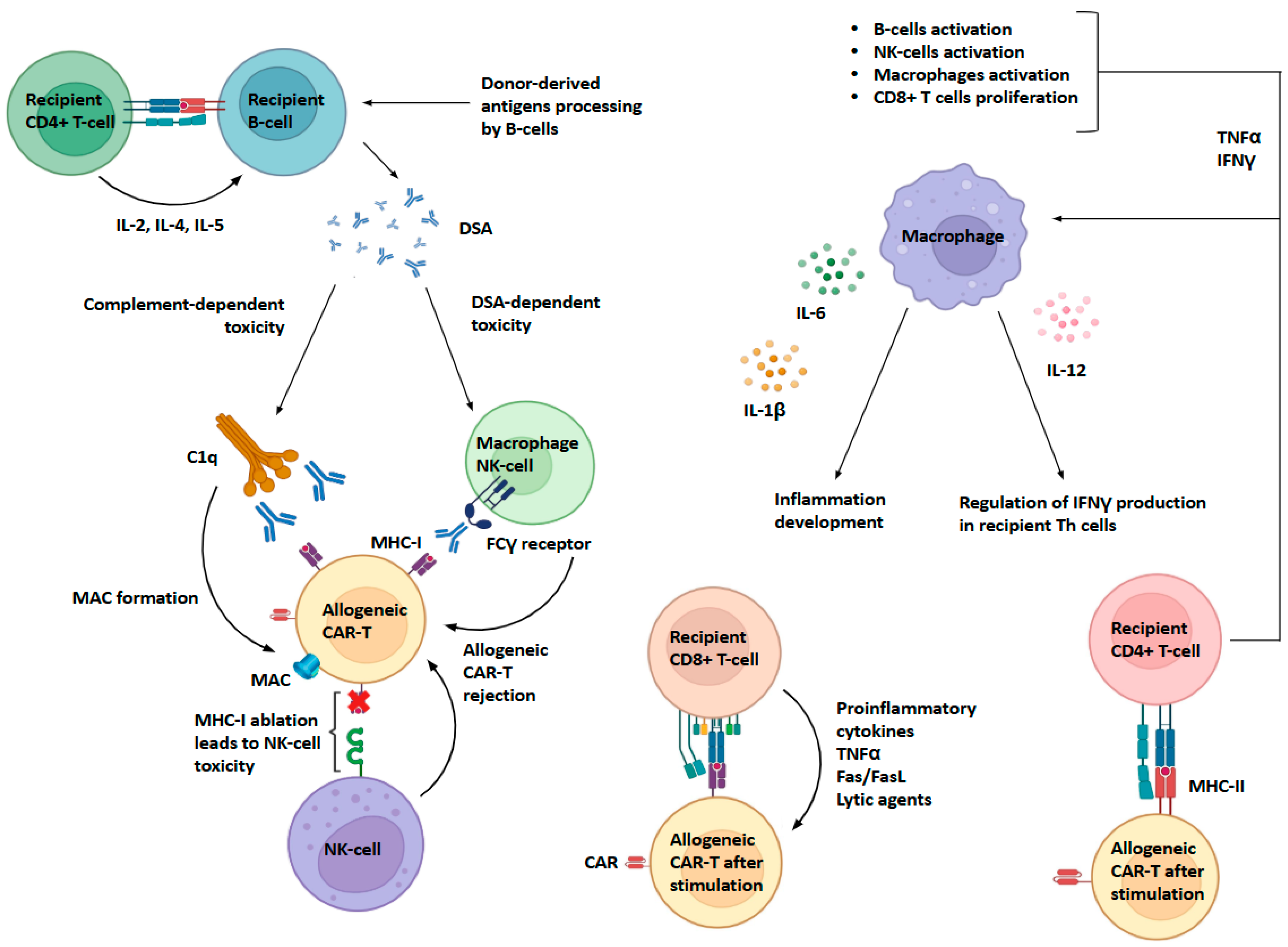
4. Problems with the Interaction of Allogeneic CAR-T Cells with the Host Immune System
5. Genome Editing Techniques to Generate Universal and Safe CAR-T Cells
6. Clinical Trials of Allogeneic NKG2D CAR-T Lymphocytes
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sanber, K.; Savani, B.; Jain, T. Graft-versus-host disease risk after chimeric antigen receptor T-cell therapy: The diametric opposition of T cells. Br. J. Haematol. 2021, 195, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Depil, S.; Duchateau, P.; Grupp, S.; Mufti, G.; Poirot, L. ‘Off-the-shelf’ allogeneic CAR T cells: Development and challenges. Nat. Rev. Drug Discov. 2020, 19, 185–199. [Google Scholar] [CrossRef]
- Sapio, L.; Naviglio, S. Innovation through Tradition: The Current Challenges in Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 5296. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Wu, H.; Xu, R. Advancing to the era of cancer immunotherapy. Cancer Commun. 2021, 41, 803–829. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, M.; Zhang, D.; Chen, M.; Zhu, D. Clinical cancer immunotherapy: Current progress and prospects. Front. Immunol. 2022, 13, 961805. [Google Scholar] [CrossRef]
- Christofi, T.; Baritaki, S.; Falzone, L.; Libra, M.; Zaravinos, A. Current Perspectives in Cancer Immunotherapy. Cancers 2019, 11, 1472. [Google Scholar] [CrossRef]
- Keshavarz-Fathi, M.; Rezaei, N. Cancer Immunoprevention: Current Status and Future Directions. Arch. Immunol. Ther. Experimentalis. 2021, 69, 3. [Google Scholar] [CrossRef] [PubMed]
- Ellis, G.; Sheppard, N.; Riley, J. Genetic engineering of T cells for immunotherapy. Nat. Rev. Genet. 2021, 22, 427–447. [Google Scholar] [CrossRef]
- Feldman, S.; Assadipour, Y.; Kriley, I.; Goff, S.; Rosenberg, S. Adoptive Cell Therapy--Tumor-Infiltrating Lymphocytes, T-Cell Receptors, and Chimeric Antigen Receptors. Semin. Oncol. 2015, 42, 626–639. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, J.; Rao, S.; Guo, S.; Shen, J.; Du, F.; Wu, X.; Chen, Y.; Li, M.; Chen, M.; et al. Tumor Infiltrating Lymphocyte (TIL) Therapy for Solid Tumor Treatment: Progressions and Challenges. Cancers 2022, 14, 4160. [Google Scholar] [CrossRef]
- Wei, F.; Cheng, X.; Xue, J.; Xue, S. Emerging Strategies in TCR-Engineered T Cells. Front. Immunol. 2022, 13, 850358. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.; Sterner, R. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Mazinani, M.; Rahbarizadeh, F. CAR-T cell potency: From structural elements to vector backbone components. Biomark. Res. 2022, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Lai, Y.; Yuan, W.; Li, S.; Liu, X.; Xiao, Z.; Xiao, H. mRNA-based chimeric antigen receptor T cell therapy: Basic principles, recent advances and future directions. Interdiscip. Med. 2024, 2, e20230036. [Google Scholar] [CrossRef]
- Billingsley, M.M.; Gong, N.; Mukalel, A.J.; Thatte, A.S.; El-Mayta, R.; Patel, S.K.; Metzloff, A.E.; Swingle, K.L.; Han, X.; Xue, L.; et al. In vivo mRNA CAR T cell engineering via targeted ionizable lipid nanoparticles with extrahepatic tropism. Small 2024, 20, 2304378. [Google Scholar] [CrossRef]
- Ponterio, E.; Haas, T.; De Maria, R. Oncolytic virus and CAR-T cell therapy in solid tumors. Front. Immunol. 2024, 15, 1455163. [Google Scholar] [CrossRef]
- Peter, J.; Toppeta, F.; Trubert, A.; Danhof, S.; Hudecek, M.; Däullary, T. Multi-Targeting CAR-T Cell Strategies to Overcome Immune Evasion in Lymphoid and Myeloid Malignancies. Oncol. Res. Treat. 2025, 48, 265–279. [Google Scholar] [CrossRef]
- Gómez-Melero, S.; Hassouneh, F.; Vallejo-Bermúdez, I.; Agüera-Morales, E.; Solana, R.; Caballero-Villarraso, J. Tandem CAR-T cell therapy: Recent advances and current challenges. Front. Immunol. 2025, 16, 1546172. [Google Scholar] [CrossRef]
- Allogene Therapeutics/A Study to Investigate the Safety and Preliminary Efficacy of ALLO-329, an Allogeneic CAR T-cell Therapy, in Adults with Autoimmune Disease (RESOLUTION), ClinicalTrials.gov. Identifier: NCT07085104. September 2025. Available online: https://clinicaltrials.gov/study/NCT07085104?cond=ALLO-329&rank=1 (accessed on 11 August 2025).
- Calviño, C.; Ceballos, C.; Alfonso, A.; Jauregui, P.; Calleja-Cervantes, M.E.; Martin-Uriz, P.S.; Rodriguez-Marquez, P.; Martin-Mallo, A.; Iglesias, E.; Abizanda, G.; et al. Optimization of universal allogeneic CAR-T cells combining CRISPR and transposon-based technologies for treatment of acute myeloid leukemia. Front. Immunol. 2023, 14, 1270843. [Google Scholar] [CrossRef]
- Khaliulin, M.; Valiullina, A.; Petukhov, A.; Yuan, Y.; Spada, S.; Bulatov, E. Breaking the shield of solid tumors: A combined approach for enhanced efficacy of CAR-T cells. Cancer Immunology. Immunotherapy 2024, 74, 3. [Google Scholar] [CrossRef]
- Qasim, W. Allogeneic CAR T cell therapies for leukemia. Am. J. Hematol. 2019, 94 (Suppl. S1), S50–S54. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, K.; Gottschalk, S.; Talleur, A. Allogeneic CAR cell therapy—More than a pipe dream. Front. Immunol. 2021, 11, 618427. [Google Scholar] [CrossRef]
- Locke, F.; Rossi, J.; Neelapu, S.; Jacobson, C.; Miklos, D.; Ghobadi, A.; Oluwole, O.O.; Reagan, P.M.; Lekakis, L.J.; Lin, Y.; et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020, 4, 4898–4911. [Google Scholar] [CrossRef]
- Ghassemi, S.; Nunez-Cruz, S.; O’COnnor, R.S.; Fraietta, J.A.; Patel, P.R.; Scholler, J.; Barrett, D.M.; Lundh, S.M.; Davis, M.M.; Bedoya, F.; et al. Reducing Ex Vivo Culture Improves the Antileukemic Activity of Chimeric Antigen Receptor (CAR) T Cells. Cancer Immunol. Res. 2018, 6, 1100–1109. [Google Scholar] [CrossRef]
- Singh, N.; Perazzelli, J.; Grupp, S.; Barrett, D. Early memory phenotypes drive T cell proliferation in patients with pediatric malignancies. Sci. Transl Med. 2016, 8, 320ra3. [Google Scholar] [CrossRef]
- Metelo, A.M.; Jozwik, A.; Luong, L.A.; Dominey-Foy, D.; Graham, C.; Attwood, C.; Inam, S.; Dunlop, A.; Sanchez, K.; Cuthill, K.; et al. Allogeneic Anti-BCMA CAR T Cells Are Superior to Multiple Myeloma-derived CAR T Cells in Preclinical Studies and May Be Combined with Gamma Secretase Inhibitors. Cancer Res. Commun. 2022, 2, 158–171. [Google Scholar] [CrossRef]
- Tyagarajan, S.; Spencer, T.; Smith, J. Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials. Mol. Ther. Methods Clin. Dev. 2019, 16, 136–144. [Google Scholar] [CrossRef]
- Ayala Ceja, M.; Khericha, M.; Harris, C.; Puig-Saus, C.; Chen, Y. CAR-T cell manufacturing: Major process parameters and next-generation strategies. J. Exp. Med. 2024, 221, e20230903. [Google Scholar] [CrossRef]
- Kasakovski, D.; Xu, L.; Li, Y. T cell senescence and CAR-T cell exhaustion in hematological malignancies. J. Hematol. Oncol. 2018, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Shokati, A.; Sanjari-Pour, M.; Akhavan Rahnama, M.; Hoseinzadeh, S.; Vaezi, M.; Ahmadvand, M. Allogeneic CART progress: Platforms, current progress and limitations. Front. Immunol. 2025, 16, 1557157. [Google Scholar] [CrossRef] [PubMed]
- Diorio, C.; Teachey, D.; Grupp, S. Allogeneic chimeric antigen receptor cell therapies for cancer: Progress made and remaining roadblocks. Nat. Rev. Clin. Oncol. 2025, 22, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Sung, A.; Chao, N. Concise review: Acute graft-versus-host disease: Immunobiology, prevention, and treatment. Stem Cells Transl. Med. 2013, 2, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Aftab, B.; Sasu, B.; Krishnamurthy, J.; Gschweng, E.; Alcazer, V.; Depil, S. Toward “off-the-shelf” allogeneic CAR T cells. Adv. Cell Gene Ther. 2020, 3, e86. [Google Scholar] [CrossRef]
- Yang, Y.; Bi, X.; Gergis, M.; Yi, D.; Hsu, J.; Gergis, U. Allogeneic chimeric antigen receptor T cells for hematologic malignancies. Hematol. Oncol. Stem Cell Ther. 2022, 15, 112–116. [Google Scholar] [CrossRef]
- Locke, F.; Lekakis, L.; Eradat, H.; Munoz, J.; Tees, M.; Vos, S. Phase 1 results with anti-CD19 allogeneic CAR T ALLO-501/501A in relapsed/refractory large B-cell lymphoma (r/r LBCL). J. Clin. Oncol. 2023, 41, 2517. [Google Scholar] [CrossRef]
- Hua, J.; Zhang, J.; Wu, X.; Zhou, L.; Bao, X.; Han, Y. Allogeneic Donor-Derived Anti-CD19 CAR T Cell Is a Promising Therapy for Relapsed/Refractory B-ALL After Allogeneic Hematopoietic Stem-Cell Transplantation. Clin. Lymphoma Myeloma Leuk. 2020, 20, 610–616. [Google Scholar] [CrossRef]
- Jiang, H.; Fu, D.; Bidgoli, A.; Paczesny, S. T cell subsets in graft versus host disease and graft versus tumor. Front. Immunol. 2021, 12, 761448. [Google Scholar] [CrossRef]
- Charmetant, X.; Pettigrew, G.J.; Thaunat, O. Allorecognition Unveiled: Integrating Recent Breakthroughs into the Current Paradigm. Transpl. Int. 2024, 37, 13523. [Google Scholar] [CrossRef]
- Dwyer, G.K.; Mathews, L.R.; Villegas, J.A.; Lucas, A.; de Peredo, A.G.; Blazar, B.R.; Girard, J.-P.; Poholek, A.C.; Luther, S.A.; Shlomchik, W.; et al. IL-33 acts as a costimulatory signal to generate alloreactive Th1 cells in graft-versus-host disease. J. Clin. Invest. 2022, 132, e150927. [Google Scholar] [CrossRef]
- Malard, F.; Holler, E.; Sandmaier, B.; Huang, H.; Mohty, M. Acute graft-versus-host disease. Nat. Rev. Dis. Primers 2023, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castillo, C.; Escobar, A.; García-Gómez, M.; Bachelet, V.C.; Huidobro-Toro, J.P.; Sauma, D.; Barrera-Avalos, C. P2X7 Receptor in Dendritic Cells and Macrophages: Implications in Antigen Presentation and T Lymphocyte Activation. Int. J. Mol. Sci. 2024, 25, 2495. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R.; Blazar, B.R. Acute graft-versus-host disease—Biologic process, prevention, and therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef]
- Rosenberg, N.; Kang, J. Genetic diversity and societally important disparities. Genetics 2015, 201, 1–12. [Google Scholar] [CrossRef]
- Sasu, B.; Lauron, E.; Schulz, T.; Cheng, H.-Y.; Sommer, C. Allogeneic CAR T Cell Therapy for Cancer. Annu. Rev. Cancer Biol. 2023, 8, 227–243. [Google Scholar] [CrossRef]
- Lu, Y.; Waller, E. Dichotomous Role of Interferon-γ in Allogeneic Bone Marrow Transplant. Biol. Blood Marrow Transplant. 2009, 15, 1347–1353. [Google Scholar] [CrossRef]
- Carlson, M.; West, M.; Coghill, J.; Panoskaltsis-Mortari, A.; Blazar, B.; Serody, J. In vitro–differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood 2009, 113, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chu, J.; Yu, J.; Wei, W. Cellular and molecular mechanisms in graft-versus-host disease. J. Leukoc. Biol. 2016, 99, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Martínez Bedoya, D.; Dutoit, V.; Migliorini, D. Allogeneic CAR T cells: An alternative to overcome challenges of CAR T cell therapy in glioblastoma. Front. Immunol. 2021, 12, 640082. [Google Scholar] [CrossRef]
- Duneton, C.; Pamela, D.; Mandy, L. Activation and regulation of alloreactive T cell immunity in solid organ transplantation. Nat. Rev. Nephrol. 2022, 18, 663–676. [Google Scholar] [CrossRef]
- Ghosh, A.; Smith, M.; James, S.; Davila, M.; Velardi, E.; Argyropoulos, K. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat Med. 2017, 23, 242–249. [Google Scholar] [CrossRef]
- Chong, A. B cells as antigen-presenting cells in transplantation rejection and tolerance. Cell. Immunol. 2020, 349, 104061. [Google Scholar] [CrossRef]
- Li, Y.; Fang, Y.; Niu, S.; Chen, Y.; Lyu, Z.; Yang, L. Managing allorejection in off-the-shelf CAR-engineered cell therapies. Mol. Ther. 2024, 33, 2368–2390. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.; Valenzuela, N.; Reed, E. The perfect storm: HLA antibodies, complement, FcγRs, and endothelium in transplant rejection. Trends Mol. Med. 2015, 21, 319–329. [Google Scholar] [CrossRef]
- Smirnov, S.; Petukhov, A.; Levchuk, K.; Kulemzin, S.; Staliarova, A.; Lepik, K. Strategies to Circumvent the Side-Effects of Immunotherapy Using Allogeneic CAR-T Cells and Boost Its Efficacy: Results of Recent Clinical Trials. Front. Immunol. 2021, 12, 780145. [Google Scholar] [CrossRef] [PubMed]
- Maldini, C.; Ellis, G.; Riley, J. CAR T cells for infection, autoimmunity and allotransplantation. Nat. Rev. Immunol. 2018, 18, 605–616. [Google Scholar] [CrossRef]
- Chen, S.; Brink, M. Allogeneic “Off-the-Shelf” CAR T cells: Challenges and advances. Best Pract. Res. Clin. Haematol. 2024, 37, 101566. [Google Scholar] [CrossRef]
- Patel, K.; Tariveranmoshabad, M.; Kadu, S.; Shobaki, N.; June, C. From concept to cure: The evolution of CAR-T cell therapy. Mol. Ther. 2025, 33, 2123–2140. [Google Scholar] [CrossRef]
- Obstfeld, A.; Frey, N.; Mansfield, K.; Lacey, S.; June, C.; Porter, D. Cytokine release syndrome associated with chimeric-antigen receptor T-cell therapy: Clinicopathological insights. Blood 2017, 130, 2569–2572. [Google Scholar] [CrossRef]
- Guha, T.; Wai, A.; Hausner, G. Programmable Genome Editing Tools and their Regulation for Efficient Genome Engineering. Comput. Struct. Biotechnol. J. 2017, 15, 146–160. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Cheng, C.; Cheng, A.; Zhang, X.; Li, N.; Xia, C.; Wei, X.; Liu, X.; Wang, H. CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res. 2017, 27, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Lonez, C.; Breman, E. Allogeneic CAR-T Therapy Technologies: Has the Promise Been Met? Cells 2024, 13, 146. [Google Scholar] [CrossRef] [PubMed]
- Asmamaw, M.; Zawdie, B. Mechanism and Applications of CRISPR/Cas-9-Mediated Genome Editing. Biologics 2021, 15, 353–361. [Google Scholar] [CrossRef]
- Chen, X.; Tan, B.; Xing, H.; Zhao, X.; Ping, Y.; Zhang, Z.; Huang, J.; Shi, X.; Zhang, N.; Lin, B.; et al. Allogeneic CAR-T cells with of HLA-A/B and TRAC disruption exhibit promising antitumor capacity against B cell malignancies. Cancer Immunol. Immunother. 2024, 73, 13. [Google Scholar] [CrossRef]
- Li, W.; Zhu, X.; Xu, Y.; Chen, J.; Zhang, H.; Yang, Z.; Qi, Y.; Hong, J.; Li, Y.; Wang, G.; et al. Simultaneous editing of TCR, HLA-I/II and HLA-E resulted in enhanced universal CAR-T resistance to allo-rejection. Front. Immunol. 2022, 13, 1052717. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, B.; Wu, Z.; Bo, J.; Tong, C.; Chen, D.; Wang, J.; Wang, H.; Wang, Y.; Han, W. Mutant B2M-HLA-E and B2M-HLA-G fusion proteins protects universal chimeric antigen receptor-modified T cells from allogeneic NK cell-mediated lysis. Eur. J. Immunol. 2021, 51, 2513–2521. [Google Scholar] [CrossRef]
- Winterhalter, P.; Warmuth, L.; Hilgendorf, P.; Schütz, J.; Dötsch, S.; Tonn, T.; Cicin-Sain, L.; Busch, D.H.; Schober, K. HLA reduction of human T cells facilitates generation of immunologically multicompatible cellular products. Blood Adv. 2024, 8, 3416–3426. [Google Scholar] [CrossRef]
- Mariuzza, R.; Agnihotri, P.; Orban, J. The structural basis of T-cell receptor (TCR) activation: An enduring enigma. J. Biol. Chem. 2020, 295, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Eyquem, J.; Mansilla-Soto, J.; Giavridis, T.; Stegen, S.; Hamieh, M.; Cunanan, K.; Odak, A.; Gönen, M.; Sadelain, M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 2017, 543, 113–117. [Google Scholar] [CrossRef]
- Gilham, D.; Michaux, A.; Breman, E.; Mauen, S.; Bolsée, J.; Huberty, F.; Marijsse, J.; Violle, B.; Jacques-Hespel, C.; Marchand, C.; et al. TCR inhibitory molecule as a promising allogeneic NKG2D CAR-t cell approach. J. Clin. Oncol. 2018, 36, e15042. [Google Scholar] [CrossRef]
- Razeghian, E.; Nasution, M.; Rahman, H.; Gardanova, Z.; Abdelbasset, W.; Aravindhan, S.; Bokov, D.O.; Suksatan, W.; Nakhaei, P.; Shariatzadeh, S.; et al. A deep insight into CRISPR/Cas9 application in CAR-T cell-based tumor immunotherapies. Stem Cell Res Ther. 2021, 12, 428. [Google Scholar] [CrossRef]
- Ren, J.; Liu, X.; Fang, C.; Jiang, S.; June, C.; Zhao, Y. Multiplex Genome Editing to Generate Universal CAR T Cells Resistant to PD1 Inhibition. Clin. Cancer Res. 2017, 23, 2255–2266. [Google Scholar] [CrossRef]
- Lu, X.A.; He, T.; Qi, F.F.; Liu, G.H.; Xu, W.P.; Wang, T.X.; Hu, X.L. Reagents and Methods for Knocking out TRAC and B2M in T Cells (China Patent No. 116,622,712A). Beijing Yimiao Shenzhou Pharmaceutical Technology Co Ltd. 2023. Available online: https://patents.google.com/patent/CN116622712A/en?oq=CN116622712A (accessed on 1 July 2025).
- McGuirk, J.; Tam, C.; Kröger, N.; Riedell, P.; Murthy, H.; Ho, P.; Maakaron, J.E.; Waller, E.K.; Awan, F.T.; Shaughnessy, P.J.; et al. CTX110 allogeneic CRISPR-Cas9-engineered CAR T cells in patients (Pts) with relapsed or refractory (R/R) large B-cell lymphoma (LBCL): Results from the phase 1 dose escalation carbon study. Blood 2022, 140 (Suppl. S1), 10303–10306. [Google Scholar] [CrossRef]
- Valiullina, A.; Zmievskaya, E.; Ganeeva, I.; Zhuravleva, M.; Garanina, E.; Rizvanov, A.; Petukhov, A.; Bulatov, E. Evaluation of CAR-T cells’ cytotoxicity against modified solid tumor cell lines. Biomedicines 2023, 11, 626. [Google Scholar] [CrossRef]
- Valiullina, A.; Zmievskaya, E.; Ganeeva, I.; Zhuravleva, M.; Garanina, E.; Rizvanov, A.; Petukhov, A.; Bulatov, E. Cytotoxic effect of CAR-T cells against modified MCF-7 breast cancer cell line. Mol. Biol. Res. Commun. 2023, 12, 139. [Google Scholar] [CrossRef]
- Sentman, C.; Meehan, K. NKG2D CARs as Cell Therapy for Cancer. Cancer J. 2014, 20, 156–159. [Google Scholar] [CrossRef]
- CytoMed Therapeutics Pte Ltd. Allogeneic NKG2DL-targeting CAR γδ T Cells (CTM-N2D) in Advanced Cancers (ANGELICA), ClinicalTrials.gov. Identifier: NCT05302037. 22 November 2024. Available online: https://clinicaltrials.gov/study/NCT05302037 (accessed on 26 August 2025).
- Instituto de Investigación Hospital Universitario La Paz. Phase I Clinical Trial of CART Cell Therapy for Refractory/Relapsed Acute Lymphoblastic Leukemia in Children, Adolescents and Young Adults, ClinicalTrials.gov. Identifier: NCT06709469. 29 November 2024. Available online: https://clinicaltrials.gov/study/NCT06709469 (accessed on 30 June 2025).
- Michaux, A.; Mauën, S.; Breman, E.; Dheur, M.-S.; Twyffels, L.; Saerens, L.; Jacques-Hespel, C.; Gauthy, E.; Agaugué, S.; Gilham, D.E.; et al. Clinical Grade Manufacture of CYAD-101, a NKG2D-based, First in Class, Non–Gene-edited Allogeneic CAR T-Cell Therapy. J. Immunother. 2022, 45, 150–161. [Google Scholar] [CrossRef] [PubMed]
- CytoMed Therapeutics Pte Ltd. Haplo/Allogeneic NKG2DL-Targeting Chimeric Antigen Receptor-Grafted γδ T Cells for Relapsed or Refractory Solid Tumour, ClinicalTrials.gov. Identifier: NCT04107142. 27 September 2019. Available online: https://clinicaltrials.gov/study/NCT04107142 (accessed on 30 June 2025).
- Mansoori, S.; Noei, A.; Maali, A.; Seyed-Motahari, S.; Sharifzadeh, Z. Recent updates on allogeneic CAR-T cells in hematological malignancies. Cancer Cell Int. 2024, 24, 304. [Google Scholar] [CrossRef] [PubMed]
- Abou-el-Enein, M.; Elsallab, M.; Feldman, S.A.; Fesnak, A.D.; Heslop, H.E.; Marks, P.; Brian, G.T.; Bauer, G.; Savoldo, B. Scalable manufacturing of CAR T cells for cancer immunotherapy. Blood Cancer Discov. 2021, 2, 408–422. [Google Scholar] [CrossRef] [PubMed]
| ZFN | TALEN | CRISPR/Cas9 | |
|---|---|---|---|
| Recognition site | Zinc finger protein | TALE protein | GuideRNA and tracrRNA |
| Modification pattern | Fok1 nuclease | Fok1 nuclease | Cas9 nuclease |
| Specificity | Small number of positional mismatches | Small number of positional mismatches | Positional/multiple consecutive mismatches |
| Target sequence size | 9–18 bp | 14–20 bp | 20 bp- guide + PAM sequence |
| Targeting limitations | Difficult to target non-G-rich sites | 5′ targeted base must be a T for each TALEN monomer | Recognizes 3′ G-rich Must precede a PAM sequence of 3–5 nt |
| Engineering | Requires substantial protein engineering | Requires complex molecular cloning methods | Uses standard cloning procedures |
| Delivery | Easy due to small size | Difficult due to large size | Moderate to difficult due to large size of SpCas9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhametshin, S.A.; Gilyazova, E.M.; Davletshin, D.R.; Ganeeva, I.A.; Zmievskaya, E.A.; Chasov, V.V.; Petukhov, A.V.; Valiullina, A.K.; Spada, S.; Bulatov, E.R. Allogeneic NKG2D CAR-T Cell Therapy: A Promising Approach for Treating Solid Tumors. Biomedicines 2025, 13, 2314. https://doi.org/10.3390/biomedicines13092314
Mukhametshin SA, Gilyazova EM, Davletshin DR, Ganeeva IA, Zmievskaya EA, Chasov VV, Petukhov AV, Valiullina AK, Spada S, Bulatov ER. Allogeneic NKG2D CAR-T Cell Therapy: A Promising Approach for Treating Solid Tumors. Biomedicines. 2025; 13(9):2314. https://doi.org/10.3390/biomedicines13092314
Chicago/Turabian StyleMukhametshin, Sabir A., Elvina M. Gilyazova, Damir R. Davletshin, Irina A. Ganeeva, Ekaterina A. Zmievskaya, Vitaly V. Chasov, Alexsei V. Petukhov, Aigul Kh. Valiullina, Sheila Spada, and Emil R. Bulatov. 2025. "Allogeneic NKG2D CAR-T Cell Therapy: A Promising Approach for Treating Solid Tumors" Biomedicines 13, no. 9: 2314. https://doi.org/10.3390/biomedicines13092314
APA StyleMukhametshin, S. A., Gilyazova, E. M., Davletshin, D. R., Ganeeva, I. A., Zmievskaya, E. A., Chasov, V. V., Petukhov, A. V., Valiullina, A. K., Spada, S., & Bulatov, E. R. (2025). Allogeneic NKG2D CAR-T Cell Therapy: A Promising Approach for Treating Solid Tumors. Biomedicines, 13(9), 2314. https://doi.org/10.3390/biomedicines13092314







