Calprotectin, Azurocidin, and Interleukin-8: Neutrophil Signatures with Diagnostic and Prognostic Value in Sepsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Serum Biomarker Assessment
2.2. Statistical Analysis
3. Results
3.1. Serum Biomarkers
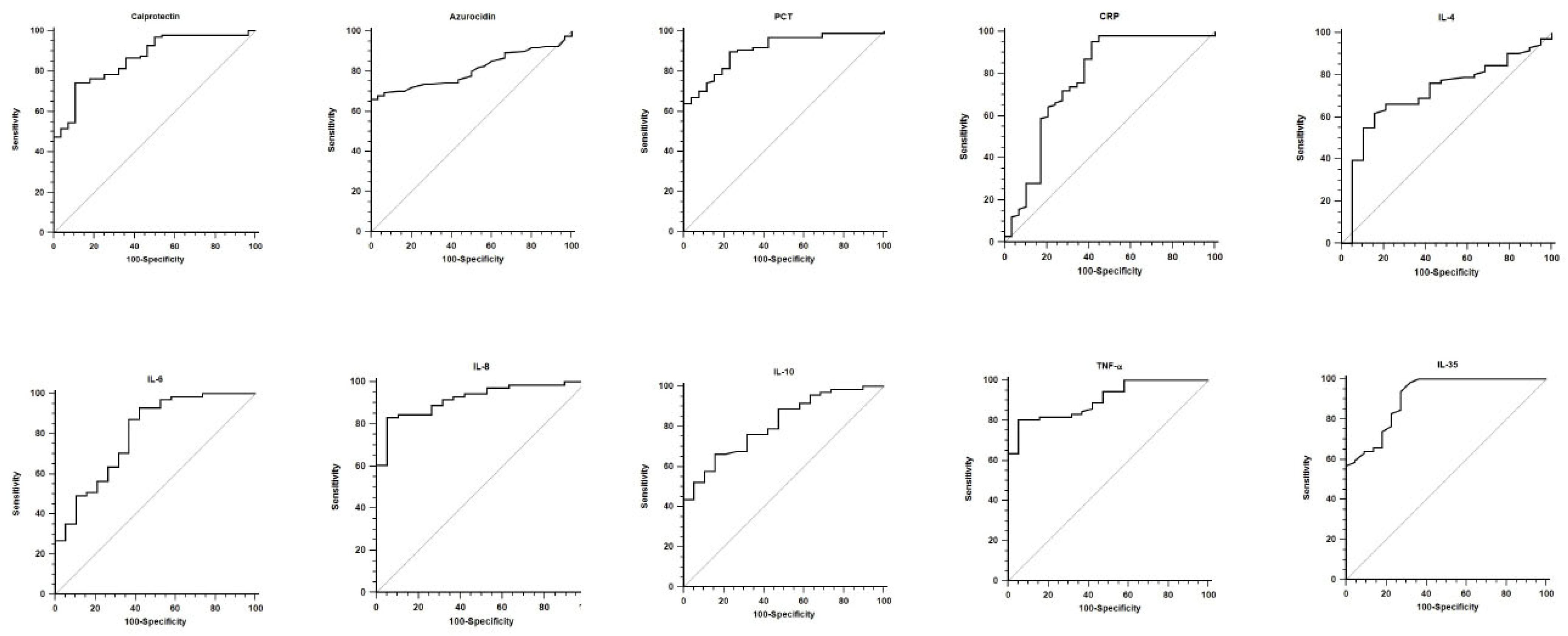
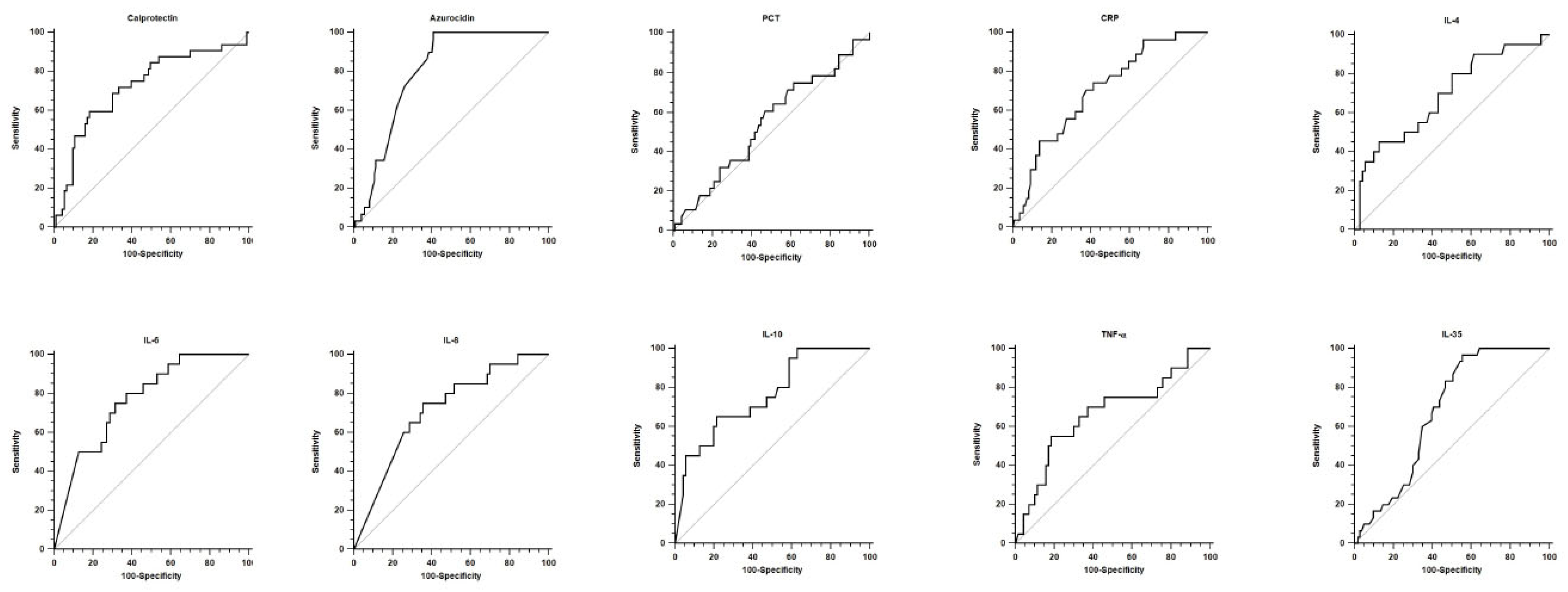
3.2. Diagnostic and Prognostic Values
4. Discussion
Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Povoa, P.; Coelho, L.; Dal-Pizzol, F.; Ferrer, R.; Huttner, A.; Conway Morris, A.; Nobre, V.; Ramirez, P.; Rouze, A.; Salluh, J.; et al. How to use biomarkers of infection or sepsis at the bedside: Guide to clinicians. Intensive Care Med. 2023, 49, 142–153. [Google Scholar] [CrossRef]
- Hung, S.K.; Lan, H.M.; Han, S.T.; Wu, C.C.; Chen, K.F. Current evidence and limitation of biomarkers for detecting sepsis and systemic infection. Biomedicines 2020, 8, 494. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, J.; Guo, F.; Longhini, F.; Gao, Z.; Huang, Y.; Qiu, H. Combination of c-reactive protein, procalcitonin and sepsis-related organ failure score for the diagnosis of sepsis in critical patients. Ann. Intensive Care 2016, 6, 51. [Google Scholar] [CrossRef]
- Taha, A.M.; Najah, Q.; Omar, M.M.; Abouelmagd, K.; Ali, M.; Hasan, M.T.; Allam, S.A.; Hamam, Y.A.; Arian, R.; Abd-ElGawad, M. Diagnostic and prognostic value of heparin-binding protein in sepsis: A systematic review and meta-analysis. Medicine 2024, 103, e38525. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.Y.; Jia, H.M.; Han, Y.Z.; Qian, B.S.; You, P.; Zhang, X.K.; Li, W.X.; Huang, L.F. Calprotectin as a diagnostic marker for sepsis: A meta-analysis. Front. Cell. Infect. Microbiol. 2022, 12, 1045636. [Google Scholar] [CrossRef]
- Eggers, K.; Sikora, K.; Lorenz, M.; Taubert, T.; Moobed, M.; Baumann, G.; Stangl, K.; Stangl, V. Rage-dependent regulation of calcium-binding proteins s100a8 and s100a9 in human thp-1. Exp. Clin. Endocrinol. Diabetes 2011, 119, 353–357. [Google Scholar] [CrossRef]
- Edgeworth, J.; Gorman, M.; Bennett, R.; Freemont, P.; Hogg, N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J. Biol. Chem. 1991, 266, 7706–7713. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The accp/sccm consensus conference committee. American college of chest physicians/society of critical care medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Tinsley, K.W.; Swanson, P.E.; Schmieg, R.E., Jr.; Hui, J.J.; Chang, K.C.; Osborne, D.F.; Freeman, B.D.; Cobb, J.P.; Buchman, T.G.; et al. Sepsis-induced apoptosis causes progressive profound depletion of b and cd4+ t lymphocytes in humans. J. Immunol. 2001, 166, 6952–6963. [Google Scholar] [CrossRef]
- Shen, X.; Cao, K.; Zhao, Y.; Du, J. Targeting neutrophils in sepsis: From mechanism to translation. Front. Pharmacol. 2021, 12, 644270. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tang, D.; Zhang, P. The diagnostic value of interleukin 35 as a septic biomarker: A meta-analysis. Front. Med. 2022, 9, 999892. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, M.; Fu, Y.J.; Jiang, Y.J.; Zhang, Z.N. Alterations in levels of cytokine following treatment to predict outcome of sepsis: A meta-analysis. Cytokine 2023, 161, 156056. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Baddam, S.; Burns, B. Systemic Inflammatory Response Syndrome; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Cione, E.; Siniscalchi, A.; Gangemi, P.; Cosco, L.; Colosimo, M.; Longhini, F.; Luciani, F.; De Sarro, G.; Berrino, L.; D’Agostino, B.; et al. Neuron-specific enolase serum levels in COVID-19 are related to the severity of lung injury. PLoS ONE 2021, 16, e0251819. [Google Scholar] [CrossRef] [PubMed]
- Perticone, M.; Zito, R.; Miceli, S.; Pinto, A.; Suraci, E.; Greco, M.; Gigliotti, S.; Hribal, M.L.; Corrao, S.; Sesti, G.; et al. Immunity, inflammation and heart failure: Their role on cardiac function and iron status. Front. Immunol. 2019, 10, 2315. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Marin, M.J.; van Wijk, X.M.R.; Chambliss, A.B. Advances in sepsis biomarkers. Adv. Clin. Chem. 2024, 119, 117–166. [Google Scholar] [CrossRef]
- Li, J.; Xiao, C.; Zheng, H. Prognostic value of inflammatory cytokine detection for sepsis patients in icu: A meta-analysis. Am. J. Transl. Res. 2024, 16, 2612–2621. [Google Scholar] [CrossRef]
- Song, J.; Moon, S.; Park, D.W.; Cho, H.J.; Kim, J.Y.; Park, J.; Cha, J.H. Biomarker combination and sofa score for the prediction of mortality in sepsis and septic shock: A prospective observational study according to the sepsis-3 definitions. Medicine 2020, 99, e20495. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; She, F.; Zhang, W.; Liu, H.; Zhao, X. Effects of neutrophil-to-lymphocyte ratio combined with interleukin-6 in predicting 28-day mortality in patients with sepsis. Front. Immunol. 2021, 12, 639735. [Google Scholar] [CrossRef]
- Behnes, M.; Bertsch, T.; Lepiorz, D.; Lang, S.; Trinkmann, F.; Brueckmann, M.; Borggrefe, M.; Hoffmann, U. Diagnostic and prognostic utility of soluble cd 14 subtype (presepsin) for severe sepsis and septic shock during the first week of intensive care treatment. Crit. Care 2014, 18, 507. [Google Scholar] [CrossRef]
- Schrijver, D.P.; Roring, R.J.; Deckers, J.; de Dreu, A.; Toner, Y.C.; Prevot, G.; Priem, B.; Munitz, J.; Nugraha, E.G.; van Elsas, Y.; et al. Resolving sepsis-induced immunoparalysis via trained immunity by targeting interleukin-4 to myeloid cells. Nat. Biomed. Eng. 2023, 7, 1097–1112. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Kong, L.; Fink, M.P.; Weissfeld, L.A.; Yealy, D.M.; Pinsky, M.R.; Fine, J.; Krichevsky, A.; Delude, R.L.; Angus, D.C. Understanding the inflammatory cytokine response in pneumonia and sepsis: Results of the genetic and inflammatory markers of sepsis (genims) study. Arch. Intern. Med. 2007, 167, 1655–1663. [Google Scholar] [CrossRef]
- Matera, G.; Puccio, R.; Giancotti, A.; Quirino, A.; Pulicari, M.C.; Zicca, E.; Caroleo, S.; Renzulli, A.; Liberto, M.C.; Foca, A. Impact of interleukin-10, soluble cd25 and interferon-gamma on the prognosis and early diagnosis of bacteremic systemic inflammatory response syndrome: A prospective observational study. Crit. Care 2013, 17, R64. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Long, G.; Luo, H.; Zhu, X.; Han, Y.; Shang, Y.; Zhang, D.; Gong, R. S100a8/a9: An emerging player in sepsis and sepsis-induced organ injury. Biomed. Pharmacother. 2023, 168, 115674. [Google Scholar] [CrossRef]
- Liu, D.; Huang, S.Y.; Sun, J.H.; Zhang, H.C.; Cai, Q.L.; Gao, C.; Li, L.; Cao, J.; Xu, F.; Zhou, Y.; et al. Sepsis-induced immunosuppression: Mechanisms, diagnosis and current treatment options. Mil. Med. Res. 2022, 9, 56. [Google Scholar] [CrossRef]
- Nedeva, C.; Menassa, J.; Duan, M.; Liu, C.; Doerflinger, M.; Kueh, A.J.; Herold, M.J.; Fonseka, P.; Phan, T.K.; Faou, P.; et al. Treml4 receptor regulates inflammation and innate immune cell death during polymicrobial sepsis. Nat. Immunol. 2020, 21, 1585–1596. [Google Scholar] [CrossRef]
- Venet, F.; Monneret, G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 2018, 14, 121–137. [Google Scholar] [CrossRef]
- Jakobsson, G.; Papareddy, P.; Andersson, H.; Mulholland, M.; Bhongir, R.; Ljungcrantz, I.; Engelbertsen, D.; Bjorkbacka, H.; Nilsson, J.; Manea, A.; et al. Therapeutic s100a8/a9 blockade inhibits myocardial and systemic inflammation and mitigates sepsis-induced myocardial dysfunction. Crit. Care 2023, 27, 374. [Google Scholar] [CrossRef]
- Riva, M.; Kallberg, E.; Bjork, P.; Hancz, D.; Vogl, T.; Roth, J.; Ivars, F.; Leanderson, T. Induction of nuclear factor-κb responses by the s100a9 protein is toll-like receptor-4-dependent. Immunology 2012, 137, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xu, F.; Lin, S.; Tao, X.; Xiang, Y.; Lai, X.; Zhang, L. Il-35 is elevated in clinical and experimental sepsis and mediates inflammation. Clin. Immunol. 2015, 161, 89–95. [Google Scholar] [CrossRef]
- Hack, C.E.; Hart, M.; van Schijndel, R.J.; Eerenberg, A.J.; Nuijens, J.H.; Thijs, L.G.; Aarden, L.A. Interleukin-8 in sepsis: Relation to shock and inflammatory mediators. Infect. Immun. 1992, 60, 2835–2842. [Google Scholar] [CrossRef]
- Holub, M.; Dzupova, O.; Ruzkova, M.; Stranikova, A.; Bartakova, E.; Maca, J.; Benes, J.; Herwald, H.; Beran, O. Selected biomarkers correlate with the origin and severity of sepsis. Mediat. Inflamm. 2018, 2018, 7028267. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Li, X.; Wang, L.; Yuan, H.; Liao, Z.; Zhou, S.; Wu, J.; Guan, X.; Liu, Y. Heparin-binding protein as a biomarker for the diagnosis of sepsis in the intensive care unit: A retrospective cross-sectional study in china. BMJ Open 2024, 14, e078687. [Google Scholar] [CrossRef]
- Wu, Y.L.; Yo, C.H.; Hsu, W.T.; Qian, F.; Wu, B.S.; Dou, Q.L.; Lee, C.C. Accuracy of heparin-binding protein in diagnosing sepsis: A systematic review and meta-analysis. Crit. Care Med. 2021, 49, e80–e90. [Google Scholar] [CrossRef]
- Xia, P.; Ji, X.; Yan, L.; Lian, S.; Chen, Z.; Luo, Y. Roles of s100a8, s100a9 and s100a12 in infection, inflammation and immunity. Immunology 2024, 171, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, C.C.; Skaar, E.P. Nutritional immunity: The battle for nutrient metals at the host-pathogen interface. Nat. Rev. Microbiol. 2022, 20, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; Marques, P.; Francisco, V.; Piqueras, L.; Sanz, M.J. Targeting systemic inflammation in metabolic disorders. A therapeutic candidate for the prevention of cardiovascular diseases? Pharmacol. Res. 2024, 200, 107058. [Google Scholar] [CrossRef]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef]
- Lopez-Candales, A.; Hernandez Burgos, P.M.; Hernandez-Suarez, D.F.; Harris, D. Linking chronic inflammation with cardiovascular disease: From normal aging to the metabolic syndrome. J. Nat. Sci. 2017, 3, e341. [Google Scholar]
- Wang, J.; He, L.; Jin, Z.; Lu, G.; Yu, S.; Hu, L.; Fang, M.; Jin, X. Immune dysfunction-associated elevated rdw, apache-ii, and sofa scores were a possible cause of 28-day mortality in sepsis patients. Infect. Drug Resist. 2024, 17, 1199–1213. [Google Scholar] [CrossRef]
- Sa-Couto, C.; Ericsson, C.; Lazarovici, M. Conducting multicenter simulation-based experimental research: Lessons drawn from the quality cpr european project. Resusc. Plus 2025, 25, 101054. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K. Multicenter studies: Relevance, design and implementation. Indian. Pediatr. 2022, 59, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Kessler, D.; Mackinnon, R.; Chang, T.P.; Nadkarni, V.M.; Hunt, E.A.; Duval-Arnould, J.; Lin, Y.; Pusic, M.; Auerbach, M. Conducting multicenter research in healthcare simulation: Lessons learned from the inspire network. Adv. Simul. 2017, 2, 6. [Google Scholar] [CrossRef]
- Hammer, G.P.; du Prel, J.B.; Blettner, M. Avoiding bias in observational studies: Part 8 in a series of articles on evaluation of scientific publications. Dtsch. Arztebl. Int. 2009, 106, 664–668. [Google Scholar] [CrossRef]
- Keller, D.; Mester, P.; Rath, U.; Krautbauer, S.; Schmid, S.; Greifenberg, V.; Muller, M.; Kunst, C.; Buechler, C.; Pavel, V. Calprotectin, a promising serological biomarker for the early diagnosis of superinfections with multidrug-resistant bacteria in patients with COVID-19. Int. J. Mol. Sci. 2024, 25, 9294. [Google Scholar] [CrossRef]
- Garcia de Guadiana-Romualdo, L.; Botella, L.A.; Rodriguez Rojas, C.; Puche Candel, A.; Jimenez Sanchez, R.; Conesa Zamora, P.; Albaladejo-Oton, M.D.; Allegue-Gallego, J.M. Mortality prediction model from combined serial lactate, procalcitonin and calprotectin levels in critically ill patients with sepsis: A retrospective study according to sepsis-3 definition. Med. Intensiv. (Engl. Ed.) 2024, 48, 629–638. [Google Scholar] [CrossRef]

| Control (n = 15) | SIRS (n = 15) | Sepsis_A (n = 92) | Sepsis_D (n = 29) | |
|---|---|---|---|---|
| Age-(yr) | 37.5 ± 15.3 | 59.5 ± 14.0 | 68.7 ± 15.5 | 71.2 ± 13.7 |
| Male sex–n (%) | 3 (20) | 8 (53) | 52 (57) | 14 (48) |
| SOFA score | 0 [0; 0] | 4 [3; 6] | 7 [5; 9] | 10 [8; 12] |
| APACHE-II | 0 [0; 0] | 15 [8; 18] | 18 [12; 21] | 23 [16; 30] |
| Comorbidities-n (%) | ||||
| Chronic Respiratory Failure | 0 (0) | 7 (7) | 3 (10) | |
| Cardiovascular disease | 1 (7) | 18 (19) | 8 (28) | |
| Arterial Hypertension | 4 (27) | 38 (41) | 15 (52) | |
| Diabetes | 2 (13) | 28 (30) | 11 (38) | |
| Others | 0 (0) | 8 (8) | 4 (14) | |
| Admission department-n (%) | ||||
| Intensive Care Unit | 3 (20) | 37 (40) | 26 (90) | |
| Surgical wards | 8 (53) | 21 (23) | 1 (3) | |
| Medical wards | 4 (27) | 34 (37) | 2 (7) | |
| Pathology of admission-n (%) | ||||
| Respiratory | 0 (0) | 47 (51) | 16 (56) | |
| Cardiovascular | 0 (0) | 16 (17) | 7 (24) | |
| Neurologic | 0 (0) | 7 (8) | 2 (7) | |
| Trauma | 10 (67) | 12 (13) | 3 (10) | |
| Others | 5 (33) | 10 (11) | 1 (3) | |
| Organ support requirments-n (%) | ||||
| Mechanical ventilation | 2 (13) | 36 (39) | 26 (90) | |
| Vasoactive drugs | 2 (13) | 35 (38) | 25 (86) | |
| Renal replacement therapy | 1 (7) | 16 (17) | 20 (69) | |
| ECMO | 0 (0) | 1 (1) | 2 (7) | |
| Source of infection–n (%) | ||||
| Lung | 50 (55) | 18 (63) | ||
| Abdomen | 3 (3) | 1 (3) | ||
| Blood | 22 (24) | 6 (21) | ||
| Endocarditis | 14 (15) | 3 (10) | ||
| Urinary tract | 3 (3) | 1 (3) | ||
| Gram-negative pathogen isolations-n (%) | ||||
| Escherichia coli | 27 (30) | 10 (34) | ||
| Klebsiella pneumoniae | 34 (37) | 15 (52) | ||
| Acinetobacter baumannii | 14 (15) | 3 (10) | ||
| Pseudomonas aeruginosa | 14 (15) | 7 (24) | ||
| Others bacilli | 3 (3) | 1 (3) | ||
| Control (n = 15) | SIRS (n = 15) | Sepsis_A (n = 92) | Sepsis_D (n = 29) | Kruskal–Wallis p-Values (H Statistic) | Dunn’s Test p-Values | |
|---|---|---|---|---|---|---|
| Calprotectin (ng/mL) | 3.19 [2.60; 4.88] | 9.12 [7.47; 12.28] | 13.81 [8.69; 22.71] | 24.04 [11.76; 30.83] | <0.001 (H = 43.70) |
|
| Azurocidin (ng/mL) | 0.052 [0.042; 0.060] | 0.056 [0.044; 0.064] | 0.208 [0.052; 0.223] | 0.223 [0.213; 0.246] | <0.001 (H = 41.63) |
|
| PCT (ng/mL) | 0.10 [0.10; 0.20] | 2.92 [1.32; 5.76] | 17.5 [5.35; 36.9] | 10.8 [2.8; 28.6] | <0.001 (H = 46.43) |
|
| CRP (mg/L) | 1 [1; 2] | 114 [76; 230] | 138 [73; 212] | 196 [119; 289] | <0.001 (H = 42.06) |
|
| IL-4 (pg/mL) | 1.28 [1.17; 1.55] | 1.22 [1.01; 1.62] | 1.82 [1.20; 2.89] | 2.43 [1.56; 6.22] | 0.008 (H = 14.53) |
|
| IL-6 (pg/mL) | 1.40 [1.05; 2.58] | 92.7 [18.4; 178.7] | 90.7 [31.5; 449.7] | 502.1 [126.4; 719.0] | <0.001 (H = 32.06) |
|
| IL-8 (pg/mL) | 12.93 [9.10; 17.77] | 57.3 [31.8; 78.7] | 413.5 [116.2; 862.0] | 862.0 [235.9; 862.0] | <0.001 (H = 36.69) |
|
| IL-10 (pg/mL) | 0.55 [0.38; 7.70] | 2.25 [0.99; 4.69] | 9.86 [2.22; 56.2] | 48.4 [4.79; 609.9] | <0.001 (H = 23.50) |
|
| TNF-a (pg/mL) | 2.77 [2.43; 3.96] | 4.22 [3.46; 4.68] | 11.71 [6.92; 22.35] | 23.10 [5.19; 52.7] | <0.001 (H = 22.33) |
|
| IL-35 (pg/mL) | 47.35 [43.30; 53.88] | 43.67 [43.30; 51.40] | 60.12 [52.13; 69.29] | 61.34 [57.68; 67.14] | <0.001 (H = 39.22) |
|
| AUC | p Value | Cut-Off | Sensitivity | Specificity | PLR | NLR | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Diagnostic accuracy | |||||||||
| Calprotectin | 0.864 [0.791–0.919] | <0.001 | 10.04 | 74.2 [64.3–82.6] | 89.3 [71.8–97.7] | 6.93 [2.4–20.3] | 0.29 [0.2–0.4] | 96.0 [88.8–99.2] | 50.0 [35.4–64.6] |
| Azurocidin | 0.808 [0.739–0.867] | <0.001 | 0.102 | 66.1 [57.0–74.5] | 100.0 [88.4–100.0] | // | 0.34 [0.3–0.4] | 100.0 [95.5–100.0] | 42.3 [30.6–54.6] |
| PCT | 0.908 [0.842–0.953] | <0.001 | 1.44 | 89.7 [81.9–94.9] | 76.9 [56.4–91.0] | 3.89 [1.9–7.9] | 0.13 [0.07–0.3] | 93.5 [86.5–97.6] | 66.7 [47.2–82.7] |
| CRP | 0.777 [0.698–0.844] | <0.001 | 20.4 | 95.3 [89.4–98.5] | 58.6 [38.9–76.5] | 2.30 [1.5–3.6] | 0.08 [0.03–0.2] | 89.5 [82.3–94.4] | 77.3 [54.6–92.2] |
| IL-4 | 0.715 [0.610–0.805] | 0.007 | 1.57 | 62.0 [49.7–73.2] | 84.2 [60.4–96.6] | 3.92 [1.4–11.3] | 0.45 [0.3–0.6] | 93.6 [82.3–98.7] | 37.2 [23.0–53.3] |
| IL-6 | 0.799 [0.701–0.876] | <0.001 | 13.79 | 93.0 [84.3–97.7] | 57.9 [33.5–79.7] | 2.21 [1.3–3.8] | 0.12 [0.05–0.3] | 89.2 [79.8–95.2] | 68.7 [41.3–89.0] |
| IL-8 | 0.919 [0.842–0.966] | <0.001 | 92.37 | 83.1 [72.3–91.0] | 94.7 [74.0–99.9] | 15.79 [2.3–106.7] | 0.18 [0.1–0.3] | 98.3 [91.1–100.0] | 60.0 [40.6–77.3] |
| IL-10 | 0.812 [0.716–0.887] | <0.001 | 4.87 | 66.2 [54.0–77.0] | 84.2 [60.4–96.6] | 4.19 [1.5–12.0] | 0.40 [0.3–0.6] | 94.0 [83.5–98.7] | 40.0 [24.9–56.7] |
| TNF-a | 0.903 [0.822–0.955] | <0.001 | 5.41 | 80.3 [69.1–88.8] | 94.7 [74.0–99.9] | 15.25 [2.3–103.1] | 0.21 [0.1–0.3] | 98.3 [90.8–100.0] | 56.2 [37.7–73.6] |
| IL-35 | 0.910 [0.848–0.953] | <0.001 | 48.83 | 93.7 [87.4–97.4] | 72.7 [49.8–89.3] | 3.44 [1.7–6.8] | 0.09 [0.04–0.2] | 94.5 [88.5–98.0] | 69.6 [47.1–86.8] |
| Prognostic accuracy | |||||||||
| Calprotectin | 0.721 [0.634–0.798] | <0.001 | 20.89 | 59.4 [40.6–76.3] | 81.7 [72.4–89.0] | 3.25 [1.9–5.4] | 0.50 [0.3–0.8] | 52.8 [35.2–69.8] | 85.4 [76.3–92.0] |
| Azurocidin | 0.796 [0.723–0.857] | <0.001 | 0.18 | 100.0 [88.1–100.0] | 59.0 [49.7–67.8] | 2.44 [2.0–3.0] | // | 36.7 [26.1–48.3] | 100.0 [95.0–100.0] |
| PCT | 0.544 [0.452–0.634] | 0.484 | 7.04 | 60.7 [40.6–78.5] | 53.1 [42.7–63.4] | 1.30 [0.9–1.9] | 0.74 [0.4–1.2] | 27.4 [16.9–40.2] | 82.3 [70.4–90.9] |
| CRP | 0.702 [0.617–0.777] | <0.001 | 135 | 74.1 [53.7–88.9] | 58.7 [48.9–68.1] | 1.79 [1.3–2.5] | 0.44 [0.2–0.9] | 30.8 [19.9–43.4] | 90.1 [80.7–95.9] |
| IL-4 | 0.689 [0.583–0.782] | 0.007 | 3.34 | 45.0 [23.1–68.5] | 87.1 [77.0–93.9] | 3.50 [1.6–7.6] | 0.63 [0.4–0.9] | 50.9 [26.0–74.0] | 74.7 [74.3–92.1] |
| IL-6 | 0.769 [0.669–0.852] | <0.001 | 142.13 | 75.0 [50.9–91.3] | 68.6 [56.4–79.1] | 2.39 [1.6–3.7] | 0.36 [0.2–0.8] | 40.5 [24.8–57.9] | 90.6 [79.3–96.9] |
| IL-8 | 0.713 [0.608–0.803] | <0.001 | 413.48 | 75.0 [50.9–91.3] | 64.3 [51.9–75.4] | 2.10 [1.4–3.1] | 0.39 [0.2–0.8] | 37.5 [22.7–54.2] | 90.0 [78.2–96.7] |
| IL-10 | 0.759 [0.658–0.843] | <0.001 | 20.17 | 65.0 [40.8–84.6] | 78.6 [67.1–87.5] | 3.03 [1.7–5.3] | 0.45 [0.2–0.8] | 46.4 [27.5–66.1] | 88.7 [78.1–95.3] |
| TNF-a | 0.663 [0.556–0.759] | 0.030 | 19.83 | 55.0 [31.5–76.9] | 81.4 [70.3–89.7] | 2.96 [1.6–5.6] | 0.55 [0.3–0.9] | 45.8 [25.6–67.2] | 86.4 [75.7–93.6] |
| IL-35 | 0.677 [0.590–0.755] | <0.001 | 53.97 | 96.7 [82.8–99.9] | 44.7 [34.9–54.8] | 1.75 [1.5–2.1] | 0.08 [0.0–0.5] | 33.7 [23.9–44.7] | 97.9 [88.7–99.9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gigliotti, S.; Manno, M.; Divenuto, F.; Pavia, G.; Peronace, C.; Trimboli, F.; Zangari, C.; Tancrè, V.; Greco, F.; Colosimo, M.; et al. Calprotectin, Azurocidin, and Interleukin-8: Neutrophil Signatures with Diagnostic and Prognostic Value in Sepsis. Biomedicines 2025, 13, 2673. https://doi.org/10.3390/biomedicines13112673
Gigliotti S, Manno M, Divenuto F, Pavia G, Peronace C, Trimboli F, Zangari C, Tancrè V, Greco F, Colosimo M, et al. Calprotectin, Azurocidin, and Interleukin-8: Neutrophil Signatures with Diagnostic and Prognostic Value in Sepsis. Biomedicines. 2025; 13(11):2673. https://doi.org/10.3390/biomedicines13112673
Chicago/Turabian StyleGigliotti, Simona, Michele Manno, Francesca Divenuto, Grazia Pavia, Cinzia Peronace, Francesca Trimboli, Concetta Zangari, Valentina Tancrè, Francesca Greco, Manuela Colosimo, and et al. 2025. "Calprotectin, Azurocidin, and Interleukin-8: Neutrophil Signatures with Diagnostic and Prognostic Value in Sepsis" Biomedicines 13, no. 11: 2673. https://doi.org/10.3390/biomedicines13112673
APA StyleGigliotti, S., Manno, M., Divenuto, F., Pavia, G., Peronace, C., Trimboli, F., Zangari, C., Tancrè, V., Greco, F., Colosimo, M., Minchella, P., Principe, L., Marascio, N., Licata, F., Bianco, A., Russo, A., Longhini, F., Quirino, A., & Matera, G. (2025). Calprotectin, Azurocidin, and Interleukin-8: Neutrophil Signatures with Diagnostic and Prognostic Value in Sepsis. Biomedicines, 13(11), 2673. https://doi.org/10.3390/biomedicines13112673









