Localization and Expression of Renin–Angiotensin System Receptors in Lung from Transplant Patients: A Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Western Blot Analysis of Pulmonary Ras Receptor Expression
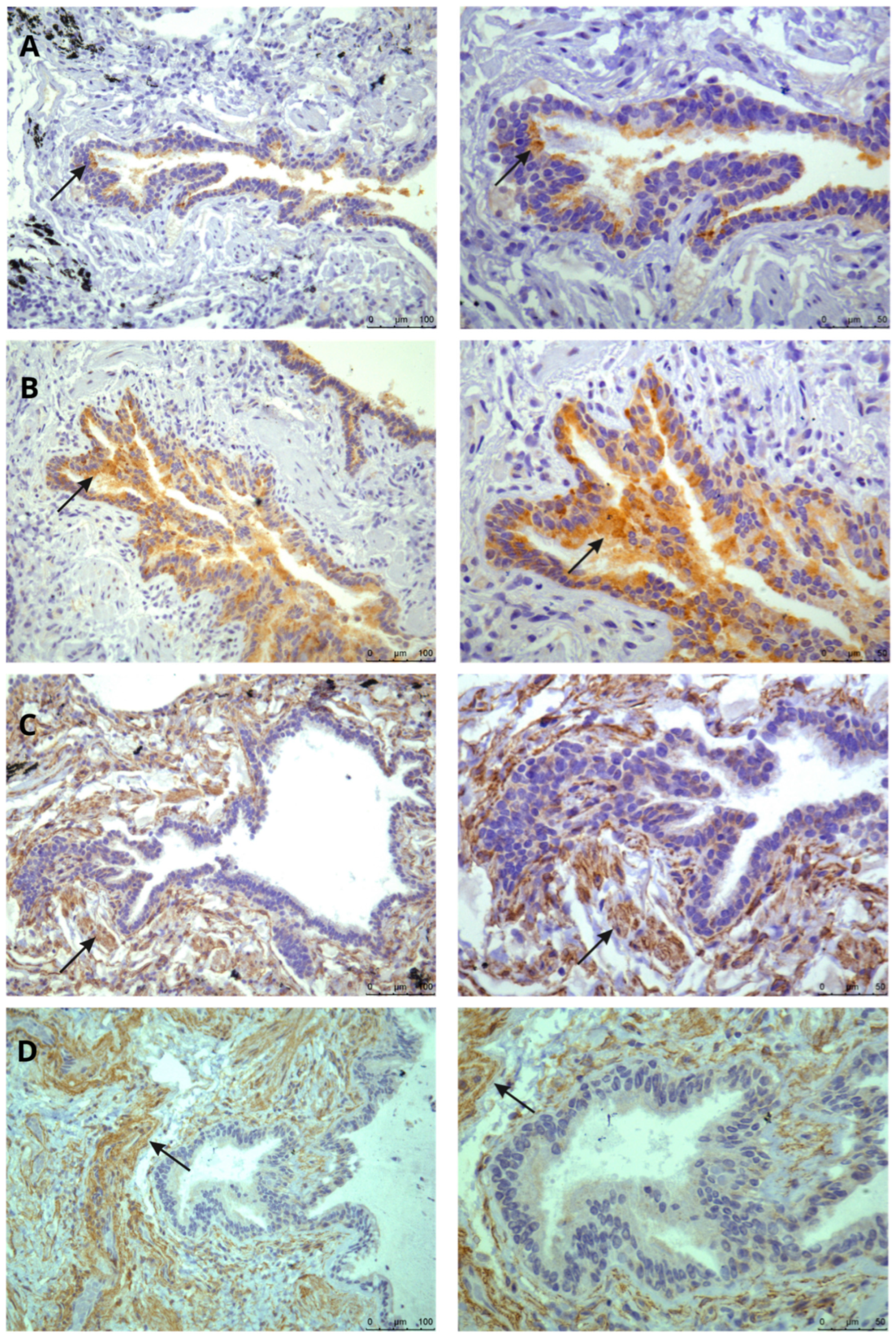
2.2. Immunohistochemical Mapping of RAS Receptors in Lung Tissue
2.3. Determination of Ang I, Ang II, Ang A, Alamandine, and Ang-(1-7) by Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
2.4. Statistical Analysis
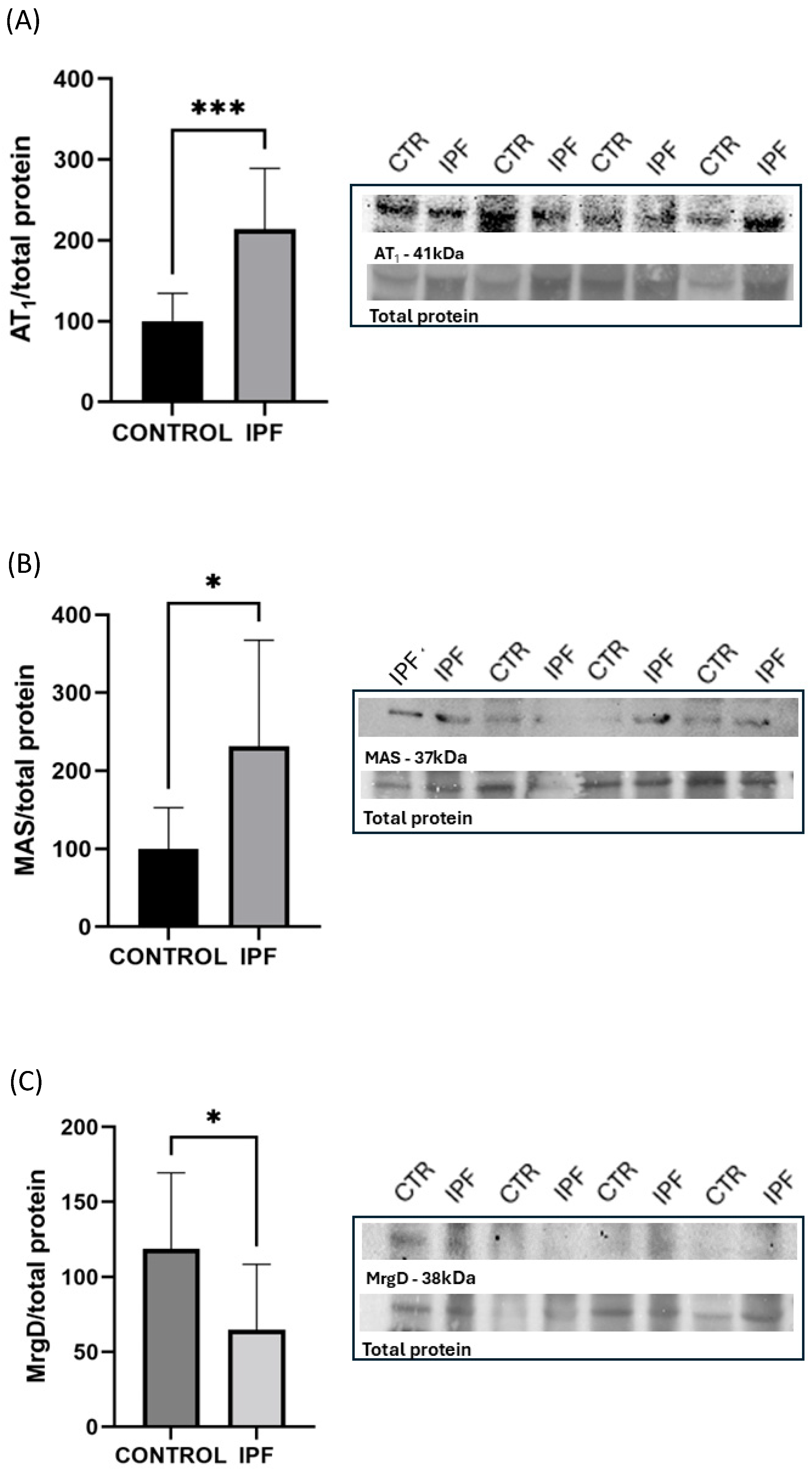
3. Results
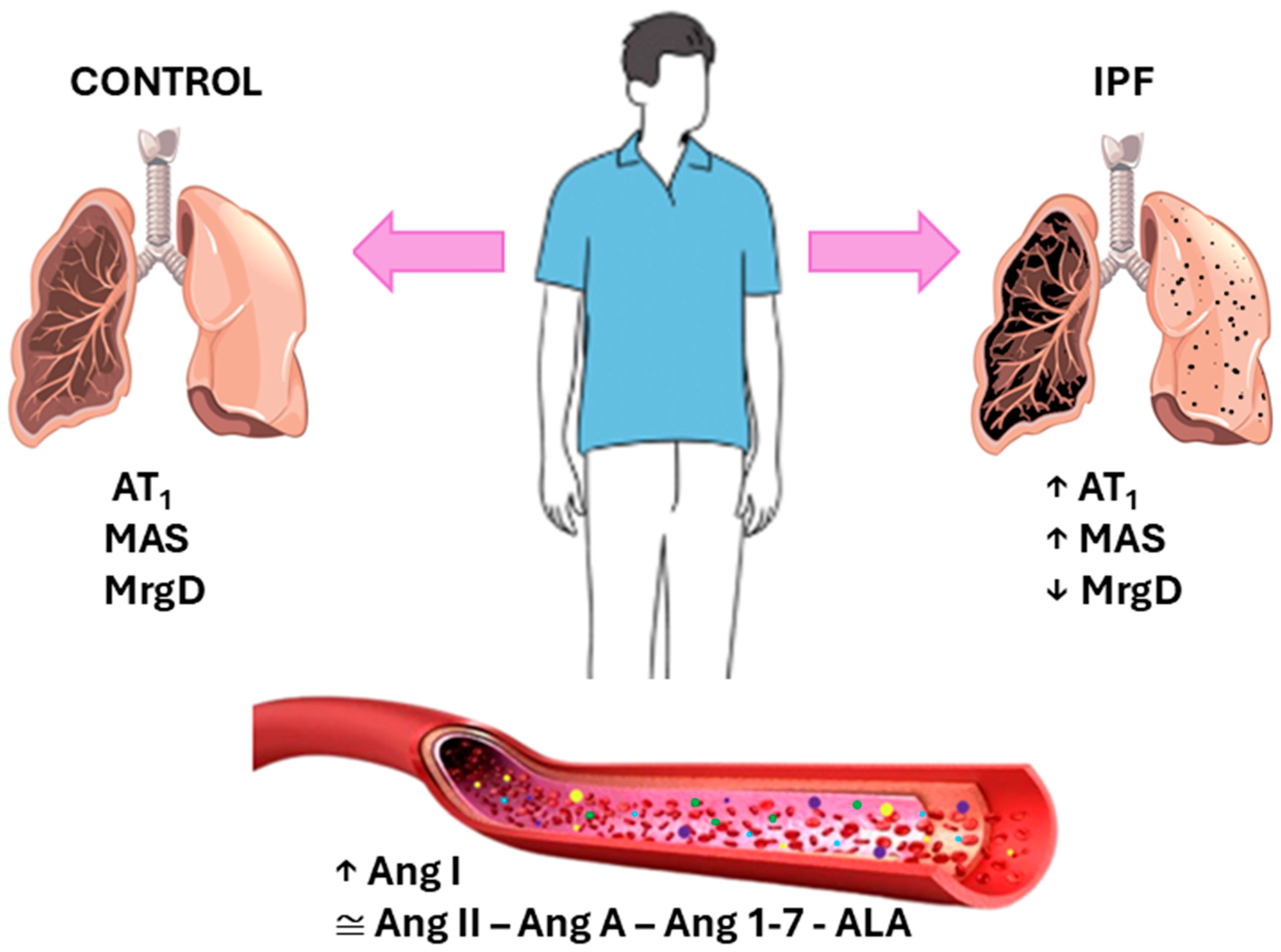
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| ALA | Alamandine |
| Ang-(1-7) | Angiotensin-1-7 |
| Ang I | Angiotensin I |
| Ang II | Angiotensin II |
| Ang A | Angiotensin A |
| AT1 | Angiotensin type 1 |
| BMI | Body mass index |
| DLCO | Carbon monoxide diffusing capacity |
| FEV1 | Expiratory volume in first second |
| FVC | Forced vital capacity |
| HR | Heart rate |
| IPF | Idiopathic pulmonary fibrosis |
| MrgD | Mas-related G-protein-coupled receptor D |
| PF | Pulmonary fibrosis |
| RAS | Renin–angiotensin system |
| RR | Respiratory rate |
| SPAP | Systolic pulmonary artery pressure |
| SPO2 | Oxygen saturation |
| 6MWT | 6-min walk test |
References
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of idiopathic pulmonary fibrosis An Official ATS/ERS/JRS/ALAT Clinical practice guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Pereiraa, C.A.C.; Corderoa, S.; Resendea, A.C. Progressive fibrotic interstitial lung disease. J. Bras. Pneumol. 2023, 49, e20230098. [Google Scholar] [CrossRef]
- Moss, B.J.; Ryter, S.W.; Rosas, I.O. Pathogenic Mechanisms Underlying Idiopathic Pulmonary Fibrosis. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 515–546. [Google Scholar] [CrossRef]
- Mikolasch, T.A.; Garthwaite, H.S.; Porter, J.C. Update in diagnosis and management of interstitial lung disease. Clin. Med. 2017, 17, 146–153. [Google Scholar] [CrossRef]
- SBPT (Sociedade Brasileira Pneumologia e Tisiologia). Fibrose Pulmonar Idiopática. 2023. Available online: https://sbpt.org.br/portal/publico-geral/doencas/fibrose-pulmonar-idiopatica/ (accessed on 13 June 2025).
- SBPT (Sociedade Brasileira Pneumologia e Tisiologia). Fibrose Pulmonar Idiopática: O diagnóstico Precoce Salva Vidas. 2021. Available online: https://sbpt.org.br/portal/fibrose-pulmonar-idiopatica-2021/ (accessed on 13 November 2024).
- Baddini-Martinez, J.; Baldi, B.G.; da Costa, C.H.; Jezler, S.; Lima, M.S.; Rufino, R. Update on diagnosis and treatment of idiopathic pulmonary fibrosis. J. Bras. Pneumol. 2015, 41, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.A.d.C.; Baddini-Martinez, J.A.; Baldi, B.G.; Jezler, S.F.d.O.; Rubin, A.S.; Alves, R.L.R.; Zonzin, G.A.; Quaresma, M.; Trampisch, M.; Rabahi, M.F. Segurança e tolerabilidade de Nintedanibe em pacientes com fibrose pulmonar idiopática no Brasil. J. Bras. Pneumol. 2019, 45, e20180414. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Wollin, L.; Fischer, A.; Quaresma, M.; Stowasser, S.; Harari, S. Fibrosing interstitial lung diseases: Knowns and unknowns. Eur. Respir. Rev. 2019, 28, 180100. [Google Scholar] [CrossRef]
- ABTO (Associação Brasileira de Transplante de Órgãos). Registro Brasileiro de Transplantes 2024, ano XXXI, n.4. São Paulo, SP-Brasil. 2024, pp. 1–88. Available online: https://site.abto.org.br/wp-content/uploads/2025/05/rbt-n4-2024-populacao.pdf (accessed on 12 June 2025).
- ABTO (Associação Brasileira de Transplante de Órgãos). Registro Brasileiro de Transplantes. Dados Númericos da doação de órgãos e transplantes realizados por estado e instituição no período: JANEIRO/MARÇO-2025. São Paulo, SP- Brasil; 2025; pp 1-21. Available online: https://site.abto.org.br/wp-content/uploads/2025/07/RBT2025-1trimestre-Populacao.pdf (accessed on 13 June 2025).
- Marut, W.; Kavian, N.; Servettaz, A.; Hua-Huy, T.; Nicco, C.; Chéreau, C.; Weill, B.; Dinh-Xuan, A.T.; Batteux, F. Amelioration of Systemic Fibrosis in Mice by Angiotensin II Receptor Blockade. Arthritis Rheum. 2013, 65, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tan, Y.; Zhao, F.; Ma, Z.; Wang, Y.; Zheng, S.; Epstein, P.N.; Yu, J.; Yin, X.; Zheng, Y.; et al. Angio-tensin II plays a critical role in diabetic pulmonary fibrosis most likely via activation of nadph oxi-dase-mediated nitrosative damage. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E132–E144. [Google Scholar] [CrossRef]
- Lautner, R.Q.; Villela, D.C.; Fraga-Silva, R.A.; Silva, N.; Verano-Braga, T.; Costa-Fraga, F.; Jankowski, J.; Jankowski, V.; Sousa, F.; Alzamora, A.; et al. Discovery and characterization of alamandine: A novel component of the renin-angiotensin system. Circ. Res. 2013, 112, 1104–1111. [Google Scholar] [CrossRef]
- Raupp, D.; Fernandes, R.S.; Antunes, K.H.; Perin, F.A.; Rigatto, K. Impact of angiotensin II type 1 and G-protein-coupled Mas receptor expression on the pulmonary performance of patients with idiopathic pulmonary fibrosis. Peptides 2020, 133, 170384. [Google Scholar] [CrossRef] [PubMed]
- Sipriani, T.S.; dos Santos, R.A.S.; Rigatto, K. The Renin-Angiotensin System: Alamandine is reduced in pa-tients with Idiopathic Pulmonary Fibrosis. J. Cardiol. Cardiovasc. Med. 2019, 4, 210–215. [Google Scholar] [CrossRef]
- Grobe, J.L.; Mecca, A.P.; Lingis, M.; Shenoy, V.; Bolton, T.A.; Machado, J.M.; Speth, R.C.; Raizada, M.K.; Katovich, M.J. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1-7). Am. J. Physiol. Circ. Physiol. 2007, 292, H736–H742. [Google Scholar] [CrossRef] [PubMed]
- Villela, D.C.; Passos-Silva, D.G.; Santos, R.A. Alamandine: A new member of the angiotensin family. Curr. Opin. Nephrol. Hypertens. 2014, 23, 130–134. [Google Scholar] [CrossRef]
- De Carvalho Santuchi, M.; Dutra, M.F.; Vago, J.P.; Lima, K.M.; Galvão, I.; De Souza-Neto, F.P.; E Silva, M.M.; Oliveira, A.C.; De Oliveira, F.C.B.; Gonçalves, R.; et al. Angiotensin-(1-7) and Alamandine Promote Anti-inflammatory Response in Macrophages In Vitro and In Vivo. Mediat. Inflamm. 2019, 2019, 2401081. [Google Scholar] [CrossRef]
- Magalhães, G.S.; Rodrigues-Machado, M.G.; Motta-Santos, D.; Silva, A.R.; Caliari, M.V.; Prata, L.O.; Abreu, S.C.; Rocco, P.R.M.; Barcelos, L.S.; Santos, R.A.S.; et al. Angiotensin-(1-7) attenuates airway remodelling and hyperresponsiveness in a model of chronic allergic lung inflammation. Br. J. Pharmacol. 2015, 172, 2330–2342. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, G.S.; Barroso, L.C.; Reis, A.C.; Rodrigues-Machado, M.G.; Gregório, J.F.; Motta-Santos, D.; Oliveira, A.C.; Perez, D.A.; Barcelos, L.S.; Teixeira, M.M.; et al. Angiotensin-(1-7) promotes resolution of eosinophilic inflammation in an experimental model of asthma. Front. Immunol. 2018, 9, 58. [Google Scholar] [CrossRef]
- Cerdán-De-Las-Heras, J.; Balbino, F.; Løkke, A.; Catalán-Matamoros, D.; Hilberg, O.; Bendstrup, E. Effect of a new tele-rehabilitation program versus standard rehabilitation in patients with chronic obstructive pulmonary disease. J. Clin. Med. 2021, 11, 11. [Google Scholar] [CrossRef]
- Alakhras, M.; Decker, P.A.; Nadrous, H.F.; Collazo-Clavell, M.; Ryu, J.H. Body mass index and mortality in patients with idiopathic pulmonary fibrosis. Chest 2007, 131, 1448–1453. [Google Scholar] [CrossRef]
- de Souza, S.M.P.; Nakasato, M.; Bruno, M.L.M.; Macedo, A. Nutritional profile of lung transplant candidates. J. Bras. Pneumol. 2009, 35, 242–247. [Google Scholar] [CrossRef]
- ABESO (Associação Brasileira para o Estudo da Obesidade e da Síndrome Metabólica). Diretrizes Brasileiras de Obesidade 2016/ABESO. Associação Brasileira para o Estudo da Obesidade e da Síndrome Metabólica. 4ª ed. São Paulo, SP-Brasil. 2016, pp. 1–188. Available online: https://abeso.org.br/wp-content/uploads/2019/12/Diretrizes-Download-Diretrizes-Brasileiras-de-Obesidade-2016.pdf (accessed on 13 June 2025).
- Morales-Blanhir, J.E.; Palafox Vidal, C.D.; Rosas Romero, M.D.J.; García Castro, M.M.; Londoño Villegas, A.; Zamboni, M. Six-minute walk test: A valuable tool for assessing pulmonary impairment. J. Bras. Pneumol. 2011, 37, 110–117. [Google Scholar] [CrossRef]
- Eduardo, D.S.; Gonçalves, N.T.; Garcia, L.C.C.; Rosa, T.d.S.; Côrrea, R.d.A.; Mancuzo, E.V. Effect of pulmonary rehabilitation on exercising tolerance in patients with advanced lung disease in waiting list for lung transplant. Rev. Médica Minas Gerais 2015, 25, 46–50. [Google Scholar] [CrossRef]
- Azevedo, K.R.S. Measurement of diffusing capacity: Interpretative strategies. Pulmão RJ 2018, 27, 45–50. [Google Scholar]
- Guimarães, V.P.; de Miranda, D.M.; Reis, M.A.S.; Andrade, T.L.; Matos, R.L.; Soares, M.R.; Pereira, C.A.C. Reference values for the carbon monoxide diffusion (Transfer factor) in a Brazilian sample of white race. J. Bras. Pneumol. 2019, 45, e20180262. [Google Scholar] [CrossRef]
- Ait Hamou, Z.; Levy, N.; Charpentier, J.; Mira, J.P.; Jamme, M.; Jozwiak, M. Use of high-flow nasal cannula oxygen and risk factors for high-flow nasal cannula oxygen failure in critically-ill patients with COVID-19. Respir. Res. 2022, 23, 329. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Chen, B.; Meliton, A.; Liu, S.Q.; Shi, Y.; Liu, T.; Deb, D.K.; Solway, J.; Li, Y.C. Chronic Activation of the renin-angiotensin system induces lung fibrosis. Sci. Rep. 2015, 5, 15561. [Google Scholar] [CrossRef] [PubMed]
- Uhal, B.D.; Li, X.; Piasecki, C.C.; Molina-Molina, M. Angiotensin signalling in pulmonary fibrosis. Int. J. Biochem. Cell Biol. 2012, 44, 465–468. [Google Scholar] [CrossRef]
- van Kats, J.P.; de Lannoy, L.M.; Danser, A.H.J.; van Meegen, J.R.; Verdouw, P.D.; Schalekamp, M.A. Angiotensin II Type 1 (AT 1) Receptor–Mediated Accumulation of Angiotensin II in Tissues and Its Intracellular Half-life In Vivo. Hypertension 1997, 30, 42–49. [Google Scholar] [CrossRef]
- Atlas, S.A. The Renin-Angiotensin Aldosterone System: Pathophysiological Role and Pharmacologic Inhibition. J. Manag. Care Pharm. 2007, 13, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Iwao, H. Molecular and Cellular Mechanisms of Angiotensin II-Mediated Cardiovascular and Renal Diseases. Pharmacol. Rev. 2000, 52, 11–34. [Google Scholar] [CrossRef]
- Park, S.; Lee, E.J. Recent advances in idiopathic pulmonary fibrosis. Tuberc. Respir. Dis. 2013, 74, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Feng, D.; Wu, Q.; Li, K.; Wang, L. Losartan Attenuates Ventilator-Induced Lung Injury. J. Surg. Res. 2008, 145, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Nadrous, H.F.; Ryu, J.H.; Douglas, W.W.; Decker, P.A.; Olson, E.J. Impact of angiotensin-converting enzyme inhibitors and statins on survival in idiopathic pulmonary fibrosis. Chest 2004, 126, 438–446. [Google Scholar] [CrossRef]
- Zhang, W.-T.; Wang, X.-J.; Xue, C.-M.; Ji, X.-Y.; Pan, L.; Weng, W.-L.; Li, Q.-Y.; Hua, G.-D.; Zhu, B.-C. The Effect of Cardiovascular Medications on Disease-Related Outcomes in Idiopathic Pulmonary Fibrosis: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 12, 771804. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ortega, M.; Lorenzo, O.; RupérEz, M.; Esteban, V.; Suzuki, Y.; Mezzano, S.; Plaza, J.; Egido, J. Role of the renin-angiotensin system in vascular diseases: Expanding the field. Hypertension 2001, 38, 1382–1387. [Google Scholar] [CrossRef]
- Magalhães, G.S.; Rodrigues-Machado, M.G.; Motta-Santos, D.; Alenina, N.; Bader, M.; Santos, R.A.; Barcelos, L.S.; Campagnole-Santos, M.J. Chronic allergic pulmonary inflammation is aggravated in angiotensin-(1–7) mas receptor knockout mice. Am. J. Physiol. Cell. Mol. Physiol. 2016, 311, L1141–L1148. [Google Scholar] [CrossRef]
- Oda, K.; Ishimoto, H.; Yamada, S.; Kushima, H.; Imanaga, T.; Harada, T.; Ishimatsu, Y.; Matsumoto, N.; Naito, K.; Yatera, K.; et al. Autopsy analyses in acute exacerbation of idiopathic pulmonary fibrosis. Respir. Res. 2014, 15, 109. [Google Scholar] [CrossRef]
- El-Hashim, A.Z.; Renno, W.M.; Raghupathy, R.; Abduo, H.T.; Akhtar, S.; Benter, I.F. Angiotensin-(1-7) inhibits allergic inflammation, via the MAS1 receptor, through suppression of ERK1/2- and NF-kB-dependent pathways. Br. J. Pharmacol. 2012, 166, 1964–1976. [Google Scholar] [CrossRef]
| Variables | Control (n = 9) | IPF (n = 10) | p |
|---|---|---|---|
| Age (years) | 68 ± 13 | 59 ± 9 | 0.092 |
| Male | 4 | 7 | 0.369 |
| Caucasian | 9 | 8 | 0.156 |
| Weight (Kg) | 61.77 ± 13.8 | 74.20 ± 7.98 | 0.026 * |
| Height (m) | 1.63 ± 7.53 | 1.69 ± 7.04 | 0.072 |
| BMI (Kg/m2) | 23.11 ± 4.57 | 25.81 ± 1.73 | 0.100 |
| Previous smoking | 6 | 6 | |
| Never smoked | 3 | 4 | 0.999 |
| Variables | Control (n = 9) | IPF (n = 10) | p |
|---|---|---|---|
| Oxygen use—n(%) | 0 | 6 (60) | 0.0108 |
| Spirometry | |||
| FVC (%) | 97 ± 14.51 | 48.72 ± 12.69 | 0.0001 **** |
| FEV1 (%) | 90.56 ± 11.33 | 51.29 ± 14.58 | 0.0001 **** |
| Echocardiogram | |||
| EF(%) | 67.10 ± 7.34 | ||
| SPAP (mmHg) | 53 ± 29.89 | ||
| Lung scintigtaphy | |||
| DLCO(%) | 32.83 ± 13.43 | ||
| TC6M | |||
| Distance traveled (m) | 393.67 ± 73.71 | ||
| Estimated distance (m) | 556.67 ± 64.97 | ||
| % predicted | 70.88 ± 11.24 | ||
| HR—pre—post (bpm) | 81.44 ± 12.47—120.6 ± 13.32 | 0.0001 **** | |
| RR—pre—post (mpm) | 21.22 ± 6.77—37.22 ± 13.49 | 0.005 ** | |
| BORG—pre—post | 0.83 ± 1.17—3.94 ± 2.37 | 0.002 ** | |
| SPO2—pre—post | 96.11 ± 1.90—79.56 ± 6.04 | 0.0001 **** |
| RAS Peptides | Control (n = 9) | IPF (n = 10) | p |
|---|---|---|---|
| Angiotensin I | 0.1703 ± 0.06185 | 0.1193 ± 0.02392 | 0.0449 * |
| Angiotensin II | 0.06500 ± 0.01911 | 0.06088 ± 0.01027 | 0.5948 |
| Angiotensin A | 0.1733 ± 0.1543 | 0.1181 ± 0.05859 | 0.3572 |
| Angiotensin 1-7 | 0.06088 ± 0.01241 | 0.05288 ± 0.009156 | 0.1645 |
| Alamandine | 0.1782 ± 0.04416 | 0.1479 ± 0.06302 | 0.2638 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silveira, A.T.; Fagundes, L.S.; Flor, J.; Martins, I.A.; Otero, L.B.; Marques da Silva, L.T.; Maciel, L.S.; Eller, S.; Guimarães, G.R.; Perin, F.A.; et al. Localization and Expression of Renin–Angiotensin System Receptors in Lung from Transplant Patients: A Case-Control Study. Biomedicines 2025, 13, 2312. https://doi.org/10.3390/biomedicines13092312
Silveira AT, Fagundes LS, Flor J, Martins IA, Otero LB, Marques da Silva LT, Maciel LS, Eller S, Guimarães GR, Perin FA, et al. Localization and Expression of Renin–Angiotensin System Receptors in Lung from Transplant Patients: A Case-Control Study. Biomedicines. 2025; 13(9):2312. https://doi.org/10.3390/biomedicines13092312
Chicago/Turabian StyleSilveira, Andresa Thomé, Lucas Sagrillo Fagundes, Juliane Flor, Isabel Amaral Martins, Laura Bastos Otero, Laura Tibola Marques da Silva, Lorenzo Santana Maciel, Sarah Eller, Giuliano Rizzotto Guimarães, Fabíola Adelia Perin, and et al. 2025. "Localization and Expression of Renin–Angiotensin System Receptors in Lung from Transplant Patients: A Case-Control Study" Biomedicines 13, no. 9: 2312. https://doi.org/10.3390/biomedicines13092312
APA StyleSilveira, A. T., Fagundes, L. S., Flor, J., Martins, I. A., Otero, L. B., Marques da Silva, L. T., Maciel, L. S., Eller, S., Guimarães, G. R., Perin, F. A., Wink, M. R., & Rigatto, K. (2025). Localization and Expression of Renin–Angiotensin System Receptors in Lung from Transplant Patients: A Case-Control Study. Biomedicines, 13(9), 2312. https://doi.org/10.3390/biomedicines13092312








