Effects of Antifibrotic Therapy in Patients with Combined Pulmonary Fibrosis and Emphysema: A US-Based Cohort Study
Abstract
1. Introduction
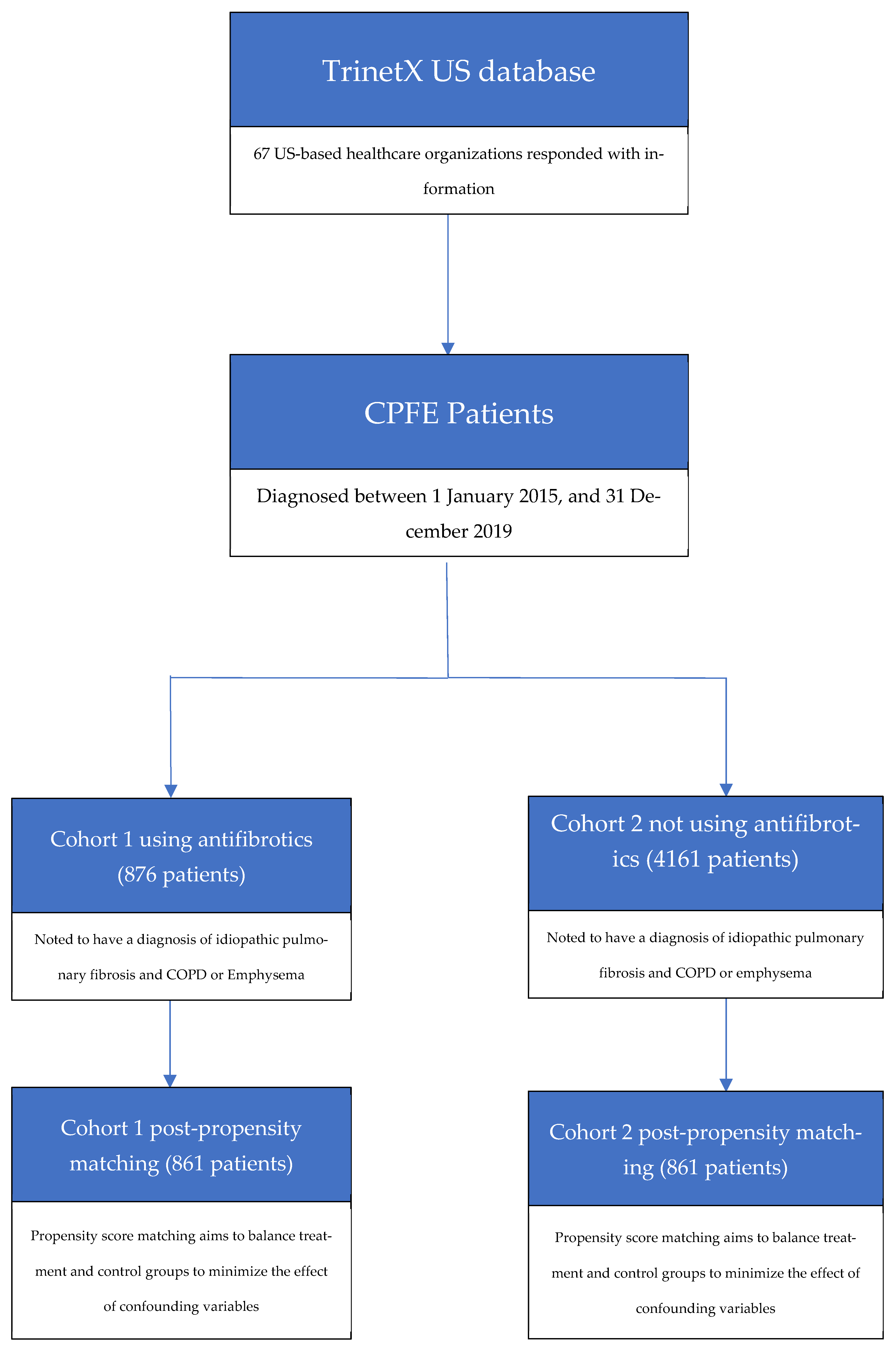
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPFE | Chronic Pulmonary Fibrosis and Emphysema |
| PF | Pulmonary Fibrosis |
| HRCT | High-Resolution Computed Tomography |
| MACE | Major Adverse Cardiac Event |
| MI | Myocardial Infarction |
| RR | Relative Risk |
| HR | Hazard Ratio |
| CT | Computed Tomography |
| CI | Confidence Interval |
| BMI | Body Mass Index |
| FEV1 | Forced Expiratory Volume |
| FVC | Forced Vital Capacity |
| CRP | C-Reactive Protein |
| ACEI | Angiotensin-Converting Enzyme |
| PSM | Propensity Score Matching |
References
- Cottin, V.; Nunes, H.; Brillet, P.Y.; Delaval, P.; Devouassaoux, G.; Tillie-Leblond, I.; Israel-Biet, D.; Court-Fortune, I.; Valeyre, D.; Cordier, J.-F. Combined pulmonary fibrosis and emphysema: A distinct underrecognised entity. Eur. Respir. J. 2005, 26, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Jankowich, M.D.; Rounds, S.I.S. Combined Pulmonary Fibrosis and Emphysema Syndrome: A Review. Chest 2012, 141, 222. [Google Scholar] [CrossRef] [PubMed]
- Hage, R.; Gautschi, F.; Steinack, C.; Schuurmans, M.M. Combined Pulmonary Fibrosis and Emphysema (CPFE) Clinical Features and Management. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 167. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, H.J.; Park, C.M.; Lim, K.Y.; Lee, J.Y.; Kim, D.J.; Yeon, J.H.; Hwang, S.-S.; Kim, D.-K.; Lee, S.-M.; et al. The impact of combined pulmonary fibrosis and emphysema on mortality. Int. J. Tuberc. Lung Dis. 2011, 15, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Sugino, K.; Ishida, F.; Kikuchi, N.; Hirota, N.; Sano, G.; Sato, K.; Isobe, K.; Sakamoto, S.; Takai, Y.; Homma, S. Comparison of clinical characteristics and prognostic factors of combined pulmonary fibrosis and emphysema versus idiopathic pulmonary fibrosis alone. Respirology 2014, 19, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Le Pavec, J.; Prévot, G.; Mal, H.; Humbert, M.; Simonneau, G.; Cordier, J.-F. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur. Respir. J. 2009, 35, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.J.; Do, K.H.; Lee, J.B.; Alblushi, S.; Lee, S.M. Lung Cancer in Combined Pulmonary Fibrosis and Emphysema: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0161437. [Google Scholar] [CrossRef]
- Cottin, V.; Inoue, Y.; Selman, M.; Ryerson, C.J.; Wells, A.U.; Agusti, A.; Wong, A.W.; Corte, T.J.; Flaherty, K.R.; Han, M.K.; et al. Syndrome of Combined Pulmonary Fibrosis and Emphysema: An official research statement from American Thoracic Society (ATS), European Respiratory Society (ERS), Japanese Respiratory Society (JRS), and Asociación Latinoamericana de Tórax (ALAT). Am. J. Respir. Crit. Care Med. 2022, 206, e7. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Correction: Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082, Erratum in N. Engl. J. Med. 2015, 373, 782. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa1402584 (accessed on 10 April 2025). [PubMed]
- King, T.E.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A Phase 3 Trial of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Palchuk, M.B.; London, J.W.; Perez-Rey, D.; Drebert, Z.J.; Winer-Jones, J.P.; Thompson, C.N.; Esposito, J.; Claerhout, B. A global federated real-world data and analytics platform for research. JAMIA Open 2023, 6, ooad035. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.W.; Liang, J.; Cottin, V.; Ryerson, C.J. Diagnostic Features in Combined Pulmonary Fibrosis and Emphysema: A Systematic Review. Ann. Am. Thorac. Soc. 2020, 17, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, H.; Mamesaya, N.; Yamashita, S.; Kida, Y.; Kaneko, M.; Sakai, H. Combined pulmonary fibrosis and emphysema: Effect of pulmonary rehabilitation in comparison with chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2016, 3, e000099. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zhang, Y.; Chi, F.; Song, Q.; Zhang, L.; Wang, Y.; Che, C. Clinical efficacy and safety of ICS/LABA in patients with combined idiopathic pulmonary fibrosis and emphysema. Int. J. Clin. Exp. Med. 2015, 8, 8617. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4538025/ (accessed on 13 April 2025). [PubMed]
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glassberg, M.K.; Kardatzke, D.; King, T.E., Jr.; Lancaster, L.; Sahn, S.A.; Szwarcberg, J.; et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet 2011, 377, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.F.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Mejía, M.; Carrillo, G.; Rojas-Serrano, J.; Estrada, A.; Suárez, T.; Alonso, D.; Barrientos, E.; Gaxiola, M.; Navarro, C.; Selman, M. Idiopathic pulmonary fibrosis and emphysema: Decreased survival associated with severe pulmonary arterial hypertension. Chest 2009, 136, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Kurashima, K.; Takayanagi, N.; Tsuchiya, N.; Kanauchi, T.; Ueda, M.; Hoshi, T.; Miyahara, Y.; Sugita, Y. The effect of emphysema on lung function and survival in patients with idiopathic pulmonary fibrosis. Respirology 2010, 15, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Hansell, D.M.; Sverzellati, N.; Weycker, D.; Antoniou, K.M.; Atwood, M.; Oster, G.; Kirchgaessler, K.-U.; Collard, H.R.; Wells, A.U. Effect of emphysema extent on serial lung function in patients with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2017, 196, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.; Gupta, R.S.; George, P.M.; Quint, J.K. Validation of the recording of idiopathic pulmonary fibrosis in routinely collected electronic healthcare records in England. BMC Pulm. Med. 2023, 23, 256. [Google Scholar] [CrossRef] [PubMed]
| Before Propensity Score Matching | After Propensity Score Matching | |||||
|---|---|---|---|---|---|---|
| Characteristics | Cohort 1 (N = 876) | Cohort 2 (N = 4161) | p Value | Cohort 1 (N = 861) | Cohort 2 (N = 861) | p Value |
| Demographics | ||||||
| Current Age | 77.2 +/− 8.6 | 77.9 +/− 10.5 | 0.083 | 77.3 +/− 8.6 | 77.4 +/− 10.3 | 0.699 |
| Age at Index | 70.7 +/− 9.1 | 71.5 +/− 11.4 | 0.056 | 70.8 +/− 9.1 | 70.8 +/− 10.9 | 0.924 |
| White | 84.0% | 76.5% | <0.001 | 84.1% | 84.7% | 0.740 |
| Male | 68.9% | 56.2% | <0.001 | 68.5% | 67.7% | 0.717 |
| Female | 31.1% | 43.8% | <0.001 | 31.5% | 32.3% | 0.717 |
| Hispanic of Latino | 6.5% | 8.1% | 0.116 | 6.4% | 5.9% | 0.688 |
| Not Hispanic or Latino | 81.7% | 74.5% | <0.001 | 81.8% | 80.7% | 0.578 |
| Black or African American | 6.1% | 11.8% | <0.001 | 6.2% | 5.3% | 0.469 |
| Asian | 2.7% | 2.6% | 0.872 | 2.6% | 2.0% | 0.418 |
| Diagnoses | ||||||
| Asthma | 13.5% | 18.2% | 0.001 | 13.7% | 14.4% | 0.677 |
| Bronchiectasis, Uncomplicated | 29.1% | 19.6% | <0.001 | 28.5% | 28.1% | 0.872 |
| Hypertensive Chronic Kidney Disease | 9.9% | 15.1% | <0.001 | 10.1% | 8.5% | 0.245 |
| Hypertensive Heart Disease | 13.4% | 13.4% | 0.951 | 13.4% | 12.1% | 0.426 |
| Essential (Primary) Hypertension | 70.5% | 69.8% | 0.657 | 70.0% | 70.6% | 0.792 |
| Chronic Ischemic Heart Disease | 52.5% | 47.1% | 0.003 | 52.4% | 50.1% | 0.335 |
| Heart Failure | 29.7% | 35.7% | 0.001 | 30.0% | 28.0% | 0.367 |
| Diabetes Mellitus | 29.1% | 31.1% | 0.235 | 28.8% | 27.2% | 0.452 |
| Overweight, Obesity, And other Hyperalimentation | 29.3% | 25.1% | 0.009 | 29.0% | 26.0% | 0.161 |
| Hyperlipidemia, Unspecified | 56.6% | 54.0% | 0.150 | 56.0% | 56.0% | 1 |
| Neoplasms | 39.4% | 40.6% | 0.516 | 39.5% | 39.5% | 1 |
| Obstructive Sleep Apnea (Adult) (Pediatric) | 28.9% | 23.4% | 0.001 | 28.6% | 28.1% | 0.831 |
| Pulmonary Hypertension, Unspecified | 24.0% | 16.3% | <0.001 | 23.2% | 22.4% | 0.688 |
| Cerebral Infarction | 5.6% | 6.5% | 0.299 | 5.7% | 4.8% | 0.386 |
| Nontraumatic Intracerebral Hemorrhage | 1.1% | 0.5% | 0.015 | 1.2% | 1.2% | 1 |
| Medications | ||||||
| Immunosuppressants | ||||||
| Mycophenolate mofetil | 7.8% | 11.4% | 0.002 | 7.8% | 8.0% | 0.858 |
| Tacrolimus | 6.2% | 8.2% | 0.038 | 6.0% | 5.6% | 0.680 |
| Azathioprine | 5.6% | 6.5% | 0.323 | 5.5% | 5.6% | 0.916 |
| Basiliximab | 3.1% | 3.2% | 0.861 | 3.0% | 3.3% | 0.782 |
| Mycophenolic Acid | 1.7% | 3.1% | 0.027 | 1.7% | 2.1% | 0.598 |
| Cyclosporine | 1.9% | 2.3% | 0.560 | 1.9% | 2.1% | 0.729 |
| Sirolimus | 1.1% | 1.3% | 0.629 | 1.2% | 0% | 0.002 |
| Infliximab | 1.1% | 0.5% | 0.028 | 1.2% | 1.2% | 1 |
| Ustekinumab | 0% | 0.2% | 0.146 | 0% | 0% | - |
| Imiquimod | 1.1% | 0.4% | 0.010 | 1.2% | 1.2% | 1 |
| Biological Therapy | ||||||
| Etanercept | 1.1% | 0.7% | 0.172 | 1.2% | 1.2% | 1 |
| Adalimumab | 1.1% | 0.6% | 0.121 | 1.2% | 1.2% | 1 |
| Inhaled Therapies | ||||||
| Anti-Inflammatories | 60.8% | 51.2% | <0.001 | 60.7% | 60.2% | 0.805 |
| Bronchodilators, Anticholinergic | 65.9% | 59.4% | <0.001 | 65.7% | 65.0% | 0.761 |
| Bronchodilators, Sympathomimetic | 84.5% | 75.9% | <0.001 | 84.2% | 82.9% | 0.474 |
| Vilanterol | 14.5% | 9.8% | <0.001 | 14.4% | 13.8% | 0.729 |
| Cromolyn | 1.1% | 0.2% | <0.001 | 1.2% | 1.2% | 1 |
| Montelukast | 15.3% | 11.6% | 0.002 | 15.3% | 16.1% | 0.643 |
| Miscellaneous | ||||||
| Anti-Neoplastics | 9.7% | 11.1% | 0.219 | 9.6% | 8.6% | 0.451 |
| Cardiovascular | ||||||
| Anti-Lipemics | 63.0% | 55.0% | <0.001 | 62.5% | 60.4% | 0.373 |
| Antiarrhythmics | 60.8% | 58.8% | 0.265 | 60.9% | 59.8% | 0.658 |
| Diuretics | 53.5% | 55.4% | 0.309 | 53.7% | 50.9% | 0.247 |
| Beta-Blockers | 53.8% | 55.3% | 0.400 | 54.1% | 53.1% | 0.664 |
| Calcium Channel Blockers | 36.0% | 38.5% | 0.163 | 36.1% | 32.6% | 0.128 |
| Angiotensin-Converting Enzyme (ACEI) Inhibitors | 23.7% | 25.3% | 0.332 | 24.0% | 22.4% | 0.424 |
| Angiotensin II Inhibitors | 23.5% | 20.0% | 0.018 | 22.9% | 23.1% | 0.909 |
| Corticosteroids | ||||||
| Prednisone | 61.5% | 55.8% | 0.002 | 61.4% | 61.3% | 0.961 |
| Methylprednisolone | 43.4% | 41.0% | 0.189 | 43.4% | 44.8% | 0.560 |
| Dexamethasone | 25.6% | 22.7% | 0.064 | 25.4% | 26.9% | 0.476 |
| Triamcinolone | 16.4% | 17.2% | 0.606 | 16.4% | 16.3% | 0.948 |
| Hydrocortisone | 12.3% | 14.8% | 0.060 | 12.4% | 13.2% | 0.614 |
| Prednisolone | 5.0% | 5.0% | 1 | 5.0% | 4.9% | 0.911 |
| Betamethasone | 6.6% | 6.5% | 0.927 | 6.7% | 6.7% | 1 |
| Brain Natriuretic Peptide (serum) | 1465.4 +/− 2705.1 | 2663.2 +/− 6529.3 | 0.014 | 1473.6 +/− 2717.7 | 1507.6 +/− 2754.0 | 0.905 |
| Hemoglobin A1c in Blood | 6.1 +/− 1.0 | 6.3 +/− 1.2 | 0.009 | 6.1 +/− 1.0 | 6.3 +/− 1.3 | 0.124 |
| C-Reactive Protein (Serum) | 23.0 +/− 45.9 | 29.3 +/− 49.5 | 0.059 | 23.5 +/− 46.6 | 29.6 +/− 55.5 | 0.185 |
| BMI | 28.3 +/− 5.8 | 27.8 +/− 6.5 | 0.054 | 28.3 +/− 5.8 | 27.9 +/− 6.2 | 0.173 |
| 5-Year Follow-Up | Cohort 1 (Using Antifibrotics) | Cohort 2 (Not Using Antifibrotics) | ||
|---|---|---|---|---|
| Outcomes a | 5-Year Follow-Up | 5-Year Follow-Up | RR * or HR * (95% CI) p Value ** | |
| Overall Mortality | n after matching b | 856 | 851 | HR 1.14 CI (0.99, 1.33) p: 0.06 |
| Patients with outcome | 377 | 332 | ||
| Incidence of acute hypoxic respiratory failure | n after matching b | 861 | 861 | HR 1.17 CI (0.99, 1.39) p: 0.06 |
| Patients with outcome | 279 | 246 | ||
| Incidence of acute hypercapnic respiratory failure | n after matching b | 861 | 861 | HR 0.99 CI (0.67, 1.47) p: 0.97 |
| Patients with outcome | 50 | 50 | ||
| Incidence of myocardial infarction | n after matching b | 861 | 861 | HR 1.68 CI (0.88, 3.18) p: 0.10 |
| Patients with outcome | 25 | 15 | ||
| Incidence of stroke | n after matching b | 861 | 861 | HR 0.73 CI (0.51, 1.05) p: 0.08 |
| Patients with outcome | 53 | 71 | ||
| Incidence of unstable angina | n after matching b | 861 | 861 | HR 0.94 CI (0.47, 1.86) p: 0.86 |
| Patients with outcome | 16 | 17 | ||
| 3-Year Follow-Up | Cohort 1 (Using Antifibrotics) | Cohort 2 (Not Using Antifibrotics) | ||
|---|---|---|---|---|
| Outcomes a | 3-Year Follow-Up | 3-Year Follow-Up | RR * or HR * (95% CI) p Value ** | |
| Overall Mortality | n after matching b | 819 | 817 | HR 1.30 CI (1.11, 1.53) p: <0.01 |
| Patients with outcome | 362 | 277 | ||
| Incidence of acute hypoxic respiratory failure | n after matching b | 823 | 823 | HR 0.97 CI (0.81, 1.17) p: 0.82 |
| Patients with outcome | 222 | 224 | ||
| Incidence of acute hypercapnic respiratory failure | n after matching b | 823 | 823 | HR 0.81 CI (0.54, 1.22) p: 0.32 |
| Patients with outcome | 44 | 52 | ||
| Incidence of myocardial infarction | n after matching b | 823 | 823 | HR 1.20 CI (0.64, 2.25) p: 0.55 |
| Patients with outcome | 22 | 18 | ||
| Incidence of stroke | n after matching b | 823 | 823 | HR 0.90 CI (0.61, 1.33) p: 0.62 |
| Patients with outcome | 49 | 53 | ||
| Incidence of unstable angina | n after matching b | 823 | 823 | HR 0.93 CI (0.46, 1.88) p: 0.84 |
| Patients with outcome | 15 | 16 | ||
| 1-Year Follow Up | Cohort 1 (Using Antifibrotics) | Cohort 2 (Not Using Antifibrotics) | ||
|---|---|---|---|---|
| Outcomes a | 1-Year Follow-Up | 1-Year Follow-Up | RR * or HR * (95% CI) p Value ** | |
| Overall Mortality | n after matching b | 835 | 833 | HR 1.00 CI (0.84, 1.20) p: 0.93 |
| Patients with outcome | 244 | 234 | ||
| Incidence of acute hypoxic respiratory failure | n after matching b | 840 | 840 | HR 0.99 CI (0.79, 1.24) p: 0.93 |
| Patients with outcome | 150 | 150 | ||
| Incidence of acute hypercapnic respiratory failure | n after matching b | 840 | 840 | HR 0.78 CI (0.46, 1.32) p: 0.36 |
| Patients with outcome | 25 | 31 | ||
| Incidence of myocardial infarction | n after matching b | 840 | 840 | HR 1.17 CI (0.59, 2.33) p: 0.63 |
| Patients with outcome | 18 | 15 | ||
| Incidence of stroke | n after matching b | 840 | 840 | HR 0.76 CI (0.48, 1.20) p: 0.24 |
| Patients with outcome | 33 | 42 | ||
| Incidence of unstable angina | n after matching b | 840 | 840 | HR 2.67 CI (0.85, 8.41) p: 0.07 |
| Patients with outcome | 11 | 10 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, A.; Lopez, E.K.; Geller, A.; Patel, M.; Benzaquen, S. Effects of Antifibrotic Therapy in Patients with Combined Pulmonary Fibrosis and Emphysema: A US-Based Cohort Study. Biomedicines 2025, 13, 2522. https://doi.org/10.3390/biomedicines13102522
Shah A, Lopez EK, Geller A, Patel M, Benzaquen S. Effects of Antifibrotic Therapy in Patients with Combined Pulmonary Fibrosis and Emphysema: A US-Based Cohort Study. Biomedicines. 2025; 13(10):2522. https://doi.org/10.3390/biomedicines13102522
Chicago/Turabian StyleShah, Abhishek, Esteban Kosak Lopez, Andrew Geller, Maanav Patel, and Sadia Benzaquen. 2025. "Effects of Antifibrotic Therapy in Patients with Combined Pulmonary Fibrosis and Emphysema: A US-Based Cohort Study" Biomedicines 13, no. 10: 2522. https://doi.org/10.3390/biomedicines13102522
APA StyleShah, A., Lopez, E. K., Geller, A., Patel, M., & Benzaquen, S. (2025). Effects of Antifibrotic Therapy in Patients with Combined Pulmonary Fibrosis and Emphysema: A US-Based Cohort Study. Biomedicines, 13(10), 2522. https://doi.org/10.3390/biomedicines13102522






