Antenatal Sildenafil for Congenital Diaphragmatic Hernia: A Systematic Review and Bayesian Meta-Analysis of Preclinical Studies
Abstract
1. Introduction
2. Methods
2.1. Protocol
2.2. Sources and Search Strategy
2.3. Inclusion Criteria and Study Selection
2.4. Data Extraction
2.5. Risk of Bias Assessment
2.6. Bayesian Meta-Analysis
2.7. Subgroup Analysis and Publication Bias Analysis
3. Results
Bayesian Meta-Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Russo, F.M.; De Coppi, P.; Allegaert, K.; Toelen, J.; Van Der Veeken, L.; Attilakos, G.; Eastwood, M.P.; David, A.L.; Deprest, J. Current and future antenatal management of isolated congenital diaphragmatic hernia. Semin. Fetal Neonat. Med. 2017, 22, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, S.; Barbato, A.; Bush, A.; Claus, F.; Davenport, M.; Delacourt, C.; Deprest, J.; Eber, E.; Frenckner, B.; Greenough, A. Congenital diaphragmatic hernia. Eur. Respir. J. 2012, 39, 820–8292012. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, M.; Hislop, A.; Boyden, E.A.; Reid, L. Lung hypoplasia in congenital diaphragmatic hernia. A quantitative study of airway, artery, and alveolar development. Br. J. Surg. 1971, 58, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.; Jimenez, J.; Rendin, L.E.; Muller, C.; Westergren-Thorsson, G.; Deprest, J.; Toelen, J. The proportion of alveolar type 1 cells decreases in murine hypoplastic congenital diaphragmatic hernia lungs. PLoS ONE 2019, 14, e0214793, Correction in PLoS ONE 2019, 14, e0217322. [Google Scholar] [CrossRef] [PubMed]
- Geggel, R.L.; Murphy, J.D.; Langleben, D.; Crone, R.K.; Vacanti, J.P.; Reid, L.M. Congenital diaphragmatic hernia: Arterial structural changes and persistent pulmonary hypertension after surgical repair. J. Pediatr. 1985, 107, 457–464. [Google Scholar] [CrossRef]
- Snoek, K.G.; Greenough, A.; Van Rosmalen, J.; Capolupo, I.; Schaible, T.; Ali, K.; Wijnen, R.M.; Tibboel, D. Congenital diaphragmatic hernia: 10-year evaluation of survival, extracorporeal membrane oxygenation, and foetoscopic endotracheal occlusion in four high-volume centRes. Neonatology 2018, 113, 63–68. [Google Scholar] [CrossRef]
- Keijzer, R.; Liu, J.; Deimling, J.; Tibboel, D.; Post, M. Dual-hit hypothesis explains pulmonary hypoplasia in the nitrofen model of congenital diaphragmatic hernia. Am. J. Pathol. 2000, 156, 1299–1306. [Google Scholar] [CrossRef]
- Montalva, L.; Antounians, L.; Zani, A. Pulmonary hypertension secondary to congenital diaphragmatic hernia: Factors and pathways involved in pulmonary vascular remodeling. Pediatr. Res. 2019, 85, 754–768. [Google Scholar] [CrossRef]
- Karamanoukian, H.L.; Peay, T.; Love, J.E.; Abdel-Rahman, E.; Dandonna, P.; Azizkhan, R.G.; Glick, P.L. Decreased Pulmonary Nitric Oxide Synthase Activity in the Rat Model of Congenital Diaphragmatic Hernia. J. Pediatr. Surg. 1996, 31, 1016–1019. [Google Scholar] [CrossRef]
- Thebaud, B.; Petit, T.; De Lagausie, P.; Dall’Ava-Santucci, J.; Mercier, J.C.; Dinh-Xuan, A.T. Altered Guanylyl-Cyclase Activity in Vitro of Pulmonary Arteries From Fetal Lambs With Congenital Diaphragmatic Hernia. Am. J. Respir. Cell Mol. Biol. 2002, 27, 42–47. [Google Scholar] [CrossRef]
- de Buys Roessingh, A.; Fouquet, V.; Aigrain, Y.; Mercier, J.C.; de Lagausie, P.; Dinh-Xuan, A.T. Nitric Oxide Activity Through Guanylate Cyclase and Phosphodiesterase Modulation Is Impaired in Fetal Lambs with Congenital Diaphragmatic Hernia. J. Pediatr. Surg. 2011, 46, 1516–1522. [Google Scholar] [CrossRef]
- van der Horst, I.W.; Morgan, B.; Eaton, F.; Reiss, I.; Tibboel, D.; Thébaud, B. Expression and Function of Phosphodiesterases in Nitrofen-Induced Congenital Diaphragmatic Hernia in Rats. Pediatr. Pulmonol. 2010, 45, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Zhaorigetu, S.; Bair, H.; Lu, J.; Jin, D.; Olson, S.D.; Harting, M.T. Perturbations in Endothelial Dysfunction-Associated Pathways in the Nitrofen-Induced Congenital Diaphragmatic Hernia Model. J. Vasc. Res. 2018, 55, 26–34. [Google Scholar] [CrossRef]
- Acker, S.N.; Seedorf, G.J.; Abman, S.H.; Nozik-Grayck, E.; Partrick, D.A.; Gien, J. Pulmonary Artery Endothelial Cell Dysfunction and Decreased Populations of Highly Proliferative Endothelial Cells in Experimental Congenital Diaphragmatic Hernia. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L943–L952. [Google Scholar] [CrossRef] [PubMed]
- Solari, V.; Piotrowska, A.P.; Puri, P. Expression of Heme Oxygenase-1 and Endothelial Nitric Oxide Synthase in the Lung of Newborns With Congenital Diaphragmatic Hernia and Persistent Pulmonary Hypertension. J. Pediatr. Surg. 2003, 38, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Tantini, B.; Manes, A.; Fiumana, E.; Pignatti, C.; Guarnieri, C.; Zannoli, R.; Branzi, A.; Galie, N. Antiproliferative effect of sildenafil on human pulmonary artery smooth muscle cells. Basic. Res. Cardiol. 2005, 100, 131–138. [Google Scholar] [CrossRef]
- Kipfmueller, F.; Schroeder, L.; Berg, C.; Heindel, K.; Bartmann, P.; Mueller, A. Continuous intravenous sildenafil as an early treatment in neonates with congenital diaphragmatic hernia. Pediatr. Pulmonol. 2018, 53, 452–460. [Google Scholar] [CrossRef]
- Lawrence, K.M.; Monos, S.; Adams, S.; Herkert, L.; Peranteau, W.H.; Munson, D.A.; Hopper, R.K.; Avitabile, C.M.; Rintoul, N.E.; Hedrick, H.L. Inhaled Nitric Oxide Is Associated with Improved Oxygenation in a Subpopulation of Infants with Congenital Diaphragmatic Hernia and Pulmonary Hypertension. J. Pediatr. 2020, 219, 167–172. [Google Scholar] [CrossRef]
- Abman, S.H.; Hansmann, G.; Archer, S.L.; Ivy, D.D.; Adatia, I.; Chung, W.K.; Hanna, B.D.; Rosenzweig, E.B.; Raj, J.U.; Cornfield, D. Pediatric pulmonary hypertension: Guidelines from the American heart association and American thoracic society. Circulation 2015, 132, 2037–2099. [Google Scholar] [CrossRef]
- Russo, F.; Benachi, A.; Gratacos, E.; Zani, A.; Keijzer, R.; Partridge, E.; Sananes, N.; De Coppi, P.; Aertsen, M.; Nicolaides, K.H. Antenatal management of congenital diaphragmatic hernia: What’s next? Prenat. Diagn. 2022, 42, 291–300. [Google Scholar] [CrossRef]
- Luong, C.; Rey-Perra, J.; Vadivel, A.; Gilmour, G.; Sauve, Y.; Koonen, D.; Walker, D.; Todd, K.G.; Gressens, P.; Kassiri, Z.; et al. Antenatal sildenafil treatment attenuates pulmonary hypertension in experimental congenital diaphragmatic hernia. Circulation 2011, 123, 2120–2131. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Thebaud, B.; Vadivel, A.; Eaton, F.; Jain, V.; Hornberger, L.K. Doppler parameters of fetal lung hypoplasia and impact of sildenafil. Am. J. Obstet. Gynecol. 2014, 211, 263. [Google Scholar] [CrossRef]
- Mous, D.S.; Kool, H.M.; Burgisser, P.E.; Buscop-van Kempen, M.J.; Nagata, K.; Boerema-de Munck, A.; van Rosmalen, J.; Dzyubachyk, O.; Wijnen, R.M.H.; Tibboel, D.; et al. Treatment of rat congenital diaphragmatic hernia with sildenafil and NS-304, selexipag’s active compound, at the pseudoglandular stage improves lung vasculature. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 315, L276–L285. [Google Scholar] [CrossRef] [PubMed]
- Mous, D.S.; Kool, H.M.; Buscop-van Kempen, M.J.; Koning, A.H.; Dzyubachyk, O.; Wijnen, R.M.; Tibboel, D.; Rottier, R.J. Clinically relevant timing of antenatal sildenafil treatment reduces pulmonary vascular remodeling in congenital diaphragmatic hernia. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L734–L742. [Google Scholar] [CrossRef] [PubMed]
- Mesas-Burgos, C.; Pearson, E.G.; Davey, M.; Riley, J.; Jia, H.; Laje, P.; Flake, A.W.; Peranteau, W.H. Improved pulmonary function in the nitrofen model of congenital diaphragmatic hernia following prenatal maternal dexamethasone and/or sildenafil. Pediatr. Res. 2016, 80, 577–585. [Google Scholar] [CrossRef]
- Russo, F.M.; Toelen, J.; Eastwood, M.P.; Jimenez, J.; Miyague, A.H.; Vande Velde, G.; DeKoninck, P.; Himmelreich, U.; Vergani, P.; Allegaert, K.; et al. Transplacental sildenafil rescues lung abnormalities in the rabbit model of diaphragmatic hernia. Thorax 2016, 71, 517–525. [Google Scholar] [CrossRef]
- Kashyap, A.J.; Dekoninck, P.L.J.; Rodgers, K.A.; Thio, M.; McGillick, E.V.; Amberg, B.J.; Skinner, S.M.; Moxham, A.M.; Russo, F.M.; Deprest, J.A.; et al. Antenatal sildenafil treatment improves neonatal pulmonary hemodynamics and gas exchange in lambs with diaphragmatic hernia. Ultrasound Obstet. Gynecol. 2019, 54, 506–516. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- de Vries, R.B.; Hooijmans, C.R.; Tillema, A.; Leenaars, M.; Ritskes-Hoitinga, M. Updated version of the Embase search filter for animal studies. Lab. Anim. 2014, 48, 88. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Tillema, A.; Leenaars, M.; Ritskes-Hoitinga, M. Enhancing search efficiency by means of a search filter for finding all studies on animal experimentation in PubMed. Lab. Anim. 2010, 44, 170–175. [Google Scholar] [CrossRef]
- Schmitt, G.; Barrow, P. Considerations for and against dosing rodent pups before 7 days of age in juvenile toxicology studies. Reprod. Toxicol. 2022, 112, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Holmøy, I.; Waage, S.; Granquist, E.; L’Abée-Lund, T.; Ersdal, C.; Hektoen, L.; Sørby, R. Early neonatal lamb mortality: Postmortem findings. Animal 2017, 11, 295–305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trejo-Muñoz, L.; Navarrete, E.; Montúfar-Chaveznava, R.; Caldelas, I. Determining the period, phase and anticipatory component of activity and temperature patterns in newborn rabbits that were maintained under a daily nursing schedule and fasting conditions. Physiol. Behav. 2012, 106, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Systemat Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Rohatgi, A. WebPlotDigitizer. Available online: https://automeris.io/WebPlotDigitizer (accessed on 6 July 2024).
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Kilkenny, C.; Parsons, N.; Kadyszewski, E.; Festing, M.F.; Cuthill, I.C.; Fry, D.; Hutton, J.; Altman, D.G. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS ONE 2009, 4, e7824. [Google Scholar] [CrossRef]
- Bartoš, F.; Gronau, Q.F.; Timmers, B.; Otte, W.M.; Ly, A.; Wagenmakers, E.J. Bayesian model-averaged meta-analysis in medicine. Stat. Med. 2021, 40, 6743–6761. [Google Scholar] [CrossRef]
- Gronau, Q.F.; Heck, D.W.; Berkhout, S.W.; Haaf, J.M.; Wagenmakers, E.J. A primer on Bayesian model-averaged meta-analysis. Adv. Methods Prac. Psychol. Sci. 2021, 4, 25152459211031256. [Google Scholar] [CrossRef]
- Heck, D.W.; Gronau, Q.F.; Wagenmakers, E. metaBMA: Bayesian Model Averaging for Random and Fixed Effects Meta-Analysis, version 0.6.7; Computer software. 2019. Available online: https://cran.r-project.org/web/packages/metaBMA/index.html (accessed on 6 July 2024).
- van Doorn, J.; van den Bergh, D.; Böhm, U.; Dablander, F.; Derks, K.; Draws, T.; Etz, A.; Evans, N.J.; Gronau, Q.F.; Haaf, J.M. The JASP guidelines for conducting and reporting a Bayesian analysis. Psychol. Bull. Rev. 2021, 28, 813–826. [Google Scholar] [CrossRef]
- Maier, M.; Bartoš, F.; Wagenmakers, E.J. Robust Bayesian meta-analysis: Addressing publication bias with model-averaging. Psychol. Methods 2023, 28, 107. [Google Scholar] [CrossRef]
- Bartoš, F.; Maier, M.; Wagenmakers, E.J.; Doucouliagos, H.; Stanley, T. Robust Bayesian meta-analysis: Model-averaging across complementary publication bias adjustment methods. Res. Synth. Methods 2023, 14, 99–116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, M.; Wagenmakers, E.J. Bayesian Cognitive Modeling: A Practical Course; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Bartoš, F.; Otte, W.M.; Gronau, Q.F.; Timmers, B.; Ly, A.; Wagenmakers, E.J. Empirical prior distributions for Bayesian meta-analyses of binary and time to event outcomes. arXiv 2023, arXiv:2306.11468. [Google Scholar] [CrossRef]
- Lemus-Varela, M.D.L.; Soliz, A.; Gómez-Meda, B.C.; Zamora-Pérez, A.L.; Ornelas-Aguirre, J.M.; Melnikov, V.; Torres-Mendoza, B.M.; Zúñiga-González, G.M. Antenatal use of bosentan and/or sildenafil attenuates pulmonary features in rats with congenital diaphragmatic hernia. World J. Pediatr. 2014, 10, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.M.; Da, M.G.M.C.M.; Jimenez, J.; Lesage, F.; Eastwood, M.P.; Toelen, J.; Deprest, J. Complementary effect of maternal sildenafil and fetal tracheal occlusion improves lung development in the rabbit model of congenital diaphragmatic hernia. Ann. Surg. 2022, 275, e586–e595. [Google Scholar] [CrossRef] [PubMed]
- Toso, A.; Aránguiz, O.; Céspedes, C.; Navarrete, O.; Hernández, C.; Vio, C.P.; Luco, M.; Casanello, P.; Kattan, J. Congenital diaphragmatic hernia: Phosphodiesterase-5 and arginase inhibitors prevent pulmonary vascular hypoplasia in rat lungs. Pediatr. Res. 2024, 95, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kreger, A.M.; Gittes, G.K. Intra-amniotic sildenafil treatment improves lung blood flow and pulmonary hypertension in congenital diaphragmatic hernia rats. Front. Bioeng. Biotechnol. 2023, 11, 1195623. [Google Scholar] [CrossRef]
- Liao, J.; Liu, W.; Zhang, L.; Li, Q.; Hou, F. Involvement of YAP and LATS1 in lung development in a rat model of nitrofen-induced congenital diaphragmatic hernia. Trop. J. Pharm. Res. 2020, 19, 541–547. [Google Scholar] [CrossRef]
- Okolo, F.C.; Zhang, G.; Rhodes, J.; Potoka, D.A. Intra-amniotic sildenafil treatment modulates vascular smooth muscle cell phenotype in the nitrofen model of congenital diaphragmatic hernia. Sci. Rep. 2018, 8, 17668. [Google Scholar] [CrossRef]
- Makanga, M.; Maruyama, H.; Dewachter, C.; Da Costa, A.M.; Hupkens, E.; De Medina, G.; Naeije, R.; Dewachter, L. Prevention of pulmonary hypoplasia and pulmonary vascular remodeling by antenatal simvastatin treatment in nitrofen-nduced congenital diaphragmatic hernia. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L672–L682. [Google Scholar] [CrossRef]
- Kattan, J.; Céspedes, C.; González, A.; Vio, C.P. Sildenafil stimulates and dexamethasone inhibits pulmonary vascular development in congenital diaphragmatic hernia rat lungs. Neonatology 2014, 106, 74–80. [Google Scholar] [CrossRef]
- Yoshida, S.; Eichelberger, O.; Ulis, M.; Kreger, A.M.; Gittes, G.K.; Church, J.T. Intra-Amniotic Sildenafil and Rosiglitazone Late in Gestation Ameliorate the Pulmonary Hypertension Phenotype in Congenital Diaphragmatic Hernia. J. Pediatr. Surg. 2024, 59, 1515–1525. [Google Scholar] [CrossRef]
- Okolo, F.; Zhang, G.; Rhodes, J.; Gittes, G.K.; Potoka, D.A. Intra-amniotic sildenafil treatment promotes lung growth and attenuates vascular remodeling in an experimental model of congenital diaphragmatic hernia. Fetal Diagn. Ther. 2020, 47, 787–799. [Google Scholar] [CrossRef]
- Shue, E.H.; Schecter, S.C.; Gong, W.; Etemadi, M.; Johengen, M.; Iqbal, C.; Derderian, S.C.; Oishi, P.; Fineman, J.R.; Miniati, D. Antenatal maternally-administered phosphodiesterase type 5 inhibitors normalize eNOS expression in the fetal lamb model of congenital diaphragmatic hernia. J. Pediatr. Surg. 2014, 49, 39–45. [Google Scholar] [CrossRef][Green Version]
- Hooijmans, C.R.; De Vries, R.B.; Ritskes-Hoitinga, M.; Rovers, M.M.; Leeflang, M.M.; IntHout, J.; Wever, K.E.; Hooft, L.; De Beer, H.; Kuijpers, T. Facilitating healthcare decisions by assessing the certainty in the evidence from preclinical animal studies. PLoS ONE 2018, 13, e0187271. [Google Scholar] [CrossRef] [PubMed]
- Hunniford, V.T.; Montroy, J.; Fergusson, D.A.; Avey, M.T.; Wever, K.E.; McCann, S.K.; Foster, M.; Fox, G.; Lafreniere, M.; Ghaly, M. Epidemiology and reporting characteristics of preclinical systematic reviews. PLoS Biol. 2021, 19, e3001177. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, M.P.; Russo, F.M.; Toelen, J.; Deprest, J. Medical interventions to reverse pulmonary hypoplasia in the animal model of congenital diaphragmatic hernia: A systematic review. Pediatr. Pulmonol. 2015, 50, 820–838. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yamamoto, H.; Gratacos, E.; Ge, X.; Verbeken, E.; Sueishi, K.; Hashimoto, S.; Vanamo, K.; Lerut, T.; Deprest, J. Lung development following diaphragmatic hernia in the fetal rabbit. Hum. Reprod. 2000, 15, 2483–2488. [Google Scholar] [CrossRef]
- Antounians, L.; Figueira, R.L.; Sbragia, L.; Zani, A. Congenital diaphragmatic hernia: State of the art in translating experimental research to the bedside. Eur. J. Pediatr. Surg. 2019, 29, 317–327. [Google Scholar] [CrossRef]
- Chiu, P.P.L. New insights into congenital diaphragmatic hernia—A surgeon’s introduction to CDH animal models. Front. Pediatr. 2014, 2, 36. [Google Scholar] [CrossRef]
- Jesudason, E.C. Small lungs and suspect smooth muscle: Congenital diaphragmatic hernia and the smooth muscle hypothesis. J. Pediatr. Surg. 2006, 41, 431–435. [Google Scholar] [CrossRef]
- van Loenhout, R.B.; Tseu, I.; Fox, E.K.; Huang, Z.; Tibboel, D.; Post, M.; Keijzer, R. The pulmonary mesenchymal tissue layer is defective in an in vitro recombinant model of nitrofen-induced lung hypoplasia. Am. J. Pathol. 2012, 180, 48–60. [Google Scholar] [CrossRef]
- Kunisaki, S.M.; Jiang, G.; Biancotti, J.C.; Ho, K.K.Y.; Dye, B.R.; Liu, A.P.; Spence, J.R. Human induced pluripotent stem cell-derived lung organoids in an ex vivo model of the congenital diaphragmatic hernia fetal lung. Stem Cells Transl. Med. 2021, 10, 98–114. [Google Scholar] [CrossRef]
- Delorimier, A.A.; Parker, H.R. Hypoplastic lungs in fetal lambs with surgically produced congenital diaphragmatic hernia. Surgery 1967, 62, 12–17. [Google Scholar]
- van Loenhout, R.B.; Tibboel, D.; Post, M.; Keijzer, R. Congenital diaphragmatic hernia: Comparison of animal models and relevance to the human situation. Neonatology 2009, 96, 137–149. [Google Scholar] [CrossRef]
- Montalva, L.; Zani, A. Assessment of the nitrofen model of congenital diaphragmatic hernia and of the dysregulated factors involved in pulmonary hypoplasia. Pediatr. Surg. Int. 2019, 35, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Kluth, D.; Kangah, R.; Reich, P.; Tenbrinck, R.; Tibboel, D.; Lambrecht, W. Nitrofen-induced diaphragmatic hernias in rats: An animal model. J. Pediatr. Surg. 1990, 25, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D. Overview of lung development in the newborn human. Neonatology 2017, 111, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.J.; Bowen, S.; Aoki, T.; Rebentisch, A.; Dawson, E.; Pettet, L.; Emerson, H.; Yu, B.; Wang, Z.; Yang, H. Former-preterm lambs have persistent alveolar simplification at 2 and 5 months corrected postnatal age. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 315, L816–L833. [Google Scholar] [CrossRef]
- Kovar, J.; Sly, P.D.; Willet, K.E. Postnatal alveolar development of the rabbit. J. Appl. Physiol. 2002, 93, 629–635. [Google Scholar] [CrossRef]
- Tschanz, S.A.; Salm, L.A.; Roth-Kleiner, M.; Barré, S.F.; Burri, P.H.; Schittny, J.C. Rat lungs show a biphasic formation of new alveoli during postnatal development. J. Appl. Physiol. 2014, 117, 89–95. [Google Scholar] [CrossRef]
- Brookes, S.T.; Whitely, E.; Egger, M.; Smith, G.D.; Mulheran, P.A.; Peters, T.J. Subgroup analyses in randomized trials: Risks of subgroup-specific analyses: Power and sample size for the interaction test. J. Clin. Epidemiol. 2004, 57, 229–236. [Google Scholar] [PubMed]
- Xiao, R.; Goldklang, M.P.; D’Armiento, J.M. Parenchymal airspace profiling: Sensitive quantification and characterization of lung structure evaluating parenchymal destruction. Am. J. Respir. Cell Mol. Biol. 2016, 55, 708–715. [Google Scholar] [CrossRef]
- Salaets, T.; Tack, B.; Gie, A.; Pavie, B.; Sindhwani, N.; Jimenez, J.; Regin, Y.; Allegaert, K.; Deprest, J.; Toelen, J. A semi-automated method for unbiased alveolar morphometry: Validation in a bronchopulmonary dysplasia model. PLoS ONE 2020, 15, e0239562. [Google Scholar] [CrossRef]
- Ochs, M.; Mühlfeld, C. Quantitative microscopy of the lung: A problem-based approach. Part 1: Basic principles of lung stereology. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L15–L22. [Google Scholar] [CrossRef]
- Kardon, G.; Ackerman, K.G.; McCulley, D.J.; Shen, Y.; Wynn, J.; Shang, L.; Bogenschutz, E.; Sun, X.; Chung, W.K. Congenital diaphragmatic hernias: From genes to mechanisms to therapies. Dis. Models Mech. 2017, 10, 955–970. [Google Scholar] [CrossRef]
- Merrell, A.J.; Ellis, B.J.; Fox, Z.D.; Lawson, J.A.; Weiss, J.A.; Kardon, G. Muscle connective tissue controls development of the diaphragm and is a source of congenital diaphragmatic hernias. Nat. Genet. 2015, 47, 496–504. [Google Scholar] [CrossRef]
- Abman, S.H.; Baker, S.H.A.M.C.; Balasubramaniam, M.V. Growth and Development of the Lung Circulation: Mechanisms and Clinical Implications. In The Newborn Lung: Neonatology Questions and Controversies; Bancalari, E., Ed.; Saunders: Philadelphia, PA, USA, 2008; pp. 50–72. ISBN 978-1-4160-3166-6. [Google Scholar] [CrossRef]
- Russo, F.M.; De Bie, F.; Hodges, R.; Flake, A.; Deprest, J. Sildenafil for antenatal treatment of congenital diaphragmatic hernia: From bench to bedside. Curr. Pharm. Des. 2019, 25, 601–608. [Google Scholar] [CrossRef]
- Loukogeorgakis, S.P.; Michielin, F.; Al-Juffali, N.; Jimenez, J.; Shibuya, S.; Allen-Hyttinen, J.; Eastwood, M.P.; Alhendi, A.S.; Davidson, J.; Naldi, E. Prenatal VEGF Nano-Delivery Reverses Congenital Diaphragmatic Hernia-Associated Pulmonary Abnormalities. Am. J. Respir. Crit. Care Med. 2025, 211, 992–1006. [Google Scholar] [CrossRef]
- Yun, E.J.; Lorizio, W.; Seedorf, G.; Abman, S.H.; Vu, T.H. VEGF and endothelium-derived retinoic acid regulate lung vascular and alveolar development. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L287–L298. [Google Scholar] [CrossRef]
- Kelter, R. Reducing the false discovery rate of preclinical animal research with Bayesian statistical decision criteria. Stat. Methods Med. Res. 2023, 32, 1880–1901. [Google Scholar] [CrossRef]
- Walley, R.; Sherington, J.; Rastrick, J.; Detrait, E.; Hanon, E.; Watt, G. Using Bayesian analysis in repeated preclinical in vivo studies for a more effective use of animals. Pharm. Stat. 2016, 15, 277–285. [Google Scholar] [CrossRef]
- Stern, H.S. A test by any other name: P values, Bayes factors, and statistical inference. Multivar. Behav. Res. 2016, 51, 23–29. [Google Scholar] [CrossRef]
- Jeon, M.; De Boeck, P. Decision qualities of Bayes factor and p value-based hypothesis testing. Psychol. Methods 2017, 22, 340. [Google Scholar] [CrossRef]
- Kousholt, B.S.; Præstegaard, K.F.; Stone, J.C.; Thomsen, A.F.; Johansen, T.T.; Ritskes-Hoitinga, M.; Wegener, G. Reporting quality in preclinical animal experimental research in 2009 and 2018: A nationwide systematic investigation. PLoS ONE 2022, 17, e0275962. [Google Scholar] [CrossRef] [PubMed]
- Avey, M.T.; Moher, D.; Sullivan, K.J.; Fergusson, D.; Griffin, G.; Grimshaw, J.M.; Hutton, B.; Lalu, M.M.; Macleod, M.; Marshall, J. The devil is in the details: Incomplete reporting in preclinical animal research. PLoS ONE 2016, 11, e0166733. [Google Scholar] [CrossRef]
- Russo, F.M.; Benachi, A.; Van Mieghem, T.; De Hoon, J.; Van Calsteren, K.; Annaert, P.; Tréluyer, J.M.; Allegaert, K.; Deprest, J. Antenatal sildenafil administration to prevent pulmonary hypertension in congenital diaphragmatic hernia (SToP-PH): Study protocol for a phase I/IIb placenta transfer and safety study. Trials 2018, 19, 524. [Google Scholar] [CrossRef]
- Pels, A.; Derks, J.; Elvan-Taspinar, A.; Van Drongelen, J.; De Boer, M.; Duvekot, H.; Van Laar, J.; Van Eyck, J.; Al-Nasiry, S.; Sueters, M. Maternal sildenafil vs placebo in pregnant women with severe early-onset fetal growth restriction: A randomized clinical trial. JAMA Netw. Open 2020, 3, e205323. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.; Cornforth, C.; Jackson, R.; Harrold, J.; Turner, M.A.; Kenny, L.; Baker, P.N.; Johnstone, E.D.; Khalil, A.; von Dadelszen, P. The efficacy of sildenafil therapy in dismal prognosis early-onset intrauterine growth restriction: The STRIDER RCT. Effic. Mech. Eval. 2024, 11. [Google Scholar] [CrossRef] [PubMed]
- von Dadelszen, P.; Audibert, F.; Bujold, E.; Bone, J.N.; Sandhu, A.; Li, J.; Kariya, C.; Chung, Y.; Lee, T.; Au, K. Halting the Canadian STRIDER randomised controlled trial of sildenafil for severe, early-onset fetal growth restriction: Ethical, methodological, and pragmatic considerations. BMC Res. Notes 2022, 15, 244. [Google Scholar] [CrossRef]
- McKinlay, C.J.; Anderson, C.; Cheong, J.L.; Gordon, A.; Harris, S.L.; Hurrion, E.M.; Ireland, S.; Koorts, P.; Lui, K.; Mackay, L. Childhood outcomes after maternal antenatal sildenafil treatment for severe early-onset fetal growth restriction: A randomized trial (STRIDER NZAus). J. Perinatol. 2024, 44, 396–403. [Google Scholar] [CrossRef]
- Turner, J.M.; Russo, F.; Deprest, J.; Mol, B.W.; Kumar, S. Phosphodiesterase-5 inhibitors in pregnancy: Systematic review and meta-analysis of maternal and perinatal safety and clinical outcomes. BJOG Int. J. Obstet. Gynaecol. 2022, 129, 1817–1831. [Google Scholar] [CrossRef]
- Russo, F.M.; Hooper, S.; Tibboel, D.; DeKoninck, P.; Benachi, A.; Tréluyer, J.M.; Allegaert, K.; Kumar, S.; Deprest, J. Antenatal therapy with sildenafil: Don’t throw the baby out with the bathwater. Ultrasound Obstet. Gynecol. 2019, 53, 274. [Google Scholar] [CrossRef]
- Kumar, S.; Tarnow-Mordi, W.; Mol, B.W.; Flenady, V.; Liley, H.G.; Badawi, N.; Walker, S.; Hyett, J.; Seidler, A.L.; Callander, E.; et al. Intrapartum Sildenafil to Improve Perinatal Outcomes: A Randomized Clinical Trial. JAMA 2025, 334, 149–159. [Google Scholar] [CrossRef]
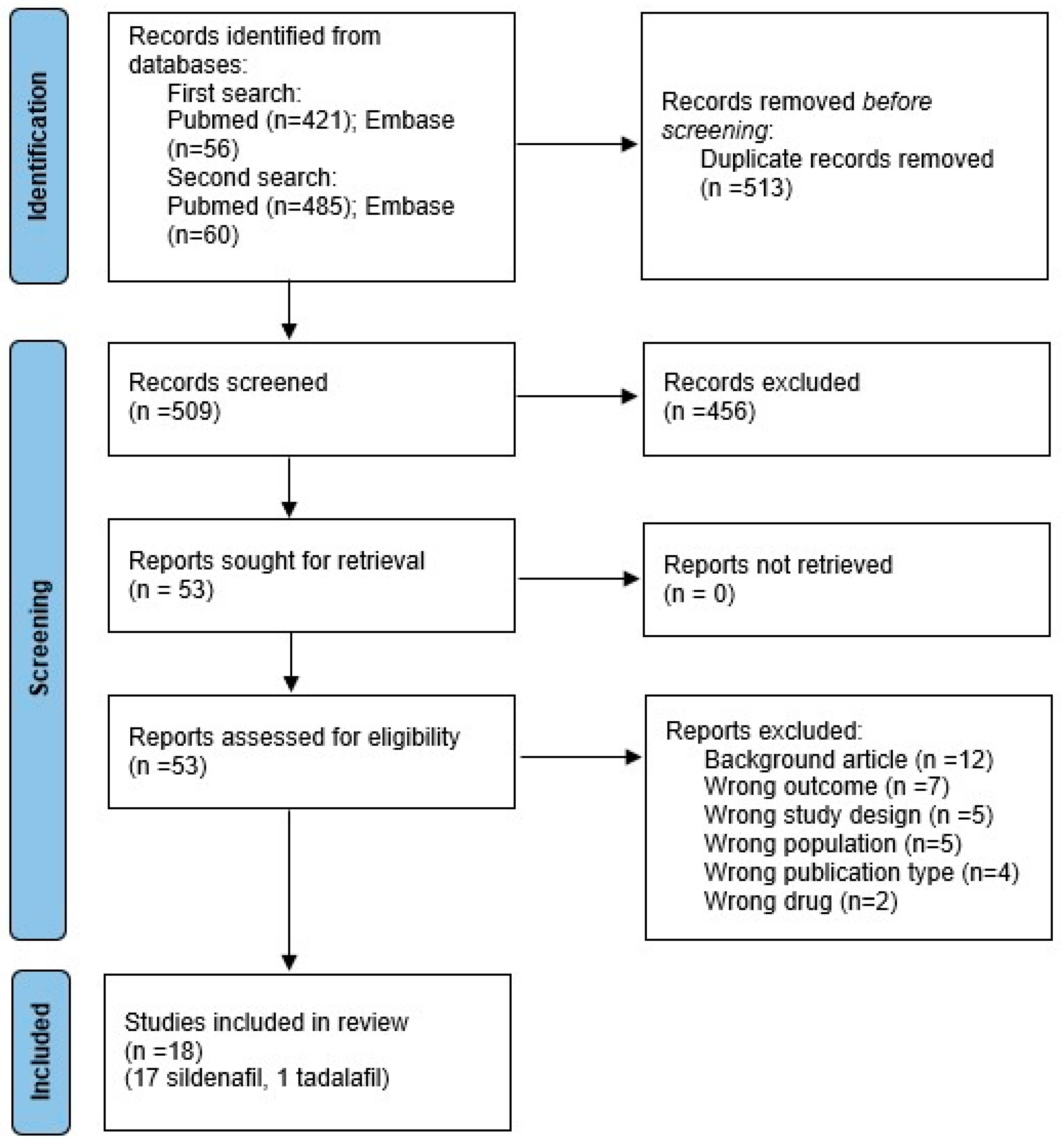

| First Author, Year | Model-Species | Time of Induction CDH | Day of Harvesting | Drug | Day of Administration | Dose | Route |
|---|---|---|---|---|---|---|---|
| Kashyap, 2019 [27] | Surgery-Lamb | E80 | E138 | Sildenafil | E105–E138 | 0.21 mg/kg/h | IV |
| Kattan, 2014 [53] | Nitrofen-Rat | E9.5 | E22 | Sildenafil | E11.5–E21.5 | 90 mg/kg | oral |
| Lemus-Varela, 2014 [46] | Nitrofen-Rat | E9 | E21 | Sildenafil | E16–E20 | 100 mg/kg | oral |
| Liao, 2020 [50] | Nitrofen-Rat | E9.5 | E21.5 | Sildenafil | E11.5–E21.5 | 100 mg/kg | oral |
| Luong, 2011 [21] | Nitrofen-Rat | E9.5 | E21.5 | Sildenafil | E11.5–E20.5 | 100 mg/kg | SC |
| Makanga, 2015 [52] | Nitrofen-Rat | E9.5 | E21.5 | Sildenafil | E11.5–E21.5 | 100 mg/kg | oral |
| Mesas-Burgos, 2016 [25] | Nitrofen-Rat | E9.5 | E21.5 | Sildenafil | E11.5–E20.5 | 100 mg/kg | SC |
| Mous, 2016 [24] | Nitrofen-Rat | E9.5 | E21 | Sildenafil | E17.5–E20.5 | 100 mg/kg | oral |
| Mous, 2018 [23] | Nitrofen-Rat | E9.5 | E21 | Sildenafil | E17.5–E20.5 | 100 mg/kg | oral |
| Okolo, 2018 [51] | Nitrofen-Mouse | E8.5 | E18.5 | Sildenafil | E12.5 | 10 µg | IA |
| Okolo, 2020 [55] | Nitrofen-Mouse | E8.5 | P0 | Sildenafil | E10.5 (early) E15.5 (late) | 4 µg | IA |
| Russo, 2016 [26] | Surgery-Rabbit | E23 | E31 | Sildenafil | E24–E30 | 10 mg/kg | SC |
| Russo, 2022 [47] | Surgery-Rabbit | E23 | E30 | Sildenafil | E24–E30 | 10 mg/kg | SC |
| Toso, 2024 [48] | Nitrofen-Rat | E9.5 | E21 | Sildenafil | E14–E21 | 100 mg/kg | oral |
| Shue, 2014 [56] | Surgery-Lamb | E85–90 | E-135 | Tadalafil | E85–90–E135 | 1–2 mg/kg | oral |
| Yamamoto, 2014 [22] | Nitrofen-Rat | E9.5 | E21.5 | Sildenafil | E11.5–E20.5 | 100 mg/kg | SC |
| Yoshida, 2023 [49] | Nitrofen-Rat | E9.5 | E20.5 | Sildenafil | E13.5 (early) E15.5 (late) | 24 µg (early) 80 µg (late) | IA |
| Yoshida, 2024 [54] | Nitrofen-Rat | E9.5 | E21.5 | Sildenafil | E19.5 | 80 µg | IA |
| Study | 1. Adequate Sequence Generation | 2. Baseline Characteristics Comparable | 3. Allocation Concealment | 4. Random Housing | 5. Investigators/Caregivers Blinding | 6. Random Outcome Assessment | 7. Blinding Outcome Assessment | 8. Incomplete Outcome Data Adequate Addressed | 9. Free of Reporting Bias | 10. Other Biases Absent | Randomization Mentioned? | Blinding Mentioned? | Sample Size and Calculation Mentioned? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kashyap, 2019 [27] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | No | No | No |
| Kattan, 2014 [53] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | No | No | No |
| Lemus-Varela, 2014 [46] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Yes | Unclear | Yes | Yes | No |
| Liao, 2020 [50] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | No | No |
| Luong, 2011 [21] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | No | No |
| Makanga, 2015 [52] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Unclear | Yes | Yes | Yes | No |
| Mesas-Burgos, 2016 [25] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Unclear | Unclear | Yes | Yes | No |
| Mous, 2016 [24] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | No | No | No |
| Mous, 2018 [23] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | No | No | No |
| Okolo, 2018 [51] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | No | No | No |
| Okolo, 2020 [55] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | No | No | No |
| Russo, 2016 [26] | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes | Yes |
| Russo, 2022 [47] | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes | Yes |
| Shue, 2014 [56] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | No | No |
| Toso, 2024 [48] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes | No |
| Yamamoto, 2014 [22] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Unclear | Yes | Yes | Yes | No |
| Yoshida, 2023 [49] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | No | No |
| Yoshida, 2024 [54] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | No | No |
| Outcome | Subgroup | K | Effect Size | 95% CrI | Tau | 95% CrI | BF10 | p-Value Effect | Evidence for Effect | BFrf | p-Value Heterogeneity | Evidence for Heterogeneity | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | Lower Limit | Upper Limit | |||||||||||
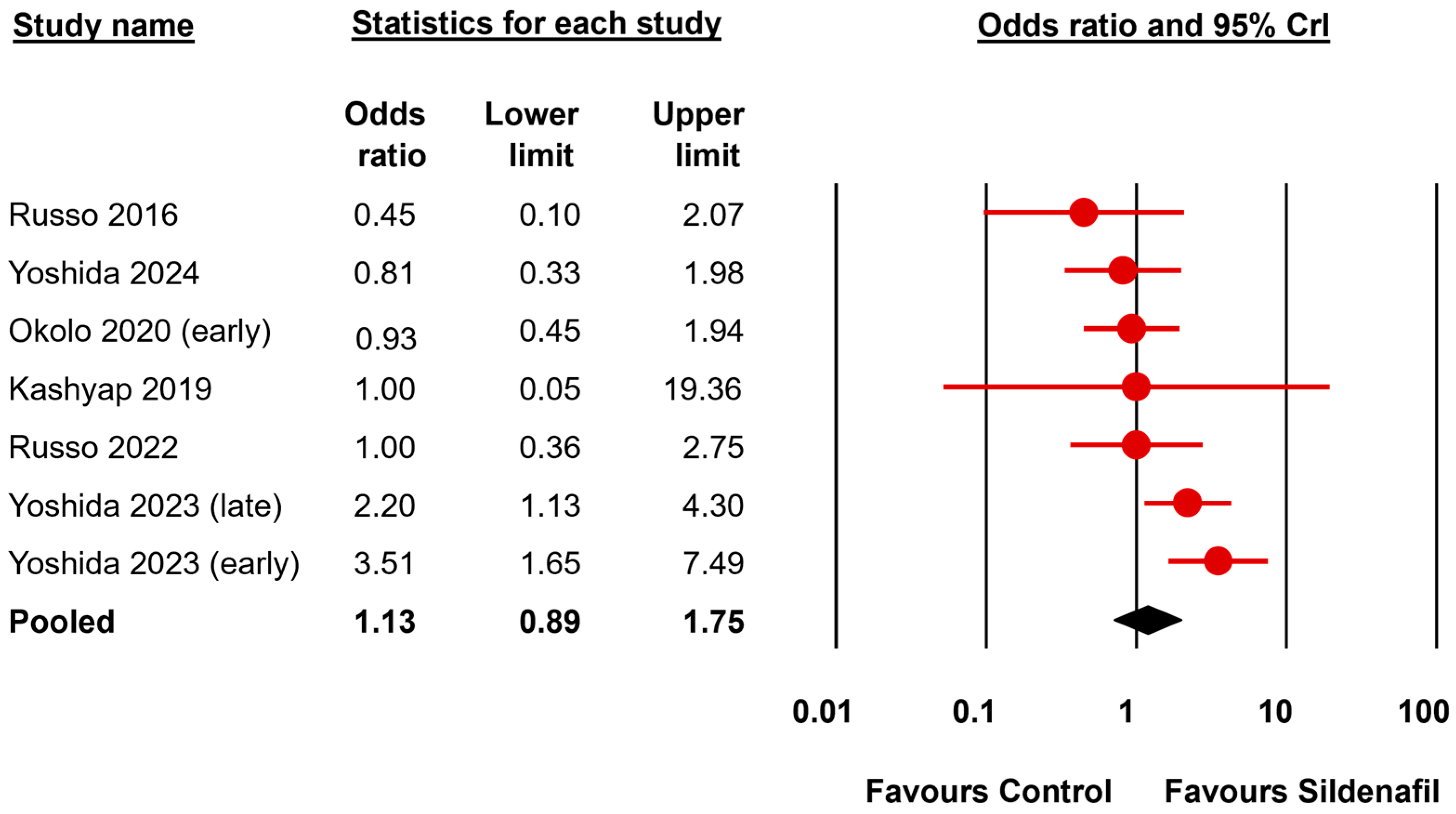
| Fetal survival | All | 7 | 1.13 a | 0.89 | 1.75 | 1.40 | 1.00 | 2.62 | 1.25 | 0.305 | undecided for | 3.83 | 0.050 | moderate for |
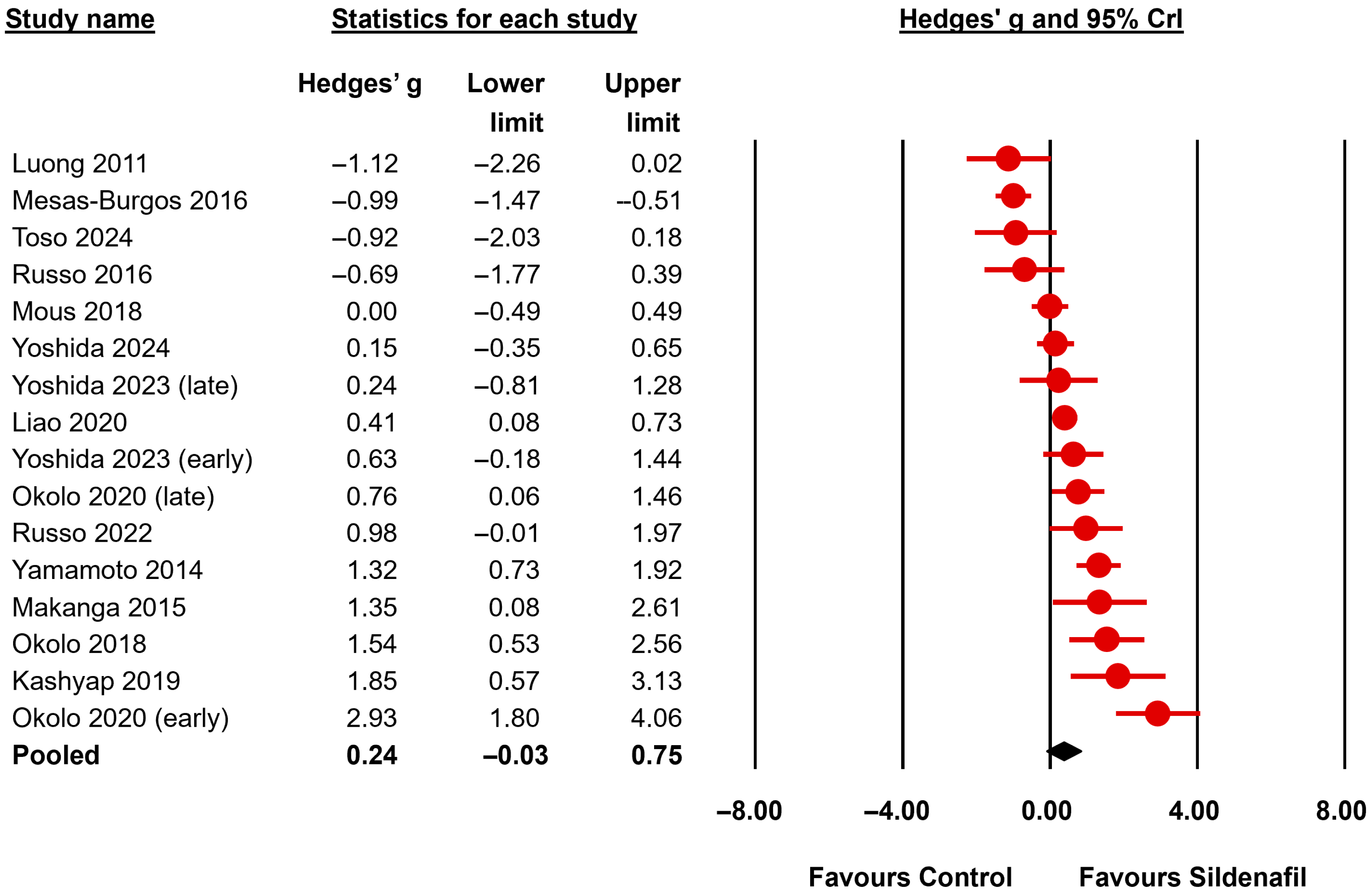
| LBWR | All | 16 | 0.24 b | −0.03 | 0.75 | 0.87 | 0.53 | 1.36 | 2.04 | 0.051 | undecided for | >106 | <0.001 | extreme for |
| Nitrofen | 13 | 0.18 b | −0.09 | 0.71 | 0.88 | 0.52 | 1.41 | 1.38 | 0.076 | undecided for | >106 | <0.001 | extreme for | |
| Distal airway complexity (MTBD) | All | 3 | −0.64 b | −1.21 | −0.07 | 0.36 | 0.07 | 1.24 | 7.95 | <0.001 | moderate for | 0.93 | 0.415 | undecided against |
| Mean saccular airspace diameter (D2 score) | All | 2 | 1.09 b | −0.10 | 1.82 | 0.69 | 0.09 | 2.40 | 7.61 | <0.001 | moderate for | 1.72 | 0.906 | Undecided for |
| Mean linear intercept | All | 4 | −0.01 b | −0.53 | 0.45 | 0.79 | 0.26 | 1.80 | 0.67 | 0.731 | undecided against | 287.52 | <0.001 | extreme for |
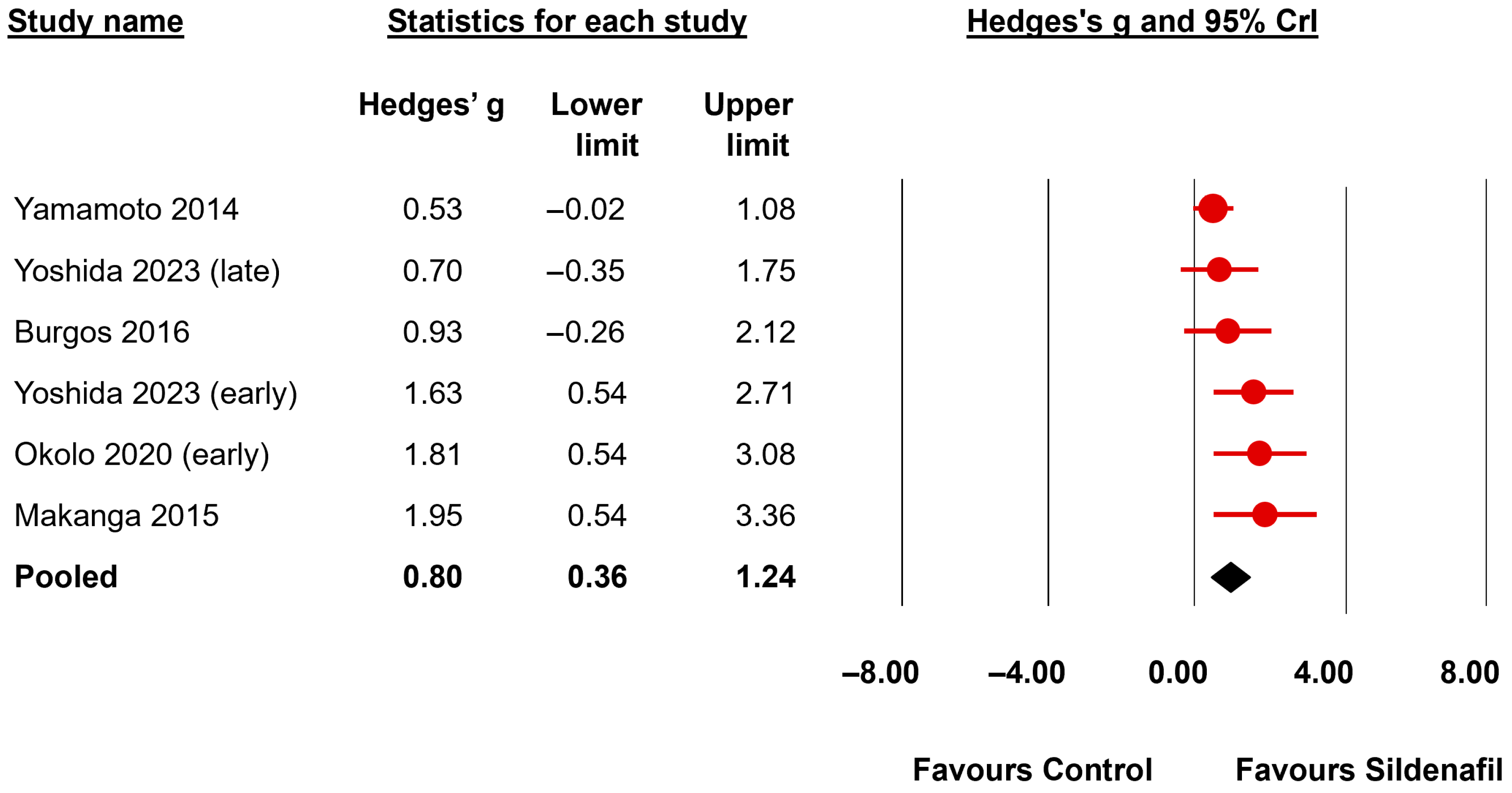
| Radial alveolar count | All | 6 | 0.80 b | 0.36 | 1.24 | 0.14 | 0.00 | 0.67 | 167.57 | <0.001 | very strong for | 0.89 | 0.175 | undecided against |
| Interalveolar septa thickness | All | 4 | −1.11 b | −1.57 | −0.47 | 0.36 | 0.07 | 1.36 | 56.86 | <0.001 | very strong for | 0.83 | 0.304 | undecided against |
| Total lung capacity | All | 2 | 0.83 b | −0.01 | 1.70 | 0.53 | 0.08 | 1.96 | 6.91 | <0.001 | moderate for | 1.25 | 0.973 | undecided for |
| Compliance | All | 2 | 0.73 b | −0.07 | 1.56 | 0.49 | 0.08 | 1.76 | 5.19 | <0.001 | moderate for | 1.22 | 0.675 | undecided for |
| Elastance | All | 2 | −0.54 b | −1.29 | 0.18 | 0.43 | 0.08 | 1.50 | 2.54 | 0.009 | undecided for | 1.15 | 0.373 | undecided for |
| Outcome | Subgroup | K | Hedges’ g | 95% CrI | Tau | 95% CrI | BF10 | p-Value Effect | Evidence for Effect | BFrf | p-Value Heterogeneity | Evidence for Heterogeneity | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | Lower Limit | Upper Limit | |||||||||||
| Medial wall thickness | All | 12 | −1.29 | −1.74 | −0.74 | 0.66 | 0.36 | 1.15 | 1499 | <0.001 | extreme for | >106 | <0.001 | extreme for |
| Nitrofen | 10 | −1.12 | −1.59 | −0.55 | 0.62 | 0.34 | 1.12 | 332.4 | <0.001 | extreme for | >106 | <0.001 | extreme for | |
| Proportionate medial thickness | All | 3 | −1.94 | −3.18 | 0.18 | 1.37 | 0.10 | 4.59 | 6.75 | <0.001 | moderate for | 2.33 | 0.024 | undecided for |
| Proportionate adventitial thickness | All | 2 | −0.46 | −1.18 | 0.23 | 0.42 | 0.08 | 1.48 | 1.90 | 0.081 | undecided for | 1.11 | 0.176 | undecided for |
| Pulmonary vascular volume | All | 2 | 0.06 | −0.61 | 0.74 | 0.33 | 0.07 | 1.09 | 0.74 | 0.867 | undecided against | 0.85 | 0.214 | undecided against |
| VEGF expression | All | 5 | 1.29 | 0.01 | 2.29 | 0.72 | 0.01 | 2.69 | 10.62 | 0.001 | strong for | 3.36 | 0.004 | moderate for |
| eNOS expression | All | 2 | 0.60 | −0.32 | 2.07 | 1.06 | 0.00 | 3.64 | 2.66 | 0.142 | undecided for | 5.16 | 0.007 | moderate for |
| RV/LV + S | All | 4 | −0.32 | −0.93 | 0.23 | 0.32 | 0.07 | 0.96 | 1.20 | 0.128 | undecided for | 0.90 | 0.293 | undecided against |
| RV wall thickness | All | 2 | −1.39 | −3.56 | 0.48 | 2.08 | 0.13 | 6.01 | 2.33 | 0.008 | undecided for | 6.17 | <0.001 | moderate for |
| Doppler AT/ET | All | 2 | 0.94 | −0.05 | 1.83 | 0.61 | 0.08 | 2.34 | 8.30 | <0.001 | moderate for | 1.32 | 0.778 | undecided for |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hundscheid, T.M.; Amodeo, I.; Cavallaro, G.; Hooijmans, C.R.; Bartoš, F.; Villamor, E. Antenatal Sildenafil for Congenital Diaphragmatic Hernia: A Systematic Review and Bayesian Meta-Analysis of Preclinical Studies. Biomedicines 2025, 13, 2274. https://doi.org/10.3390/biomedicines13092274
Hundscheid TM, Amodeo I, Cavallaro G, Hooijmans CR, Bartoš F, Villamor E. Antenatal Sildenafil for Congenital Diaphragmatic Hernia: A Systematic Review and Bayesian Meta-Analysis of Preclinical Studies. Biomedicines. 2025; 13(9):2274. https://doi.org/10.3390/biomedicines13092274
Chicago/Turabian StyleHundscheid, Tamara M., Ilaria Amodeo, Giacomo Cavallaro, Carlijn R. Hooijmans, František Bartoš, and Eduardo Villamor. 2025. "Antenatal Sildenafil for Congenital Diaphragmatic Hernia: A Systematic Review and Bayesian Meta-Analysis of Preclinical Studies" Biomedicines 13, no. 9: 2274. https://doi.org/10.3390/biomedicines13092274
APA StyleHundscheid, T. M., Amodeo, I., Cavallaro, G., Hooijmans, C. R., Bartoš, F., & Villamor, E. (2025). Antenatal Sildenafil for Congenital Diaphragmatic Hernia: A Systematic Review and Bayesian Meta-Analysis of Preclinical Studies. Biomedicines, 13(9), 2274. https://doi.org/10.3390/biomedicines13092274







