Neuropsychomotor Development of Children Exposed to SARS-CoV-2 in Utero During COVID-19 Pandemic
Abstract
1. Introduction
2. Material and Methods
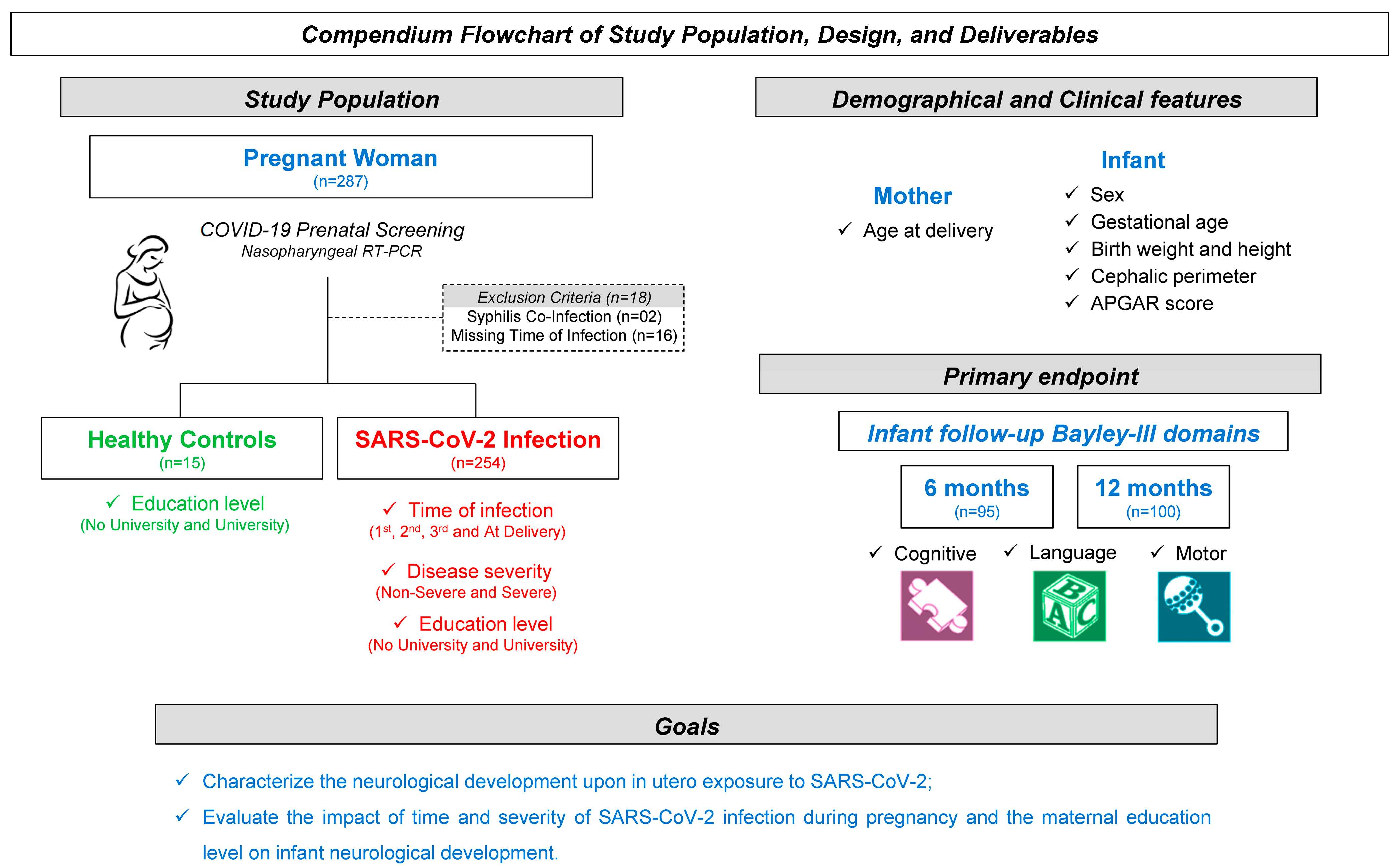
2.1. Population
2.2. Data Collection Procedures, Instruments, and Analyzed Variables
2.3. Statistical Analysis
3. Results
3.1. Characterization of Study Population
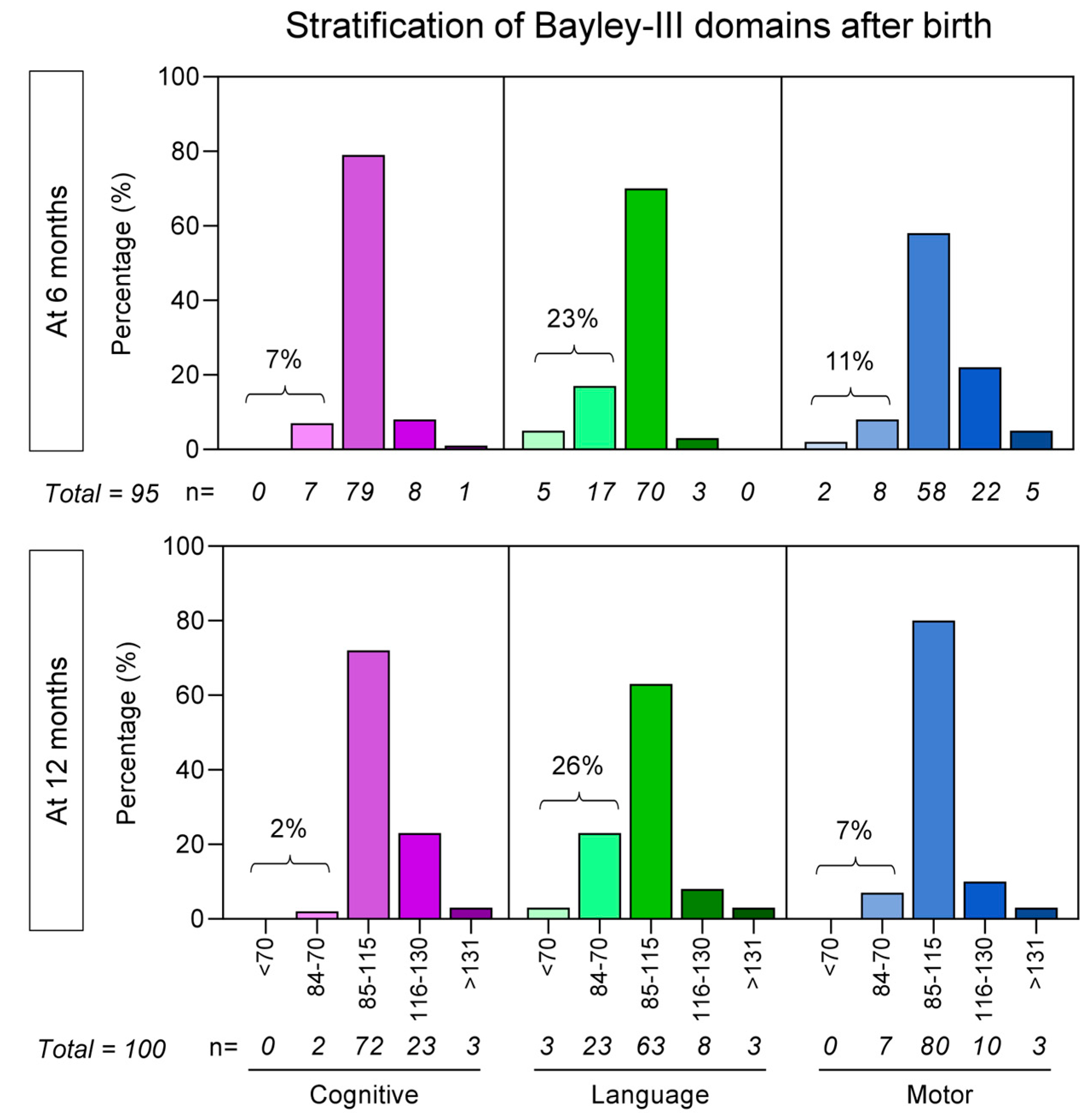
3.2. Bayley-III Developmental Delays Regardless of Unaltered APGAR Scores
3.3. Bayley-III Domains According to the Time of Maternal SARS-CoV-2 Infection
3.4. Bayley-III Domains According to the Severity of Maternal SARS-CoV-2 Infection
3.5. Bayley-III Domains According to the Caregiver Educational Level
3.6. Regression Models of Association Between Bayley-III Domains and Maternal Features
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez-López, F.R.; Savirón-Cornudella, R.; Chedraui, P.; López-Baena, M.T.; Pérez-Roncero, G.; Sanz-Arenal, A.; Narváez-Salazar, M.; Dieste-Pérez, P.; Tajada, M. Obstetric and perinatal outcomes of pregnancies with COVID 19: A systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2022, 35, 9742–9758. [Google Scholar] [CrossRef]
- Ayesa-Arriola, R.; Castro Quintas, Á.; Ortiz-García de la Foz, V.; Miguel Corredera, M.; San Martín González, N.; Murillo-García, N.; Neergaard, K.; Fañanás Saura, L.; de Las Cuevas-Terán, I. Exploring the impact of COVID-19 on newborn neurodevelopment: A pilot study. Sci. Rep. 2023, 13, 2983. [Google Scholar] [CrossRef]
- Fernandes, G.M.; Motta, F.; Sasaki, L.M.P.; de Arruda, J.C.; da Motta, L.V.; de Sousa, J.M.; Silva, G.C.N.; da Silva, L.A.; da Motta, M.M.G.; Leite, A.P.; et al. Pregnancy Outcomes and Child Development Effects of SARS-CoV-2 Infection (PROUDEST Trial): Protocol for a Multicenter, Prospective Cohort Study. JMIR Res. Protoc. 2021, 10, e26477. [Google Scholar] [CrossRef]
- Auriti, C.; Bucci, S.; De Rose, D.U.; Coltella, L.; Santisi, A.; Martini, L.; Maddaloni, C.; Bersani, I.; Lozzi, S.; Campi, F.; et al. Maternal-Fetal Infections (Cytomegalovirus, Toxoplasma, Syphilis): Short-Term and Long-Term Neurodevelopmental Outcomes in Children Infected and Uninfected at Birth. Pathogens 2022, 11, 1278. [Google Scholar] [CrossRef]
- Madaschi, V.; Paula, C.S. Medidas de avaliação do desenvolvimento infantil: Uma revisão da literatura nos últimos cinco anos. Cad. Pós Grad. Disturbios Desenvolv. 2011, 11, 52–56. Available online: https://editorarevistas.mackenzie.br/index.php/cpgdd/article/view/11173/6936 (accessed on 15 May 2025).
- Mulkey, S.B.; Arroyave-Wessel, M.; Peyton, C.; Bulas, D.I.; Fourzali, Y.; Jiang, J.; Russo, S.; McCarter, R.; Msall, M.E.; du Plessis, A.J.; et al. Neurodevelopmental Abnormalities in Children With In Utero Zika Virus Exposure Without Congenital Zika Syndrome. JAMA Pediatr. 2020, 174, 269–276. [Google Scholar] [CrossRef]
- Santos, C.A.D.; Paula, A.P.; Filho, G.G.F.; Alves, M.M.; Nery, A.F.; Pontes, M.G.A.; Macedo, E.Y.L.; Oliveira, R.M., Jr.; Freitas, S.M.; Lima, S.; et al. Developmental impairment in children exposed during pregnancy to maternal SARS-COV2: A Brazilian cohort study. Int. J. Infect. Dis. 2024, 139, 146–152. [Google Scholar] [CrossRef]
- Hardie, I.; Marryat, L.; Murray, A.; King, J.; Okelo, K.; Boardman, J.P.; Lombardo, M.V.; Stock, S.J.; Wood, R.; Auyeung, B. Early childhood developmental concerns following SARS-CoV-2 infection and COVID-19 vaccination during pregnancy: A Scottish population-level retrospective cohort study. Lancet Child Adolesc. Health 2025, 9, 162–171. [Google Scholar] [CrossRef]
- Alves de Araujo, D., Jr.; Motta, F.; Fernandes, G.M.; Castro, M.E.C.; Sasaki, L.M.P.; Luna, L.P.; Rodrigues, T.S.; Kurizky, P.S.; Soares, A.A.S.M.; Nobrega, O.T.; et al. Neuroimaging assessment of pediatric cerebral changes associated with SARS-CoV-2 infection during pregnancy. Front. Pediatr. 2023, 11, 1194114. [Google Scholar] [CrossRef]
- Araújo, L.A.; Veloso, C.F.; Souza, M.C.; Azevedo, J.M.C.; Tarro, G. The potential impact of the COVID-19 pandemic on child growth and development: A systematic review. J. Pediatr. 2021, 97, 369–377. [Google Scholar] [CrossRef]
- Shuffrey, L.C.; Firestein, M.R.; Kyle, M.H.; Casaletto, E.; Levin-Schwartz, A.; Kuo, C.; Marder, J.; Abughali, M.; Kernie, S.G.; Glicksberg, B.S.; et al. Association of Birth During the COVID-19 Pandemic With Neurodevelopmental Status at 6 Months in Infants With and Without In Utero Exposure to Maternal SARS-CoV-2 Infection. JAMA Pediatr. 2022, 176, e215563. [Google Scholar] [CrossRef]
- Firestein, M.R.; Shuffrey, L.C.; Hu, Y.; Casaletto, E.; Ment, L.R.K.K.; Scheinost, D.; Rothman, S.M.; Liston, C.; Canetta, S.E.; Bansal, R.; et al. Assessment of Neurodevelopment in Infants With and Without Exposure to Asymptomatic or Mild Maternal SARS-CoV-2 Infection During Pregnancy. JAMA Netw. Open 2023, 6, e237396. [Google Scholar] [CrossRef]
- Sadhwani, A.; Asaro, L.A.; Goldberg, C.S.; Ware, J.; Butcher, J.; Gaies, M.; Smith, C.; Alexander, J.L.; Wypij, D.; Agus, M.S.D. Impact of tight glycemic control and hypoglycemia after pediatric cardiac surgery on neurodevelopmental outcomes at three years of age: Findings from a randomized clinical trial. BMC Pediatr. 2022, 22, 531. [Google Scholar] [CrossRef]
- Madaschi, V. Tradução, Adaptação Transcultural e Evidências de Validade das Escalas Bayley III de Desenvolvimento Infantil em uma População do Município de Barueri, São Paulo. Dissertação de Mestrado, Universidade Presbiteriana Mackenzie, São Paulo, 2012. Available online: https://adelpha-api.mackenzie.br/server/api/core/bitstreams/766953f4-f9c2-4db5-9a15-6712ad49acc1/content (accessed on 18 May 2025).
- Szele, A.S.; Gáll, J.M.; Nagy, B.E. Effect of medically assisted reproduction (MAR) and pregnancy planning on bayley-III screening test subscales in preterm infants at 12 months of corrected age: A cross-sectional study. Ital. J. Pediatr. 2022, 48, 69. [Google Scholar] [CrossRef]
- Vandormael, C.; Schoenhals, L.; Hüppi, P.S.; Filippa, M.; Borradori Tolsa, C. Language in Preterm Born Children: Atypical Development and Effects of Early Interventions on Neuroplasticity. Neural Plast. 2019, 2019, 6873270. [Google Scholar] [CrossRef]
- Bayley, N. Bayley Scales of Infant and Toddler Development, 3rd ed.; Bayley-III®: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- Deoni, S.C.; Beauchemin, J.; Volpe, A.; D’Sa, V. Resonance Consortium. Impact of the COVID-19 pandemic on early child cognitive development: Initial findings in a longitudinal observational study of child health. medRxiv 2021. [Google Scholar] [CrossRef]
- Johnson, S.; Moore, T.; Marlow, N. Using the bayley-III to assess neurodevelopmental delay: Which cut-off should be used? Pediatr. Res. 2014, 75, 670–674. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, F.; Wang, R.; Chanda, R.; Suen, L.K.; Zhou, Y.; Hou, W.; Sun, C. The deadly coronaviruses: The 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J. Autoimmun. 2020, 109, 102434. [Google Scholar] [CrossRef]
- Fegert, J.M.; Vitiello, B.; Plener, P.L.; Clemens, V. Challenges and burden of the Coronavirus 2019 (COVID-19) pandemic for child and adolescent mental health: A narrative review to highlight clinical and research needs in the acute phase and the long return to normality. Child Adolesc. Psychiatry Ment. Health 2020, 14, 20. [Google Scholar] [CrossRef]
- Houweling, T.A.J.; Oude Groeniger, J.; Jansen, P.W.; van Lier, P.; Horoz, N.; Buil, M.; van Lenthe, F.J. Trajectories of socioeconomic inequality in early child development: A cohort analysis. Int. J. Equity Health 2022, 21, 79. [Google Scholar] [CrossRef]
| Clinical and Demographic Parameters | Healthy Controls * (n = 15) | SARS-CoV-2 Infection (n = 254) |
|---|---|---|
| Full term, n (%) | 12/15 (80.0) | 220/247 (89.1) |
| Gestational age (weeks), mean ± SD | 38.6 (±2.3) | 38.2 (±1.7) |
| Female, n (%) | 10/15 (56.7) | 129/254 (50.8) |
| Birth weight (grams) | 3241.4 (±523.0) | 3131.7 (±533.4) |
| Appropriate weight for gestational age, n (%) | 14/15 (93.3) | 199/247 (80.6) |
| Birth height (cm), mean ± SD | 48.5 (±3.12) | 48.2 (±2.57) |
| Cephalic perimeter (cm), mean ± SD | 35.0 (±1.01) | 34.4 (±1.98) |
| Mother age at delivery (years), mean ± SD | 31.7 (±3.65) | 34.5 (±3.19) |
| APGAR 1st-minute score | ||
| Score (mean ± SD) | 7.1 (±2.0) | 8.0 (±1.2) |
| ≤7 | 5/14 (35.7) | 50/241 (20.7) |
| >7 | 9/14 (64.3) | 191/241 (79.3) |
| APGAR 5th-minute score | ||
| Score (mean ± SD) | 8.8 (±0.70) | 8.9 (±0.69) |
| ≤7 | 1/14 (7.1) | 10/241 (4.1) |
| >7 | 13/14 (92.9) | 231/241 (95.9) |
| Bayley-III Scale at 6 months of age | ||
| Normal, n (%) | 5/5 (100.0) | 59/95 (62.1) |
| Delay, n (%) | 0/5 (0.0) | 36/95 (37.9) |
| Cognitive, n (%) | 0/5 (0.0) | 20/36 (55.6) |
| Language, n (%) | 0/5 (0.0) | 23/36 (63.9) |
| Motor, n (%) | 0/5 (0.0) | 12/36 (33.3) |
| Bayley-III Scale at 12 months of age | ||
| Normal, n (%) | 3/3 (100.0) | 67/100 (67.0) |
| Delay, n (%) | 0/3 (0.0) | 33/100 (33.0) |
| Cognitive, n (%) | 0/3 (0.0) | 4/33 (12.2) |
| Language, n (%) | 0/3 (0.0) | 26/33 (78.8) |
| Motor, n (%) | 0/3 (0.0) | 13/33 (39.4) |
| Time of Maternal SARS-CoV-2 Infection | ||
| 1st trimester, n (%) | n/a | 50/254 (19.7) |
| 2nd trimester, n (%) | n/a | 78/254 (30.7) |
| 3rd trimester, n (%) | n/a | 105/254 (41.3) |
| At delivery, n (%) | n/a | 21/254 (8.3) |
| Severity of Maternal SARS-CoV-2 Infection | ||
| Non-severe, n (%) | n/a | 212/250 (84.8) |
| Severe, n (%) | n/a | 42/250 (16.8) |
| Caregiver Education Level | ||
| No university degree, n (%) | 11/15 (73.3) | 173/243 (71.2) |
| University degree, n (%) | 4/15 (26.7) | 70/243 (28.8) |
| Bayley-III Domains * | Time of Maternal SARS-CoV-2 Infection | p-Values | ||||
|---|---|---|---|---|---|---|
| HC | 1st Trimester | 2nd Trimester | 3rd Trimester | At Delivery | ANOVA or χ2 | |
| 6 months after birth | ||||||
| Cognitive, mean (±SD) | 110.0 (±11) | 111.7 (±23) | 102.0 (±12) | 98.3 (±12) 1st | 90.0 (±10) 1st | 0.0037 |
| Normal, n (%) | 5/5 (100.0) | 21/23 (91.3) | 23/29 (79.3) | 28/38 (73.7) | 3/5 (60.0) | |
| Altered, n (%) | 0/5 (0.0) | 2/23 (8.7) # | 6/29 (20.7) #, 1st | 10/38 (26.3) #, 1st | 2/5 (40.0) #, 1st, 2nd, 3rd | 0.0001 |
| Language, mean (±SD) | 92.0 (±2) | 94.4 (±17) | 95.5 (±12) | 93.3 (±14) | 88.8 (±22) | 0.8994 |
| Normal, n (%) | 5/5 (100.0) | 21/23 (91.3) | 23/29 (79.3) | 29/38 (73.7) | 3/5 (60.0) | |
| Altered, n (%) | 0/5 (0.0) | 2/23 (8.7) # | 6/29 (20.7) # | 9/38 (26.3) # | 2/5 (40.0) #, 1st, 2nd, 3rd | 0.0001 |
| Motor, mean (±SD) | 112.4 (±12) | 106.6 (±21) | 107.3 (±16) | 104.3 (±16) | 92.2 (±17) | 0.2961 |
| Normal, n (%) | 5/5 (100.0) | 19/23 (82.6) | 26/29 (89.7) | 35/38 (92.1) | 3/5 (60.0) | |
| Altered, n (%) | 0/5 (0.0) | 4/23 (17.4) # | 3/29 (10.3) # | 3/38 (7.9) # | 2/5 (40.0) #, 1st, 2nd, 3rd | 0.0001 |
| 12 months after birth | ||||||
| Cognitive, mean (±SD) | 115.0 (±9) | 113.8 (±15) | 108.2 (±12) | 110.6 (±12) | 103.8 (±19) | 0.3503 |
| Normal, n (%) | 3/3 (100.0) | 15/15 (100.0) | 33/34 (97.1) | 44/45 (97.8) | 4/6 (66.7) | |
| Altered, n (%) | 0/3 (0.0) | 0/15 (0.0) | 1/34 (2.9) | 1/45 (2.3) | 2/6 (33.3) #, 1st, 2nd, 3rd | 0.0001 |
| Language, mean (±SD) | 98.0 (±5) | 107.0 (±12) | 92.5 (±15) | 98.2 (±17) | 83.5 (±19) 1st | 0.0161 |
| Normal, n (%) | 3/3 (100.0) | 15/15 (100.0) | 22/34 (64.7) | 34/45 (75.6) | 3/6 (50.0) | |
| Altered, n (%) | 0/3 (0.0) | 0/15 (0.0) | 12/34 (35.3) # | 11/45 (24.4) # | 3/6 (50.0) #, 1st, 2nd, 3rd | 0.0001 |
| Motor, mean (±SD) | 95.0 (±5) | 97.3 (±13) | 101.5 (±12) | 103.5 (±16) | 102.4 (±18) | 0.5351 |
| Normal, n (%) | 3/3 (100.0) | 12/15 (80.0) | 31/34 (91.2) | 39/45 (86.7) | 5/6 (83.0) | |
| Altered, n (%) | 0/3 (0.0) | 3/15 (20.0) # | 3/34 (8.8) # | 6/45 (13.3) # | 1/6 (17.0) #, 2nd, 3rd | 0.0001 |
| Bayley-III Domains * | Severity of Maternal SARS-CoV-2 Infection | p-Values | ||
|---|---|---|---|---|
| HC | Non-Severe | Severe | ANOVA or χ2 | |
| 6 months after birth | ||||
| Cognitive, mean (±SD) | 110.0 (±11) | 102.6 (±17) | 97.9 (±15) | 0.3477 |
| Normal, n (%) | 5/5 (100.0) | 65/80 (81.3) | 10/14 (71.4) | |
| Altered, n (%) | 0/5 (0.0) | 15/80 (18.7) # | 4/14 (28.6) # | 0.0001 |
| Language, mean (±SD) | 91.6 (±1) | 94.7 (±15) | 89.8 (±14) | 0.4640 |
| Normal, n (%) | 5/5 (100.0) | 62/80 (77.5) | 9/14 (64.3) | |
| Altered, n (%) | 0/5 (0.0) | 18/80 (22.5) # | 5/14 (35.7) #,N-S | 0.0001 |
| Motor, mean (±SD) | 112.4 (±12) | 106.5 (±17) | 96.8 (±17) | 0.0972 |
| Normal, n (%) | 5/5 (100.0) | 71/80 (88.8) | 11/14 (78.6) | |
| Altered, n (%) | 0/5 (0.0) | 9/80 (11.2) # | 3/14 (21.4) #,N-S | 0.0001 |
| 12 months after birth | ||||
| Cognitive, mean (±SD) | 115.0 (±9) | 109.6 (±13) | 110.0 (±13) | 0.7815 |
| Normal, n (%) | 3/3 (100.0) | 82/85 (96.5) | 11/12 (91.7) | |
| Altered, n (%) | 0/3 (0.0) | 3/85 (3.5) # | 1/12 (8.3) # | 0.0095 |
| Language, mean (±SD) | 98.0 (±5) | 96.5 (±17) | 100.3 (±16) | 0.7444 |
| Normal, n (%) | 3/3 (100.0) | 61/85 (71.8) | 11/12 (91.7) | |
| Altered, n (%) | 0/3 (0.0) | 24/85 (28.2) # | 1/12 (8.3) #,N-S | 0.0001 |
| Motor, mean (±SD) | 95.0 (±5) | 101.4 (±14) | 107.8 (±15) | 0.2238 |
| Normal, n (%) | 3/3 (100.0) | 72/85 (84.7) | 12/12 (100.0) | |
| Altered, n (%) | 0/3 (0.0) | 13/85 (15.3) # | 0/12 (0.0) #,N-S | 0.0001 |
| Bayley-III Domains * | Caregiver Educational Level | p-Values | ||
|---|---|---|---|---|
| HC | No University Degree | University Degree | ANOVA or χ2 | |
| 6 months after birth | ||||
| Cognitive, mean (±SD) | 110.0 (±11) | 102.0 (±18) | 103.0 (±15) | 0.5784 |
| Normal, n (%) | 5/5 (100.0) | 55/66 (83.3) | 19/25 (76.0) | |
| Altered, n (%) | 0/5 (0.0) | 11/66 (16.7) # | 6/25 (24.0) # | 0.0001 |
| Language, mean (±SD) | 91.6 (±1) | 93.3 (±13) | 95.2 (±19) | 0.8032 |
| Normal, n (%) | 5/5 (100.0) | 50/66 (75.8) | 19/25 (76.0) | |
| Altered, n (%) | 0/5 (0.0) | 16/66 (24.2) # | 6/25 (24.0) # | 0.0001 |
| Motor, mean (±SD) | 112.4 (±12) | 103.6 (±17) | 109.6 (±20) | 0.2356 |
| Normal, n (%) | 5/5 (100.0) | 57/66 (86.4) | 22/25 (88.0) | |
| Altered, n (%) | 0/5 (0.0) | 9/66 (13.6) # | 3/25 (12.0) # | 0.0010 |
| 12 months after birth | ||||
| Cognitive, mean (±SD) | 115.0 (±9) | 109.9 (±13) | 110.7 (±13) | 0.7926 |
| Normal, n (%) | 3/3 (100.0) | 63/66 (95.5) | 27/28 (96.4) | |
| Altered, n (%) | 0/3 (0.0) | 3/66 (4.5) | 1/28 (3.6) | 0.1466 |
| Language, mean (±SD) | 98.0 (±5) | 97.5 (±17) | 96.6 (±15) | 0.9680 |
| Normal, n (%) | 3/3 (100.0) | 49/66 (74.2) | 22/28 (78.6) | |
| Altered, n (%) | 0/3 (0.0) | 17/66 (25.8) # | 6/28 (21.4) # | 0.0001 |
| Motor, mean (±SD) | 95.0(±5) | 102.2(±15) | 103.3(±14) | 0.6285 |
| Normal, n (%) | 3/3 (100.0) | 57/66 (86.4) | 24/28 (85.7) | |
| Altered, n (%) | 0/3 (0.0) | 9/66 (13.6) # | 4/28 (14.3) # | 0.0005 |
| Bayley-III Domains * | Maternal Features ** | |||||
|---|---|---|---|---|---|---|
| Acute SARS-CoV-2 Infection at Delivery | p-Value | Severe Disease | p-Value | University Degree | p-Value | |
| 6 months after birth | ||||||
| Cognitive, Coef (SE) | −12.33 (7.58) | 0.107 | −4.71 (4.82) | 0.332 | 1.03 (3.95) | 0.795 |
| Language, Coef (SE) | −6.86 (6.63) | 0.304 | −4.89 (4.19) | 0.247 | 1.92 (3.45) | 0.579 |
| Motor, Coef (SE) | −15.80 (7.83) | 0.047 | −9.68 (4.97) | 0.055 | 5.94 (4.12) | 0.153 |
| 12 months after birth | ||||||
| Cognitive, Coef (SE) | −14.65 (5.30) | 0.007 | 0.41 (4.08) | 0.920 | 0.79 (2.98) | 0.792 |
| Language, Coef (SE) | −14.01 (6.76) | 0.041 | 3.83 (5.07) | 0.452 | −0.88 (3.67) | 0.812 |
| Motor, Coef (SE) | 0.12 (5.89) | 0.983 | 6.40 (4.29) | 0.140 | 1.07 (3.21) | 0.740 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motta, F.; Canellas-de-Castro, M.E.; Fernandes, G.M.; Sasaki, L.M.P.; de Araújo Júnior, D.A.; Zaconeta, A.M.; da Silva, Â.P.; Gomes, C.M.; Albuquerque, C.P.; Costa-Rocha, I.A.; et al. Neuropsychomotor Development of Children Exposed to SARS-CoV-2 in Utero During COVID-19 Pandemic. Biomedicines 2025, 13, 2256. https://doi.org/10.3390/biomedicines13092256
Motta F, Canellas-de-Castro ME, Fernandes GM, Sasaki LMP, de Araújo Júnior DA, Zaconeta AM, da Silva ÂP, Gomes CM, Albuquerque CP, Costa-Rocha IA, et al. Neuropsychomotor Development of Children Exposed to SARS-CoV-2 in Utero During COVID-19 Pandemic. Biomedicines. 2025; 13(9):2256. https://doi.org/10.3390/biomedicines13092256
Chicago/Turabian StyleMotta, Felipe, Maria Eduarda Canellas-de-Castro, Geraldo Magela Fernandes, Lizandra Moura Paravidine Sasaki, David Alves de Araújo Júnior, Alberto Moreno Zaconeta, Ângelo Pereira da Silva, Ciro Martins Gomes, Cleandro Pires Albuquerque, Ismael Artur Costa-Rocha, and et al. 2025. "Neuropsychomotor Development of Children Exposed to SARS-CoV-2 in Utero During COVID-19 Pandemic" Biomedicines 13, no. 9: 2256. https://doi.org/10.3390/biomedicines13092256
APA StyleMotta, F., Canellas-de-Castro, M. E., Fernandes, G. M., Sasaki, L. M. P., de Araújo Júnior, D. A., Zaconeta, A. M., da Silva, Â. P., Gomes, C. M., Albuquerque, C. P., Costa-Rocha, I. A., Santos, J. A. T., Jesus, J. A. L. D., Costa, K. N., Espindola, L. S., Mota, L. M. H. d., Lauand, L., Castro, L. C. G. d., Xavier, M. A. P., Coelho-dos-Reis, J. G. A., ... Soares, A. A. d. S. M., on behalf of PROUDEST Study—Pregnancy Outcomes and Child Development Effects of SARS-CoV-2 Infection. (2025). Neuropsychomotor Development of Children Exposed to SARS-CoV-2 in Utero During COVID-19 Pandemic. Biomedicines, 13(9), 2256. https://doi.org/10.3390/biomedicines13092256








