Persistent Pulmonary Hypertension of the Newborn: A Pragmatic Review of Pathophysiology, Diagnosis, and Advances in Management
Abstract
1. Introduction
2. Search Strategy and Selection Criteria
3. Etiology
4. Clinical Presentation and Diagnosis
5. Treatment
5.1. Inhaled Therapies
5.2. Other Pulmonary Vasodilator Therapies
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Donn, S.M. Persistent pulmonary hypertension of the newborn: Historical perspective. Semin. Fetal Neonatal Med. 2022, 27, 101323. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; McNamara, P.J. Persistent pulmonary hypertension of the newborn: Advances in diagnosis and treatment. Semin. Fetal Neonatal Med. 2022, 25, 101078. [Google Scholar] [CrossRef]
- Hansen, T.W.R.; Tissières, P. Pulmonary Vasodilators for Persistent Pulmonary Hypertension of the Newborn: Beyond Sildenafil. Pediatr. Drugs 2021, 23, 29–40. [Google Scholar]
- Sankaran, D.; Lakshminrusimha, S. Pulmonary hyperten in the newborn- etiology and pathogenesis. Semin. Fetal Neonatal Med. 2022, 27, 101381. [Google Scholar] [CrossRef]
- Steinhorn, R.H. Neonatal pulmonary hypertension. Pediatr. Crit. Care Med. 2010, 11 (Suppl. S2), S79–S84. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S.; Steinhorn, R.H. The pulmonary circulation in neonatal respiratory failure. Clin. Perinatol. 1999, 26, 623–647. [Google Scholar] [CrossRef] [PubMed]
- Abman, S.H. Recent advances in the pathogenesis and treatment of persistent pulmonary hypertension of the newborn. Neonatology 2007, 91, 283–290. [Google Scholar] [CrossRef]
- Kinsella, J.P.; Abman, S.H. Inhaled nitric oxide therapy in children. Paediatr. Respir. Rev. 2005, 6, 190–198. [Google Scholar] [CrossRef]
- Pola, T.; Gieteling, G.; Aken, J. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst. Rev. 2024, 1, CD000399. [Google Scholar]
- Morgan, C.; Zahr, R.A.; Alexander, P.M. Pulmonary Hypertension in the Neonate. Pediatr. Rev. 2018, 39, 471–482. [Google Scholar] [CrossRef]
- Mourani, P.M.; Abman, S.H. Pulmonary hypertension and vascular abnormalities in bronchopulmonary dysplasia. Clin. Perinatol. 2015, 42, 839–855. [Google Scholar] [CrossRef]
- Levy, P.T.; Patel, M.D.; Groh, G.; Choudhry, S.; Murphy, J.; Holland, M.R.; McNamara, P.J. Diagnosis and management of pulmonary hypertension in infants with bronchopulmonary dysplasia. Semin. Fetal Neonatal Med. 2022, 27, 101351. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Lakshminrusimha, S. Pathophysiology and Management of Persistent Pulmonary Hypertension of the Newborn. Clin. Perinatol. 2021, 48, 595–618. [Google Scholar] [CrossRef] [PubMed]
- de Boode, W.P.; Singh, Y.; Molnar, Z.; Schubert, U.; Savoia, M.; Sehgal, A.; Levy, P.T.; McNamara, P.J.; El-Khuffash, A. Application of Neonatologist Performed Echocardiography in the assessment and management of persistent pulmonary hypertension of the newborn. Pediatr. Res. 2018, 84 (Suppl. S1), 68–77. [Google Scholar] [CrossRef]
- Tierney, E.S.S.; Levine, J.C.; Chen, S.; Bradley, T.J.; Pearson, G.D.; Colan, S.D.; Sleeper, L.A.; Campbell, M.J.; Cohen, M.S.; De Backer, J.; et al. Echocardiographic Methods, Quality Review, and Measurement Accuracy in a Randomized Multicenter Trial of Marfan Syndrome. J. Am. Soc. Echocardiogr. 2013, 26, 657–666. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reynolds, E.W.; Ellington, J.G.; Vranicar, M.; Bada, H.S. Brain-type natriuretic peptide in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatrics 2004, 114, 1297–1304, Erratum in Pediatrics 2005, 115, 1454. [Google Scholar] [CrossRef] [PubMed]
- Jano, E.; Vaz, M.J.; Mally, P.N.; Wachtel, E.V. Pilot Study Investigating Brain Natriuretic Peptide, Troponin, Galectin-3, and miRNA-126a-5p as Biomarkers of Persistent Pulmonary Hypertension in Neonates with Hypoxic-Ischemic Injury Receiving Therapeutic Hypothermia. Am. J. Perinatol. 2024, 41, e60–e68. [Google Scholar] [CrossRef] [PubMed]
- Giesinger, R.; Rios, D.R.; McNamara, P.J.; Levy, P.T. Asphyxia, Therapeutic Hypothermia, and Pulmonary Hypertension. Clin. Perinatol. 2024, 51, 127–149. [Google Scholar] [CrossRef]
- Boyd, S.M.; Kluckow, M.; McNamara, P.J. Targeted Neonatal Echocardiography in the Management of Neonatal Pulmonary Hypertension. Clin. Perinatol. 2024, 51, 45–76. [Google Scholar] [CrossRef]
- More, K.; Soni, R.; Gupta, S. The role of bedside functional echocardiography in the assessment and management of pulmonary hypertension. Semin. Fetal Neonatal Med. 2022, 27, 101366. [Google Scholar] [CrossRef]
- Wedgwood, S.; Steinhorn, R.H.; Lakshminrusimha, S. Optimal oxygenation and role of free radicals in PPHN. Free Radic. Biol. Med. 2019, 142, 97–106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Polglase, G.R.; Blank, D.A.; Barton, S.K.; Miller, S.L.; Stojanovska, V.; Kluckow, M.; Gill, A.W.; LaRosa, D.; Te Pas, A.B.; Hooper, S.B. Physiologically based cord clamping stabilises cardiac output and reduces cerebrovascular injury in asphyxiated near-term lambs. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F530–F538. [Google Scholar] [CrossRef] [PubMed]
- Hooper, S.B.; Te Pas, A.B.; Lang, J.; van Vonderen, J.J.; Roehr, C.C.; Kluckow, M.; Gill, A.W.; Wallace, E.M.; Polglase, G.R. Cardiovascular transition at birth: A physiological sequence. Pediatr. Res. 2015, 77, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.; Carnielli, V.; Greisen, G.; Plasschaert, M.; Simeoni, U.; Speer, C.P.; Szabo, M. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2022 Update. Neonatology 2022, 129, 486–501. [Google Scholar]
- Lakshminrusimha, S.; Abman, S.H. Oxygen Targets in Neonatal Pulmonary Hypertension: Individualized, “Precision-Medicine” Approach. Clin. Perinatol. 2024, 51, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Qiang, F.; Pan, J.; Zhang, F.; Lin, Y.; Yuan, T. Comparison of Different Treatments of Persistent Pulmonary Hypertension of the Newborn: A Systematic Review and Network Meta-Analysis. Crit. Care Med. 2024, 52, e314–e322. [Google Scholar] [CrossRef]
- Cookson, M.; Kinsella, J. Inhaled Nitric Oxide in Neonatal Pulmonary Hypertension. Clin. Perinatol. 2024, 51, 95–111. [Google Scholar] [CrossRef]
- Alhumaid, S.; Alnaim, A.A.; Al Ghamdi, M.A.; Alahmari, A.A.; Alabdulqader, M.; Al Hajji Mohammed, S.M.; Alalwan, Q.M.; Al Dossary, N.; Alghazal, H.A.; Al Hassan, M.A.; et al. International treatment outcomes of neonates on extracorporeal membrane oxygenation (ECMO) with persistent pulmonary hypertension of the newborn (PPHN): A systematic review. J. Cardiothorac. Surg. 2024, 19, 493. [Google Scholar] [CrossRef] [PubMed]
- Chetan, C.; Suryawanshi, P.; Patnaik, S.; Soni, N.B.; Rath, C.; Pareek, P.; Gupta, B.; Garegrat, R.; Verma, A.; Singh, Y. Oral versus intravenous sildenafil for pulmonary hypertension in neonates: A randomized trial. BMC Pediatr. 2022, 22, 311. [Google Scholar] [CrossRef]
- Kelly, L.E.; Ohlsson, A.; Shah, P.S. Sildenafil for pulmonary hypertension in neonates. Cochrane Database Syst. Rev. 2017, 8, CD005494. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toso, A.; Aránguiz, O.; Céspedes, C.; Navarrete, O.; Hernández, C.; Vio, C.P.; Luco, M.; Casanello, P.; Kattan, J. Congenital diaphragmatic hernia: Phosphodiesterase-5 and Arginase inhibitors prevent pulmonary vascular hypoplasia in rat lungs. Pediatr. Res. 2024, 95, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Lumba, R.; Kazmi, S.H.; Vaz, M.J.; Prakash, S.S.; Bailey, S.M.; Mally, P.V.; Randis, T.M. Effects of Inhaled Iloprost for the Management of Persistent Pulmonary Hypertension of the Newborn. Am. J. Perinatol. 2022, 39, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Janjindamai, W.; Thatrimontrichai, A.; Maneenil, G.; Chanvitan, P.; Dissaneevate, S. Effectiveness and safety of intravenous iloprost for severe persistent pulmonary hypertension of the newborn. Indian. Pediatr. 2013, 50, 934–938. [Google Scholar] [CrossRef] [PubMed]
- McNamara, P.J.; Shivananda, S.P.; Sahni, M.; Freeman, D.; Taddio, A. Pharmacology of milrinone in neonates with persistent pulmonary hypertension of the newborn and suboptimal response to inhaled nitric oxide. Pediatr. Crit. Care Med. 2013, 14, 74–84. [Google Scholar] [CrossRef] [PubMed]
- James, A.T.; Corcoran, J.D.; McNamara, P.J.; Franklin, O.; El-Khuffash, A.F. The effect of milrinone on right and left ventricular function when used as a rescue therapy for term infants with pulmonary hypertension. Cardiol. Young 2016, 26, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Steinhorn, R.H.; Fineman, J.; Kusic-Pajic, A.; Cornelisse, P.; Gehin, M.; Nowbakht, P.; Pierce, C.M.; Beghetti, M. FUTURE-4 study investigators. Bosentan as Adjunctive Therapy for Persistent Pulmonary Hypertension of the Newborn: Results of the Randomized Multicenter Placebo-Controlled Exploratory Trial. J. Pediatr. 2016, 177, 90–96.e3. [Google Scholar] [CrossRef] [PubMed]
- Maneenil, G.; Thatrimontrichai, A.; Janjindamai, W.; Dissaneevate, S. Effect of bosentan therapy in persistent pulmonary hypertension of the newborn. Pediatr. Neonatol. 2018, 59, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, T.; Liang, X.; Chen, Y.; Zhou, P.; Yu, Z.; Zhong, G.; Zhang, L. Global, regional and national trends in the burden of persistent pulmonary hypertension of the newborn and essentials of its management from 1993 to 2023: A scoping review. Front. Pediatr. 2025, 13, 1502385. [Google Scholar] [CrossRef]
- Luo, K.; Tang, J.; Chen, H.; Zhang, X.; Wang, H. Vasodilators for persistent pulmonary hypertension of the newborn: A network meta-analysis. Pediatr. Pulmonol. 2024, 59, 3467–3482. [Google Scholar] [CrossRef] [PubMed]
- Bandiya, P.; Madappa, R.; Joshi, A.R. Etiology, Diagnosis and Management of Persistent Pulmonary Hypertension of the Newborn in Resource-limited Settings. Clin. Perinatol. 2024, 51, 237–252. [Google Scholar] [CrossRef]
- Ysphaneendramallimoggala; Biswas, M.; Anburaj, S.E.; Iqbal, F.; Suryakanth, V.B.; Lewis, L.E.S. Thiamine: An indispensable regulator of paediatric neuro-cardiovascular health and diseases. Eur. J. Pediatr. 2024, 183, 4597–4610. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.; Zaki El Feky, O.A.E.F.; Saad El Din El Ganady, H.M.; Abd El Halim, W.A.E. Comparative study between nebulized and intravenous magnesium sulfate for treatment of persistent pulmonary hypertension in neonates. J. Neonatal Perinat. Med. 2025, 18, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.M.; Ghazal, H.A.E.; Abdel-Mohsen, A.M.; Rezk, M.A. Nebulized nitroglycerin as an adjuvant drug in management of persistent pulmonary hypertension of newborns: A randomized controlled trial. Eur. J. Pediatr. 2025, 184, 586. [Google Scholar] [CrossRef]
- Hansmann, G.; Koestenberger, M.; Alastalo, T.P.; Apitz, C.; Austin, E.D.; Bonnet, D.; Budts, W.; D’Alto, M.; Gatzoulis, M.A.; Hasan, B.S.; et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J. Heart Lung Transplant. 2019, 38, 879–901. [Google Scholar] [CrossRef]
- Ball, M.K.; Seabrook, R.B.; Bonachea, E.M.; Chen, B.; Fathi, O.; Nankervis, C.A.; Osman, A.; Schlegel, A.B.; Magers, J.; Kulpa, T. Evidence-Based Guidelines for Acute Stabilization and Management of Neonates with Persistent Pulmonary Hypertension of the Newborn. Am. J. Perinatol. 2023, 40, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
- Kaplish, D.; Vagha, J.D.; Rathod, S.; Jain, A. Current Pharmaceutical Strategies in the Management of Persistent Pulmonary Hypertension of the Newborn (PPHN): A Comprehensive Review of Therapeutic Agents. Cureus 2024, 16, e70307. [Google Scholar] [CrossRef] [PubMed]
- Atlan, L.; Berthomieu, L.; Karsenty, C.; Gascoin, G.; Arnaud, C.; Breinig, S. Neurodevelopmental outcome in children between one and five years after persistent pulmonary hypertension of term and near-term newborns. Front. Pediatr. 2024, 12, 1450916. [Google Scholar] [CrossRef] [PubMed]

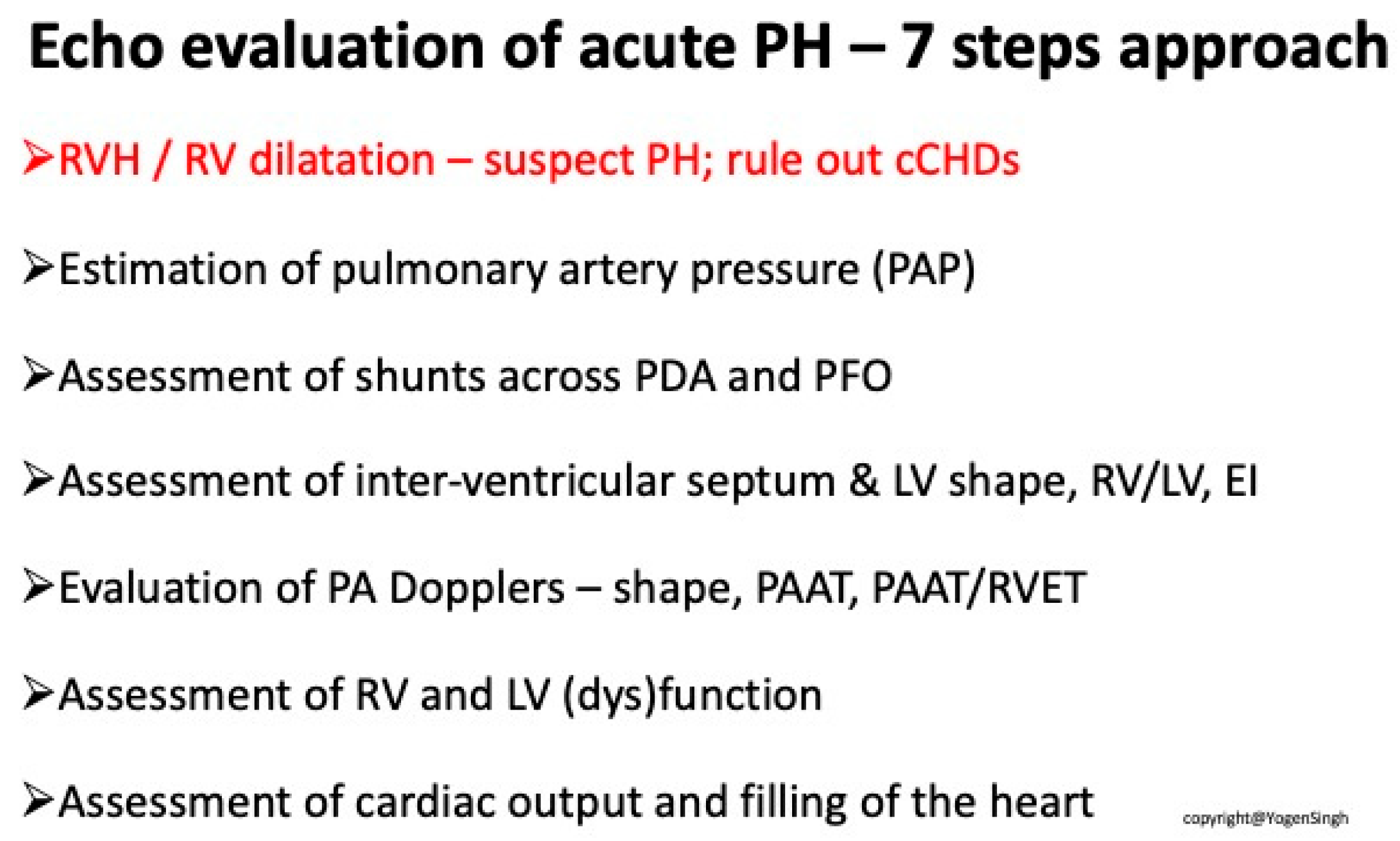
| Aspect of Study | Description | Significance |
|---|---|---|
| Heart Morphology | Exclusion of structural heart defects (note on Total Anomalous Pulmonary Venous Connection, TAPVC) | Assessment for indications for other treatment such as prostaglandin E |
| Heart Function | On visual inspection “eyeballing” Evaluation of the size, wall thickness, and contractile function of the right ventricle | Identification of right ventricular enlargement and overload |
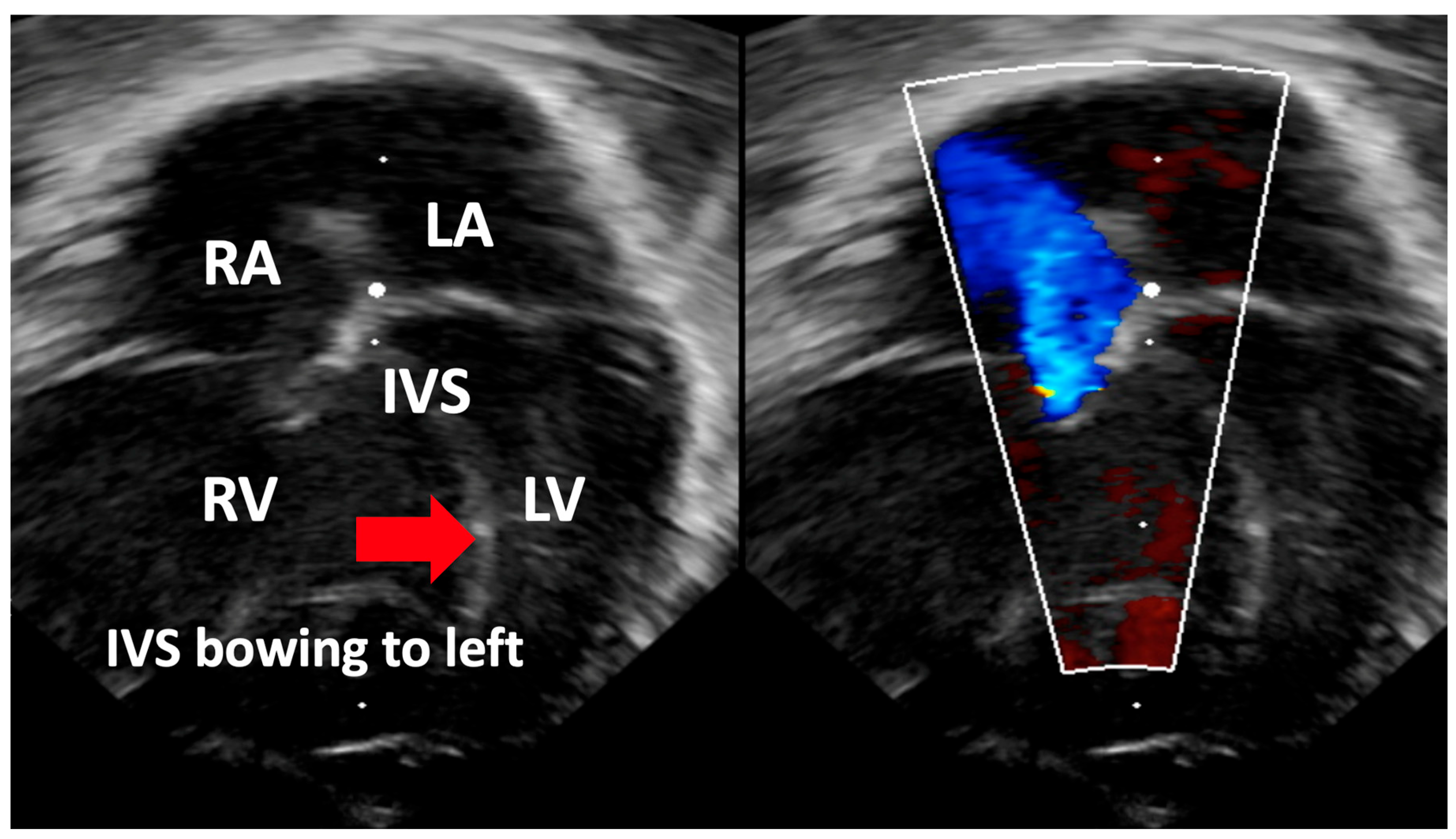
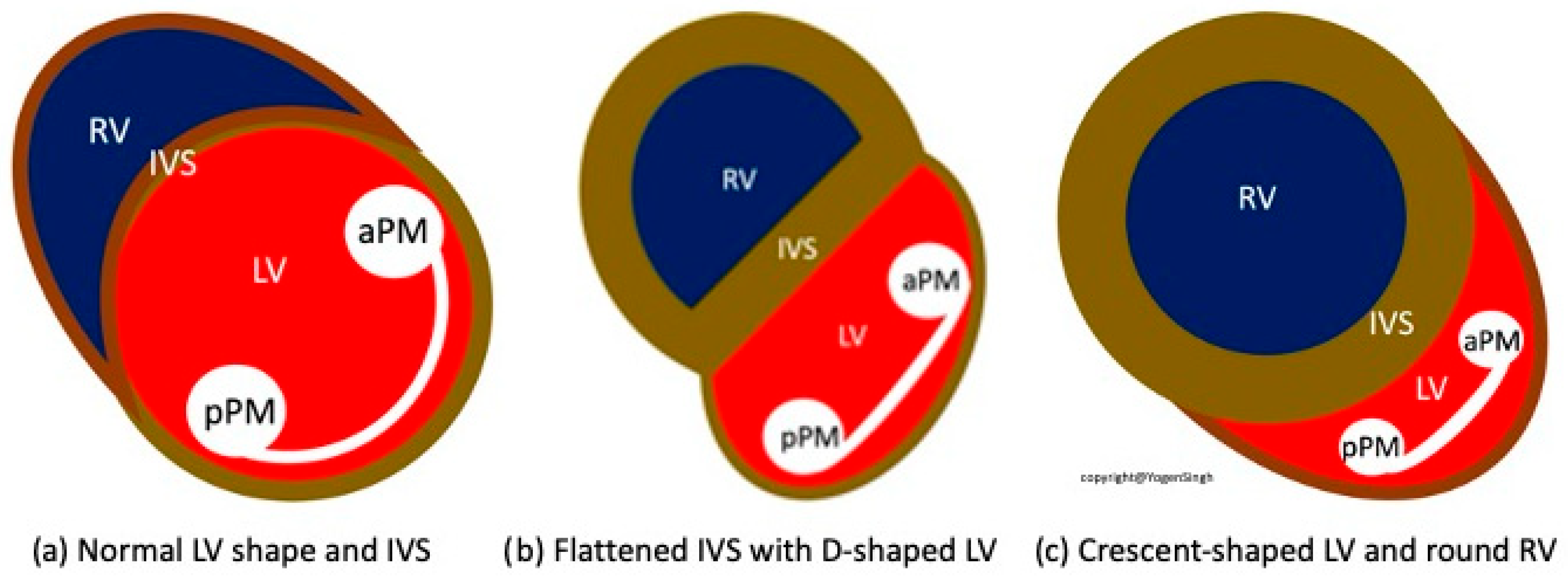
| Interventricular Septal (IVS) | Evaluation of interventricular septal flattening | Helps in estimating severity of PPHN, especially in absence of tricuspid regurgitation or PDA (Figure 4) O-shaped LV suggests normal or mildly increased pressure pulmonary artery pressure D-shaped LV with flattening of IVS indicates pulmonary artery pressure 50–100% of systemic pressure Crescent-shaped LV indicates pulmonary pressure > systemic pressure |
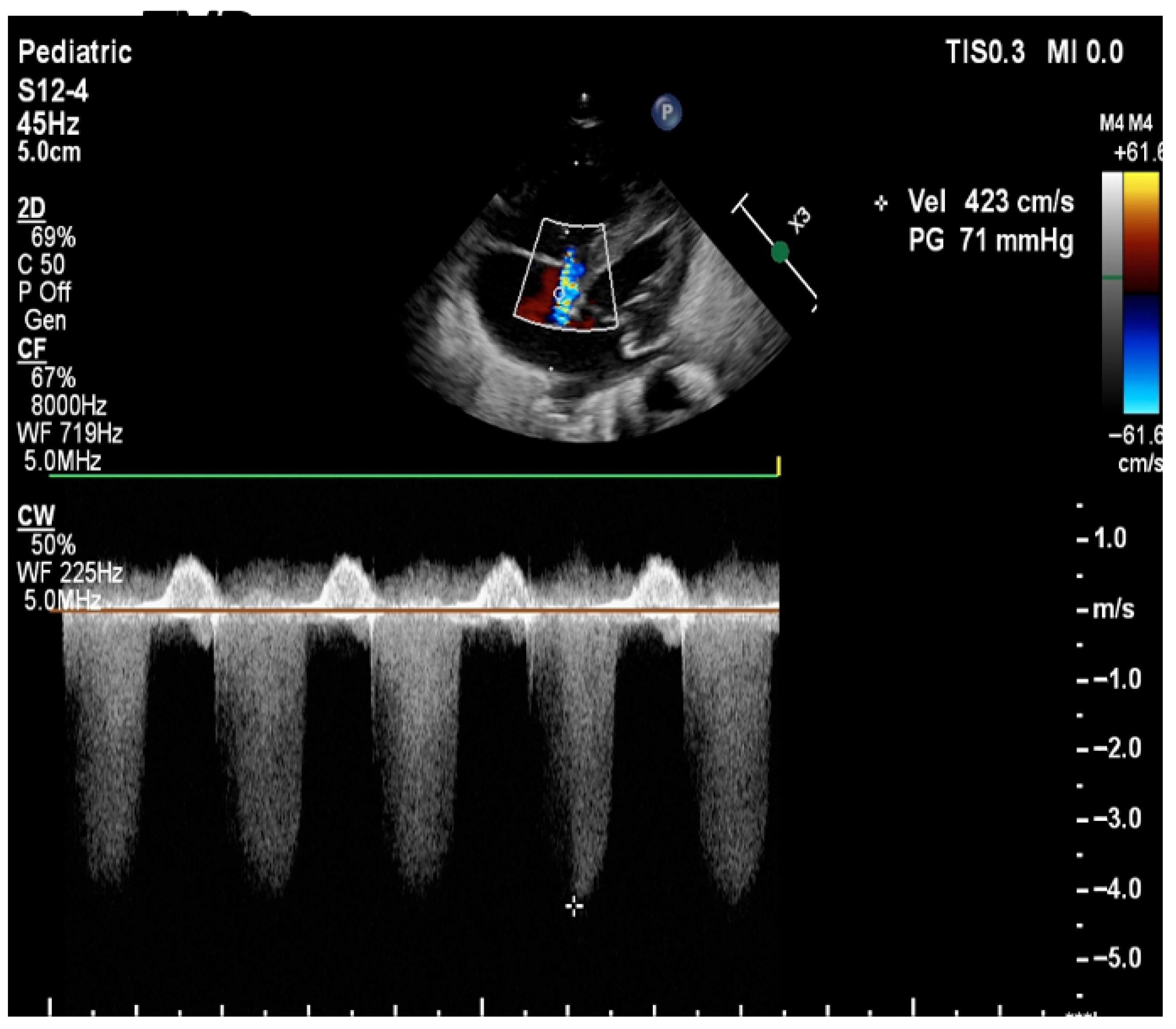
| Pulmonary Artery Pressure Measurement | Estimation based on the velocity of blood flow through regurgitant flow across the tricuspid valve using the Bernoulli equation | SPAP = 4 (TR Vmax2+ right atrial pressure (RAP) V: the velocity of regurgitation through the tricuspid valve RAP: the estimated right atrial pressure (3–10 mmHg). Pressures in the right ventricle and pulmonary artery are crucial for assessing the degree of pulmonary hypertension TV is present in approximately 60–85% of patients with PPHN. Absence of TR or minimal TR does not rule out PPHN! |
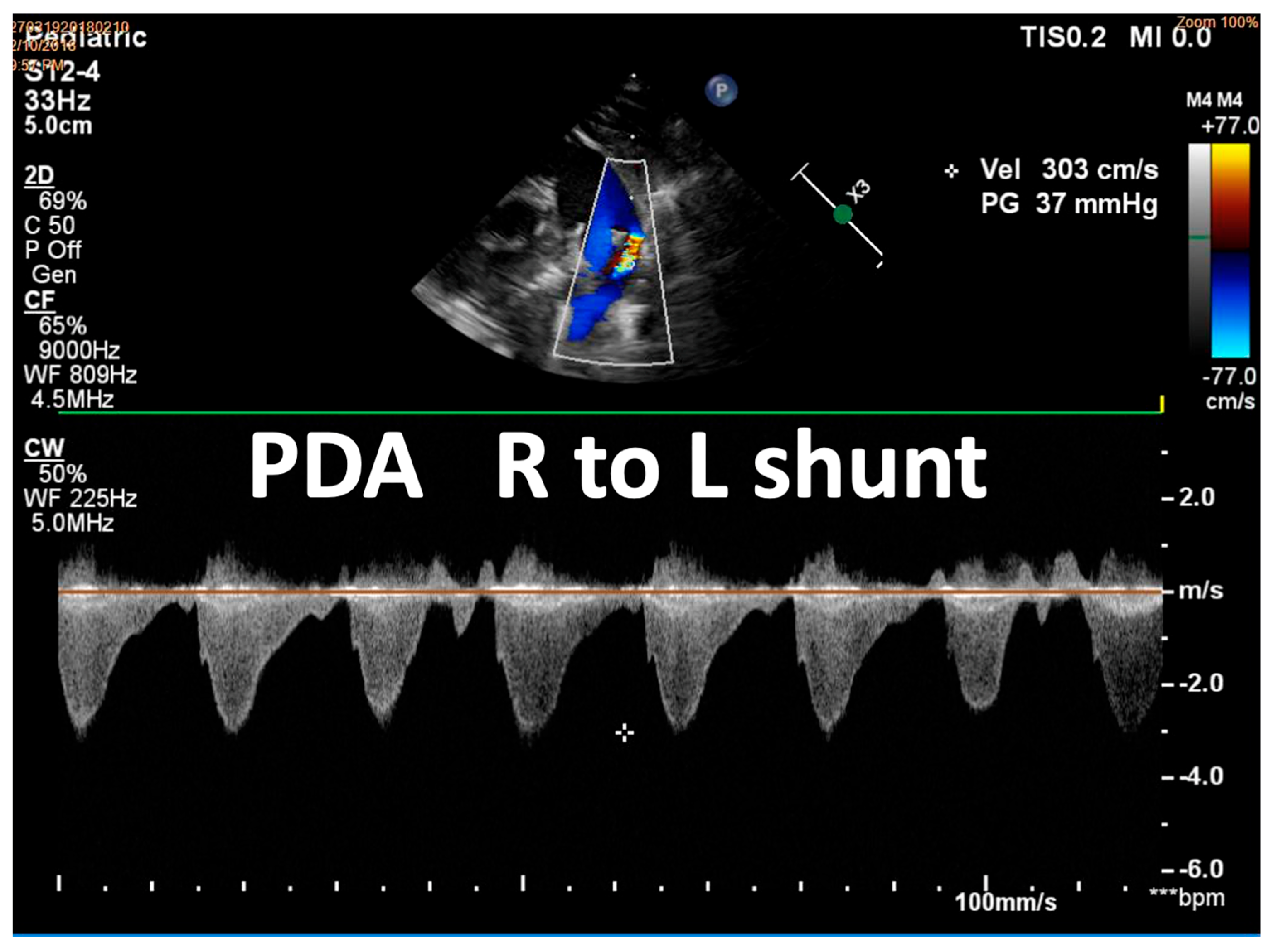
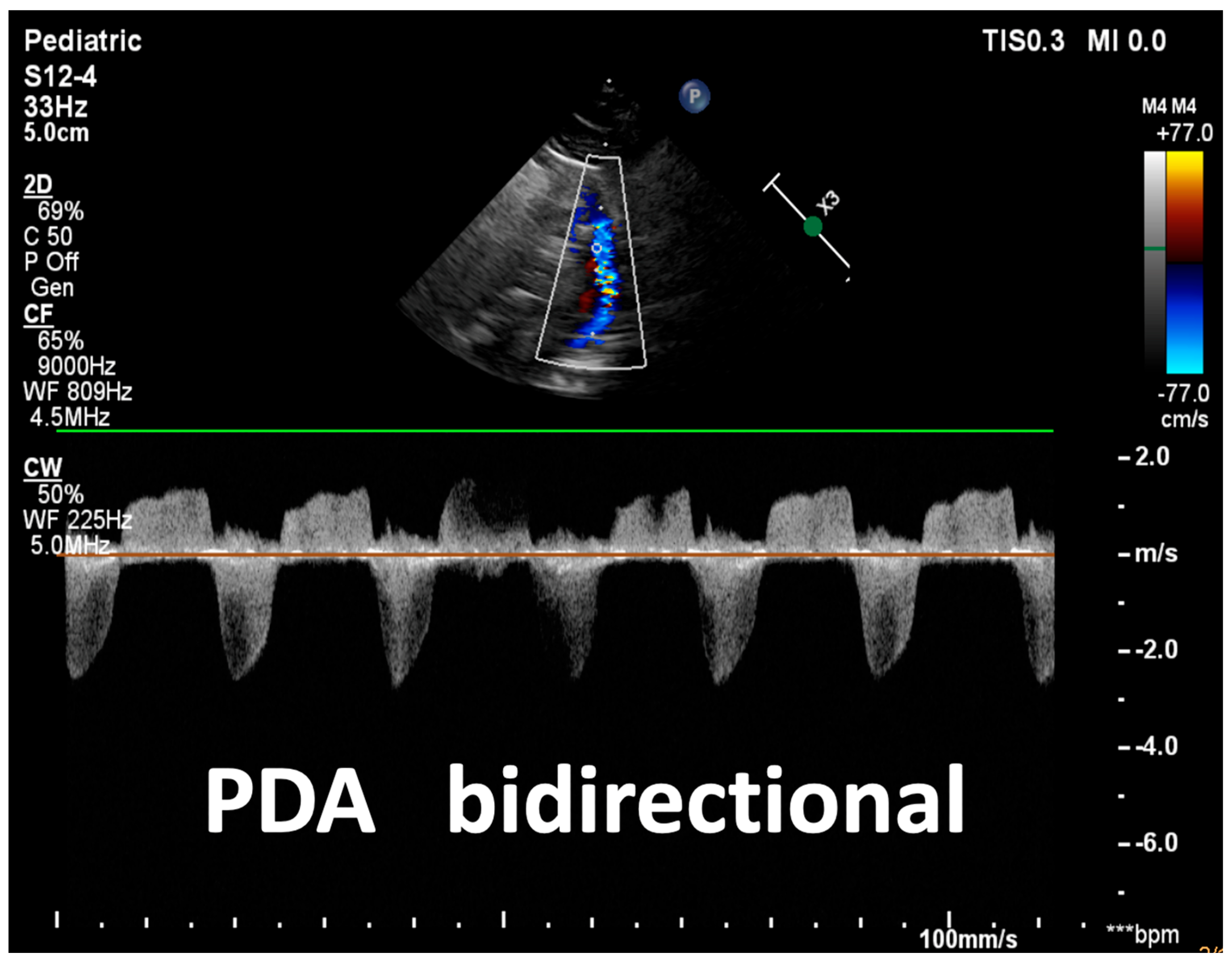
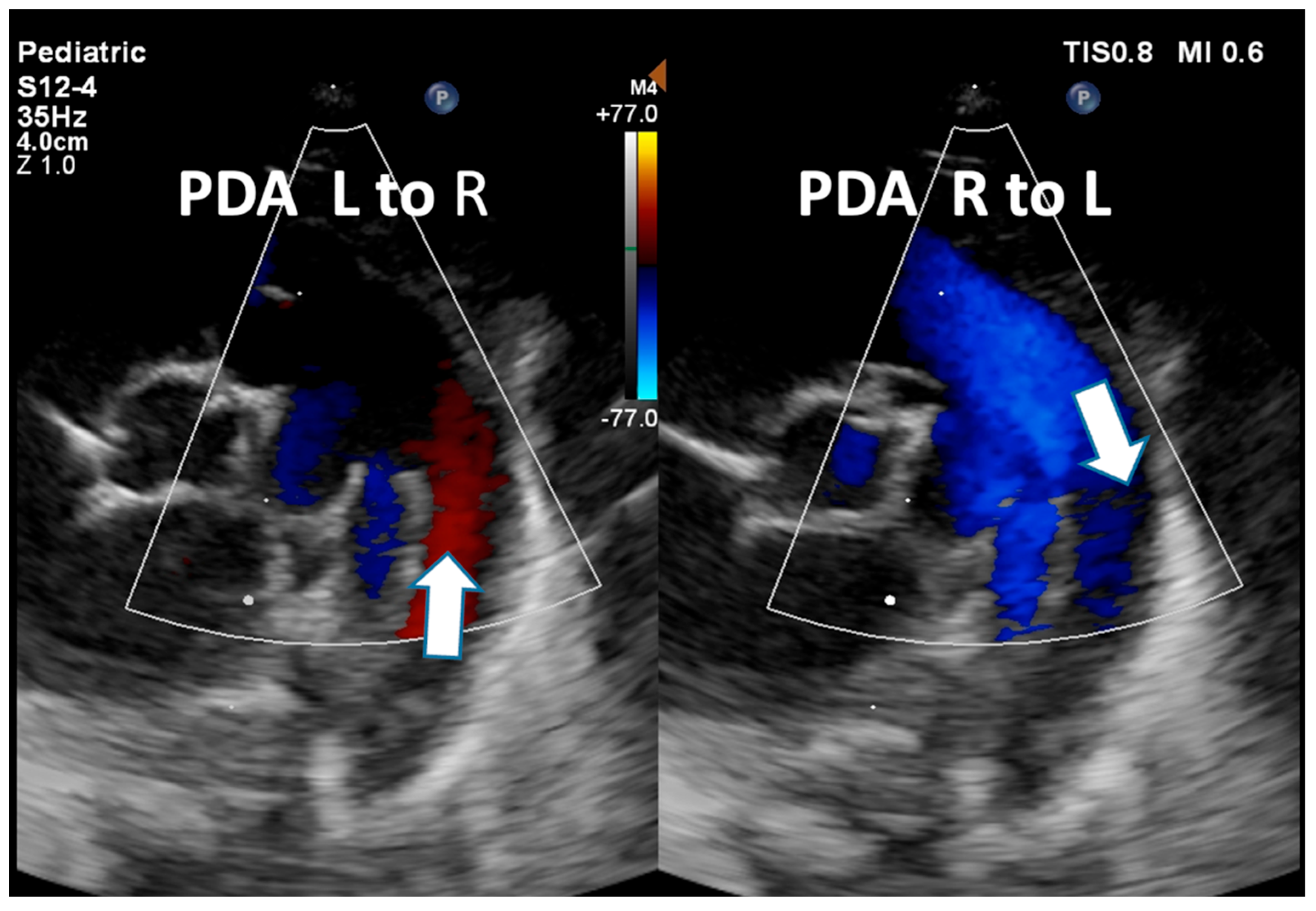
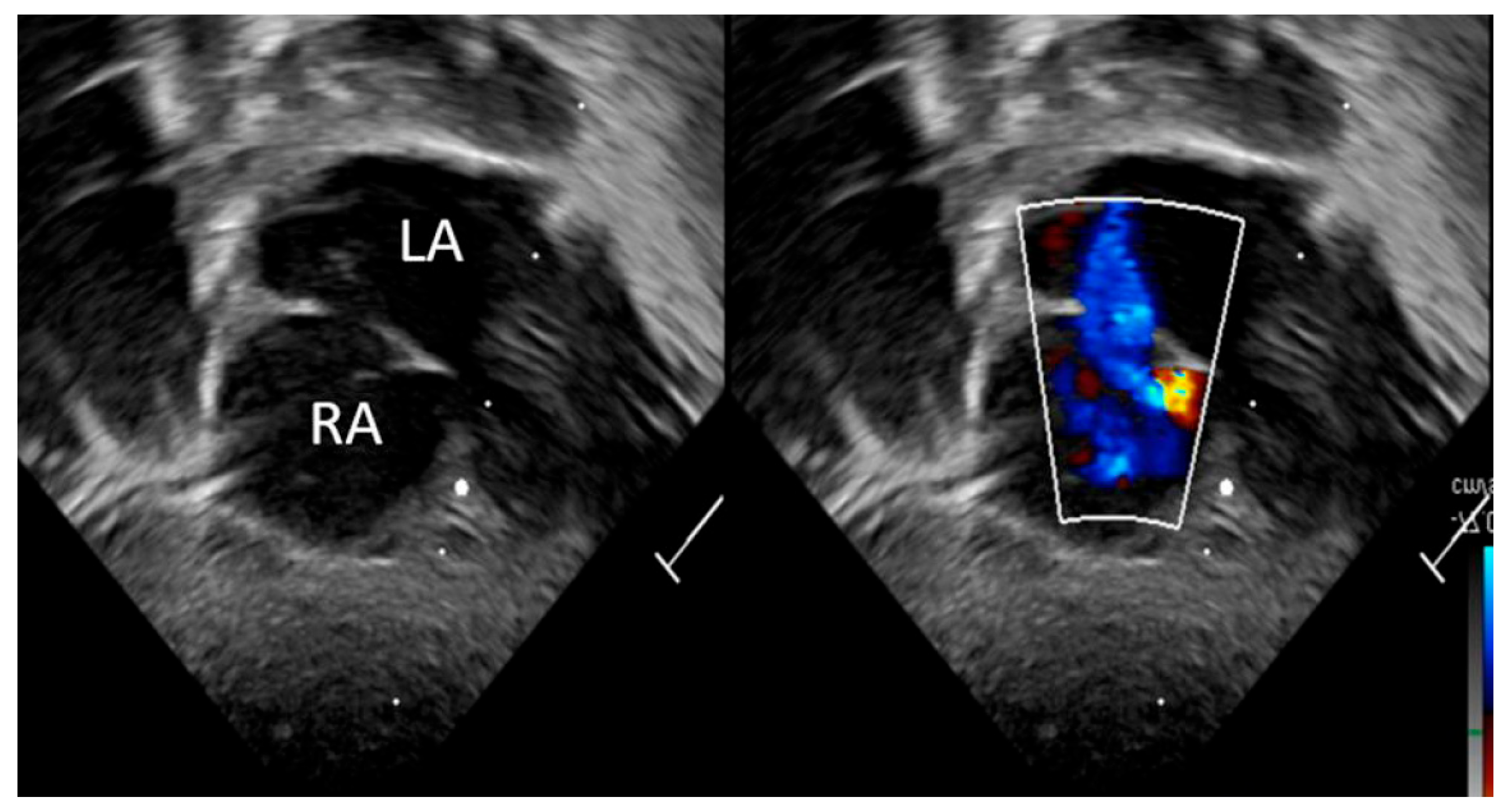
| Detection of Shunt | Identification and assessment of flow through the patent foramen ovale (PFO) and ductus arteriosus (PDA) | Indicates the presence and nature of pathological flow: left-to-right, bidirectional (often in moderate PPHN), or right-to-left (in very severe PPHN) |
| Right Ventricle Assessment | On visual inspection Movement of the tricuspid valve annulus, TAPSE (Tricuspid Annular Plane Systolic Excursion) Tei index using Tissue Doppler Imaging | Helps evaluate function of the right ventricle |
| Left Ventricle Assessment | On visual inspection, fraction shortening, ejection fraction, Tei index using Tissue Doppler Imaging | Helps evaluate function of the left ventricle |
| Assessment of Cardiac Filling | Inferior vena cava size and collapsibility | Assessment of preload |
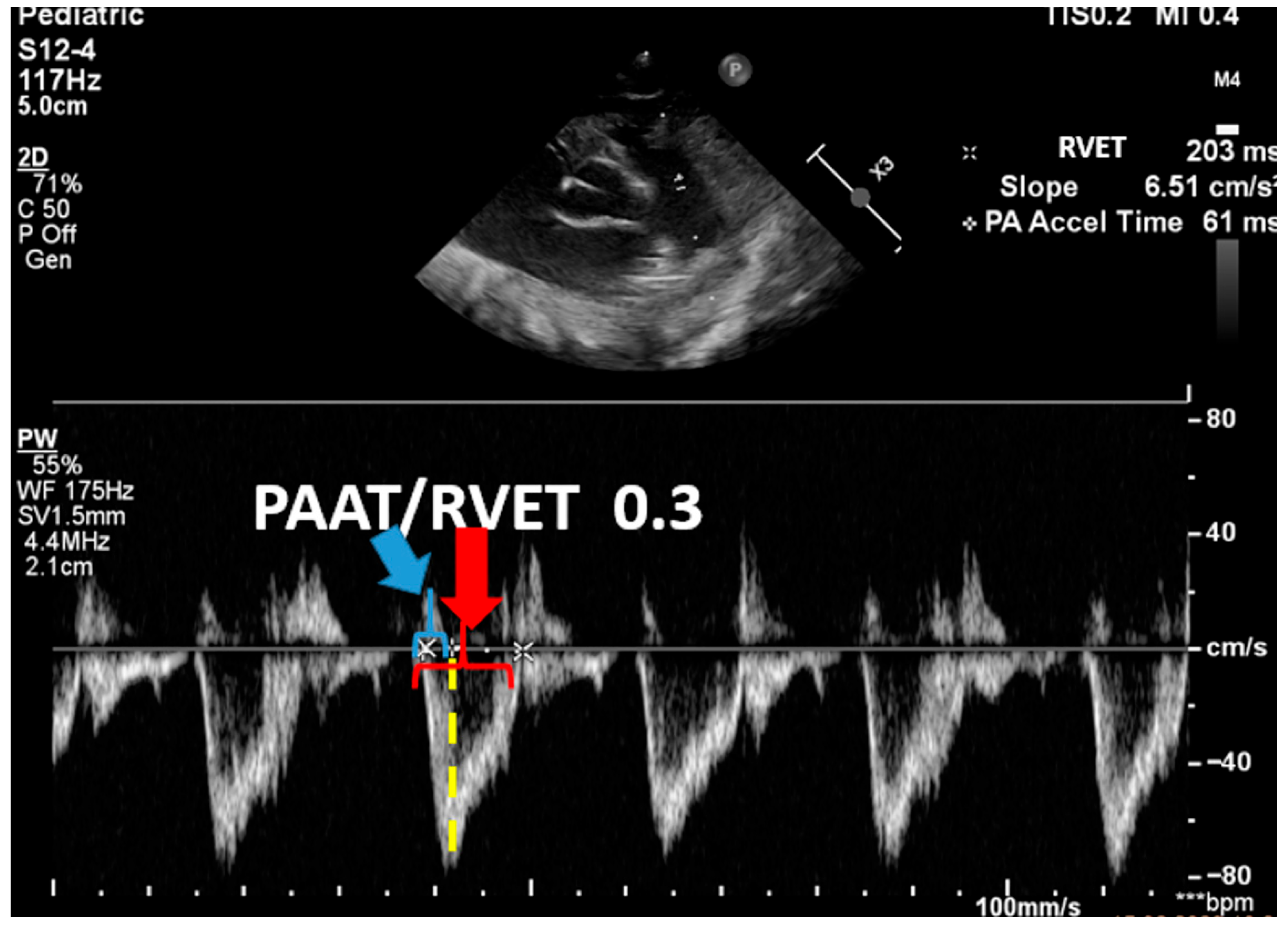
| Advanced ECHO and Hemodynamic Evaluation | RV fractional area change (FAC %) Pulmonary arterial acceleration time (PAAT) and PAAT/RV ejection time ratio Speckle tracking and strain rate Estimation of left and right cardiac output and serial assessment to see the response to therapy | Helps evaluate cardiac function and response to therapy |
| Step in Management | Actions and Considerations |
|---|---|
| Initial Assessment and Stabilization | Assess the newborn after birth for breathing, skin color, activity, and oxygenation. Ensure airway patency, provide adequate ventilation, and optimize oxygen concentration. |
| Diagnostics | Pulse Oximetry: Monitor oxygen saturation. Echocardiography: Assess heart function and exclude structural abnormalities. Arterial Blood Gas Analysis: Evaluate hypoxemia and acid–base balance. Chest X-Ray: Assess lung condition and exclude other causes of respiratory distress. |
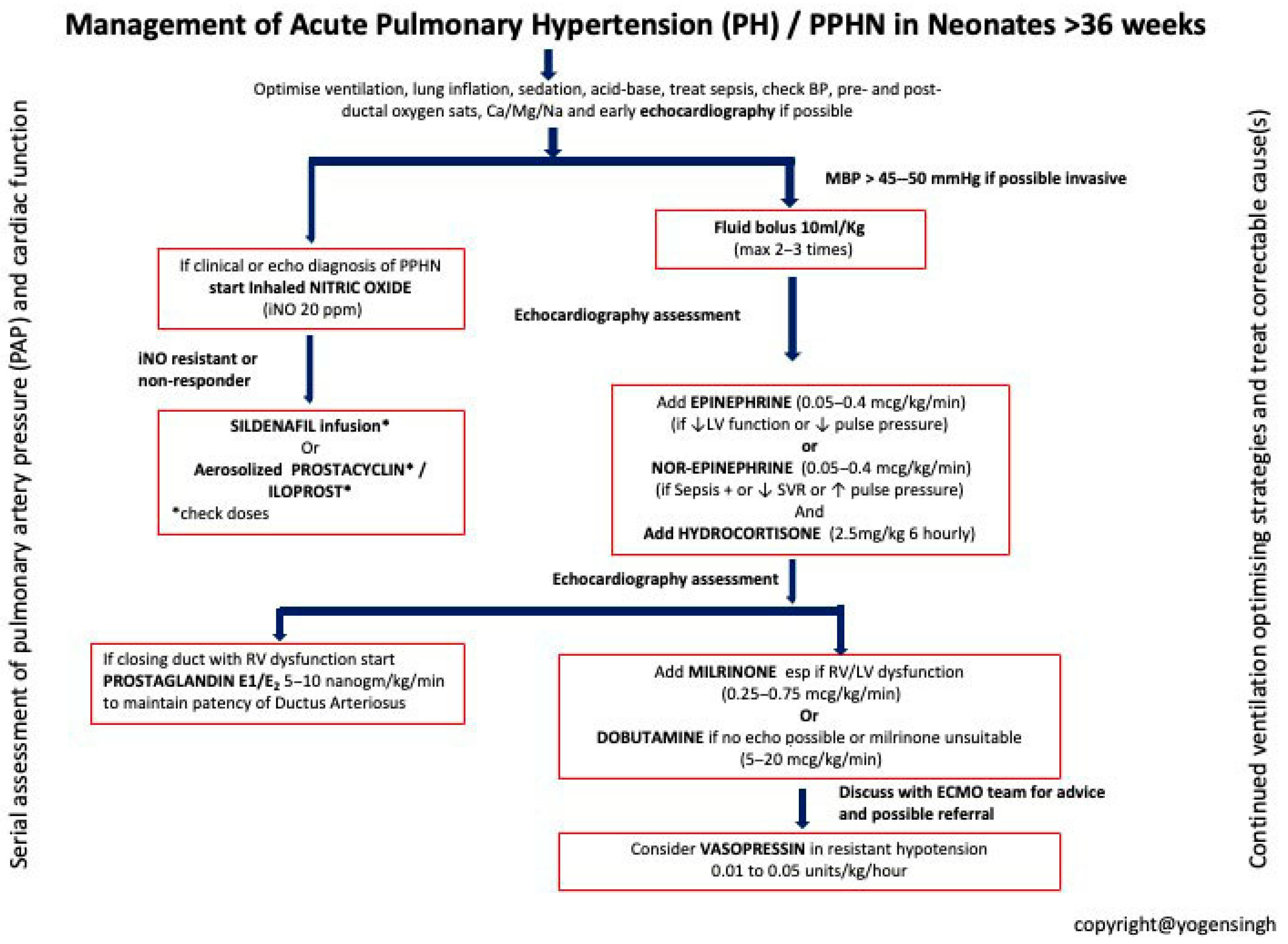
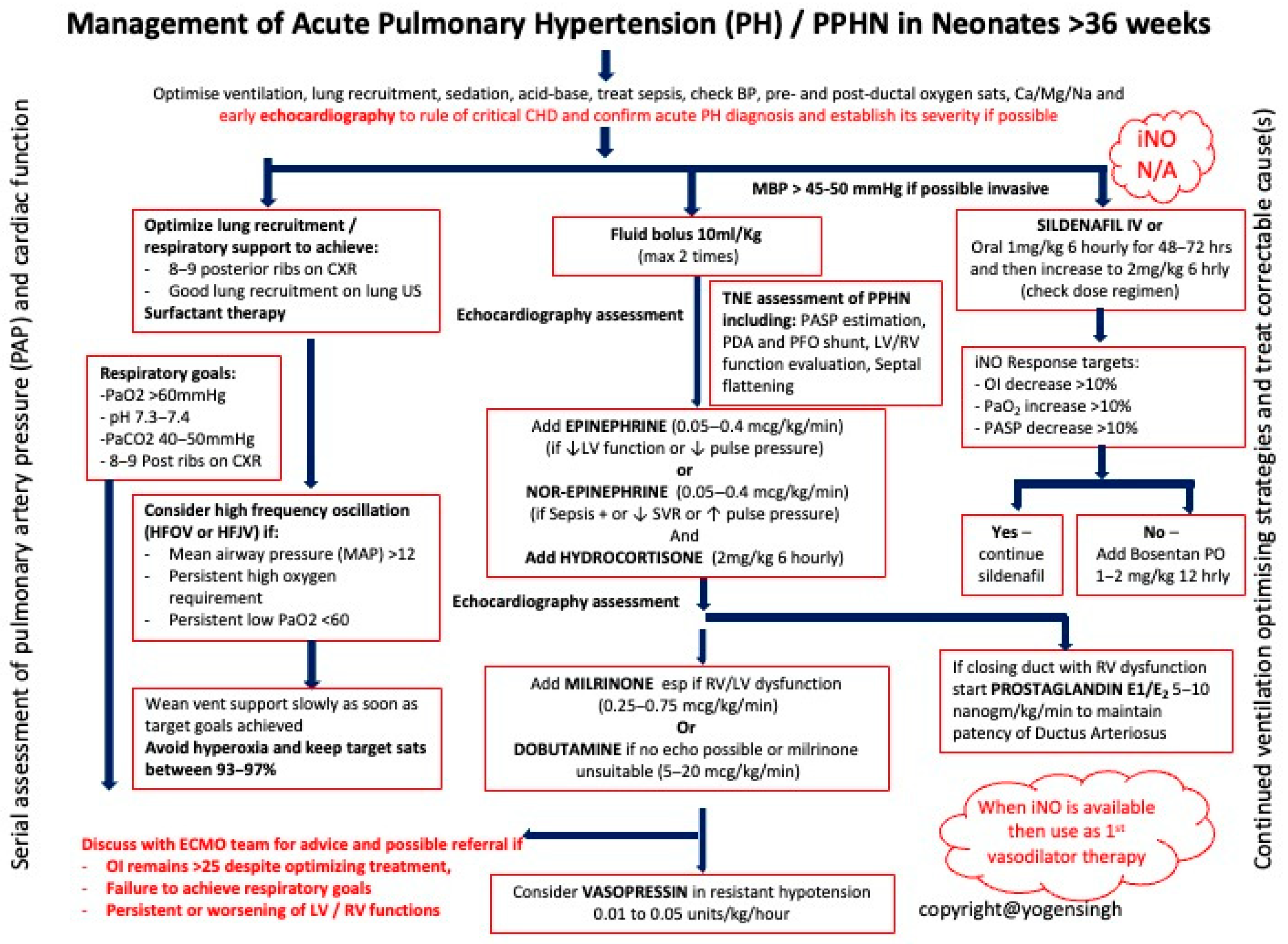
| Intensive Therapy | Optimized Ventilation: Use gentle mechanical ventilation to minimize barotrauma. Inhaled Nitric Oxide (iNO): First-line therapy for PPHN to decrease pulmonary vascular resistance. Surfactant Therapy: Administer if respiratory distress syndrome (RDS) is present. Sedation and Hemodynamic Support: Manage discomfort and maintain hemodynamic stability. Metabolic Correction: Address electrolyte and acid–base imbalances. |
| Alternative and Escalation Therapies | ECMO (Extracorporeal Membrane Oxygenation): Consider for severe PPHN unresponsive to other treatments. Vasodilator Drugs: Consider sildenafil and other vasodilators to reduce pulmonary artery pressure. |
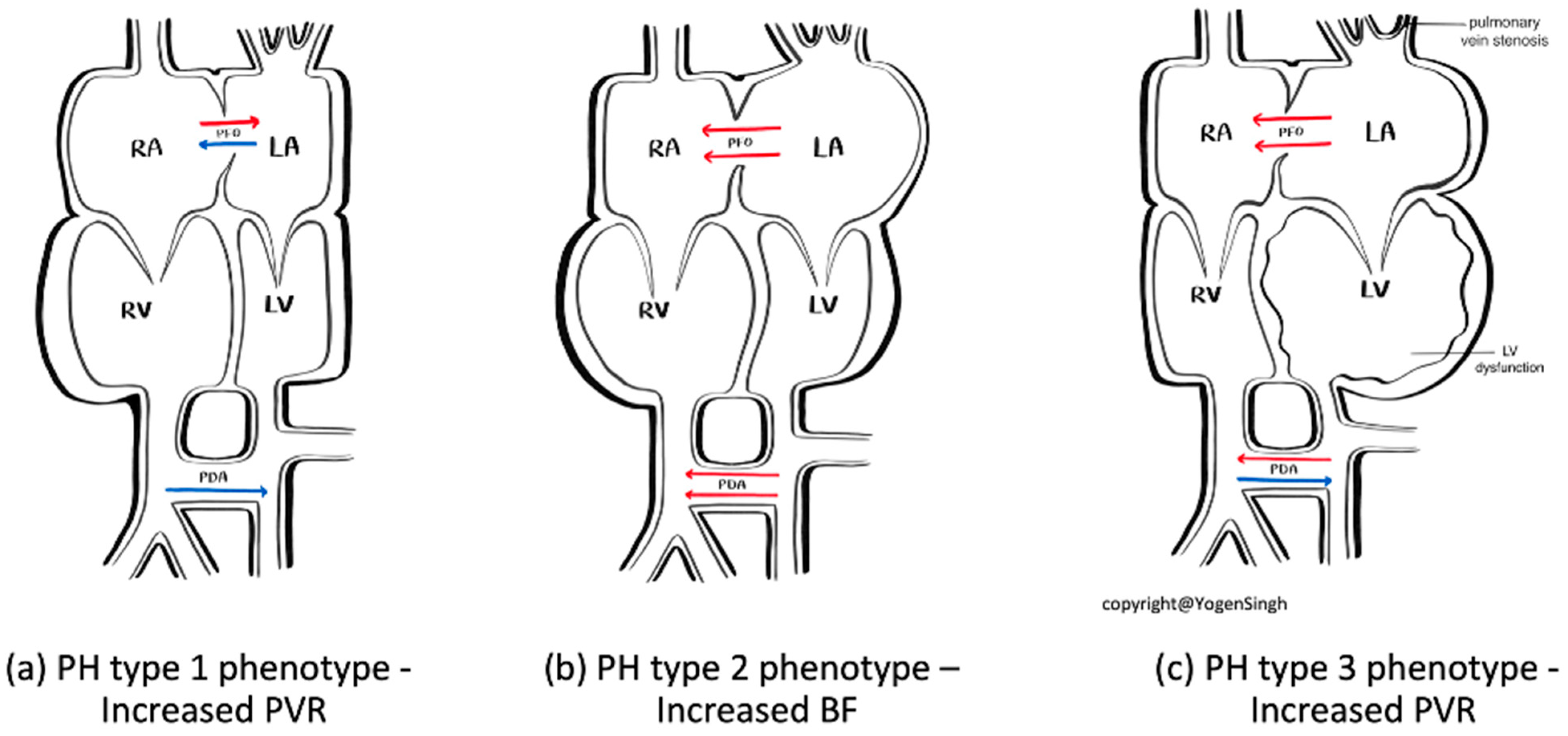
| PH Type 1 Phenotype—Increased PVR | PH Type 2 Phenotype—Increased BF | PH Type 3 Phenotype—Increased PVR | |
|---|---|---|---|
| Diagnosis clues | History of pre-disposing factors such as MAS, perinatal asphyxia, prolonged premature rupture of membranes | History of conditions leading to increased pulmonary blood flow such as persistent patent ductus arteriosus, ASD, VSD, congenital arterio-venous malformation, etc. | History of pre-disposing factors such evolving BPD, especially severe IUGR with BPD, worsening LV dysfunction |
| Significant difference between pre- and post-ductal sats difference | No significant difference between pre- and post-ductal sats difference | Difference between pre- and post-ductal sats difference when PDA open but often closed | |
| Oligemic lung fields on chest X-ray | Plethoric lung fields on chest X-ray | Non-homogenous lung fields on chest X-ray | |
| Classical signs of pulmonary hypertension on echo (see text) | Volume overloading of right side of heart, specific lesions leading to significant left to right shunt | Signs of pulmonary vein stenosis or severe LV dysfunction on echo | |
| Guide to targeted specific therapy | Optimize ventilation, pulmonary vasodilators, optimize hemodynamic support | Optimize ventilation, diuretics and treatment of specific lesion, optimize hemodynamic support | Optimize ventilation, treatment of specific lesion such pulmonary vein stenosis or improve LV function with lusitropic drugs such as milrinone |
| Drug/Dose | Action Mechanism | Side Effects | Indications |
|---|---|---|---|
| First-line therapies | |||
| Inhaled Nitric Oxide (iNO) 5–20 ppm | Activates soluble guanylate cyclase in vascular smooth muscle → selective pulmonary vasodilation | Methemoglobinemia (dose-related), decreased platelet aggregation | Hypoxemic respiratory failure (OI ≥ 25 or PaO2 < 100 mmHg on 100% FiO2) |
| Sildenafil IV: Loading 0.4 mg/kg over 3 h; Maintenance 0.067 mg/kg/h Oral: Initial 0.5 mg/kg; Maintenance 1–3 mg/kg q6h | Selective PDE5 inhibitor → increases cGMP, pulmonary vasodilation | Hypotension (more with IV), hypoxemia | Adjunct or alternative to iNO; first-line in limited-resource settings without iNO |
| Adjunctive therapies | |||
| Milrinone IV: 0.2–1 µg/kg/min (continuous infusion) | PDE3 inhibitor → ↑cAMP → inotropy + vasodilation | Hypotension, arrhythmia, thrombocytopenia [ADD: caution—ensure preload, consider inotropic support] | iNO non-responders with low cardiac output; improves oxygenation and echo indices [ADD: evidence mainly from small neonatal studies] |
| Bosentan Oral: 1–2 mg/kg q12h | Endothelin-1 receptor antagonist (ETA + ETB) | Hepatotoxicity (↑LFTs), anemia | Adjunct in refractory PPHN [ADD: evidence from small cohorts + one exploratory RCT; onset hours–days; monitor LFTs/hemoglobin] |
| Rescue/Alternative therapies | |||
| Prostanoids (Iloprost, Epoprostenol, Treprostinil) Iloprost: Inhaled 1–2.5 µg/kg q2–4h Epoprostenol: IV 1–2 ng/kg/min, max 50–80 ng/kg/min Treprostinil: IV/SC as above | Prostacyclin analogs → activate cAMP pathway → vasodilation, antiplatelet effects | Hypotension, flushing, diarrhea [ADD: evidence limited to small neonatal series; delivery logistics differ for ventilated vs. non-ventilated infants] | Rescue therapy in severe, refractory PPHN |
| Prostaglandin E1 (PGE1) IV: 5–10 ng/kg/min | Maintains ductal patency → allows right-to-left shunting and reduces RV afterload | Apnea, fever, hypotension | [DEL: RV dysfunction with closing DA maintaining ductal patency to offload the RV] [ADD: Selected PPHN cases with RV dysfunction, where ductal patency supports RV offloading and systemic circulation] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chojnacka, K.; Singh, Y.; Gahlaut, S.; Blaz, W.; Jerzak, A.; Szczapa, T. Persistent Pulmonary Hypertension of the Newborn: A Pragmatic Review of Pathophysiology, Diagnosis, and Advances in Management. Biomedicines 2025, 13, 2332. https://doi.org/10.3390/biomedicines13102332
Chojnacka K, Singh Y, Gahlaut S, Blaz W, Jerzak A, Szczapa T. Persistent Pulmonary Hypertension of the Newborn: A Pragmatic Review of Pathophysiology, Diagnosis, and Advances in Management. Biomedicines. 2025; 13(10):2332. https://doi.org/10.3390/biomedicines13102332
Chicago/Turabian StyleChojnacka, Karolina, Yogen Singh, Sheen Gahlaut, Witold Blaz, Agata Jerzak, and Tomasz Szczapa. 2025. "Persistent Pulmonary Hypertension of the Newborn: A Pragmatic Review of Pathophysiology, Diagnosis, and Advances in Management" Biomedicines 13, no. 10: 2332. https://doi.org/10.3390/biomedicines13102332
APA StyleChojnacka, K., Singh, Y., Gahlaut, S., Blaz, W., Jerzak, A., & Szczapa, T. (2025). Persistent Pulmonary Hypertension of the Newborn: A Pragmatic Review of Pathophysiology, Diagnosis, and Advances in Management. Biomedicines, 13(10), 2332. https://doi.org/10.3390/biomedicines13102332





