Impact of Pulmonary Hypertension and Patent Ductus Arteriosus in Preterm Infants with Presumed Pulmonary Hypoplasia †
Abstract
1. Introduction
2. Materials and Methods
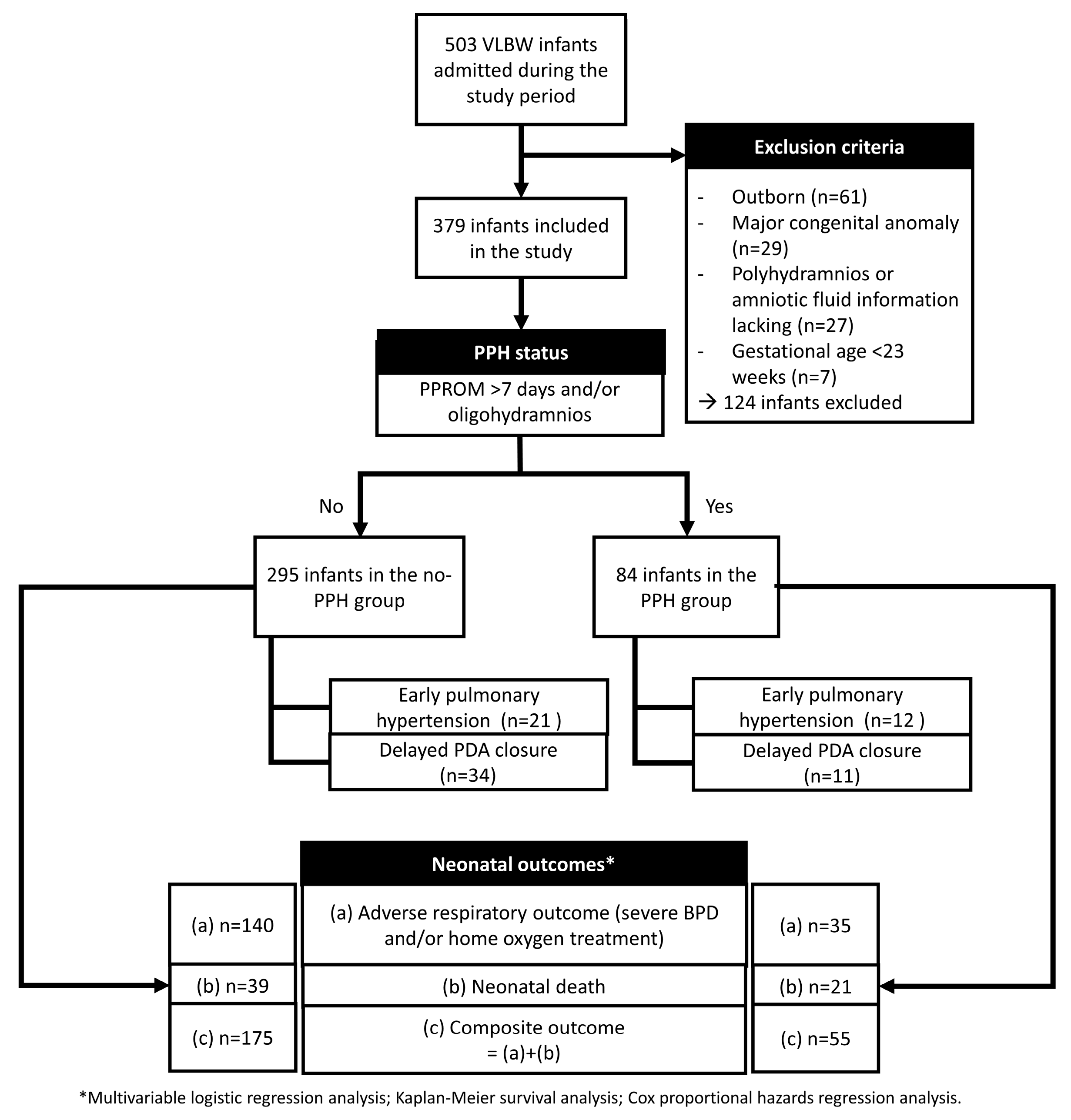
2.1. Patient Inclusion and Data Collection
2.2. Statistical Analysis
3. Results
Patient Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ART | Assisted reproduction therapy |
| CA | Corrected age |
| COX-I | Cyclo-oxygenase inhibitor |
| hsPDA | Hemodynamically significant patent ductus arteriosus |
| MV | Mechanical ventilation |
| PNA | Postanal age |
| PPH | Presumed pulmonary hypoplasia |
| PPROM | Preterm premature rupture of membranes |
| SGA | Small-for-gestational age |
| Tx | Treatment |
References
- Cotten, C.M. Pulmonary hypoplasia. Semin. Fetal Neonatal Med. 2017, 22, 250–255. [Google Scholar] [CrossRef]
- Laudy, J.A.; Wladimiroff, J.W. The fetal lung. 2: Pulmonary hypoplasia. Ultrasound Obstet. Gynecol. 2000, 16, 482–494. [Google Scholar] [CrossRef]
- Chock, V.Y.; Van Meurs, K.P.; Hintz, S.R.; Ehrenkranz, R.A.; Lemons, J.A.; Kendrick, D.E.; Stevenson, D.K.; Network, N.N.R. Inhaled nitric oxide for preterm premature rupture of membranes, oligohydramnios, and pulmonary hypoplasia. Am. J. Perinatol. 2009, 26, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S.; Keszler, M. Persistent Pulmonary Hypertension of the Newborn. Neoreviews 2015, 16, e680–e692. [Google Scholar] [CrossRef] [PubMed]
- Vettukattil, J.J. Pathophysiology of Patent Ductus Arteriosus in the Preterm Infant. Curr. Pediatr. Rev. 2016, 12, 120–122. [Google Scholar] [CrossRef]
- Scheneider, D.J.; Moore, J.W. Patent ductus arteriosus. Circulation 2006, 114, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Arjaans, S.; Fries, M.W.F.; Schoots, M.H.; Schilte, C.F.M.; Roofthooft, M.T.R.; Vrijlandt, E.; Bos, A.F.; Kooi, E.M.W.; Berger, R.M.F. Clinical Significance of Early Pulmonary Hypertension in Preterm Infants. J. Pediatr. 2022, 251, 74–81.e3. [Google Scholar] [CrossRef]
- Nakanishi, H.; Suenaga, H.; Uchiyama, A.; Kusuda, S.; Neonatal Research Network, J. Persistent pulmonary hypertension of the newborn in extremely preterm infants: A Japanese cohort study. Arch. Dis. Child Fetal Neonatal Ed. 2018, 103, F554–F561. [Google Scholar] [CrossRef]
- Hamrick, S.E.G.; Sallmon, H.; Rose, A.T.; Porras, D.; Shelton, E.L.; Reese, J.; Hansmann, G. Patent Ductus Arteriosus of the Preterm Infant. Pediatrics 2020, 146, e20201209. [Google Scholar] [CrossRef]
- Relangi, D.; Somashekar, S.; Jain, D.; Vanbuskirk, S.; Bancalari, E.; Sosenko, I.; Claure, N. Changes in Patent Ductus Arteriosus Treatment Strategy and Respiratory Outcomes in Premature Infants. J. Pediatr. 2021, 235, 58–62. [Google Scholar] [CrossRef]
- Park, G.Y.; Park, W.S.; Sung, S.I.; Kim, M.S.; Lee, M.H.; Jeon, G.W.; Kim, S.S.; Chang, Y.S. Neonatal outcome comparisons between preterm infants with or without early pulmonary hypertension following prolonged preterm premature rupture of membranes before 25 gestational weeks in Korean Neonatal Network. J. Matern.-Fetal Neonatal Med. 2022, 35, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Bae, J.G.; Chang, Y.S. Neonatal Outcomes according to the Latent Period from Membrane Rupture to Delivery among Extremely Preterm Infants Exposed to Preterm Premature Rupture of Membrane: A Nationwide Cohort Study. J. Korean Med. Sci. 2021, 36, e93. [Google Scholar] [CrossRef] [PubMed]
- Fraisse, A.; Geva, T.; Gaudart, J.; Wessel, D.L. Doppler echocardiographic predictors of outcome in newborns with persistent pulmonary hypertension. Cardiol. Young 2004, 14, 277–283. [Google Scholar] [CrossRef]
- Nair, J.; Lakshminrusimha, S. Update on PPHN: Mechanisms and treatment. Semin. Perinatol. 2014, 38, 78–91. [Google Scholar] [CrossRef]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef]
- Winn, H.N.; Chen, M.; Amon, E.; Leet, T.L.; Shumway, J.B.; Mostello, D. Neonatal pulmonary hypoplasia and perinatal mortality in patients with midtrimester rupture of amniotic membranes—A critical analysis. Am. J. Obstet. Gynecol. 2000, 182, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Najrana, T.; Ramos, L.M.; Abu Eid, R.; Sanchez-Esteban, J. Oligohydramnios compromises lung cells size and interferes with epithelial-endothelial development. Pediatr. Pulmonol. 2017, 52, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Chou, H.C.; Wang, L.F.; Lang, Y.D. Experimental oligohydramnios decreases collagen in hypoplastic fetal rat lungs. Exp. Biol. Med. 2008, 233, 1334–1340. [Google Scholar] [CrossRef]
- Hesson, A.; Langen, E. Outcomes in oligohydramnios: The role of etiology in predicting pulmonary morbidity/mortality. J. Perinat. Med. 2018, 46, 948–950. [Google Scholar] [CrossRef]
- Peipert, J.F.; Donnenfeld, A.E. Oligohydramnios: A review. Obstet. Gynecol. Surv. 1991, 46, 325–339. [Google Scholar] [CrossRef]
- Thibeault, D.W.; Beatty, E.C., Jr.; Hall, R.T.; Bowen, S.K.; O’Neill, D.H. Neonatal pulmonary hypoplasia with premature rupture of fetal membranes and oligohydramnios. J. Pediatr. 1985, 107, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Triebwasser, J.E.; Treadwell, M.C. Prenatal prediction of pulmonary hypoplasia. Semin. Fetal Neonatal Med. 2017, 22, 245–249. [Google Scholar] [CrossRef] [PubMed]
- van Teeffelen, A.S.; Van Der Heijden, J.; Oei, S.G.; Porath, M.M.; Willekes, C.; Opmeer, B.; Mol, B.W. Accuracy of imaging parameters in the prediction of lethal pulmonary hypoplasia secondary to mid-trimester prelabor rupture of fetal membranes: A systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2012, 39, 495–499. [Google Scholar] [CrossRef]
- Asabe, K.; Oka, Y.; Ka, H.; Shirakusa, T. Ultrastructural evaluation of type II pneumatocytes in the hypoplastic lung of rabbit fetuses induced by oligohydramnios. J. Obstet. Gynaecol. Res. 2007, 33, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Tomo, C.K.; Balogun, O.O.; Davidson, J.; Guinsburg, R.; Almeida, M.F.B.d.; Lopes, J.M.d.A.; Barros, M.C.d.M.; Takehara, K.; Mikami, M.; Isayama, T. Comparison of mortality and survival without major morbidities of very preterm infants with very low birth weight from Japan and Brazil. Rev. Paul. Pediatr. 2022, 41, e2021389. [Google Scholar] [CrossRef]
- Acun, C.; Nusairat, L.; Kadri, A.; Nusairat, A.; Yeaney, N.; Abu Shaweesh, J.; Aly, H. Pneumothorax prevalence and mortality per gestational age in the newborn. Pediatr. Pulmonol. 2021, 56, 2583–2588. [Google Scholar] [CrossRef]
- Kettle, R.; Subhedar, N.V.; European iNO Registry. Nitric Oxide in Pulmonary Hypoplasia: Results from the European iNO Registry. Neonatology 2019, 116, 341–346. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, H.; Zhao, Z.; Du, J.; Bai, R.; McNamara, P.J. Impact of patent ductus arteriosus shunt size and duration on risk of death or severe respiratory morbidity in preterm infants born in China. Eur. J. Pediatr. 2022, 181, 3131–3140. [Google Scholar] [CrossRef]
- Mirza, H.; Garcia, J.; McKinley, G.; Hubbard, L.; Sensing, W.; Schneider, J.; Oh, W.; Wadhawan, R. Duration of significant patent ductus arteriosus and bronchopulmonary dysplasia in extremely preterm infants. J. Perinatol. 2019, 39, 1648–1655. [Google Scholar] [CrossRef]
- Philip, R.; Waller, B.R.; Chilakala, S.; Graham, B.; Stecchi, N.; Apalodimas, L.; Cunningham, J.; Washington, K.; Sathanandam, S. Hemodynamic and clinical consequences of early versus delayed closure of patent ductus arteriosus in extremely low birth weight infants. J. Perinatol. 2021, 41, 100–108. [Google Scholar] [CrossRef]
- Bixler, G.M.; Powers, G.C.; Clark, R.H.; Walker, M.W.; Tolia, V.N. Changes in the Diagnosis and Management of Patent Ductus Arteriosus from 2006 to 2015 in United States Neonatal Intensive Care Units. J. Pediatr. 2017, 189, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Hundscheid, T.; Onland, W.; van Overmeire, B.; Dijk, P.; van Kaam, A.; Dijkman, K.P.; Kooi, E.M.W.; Villamor, E.; Kroon, A.A.; Visser, R.; et al. Early treatment versus expectative management of patent ductus arteriosus in preterm infants: A multicentre, randomised, non-inferiority trial in Europe (BeNeDuctus trial). BMC Pediatr. 2018, 18, 262. [Google Scholar] [CrossRef] [PubMed]
- Ngo, S.; Profit, J.; Gould, J.B.; Lee, H.C. Trends in Patent Ductus Arteriosus Diagnosis and Management for Very Low Birth Weight Infants. Pediatrics 2017, 139, e20162390. [Google Scholar] [CrossRef] [PubMed]
- Mashally, S.; Nield, L.E.; McNamara, P.J.; Martins, F.F.; El-Khuffash, A.; Jain, A.; Weisz, D.E. Late oral acetaminophen versus immediate surgical ligation in preterm infants with persistent large patent ductus arteriosus. J. Thorac. Cardiovasc. Surg. 2018, 156, 1937–1944. [Google Scholar] [CrossRef]
- Schena, F.; Francescato, G.; Cappelleri, A.; Picciolli, I.; Mayer, A.; Mosca, F.; Fumagalli, M. Association between hemodynamically significant patent ductus arteriosus and bronchopulmonary dysplasia. J. Pediatr. 2015, 166, 1488–1492. [Google Scholar] [CrossRef]
- McNamara, P.J.; Sehgal, A. Towards rational management of the patent ductus arteriosus: The need for disease staging. Arch. Dis. Child Fetal Neonatal Ed. 2007, 92, F424–F427. [Google Scholar] [CrossRef]
- Seo, Y.; Oh, M.-Y.; Kim, S.; Kim, M.S.; Yum, S.K. Morbidities associated with pulmonary circulation and adverse respiratory outcomes in very-low-birthweight infants affected by pulmonary hypoplasia. In Proceedings of the 9th Congress of the European Academy of Paediatric Societies, Barcelona, Spain, 7–11 October 2022; pp. 2381–2382. [Google Scholar] [CrossRef]

| No PPH (n = 295) | PPH (n = 84) | p | |
|---|---|---|---|
| Gestational age (weeks) | 28.1 [26.1–30.1] | 27.7 [25.4–30.1] | 0.220 |
| Birthweight (g) | 1070 [820–1290] | 931 [730–1227] | 0.009 |
| Male | 144 (48.8%) | 39 (46.4%) | 0.700 |
| SGA | 48 (16.3%) | 21 (25.0%) | 0.067 |
| Multiple births | 118 (40.0%) | 23 (27.4%) | 0.035 |
| Cesarean delivery | 259 (87.8%) | 76 (90.5%) | 0.499 |
| 1 min Apgar | 3 [2–5] | 3 [1–4] | 0.060 |
| 5 min Apgar | 6 [5–7] | 6 [4–7] | 0.146 |
| Maternal age (years) | 34 [31–36] | 33 [30–36] | 0.156 |
| Primiparity | 179 (60.7%) | 47 (56.0%) | 0.436 |
| Maternal diabetes | 29 (9.8%) | 5 (6.0%) | 0.272 |
| Maternal hypertension | 46 (15.6%) | 14 (16.7%) | 0.812 |
| Histologic chorioamnionitis | 97 (33.3%) | 37 (44.0%) | 0.071 |
| Conceived via ART | 67 (22.7%) | 16 (19.0%) | 0.474 |
| PPROM | 94 (31.9%) | 49 (58.3%) | <0.001 |
| PPROM (hours) 1 | 18 [6–61] | 188 [45–521] | <0.001 |
| PPROM onset (weeks) 1 | 26.8 [25.3–29.0] | 23.9 [22.6–27.6] | <0.001 |
| PPROM before 25 weeks of gestation 1 | 20 (21.3%) | 30 (61.2%) | <0.001 |
| Oligohydramnios | 0 (0.0%) | 68 (81.0%) | <0.001 |
| ACS administration | 203 (69.0%) | 67 (79.8%) | 0.055 |
| No PPH (n = 295) | PPH (n = 84) | p | |
|---|---|---|---|
| RDS | 265 (89.8%) | 75 (89.3%) | 0.885 |
| Surfactant ≥ 2 doses | 82 (27.9%) | 20 (23.8%) | 0.457 |
| Air leak syndrome | 24 (8.1%) | 16 (19.0%) | 0.004 |
| Massive pulmonary hemorrhage | 48 (16.3%) | 18 (21.4%) | 0.271 |
| Pulmonary hypertension, total | 46 (15.6%) | 24 (28.6%) | 0.007 |
| Pulmonary hypertension depending on onset | |||
| Early pulmonary hypertension * | 21 (7.1%) | 12 (14.3%) | 0.040 |
| Late pulmonary hypertension | 25 (8.5%) | 12 (14.3%) | 0.113 |
| HSPDA ** | 78 (26.9%) | 16 (20.3%) | 0.230 |
| COX-I use | 25 (32.1%) | 2 (12.5%) | 0.140 † |
| COX-I failure | 8 (32.0%) | 0 (0.0%) | >0.99 † |
| Surgical ligation | 61 (78.2%) | 14 (87.5%) | 0.512 † |
| PNA at initial Tx. | 18 [9–26] | 33 [20–47] | 0.09 |
| CA at initial Tx. | 29.7 [28.1–31.3] | 30.9 [28.4–32.0] | 0.199 |
| PNA at final Tx. | 19 [10–29] | 33 [20–47] | 0.025 |
| CA at final Tx. | 29.9 [28.1–31.4] | 30.9 [28.4–32.0] | 0.324 |
| PDA closure ≥ 21 d | 34 (11.5%) | 11 (13.1%) | 0.695 |
| No PPH (n = 295) | PPH (n = 84) | p | |
|---|---|---|---|
| MV duration | 11 [3–37] | 10 [2–44] | 0.890 |
| Severe BPD * | 95 (37.1%) | 23 (35.9%) | 0.862 |
| Length of stay | 56 [39–82] | 53 [24–95] | 0.382 |
| CA at discharge | 37.3 [35.7–39.9] | 37.1 [34.9–40.0] | 0.598 |
| Home oxygen treatment ** | 89 (36.6%) | 21 (36.8%) | 0.976 |
| Death before 28 days after birth | 39 (13.3%) | 13 (15.5%) | 0.612 |
| Death before discharge † | 39 (13.2%) | 21 (25.0%) | 0.009 |
| Early Pulmonary Hypertension | Delayed PDA Closure | |||
|---|---|---|---|---|
| Included infants, total | p | OR (95%CI) | p | aOR * (95%CI) |
| Adverse respiratory outcome | 0.293 | 2.129 (0.520–8.713) | 0.002 | 4.849 (1.783–13.189) |
| Neonatal death | <0.001 | 11.165 (3.657–34.088) | <0.001 | 0.020 (0.002–0.176) |
| Composite outcome | 0.024 | 5.800 (1.263–26.633) | 0.044 | 2.906 (1.030–8.197) |
| No-PPH infants | p | OR (95%CI) | p | aOR * (95%CI) |
| Adverse respiratory outcome | 0.310 | 2.379 (0.446–12.683) | 0.005 | 4.929 (1.613–15.055) |
| Neonatal death | <0.001 | 11.575 (2.988–44.833) | 0.006 | 0.036 (0.003–0.383) |
| Composite outcome | 0.068 | 6.920 (0.867–55.267) | 0.041 | 3.320 (1.048–10.515) |
| PPH infants | p | OR (95%CI) | p | aOR * (95%CI) |
| Adverse respiratory outcome | 0.388 | 4.158 (0.164–105.578) | 0.544 | 2.166 (0.179–26.279) |
| Neonatal death | 0.025 | 9.981 (1.334–74.647) | >0.990 | - ** |
| Composite outcome | 0.091 | 8.390 (0.710–99.135) | 0.962 | 1.059 (0.099–11.360) |
| Model # | Variables | Hazard Risk (95%CI) | p-Value |
|---|---|---|---|
| Model 1 | Birth weight (100 g) | 0.703 (0.629–0.785) | <0.001 |
| Multiple births | 0.669 (0.395–1.134) | 0.128 | |
| Air leak syndrome | 0.263 (0.146–0.472) | <0.001 | |
| PPH | 0.912 (0.518–1.607) | 0.768 | |
| Model 2 | Birth weight (100 g) | 0.704 (0.630–0.787) | <0.001 |
| Multiple births | 0.671 (0.396–1.138) | 0.130 | |
| Air leak syndrome | 0.261 (0.145–0.470) | <0.001 | |
| PPH | 0.915 (0.519–1.615) | 0.778 | |
| Delayed PDA closure | 1.153 (0.910–1.462) | 0.215 | |
| Model 3 | Birth weight (100 g) | 0.702 (0.627–0.785) | <0.001 |
| Multiple births | 0.662 (0.391–1.121) | 0.126 | |
| Air leak syndrome | 0.263 (0.146–0.474) | <0.001 | |
| PPH | 0.912 (0.518–1.608) | 0.768 | |
| Early pulmonary hypertension | 0.983 (0.712–1.356) | 0.915 | |
| Model 4 | Birth weight (100 g) | 0.705 (0.629–0.791) | <0.001 |
| Multiple births | 0.673 (0.397–1.141) | 0.132 | |
| Air leak syndrome | 0.259 (0.143–0.471) | <0.001 | |
| PPH | 0.916 (0.515–1.629) | 0.775 | |
| Delayed PDA closure | 1.154 (0.910–1.465) | 0.215 | |
| Early pulmonary hypertension | 0.986 (0.715–1.361) | 0.936 | |
| Model 5 | Birth weight (100 g) | 0.707 (0.632–0.791) | <0.001 |
| Multiple births | 0.663 (0.395–1.113) | 0.128 | |
| Air leak syndrome | 0.263 (0.146–0.474) | <0.001 | |
| PPH | 0.911 (0.518–1.603) | 0.765 | |
| Early pulmonary hypertension | 0.971 (0.704–1.339) | 0.868 | |
| PPH × Early pulmonary hypertension | 0.973 (0.469–2.021) | 0.943 | |
| Model 6 | Birth weight (100 g) | 0.713 (0.637–0.797) | <0.001 |
| Multiple births | 0.661 (0.390–1.121) | 0.123 | |
| Air leak syndrome | 0.266 (0.148–0.478) | <0.001 | |
| PPH | 0.920 (0.519–1.630) | 0.781 | |
| Delayed PDA closure | 1.174 (0.924–1.492) | 0.180 | |
| PPH × Delayed PDA closure | 0.805 (0.406–1.596) | 0.531 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Seo, Y.; Oh, M.-Y.; Kim, M.S.; Yum, S.K. Impact of Pulmonary Hypertension and Patent Ductus Arteriosus in Preterm Infants with Presumed Pulmonary Hypoplasia. Biomedicines 2025, 13, 1725. https://doi.org/10.3390/biomedicines13071725
Kim S, Seo Y, Oh M-Y, Kim MS, Yum SK. Impact of Pulmonary Hypertension and Patent Ductus Arteriosus in Preterm Infants with Presumed Pulmonary Hypoplasia. Biomedicines. 2025; 13(7):1725. https://doi.org/10.3390/biomedicines13071725
Chicago/Turabian StyleKim, Sol, Yumi Seo, Moon-Yeon Oh, Min Soo Kim, and Sook Kyung Yum. 2025. "Impact of Pulmonary Hypertension and Patent Ductus Arteriosus in Preterm Infants with Presumed Pulmonary Hypoplasia" Biomedicines 13, no. 7: 1725. https://doi.org/10.3390/biomedicines13071725
APA StyleKim, S., Seo, Y., Oh, M.-Y., Kim, M. S., & Yum, S. K. (2025). Impact of Pulmonary Hypertension and Patent Ductus Arteriosus in Preterm Infants with Presumed Pulmonary Hypoplasia. Biomedicines, 13(7), 1725. https://doi.org/10.3390/biomedicines13071725





