Impact of Biologics on Comorbidities in Patients with Psoriasis or Psoriatic Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Design and Patients
2.3. Outcomes
2.4. Covariates
2.5. Statistical Analysis
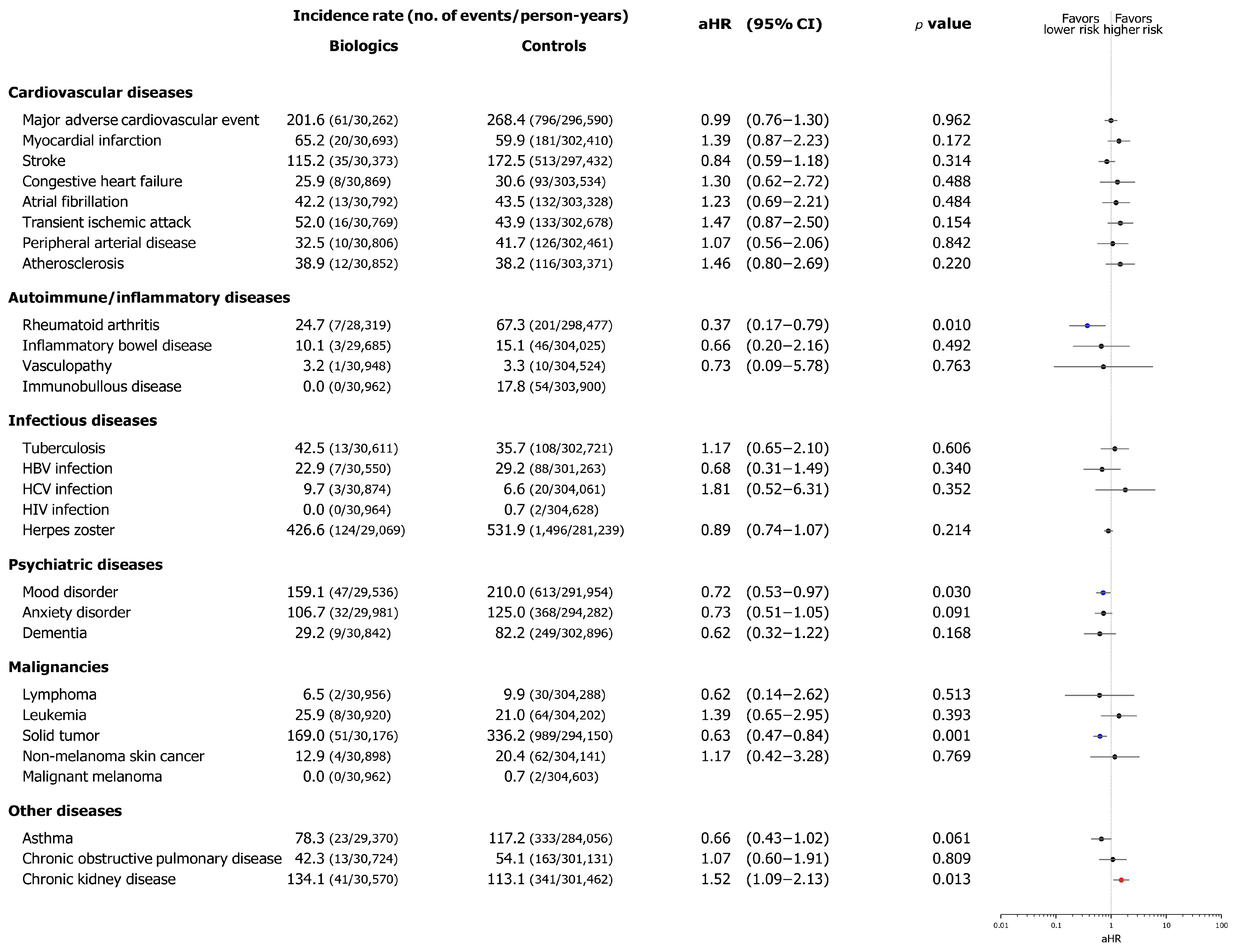
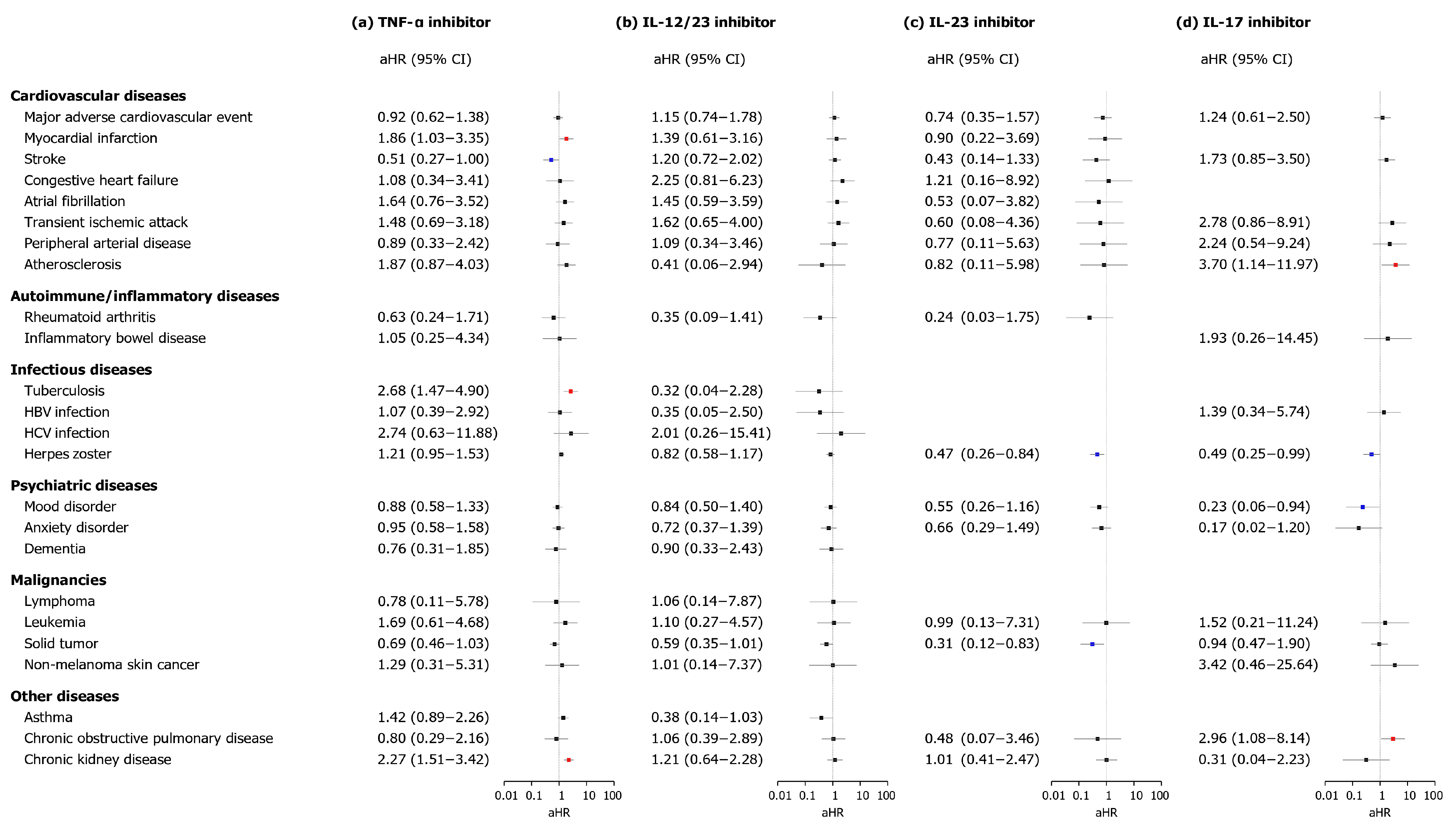
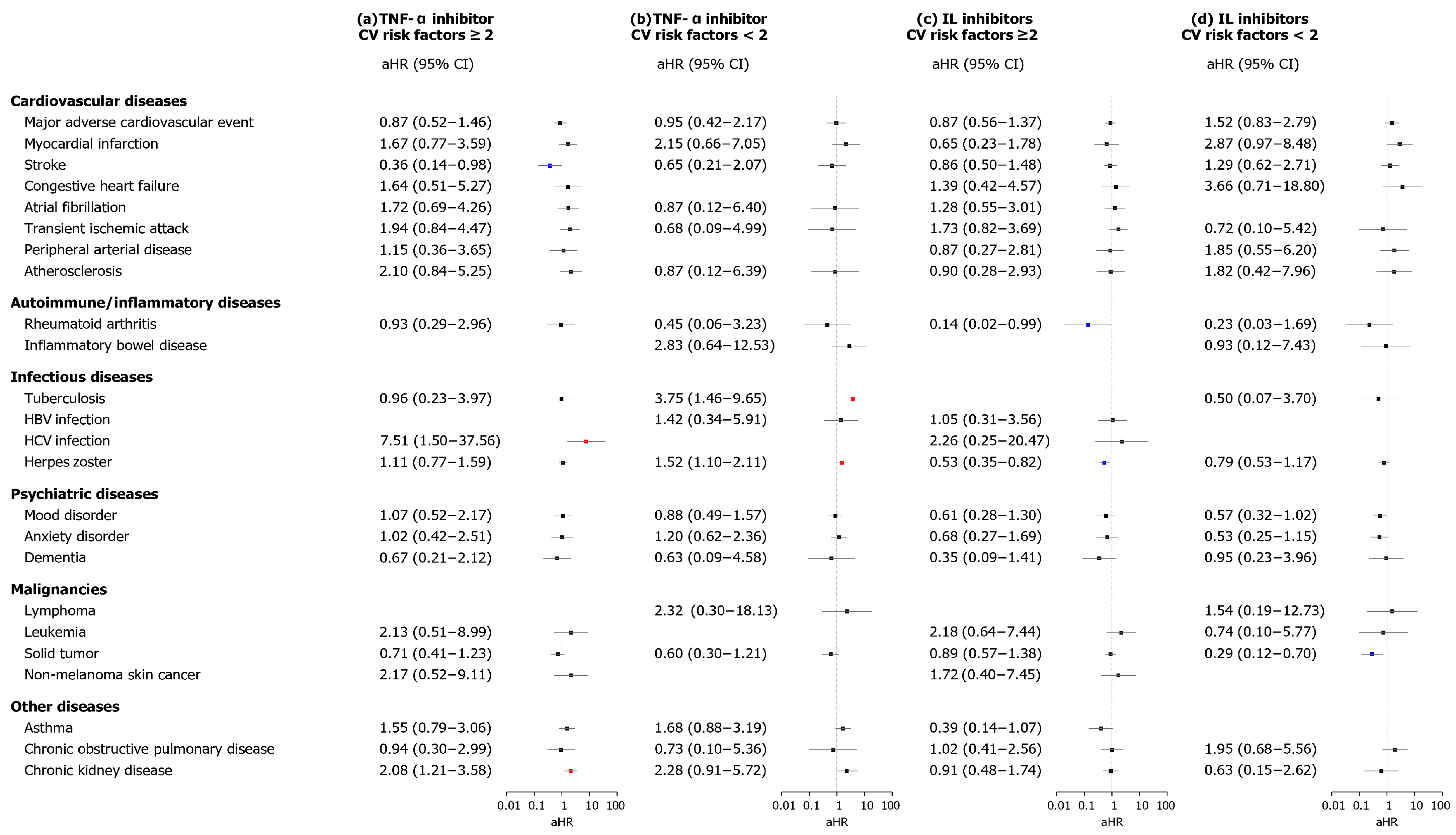
3. Results
3.1. Study Population
3.2. Prevalence of Comorbid Diseases at Baseline
3.3. Incidence and Risk of Comorbid Diseases Following the Index Date
3.4. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PsO | Psoriasis |
| PsA | Psoriatic arthritis |
| TNF | Tumor necrosis factor |
| CV | Cardiovascular |
| CsA | Cyclosporine A |
| MTX | Methotrexate |
| NHIS | National Health Insurance Service |
| MACE | Major cardiovascular event |
| MI | Myocardial infarction |
| RA | Rheumatoid arthritis |
| IBD | Inflammatory bowel disease |
| TB | Tuberculosis |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| NMSC | Non-melanoma skin cancer |
| COPD | Chronic obstructive pulmonary disease |
| CKD | Chronic kidney disease |
| HTN | Hypertension |
| aORs | Adjusted odds ratios |
| aHRs | Adjusted hazard ratios |
| CIs | Confidence intervals |
References
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Mechanisms of disease: Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Elmets, C.A.; Leonardi, C.L.; Davis, D.M.R.; Gelfand, J.M.; Lichten, J.; Mehta, N.N.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Elewski, B.E.; et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J. Am. Acad. Dermatol. 2019, 80, 1073–1113. [Google Scholar] [CrossRef] [PubMed]
- Ritchlin, C.T.; Colbert, R.A.; Gladman, D.D. Psoriatic Arthritis. N. Engl. J. Med. 2017, 376, 957–970. [Google Scholar] [CrossRef]
- Alinaghi, F.; Calov, M.; Kristensen, L.E.; Gladman, D.D.; Coates, L.C.; Jullien, D.; Gottlieb, A.B.; Gisondi, P.; Wu, J.J.; Thyssen, J.P.; et al. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. J. Am. Acad. Dermatol. 2019, 80, 251–265.e19. [Google Scholar] [CrossRef]
- Han, J.H.; Lee, J.H.; Han, K.D.; Seo, H.M.; Bang, C.H.; Park, Y.M.; Lee, J.Y.; Park, Y.G. Epidemiology and Medication Trends in Patients with Psoriasis: A Nationwide Population-based Cohort Study from Korea. Acta Derm. Venereol. 2018, 98, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Coates, L.C.; Soriano, E.R.; Corp, N.; Bertheussen, H.; Callis Duffin, K.; Campanholo, C.B.; Chau, J.; Eder, L.; Fernández-Ávila, D.G.; FitzGerald, O.; et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): Updated treatment recommendations for psoriatic arthritis 2021. Nat. Rev. Rheumatol. 2022, 18, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and comorbid diseases: Epidemiology. J. Am. Acad. Dermatol. 2017, 76, 377–390. [Google Scholar] [CrossRef]
- Yamazaki, F. Psoriasis: Comorbidities. J. Dermatol. 2021, 48, 732–740. [Google Scholar] [CrossRef]
- Bu, J.; Ding, R.; Zhou, L.; Chen, X.; Shen, E. Epidemiology of Psoriasis and Comorbid Diseases: A Narrative Review. Front. Immunol. 2022, 13, 880201. [Google Scholar] [CrossRef]
- Vaengebjerg, S.; Skov, L.; Egeberg, A.; Loft, N.D. Prevalence, Incidence, and Risk of Cancer in Patients With Psoriasis and Psoriatic Arthritis: A Systematic Review and Meta-analysis. JAMA Dermatol. 2020, 156, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Chiesa Fuxench, Z.C.; Shin, D.B.; Ogdie Beatty, A.; Gelfand, J.M. The Risk of Cancer in Patients with Psoriasis: A Population-Based Cohort Study in the Health Improvement Network. JAMA Dermatol. 2016, 152, 282–290. [Google Scholar] [CrossRef]
- Rapp, S.R.; Feldman, S.R.; Exum, M.L.; Fleischer, A.B., Jr.; Reboussin, D.M. Psoriasis causes as much disability as other major medical diseases. J. Am. Acad. Dermatol. 1999, 41, 401–407. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Jungo, P.; Maul, J.T.; Djamei, V.; von Felten, S.; Kolios, A.G.A.; Czernielewsk, J.; Yawalkar, N.; Odermatt, O.; Laffitte, E.; Anliker, M.; et al. Superiority in Quality of Life Improvement of Biologics over Conventional Systemic Drugs in a Swiss Real-Life Psoriasis Registry. Dermatology 2016, 232, 655–663. [Google Scholar] [CrossRef]
- Sakkas, L.I.; Zafiriou, E.; Bogdanos, D.P. Mini Review: New Treatments in Psoriatic Arthritis. Focus on the IL-23/17 Axis. Front. Pharmacol. 2019, 10, 872. [Google Scholar] [CrossRef]
- Kamata, M.; Tada, Y. Efficacy and Safety of Biologics for Psoriasis and Psoriatic Arthritis and Their Impact on Comorbidities: A Literature Review. Int. J. Mol. Sci. 2020, 21, 1690. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Yu, Q.; Shi, Y. Biologic and Small-Molecule Therapies for Moderate-to-Severe Psoriasis: Focus on Psoriasis Comorbidities. BioDrugs 2023, 37, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Darwin, E.; Lebwohl, M.; Han, G. Biologic Vs Conventional Therapies: Comparing Risk of Psoriasis-Associated Comorbidities. J. Drugs Dermatol. 2023, 22, 621–622. [Google Scholar] [CrossRef]
- Rungapiromnan, W.; Yiu, Z.Z.N.; Warren, R.B.; Griffiths, C.E.M.; Ashcroft, D.M. Impact of biologic therapies on risk of major adverse cardiovascular events in patients with psoriasis: Systematic review and meta-analysis of randomized controlled trials. Br. J. Dermatol. 2017, 176, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Poizeau, F.; Nowak, E.; Kerbrat, S.; Le Nautout, B.; Droitcourt, C.; Drici, M.D.; Sbidian, E.; Guillot, B.; Bachelez, H.; Ait-Oufella, H.; et al. Association Between Early Severe Cardiovascular Events and the Initiation of Treatment with the Anti-Interleukin 12/23p40 Antibody Ustekinumab. JAMA Dermatol. 2020, 156, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Yeung, H.; Takeshita, J.; Mehta, N.N.; Kimmel, S.E.; Ogdie, A.; Margolis, D.J.; Shin, D.B.; Attor, R.; Troxel, A.B.; Gelfand, J.M. Psoriasis severity and the prevalence of major medical comorbidity: A population-based study. JAMA Dermatol. 2013, 149, 1173–1179. [Google Scholar] [CrossRef]
- Wu, J.J.; Guérin, A.; Sundaram, M.; Dea, K.; Cloutier, M.; Mulani, P. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J. Am. Acad. Dermatol. 2017, 76, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Low, A.S.; Symmons, D.P.; Lunt, M.; Mercer, L.K.; Gale, C.P.; Watson, K.D.; Dixon, W.G.; Hyrich, K.L. Relationship between exposure to tumour necrosis factor inhibitor therapy and incidence and severity of myocardial infarction in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 654–660. [Google Scholar] [CrossRef]
- Lee, J.L.; Sinnathurai, P.; Buchbinder, R.; Hill, C.; Lassere, M.; March, L. Biologics and cardiovascular events in inflammatory arthritis: A prospective national cohort study. Arthritis Res. Ther. 2018, 20, 171. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Sundaram, M.; Cloutier, M.; Gauthier-Loiselle, M.; Guérin, A.; Singh, R.; Ganguli, A. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: An observational cohort study. J. Am. Acad. Dermatol. 2018, 79, 60–68. [Google Scholar] [CrossRef]
- Wan, J.; Wang, S.; Haynes, K.; Denburg, M.R.; Shin, D.B.; Gelfand, J.M. Risk of moderate to advanced kidney disease in patients with psoriasis: Population based cohort study. BMJ 2013, 347, f5961. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef]
- Singh, S.; Taylor, C.; Kornmehl, H.; Armstrong, A.W. Psoriasis and suicidality: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017, 77, 425–440.e422. [Google Scholar] [CrossRef]
- Strober, B.; Soliman, A.M.; Truong, B.; Patel, M.B.; Barqawi, Y.K.; Gisondi, P. Association Between Biologic Exposure and the Risk of Depression in Patients with Psoriasis: A Retrospective Analysis of Large US Administrative Claims Data. Am. J. Clin. Dermatol. 2024, 25, 853–856. [Google Scholar] [CrossRef]
- Habiba, U.e.; Rafiq, M.; Khawar, M.B.; Nazir, B.; Haider, G.; Nazir, N. The multifaceted role of IL-12 in cancer. Adv. Cancer Biol.-Metastasis 2022, 5, 100053. [Google Scholar] [CrossRef]
- Yue, T.; Zheng, X.; Dou, Y.; Zheng, X.; Sun, R.; Tian, Z.; Wei, H. Interleukin 12 shows a better curative effect on lung cancer than paclitaxel and cisplatin doublet chemotherapy. BMC Cancer 2016, 16, 665. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.K.; Su, W.P.; Schroeter, A.L.; Sabers, C.J.; Abraham, R.T.; Pittelkow, M.R. Cyclosporine in the treatment of dermatologic disease: An update. Mayo Clin. Proc. 1996, 71, 1182–1191. [Google Scholar] [CrossRef]
- Song, W.J.; Oh, S.; Yoon, H.S. Association between biologic and nonbiologic systemic therapy for psoriasis and psoriatic arthritis and the risk of new-onset and recurrent major adverse cardiovascular events: A retrospective cohort study. J. Am. Acad. Dermatol. 2025, 93, 141–149. [Google Scholar] [CrossRef]
- Rungapiromnan, W.; Mason, K.J.; Lunt, M.; McElhone, K.; Burden, A.D.; Rutter, M.K.; Warren, R.B.; Griffiths, C.E.M.; Ashcroft, D.M. Risk of major cardiovascular events in patients with psoriasis receiving biologic therapies: A prospective cohort study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 769–778. [Google Scholar] [CrossRef]
- Urschel, K.; Cicha, I. TNF-α in the cardiovascular system: From physiology to therapy. Int. J. Interferon Cytokine Mediat. Res. 2015, 7, 9–25. [Google Scholar] [CrossRef]
- Medler, J.; Wajant, H. Tumor necrosis factor receptor-2 (TNFR2): An overview of an emerging drug target. Expert Opin. Ther. Targets 2019, 23, 295–307. [Google Scholar] [CrossRef]
- Hussain, A.; Tarahomi, T.; Singh, L.; Bollampally, M.; Heydari-Kamjani, M.; Kesselman, M.M. Cardiovascular Risk Associated with TNF Alpha Inhibitor Use in Patients with Rheumatoid Arthritis. Cureus 2021, 13, e17938. [Google Scholar] [CrossRef]
- Taleb, S.; Tedgui, A.; Mallat, Z. IL-17 and Th17 cells in atherosclerosis: Subtle and contextual roles. Arter. Thromb. Vasc. Biol. 2015, 35, 258–264. [Google Scholar] [CrossRef]
- Fatkhullina, A.R.; Peshkova, I.O.; Dzutsev, A.; Aghayev, T.; McCulloch, J.A.; Thovarai, V.; Badger, J.H.; Vats, R.; Sundd, P.; Tang, H.Y.; et al. An Interleukin-23-Interleukin-22 Axis Regulates Intestinal Microbial Homeostasis to Protect from Diet-Induced Atherosclerosis. Immunity 2018, 49, 943–957.e9. [Google Scholar] [CrossRef] [PubMed]
- Caiazzo, G.; Fabbrocini, G.; Di Caprio, R.; Raimondo, A.; Scala, E.; Balato, N.; Balato, A. Psoriasis, Cardiovascular Events, and Biologics: Lights and Shadows. Front. Immunol. 2018, 9, 1668. [Google Scholar] [CrossRef]
- Guillet, C.; Seeli, C.; Nina, M.; Maul, L.V.; Maul, J.T. The impact of gender and sex in psoriasis: What to be aware of when treating women with psoriasis. Int. J. Womens Dermatol 2022, 8, e010. [Google Scholar] [CrossRef]
- Hägg, D.; Sundström, A.; Eriksson, M.; Schmitt-Egenolf, M. Severity of Psoriasis Differs Between Men and Women: A Study of the Clinical Outcome Measure Psoriasis Area and Severity Index (PASI) in 5438 Swedish Register Patients. Am. J. Clin. Dermatol. 2017, 18, 583–590. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Harskamp, C.T.; Dhillon, J.S.; Armstrong, E.J. Psoriasis and smoking: A systematic review and meta-analysis. Br. J. Dermatol. 2014, 170, 304–314. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Armstrong, E.J.; Fuller, E.N.; Sockolov, M.E.; Voyles, S.V. Smoking and pathogenesis of psoriasis: A review of oxidative, inflammatory and genetic mechanisms. Br. J. Dermatol. 2011, 165, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Budu-Aggrey, A.; Brumpton, B.; Tyrrell, J.; Watkins, S.; Modalsli, E.H.; Celis-Morales, C.; Ferguson, L.D.; Vie, G.; Palmer, T.; Fritsche, L.G.; et al. Evidence of a causal relationship between body mass index and psoriasis: A mendelian randomization study. PLoS Med. 2019, 16, e1002739. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.; Simon, J.C.; Saalbach, A. Psoriasis: Obesity and Fatty Acids. Front. Immunol. 2019, 10, 1807. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, A.; Raimondo, A.; Lembo, S.; Fausti, F.; Dini, V.; Costanzo, A.; Monfrecola, G.; Balato, N.; Ayala, F.; Romanelli, M.; et al. Crosstalk between skin inflammation and adipose tissue-derived products: Pathogenic evidence linking psoriasis to increased adiposity. Expert Rev. Clin. Immunol. 2016, 12, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Kong, M.; Li, X.; Zhu, X.; Wei, D.; Ni, M.; Wang, Y.; Hong, Z.; Dong, A. The Association Between Psoriasis and Risk of Cardiovascular Disease: A Mendelian Randomization Analysis. Front. Immunol. 2022, 13, 918224. [Google Scholar] [CrossRef]
- Armstrong, E.J.; Harskamp, C.T.; Armstrong, A.W. Psoriasis and major adverse cardiovascular events: A systematic review and meta-analysis of observational studies. J. Am. Heart Assoc. 2013, 2, e000062. [Google Scholar] [CrossRef] [PubMed]

| Prevalent Diseases, No. (%) | ||||||
|---|---|---|---|---|---|---|
| Comorbid Disease | Biologics (n = 8173) | Controls (n = 41,598) | aORs (95% CI) | |||
| Cardiovascular diseases | ||||||
| MACE | 147 | (1.80%) | 738 | (1.77%) | 1.01 | (0.85–1.21) |
| Myocardial infarction | 39 | (0.48%) | 204 | (0.49%) | 0.97 | (0.69–1.37) |
| Stroke | 140 | (1.71%) | 738 | (1.77%) | 0.96 | (0.80–1.16) |
| Congestive heart failure | 23 | (0.28%) | 114 | (0.27%) | 1.03 | (0.66–1.61) |
| Atrial fibrillation | 33 | (0.40%) | 117 | (0.28%) | 1.44 | (0.98–2.12) |
| Transient ischemic attack | 38 | (0.46%) | 207 | (0.50%) | 0.93 | (0.66–1.32) |
| Peripheral arterial disease | 38 | (0.46%) | 219 | (0.53%) | 0.88 | (0.62–1.25) |
| Atherosclerosis | 25 | (0.31%) | 104 | (0.25%) | 1.22 | (0.79–1.90) |
| Autoimmune/inflammatory diseases | ||||||
| Rheumatoid arthritis | 372 | (4.55%) | 595 | (1.43%) | 3.29 | (2.88–3.75) |
| Inflammatory bowel disease | 180 | (2.20%) | 61 | (0.15%) | 15.33 | (11.46–20.52) |
| Vasculopathy | 3 | (0.04%) | 15 | (0.04%) | 1.02 | (0.29–3.52) |
| Immunobullous disease | 1 | (0.01%) | 53 | (0.13%) | 0.10 | (0.01–0.69) |
| Infectious diseases | ||||||
| Tuberculosis | 58 | (0.71%) | 211 | (0.51%) | 1.40 | (1.05–1.33) |
| HBV infection | 116 | (1.42%) | 420 | (1.01%) | 1.41 | (1.15–1.74) |
| HCV infection | 19 | (0.23%) | 77 | (0.19%) | 1.26 | (0.76–2.08) |
| HIV infection | 0 | (0.00%) | 2 | (0.00%) | NA | |
| Herpes zoster | 431 | (5.27%) | 2430 | (5.84%) | 0.90 | (0.81–1.00) |
| Psychiatric diseases | ||||||
| Mood disorder | 338 | (4.14%) | 1468 | (3.53%) | 1.18 | (1.05–1.33) |
| Anxiety disorder | 227 | (2.78%) | 1215 | (2.92%) | 0.95 | (0.82–1.10) |
| Dementia | 25 | (0.31%) | 143 | (0.34%) | 0.89 | (0.58–1.36) |
| Malignancies | ||||||
| Lymphoma | 1 | (0.01%) | 51 | (0.12%) | 0.10 | (0.01–0.72) |
| Leukemia | 7 | (0.09%) | 34 | (0.08%) | 1.05 | (0.46–2.36) |
| Solid tumor | 192 | (2.35%) | 988 | (2.38%) | 0.99 | (0.85–1.16) |
| Non-melanoma skin cancer | 16 | (0.20%) | 43 | (0.10%) | 1.90 | (1.07–3.37) |
| Malignant melanoma | 1 | (0.01%) | 2 | (0.00%) | 2.55 | (0.23–28.07) |
| Other diseases | ||||||
| Asthma | 416 | (5.09%) | 2641 | (6.35%) | 0.79 | (0.71–0.88) |
| COPD | 56 | (0.69%) | 410 | (0.99%) | 0.69 | (0.52–0.92) |
| Chronic kidney disease | 58 | (0.71%) | 169 | (0.41%) | 1.75 | (1.30–2.36) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-H.; Lee, S.; Seo, H.S.; Koh, S.B.; Eom, M.; Hong, S.-P. Impact of Biologics on Comorbidities in Patients with Psoriasis or Psoriatic Arthritis. Biomedicines 2025, 13, 2219. https://doi.org/10.3390/biomedicines13092219
Lee S-H, Lee S, Seo HS, Koh SB, Eom M, Hong S-P. Impact of Biologics on Comorbidities in Patients with Psoriasis or Psoriatic Arthritis. Biomedicines. 2025; 13(9):2219. https://doi.org/10.3390/biomedicines13092219
Chicago/Turabian StyleLee, Sang-Hoon, Solam Lee, Hee Seok Seo, Sang Baek Koh, Minseob Eom, and Seung-Phil Hong. 2025. "Impact of Biologics on Comorbidities in Patients with Psoriasis or Psoriatic Arthritis" Biomedicines 13, no. 9: 2219. https://doi.org/10.3390/biomedicines13092219
APA StyleLee, S.-H., Lee, S., Seo, H. S., Koh, S. B., Eom, M., & Hong, S.-P. (2025). Impact of Biologics on Comorbidities in Patients with Psoriasis or Psoriatic Arthritis. Biomedicines, 13(9), 2219. https://doi.org/10.3390/biomedicines13092219








