Heart Rate Variability Dynamics as Predictors of Functional Recovery and Mortality After Acute Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
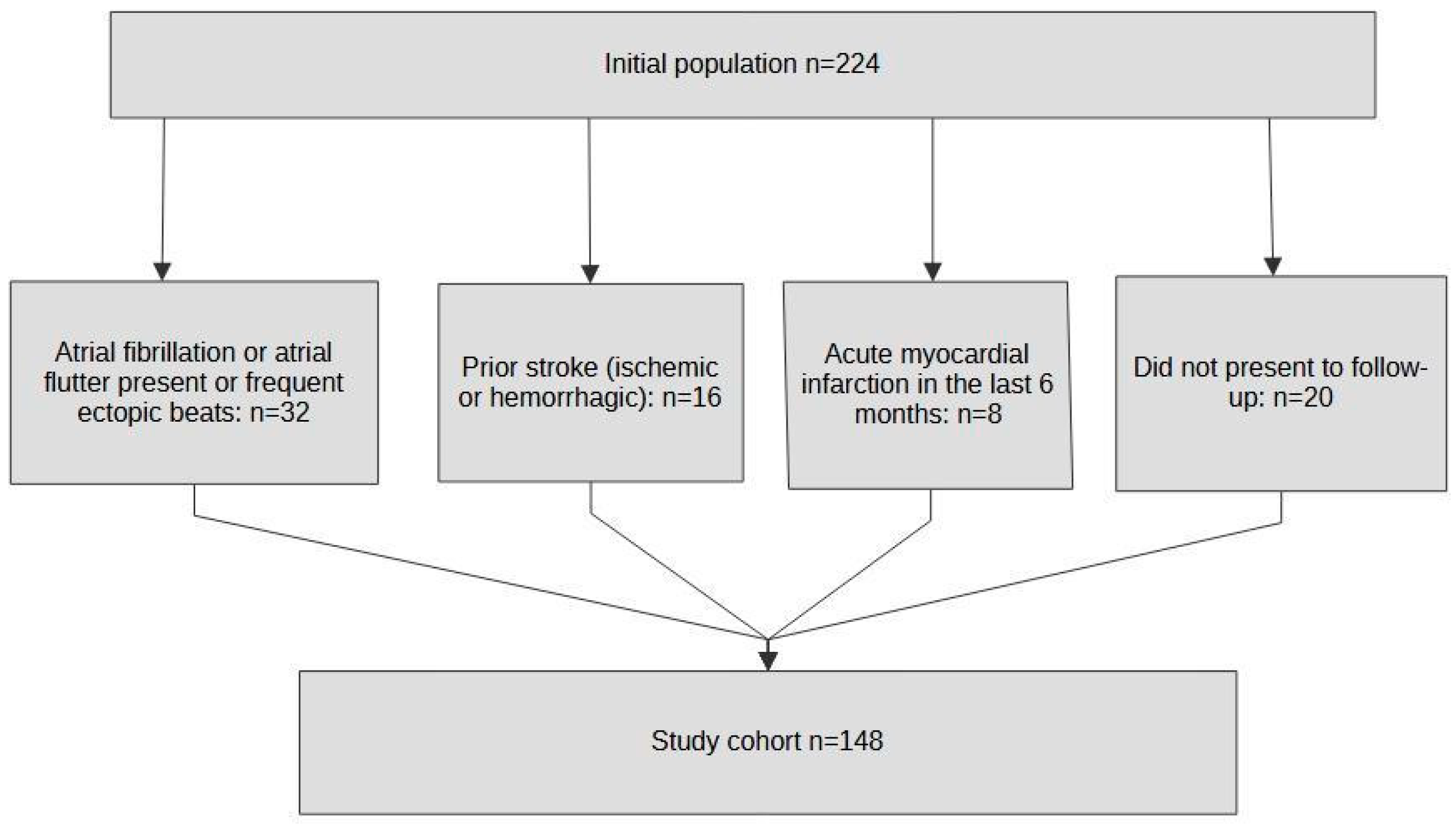
2.1. Population
2.2. Heart Rate Variability Analysis
2.3. Statistical Analysis
3. Results
3.1. Study Population Description
3.2. HRV Analysis for the Entire Study Population
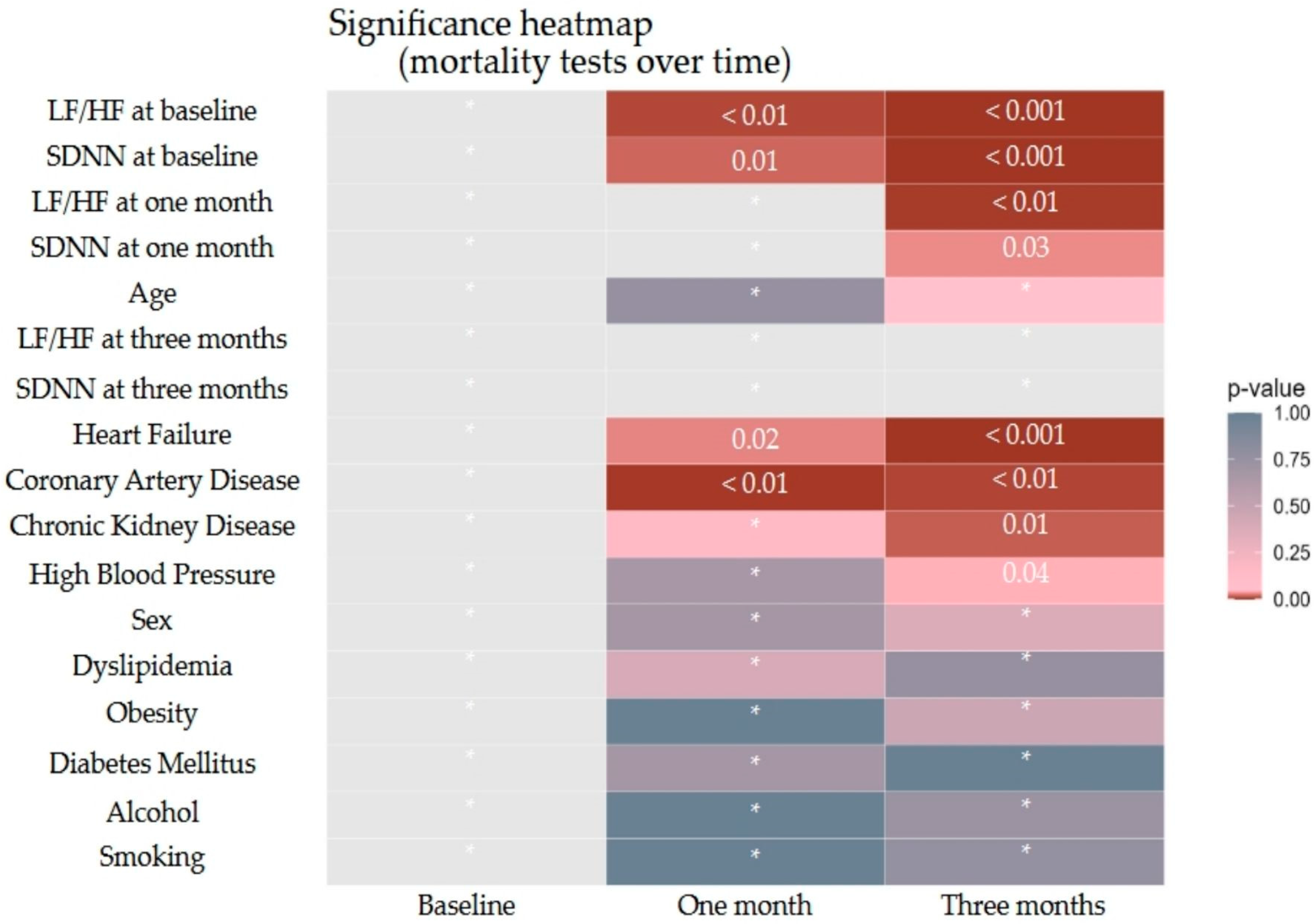
3.3. Impact of HRV on Mortality Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef]
- United Nations. World Population Ageing 2007; United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2007; ISBN 978-92-1-151432-2. [Google Scholar]
- Damkjær, M.; Simonsen, S.A.; Heiberg, A.V.; Andersen, L.; Winther, K.; Hjort, N.; Johnsen, S.P.; Møller, A.M.; Iversen, H.K.; Christensen, H.; et al. Autonomic Dysfunction after Mild Acute Ischemic Stroke and Six Months after: A Prospective Observational Cohort Study. BMC Neurol. 2023, 23, 26. [Google Scholar] [CrossRef]
- Tenberg, A.; Tahara, N.; Grewal, A.; Holwerda, S.; Jackson, C.A.; Lattanzi, S.; Lee, H.; Leigh, R.; Nakanishi, K.; Rothwell, P.M.; et al. Dysautonomia and Activity in the Early Stroke Recovery Period. Neurol. Sci. 2024, 45, 2505–2521. [Google Scholar] [CrossRef]
- Armstrong, R.; Wheen, P.; Brandon, L.; Maree, A.; Kenny, R.-A. Heart Rate: Control Mechanisms, Pathophysiology and Assessment of the Neurocardiac System in Health and Disease. QJM 2022, 115, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C.; Apostol, A.; Ivan, V.M.; Sandu, O.E.; Petre, I.; Suciu, O.; Marc, L.-E.; Maralescu, F.-M.; Lighezan, D.F. Heart Rate Variability and Global Longitudinal Strain for Prognostic Evaluation and Recovery Assessment in Conservatively Managed Post-Myocardial Infarction Patients. J. Clin. Med. 2024, 13, 5435. [Google Scholar] [CrossRef]
- Aftyka, J.; Staszewski, J.; Dębiec, A.; Pogoda-Wesołowska, A.; Żebrowski, J. Can HRV Predict Prolonged Hospitalization and Favorable or Unfavorable Short-Term Outcome in Patients with Acute Ischemic Stroke? Life 2023, 13, 856. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart Rate Variability. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Beer, R.N.; Soroker, N.; Bornstein, N.M.; Katz-Leurer, M. The Cardiac Autonomic Nervous System Response to Different Daily Demands among Patients at the Sub-Acute Phase Post Ischemic Stroke and Healthy Controls. NeuroRehabilitation 2018, 42, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Tobaldini, E.; Sacco, R.M.; Serafino, S.; Tassi, M.; Gallone, G.; Solbiati, M.; Costantino, G.; Montano, N.; Torgano, G. Cardiac Autonomic Derangement Is Associated with Worse Neurological Outcome in the Very Early Phases of Ischemic Stroke. J. Clin. Med. 2019, 8, 852. [Google Scholar] [CrossRef] [PubMed]
- De Raedt, S.; De Vos, A.; De Keyser, J. Autonomic Dysfunction in Acute Ischemic Stroke: An Underexplored Therapeutic Area? J. Neurol. Sci. 2015, 348, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Scott, C.A.; Rothwell, P.M. Association of Younger vs Older Ages with Changes in Incidence of Stroke and Other Vascular Events, 2002–2018. JAMA 2022, 328, 563–574. [Google Scholar] [CrossRef]
- Edrissi, C.; Rathfoot, C.; Knisely, K.; Sanders, C.B.; Goodwin, R.; Nathaniel, S.I.; Nathaniel, T. Age Stratification in Acute Ischemic Stroke Patients with Heart Failure. J. Clin. Med. 2023, 12, 38. [Google Scholar] [CrossRef]
- Scherbakov, N.; Haeusler, K.G.; Doehner, W. Ischemic Stroke and Heart Failure: Facts and Numbers. ESC Heart Fail. 2015, 2, 1–4. [Google Scholar] [CrossRef]
- Sacco, S.; Foschi, M.; Ornello, R.; De Santis, F.; Pofi, R.; Romoli, M. Prevention and Treatment of Ischaemic and Haemorrhagic Stroke in People with Diabetes Mellitus: A Focus on Glucose Control and Comorbidities. Diabetologia 2024, 67, 1192–1205. [Google Scholar] [CrossRef]
- Schumacher, K.; Kornej, J.; Shantsila, E.; Lip, G.Y.H. Heart Failure and Stroke. Curr. Heart Fail. Rep. 2018, 15, 287–296. [Google Scholar] [CrossRef]
- Jiang, Y.; Han, J.; Spencer, P.; Li, Y.; Vodovoz, S.J.; Ning, M.-M.; Liu, N.; Wang, X.; Dumont, A.S. Diabetes Mellitus: A Common Comorbidity Increasing Hemorrhagic Transformation after tPA Thrombolytic Therapy for Ischemic Stroke. Brain Hemorrhages 2021, 2, 116–123. [Google Scholar] [CrossRef]
- Doehner, W.; Schenkel, J.; Anker, S.D.; Springer, J.; Audebert, H.J. Overweight and Obesity Are Associated with Improved Survival, Functional Outcome, and Stroke Recurrence after Acute Stroke or Transient Ischaemic Attack: Observations from the TEMPiS Trial. Eur. Heart J. 2013, 34, 268–277. [Google Scholar] [CrossRef]
- Chaudhary, D.; Khan, A.; Gupta, M.; Hu, Y.; Li, J.; Abedi, V.; Zand, R. Obesity and Mortality after the First Ischemic Stroke: Is Obesity Paradox Real? PLoS ONE 2021, 16, e0246877. [Google Scholar] [CrossRef]
- Liu, Z.; Sanossian, N.; Starkman, S.; Avila-Rinek, G.; Eckstein, M.; Sharma, L.K.; Liebeskind, D.; Conwit, R.; Hamilton, S.; Saver, J.L.; et al. Adiposity and Outcome after Ischemic Stroke. Stroke 2021, 52, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Callaway, C.W.; Sejdić, E.; Terhorst, L.; Skidmore, E.R. Heart Rate Variability Is Associated with Motor Outcome 3-Months after Stroke. J. Stroke Cerebrovasc. Dis. 2016, 25, 129–135. [Google Scholar] [CrossRef]
- Scherbakov, N.; Barkhudaryan, A.; Ebner, N.; von Haehling, S.; Anker, S.D.; Joebges, M.; Doehner, W. Early Rehabilitation after Stroke: Relationship between the Heart Rate Variability and Functional Outcome. ESC Heart Fail. 2020, 7, 2983–2991. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Tian, G.; Leung, H.; Soo, Y.O.Y.; Chen, X.; Ip, V.H.L.; Mok, V.C.T.; Chu, W.C.W.; Wong, K.S.; Leung, T.W.H. Autonomic Dysfunction Predicts Clinical Outcomes after Acute Ischemic Stroke. Stroke 2018, 49, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-W.; Lee, M.; Huang, Y.-C.; Lee, J.-D. Initial In-Hospital Heart Rate Is Associated with Three-Month Functional Outcomes after Acute Ischemic Stroke. BMC Neurol. 2021, 21, 222. [Google Scholar] [CrossRef]
- Wu, M.-J.; Dewi, S.R.K.; Hsu, W.-T.; Hsu, T.-Y.; Liao, S.-F.; Chan, L.; Lin, M.-C. Exploring Relationships of Heart Rate Variability, Neurological Function, and Clinical Factors with Mortality and Behavioral Functional Outcome in Patients with Ischemic Stroke. Diagnostics 2024, 14, 1304. [Google Scholar] [CrossRef]
- Yperzeele, L.; van Hooff, R.J.; Nagels, G.; De Smedt, A.; De Keyser, J.; Brouns, R. Heart Rate Variability and Baroreceptor Sensitivity in Acute Stroke: A Systematic Review. Int. J. Stroke 2015, 10, 796–800. [Google Scholar] [CrossRef]
- Lago, S.; de Beukelaar, T.T.; Casetta, I.; Arcara, G.; Mantini, D. Heart Rate Variability and Autonomic Dysfunction After Stroke: Prognostic Markers for Recovery. Biomedicines 2025, 13, 1659. [Google Scholar] [CrossRef]
- Qu, Y.; Sun, Y.-Y.; Abuduxukuer, R.; Si, X.-K.; Zhang, P.; Ren, J.-X.; Fu, Y.-L.; Zhang, K.-J.; Liu, J.; Zhang, P.-D.; et al. Heart Rate Variability Parameter Changes in Patients with Acute Ischemic Stroke Undergoing Intravenous Thrombolysis. J. Am. Heart Assoc. 2023, 12, e028778. [Google Scholar] [CrossRef]
- Li, C.; Meng, X.; Pan, Y.; Li, Z.; Wang, M.; Wang, Y. The Association Between Heart Rate Variability and 90-Day Prognosis in Patients with Transient Ischemic Attack and Minor Stroke. Front. Neurol. 2021, 12, 636474. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Values, N = 148 (%) | Good Outcome at Baseline, N = 96 (%) | Poor Outcome at Baseline, N = 52 (%) |
|---|---|---|---|
| Age | 65.93 (9.19) * | 64.84 (9.27) * | 67.94 (8.77) * |
| Sex | M: 54.05 F: 45.95 | M: 51.04 F: 48.96 | M: 59.62 F: 40.38 |
| Heart Failure | 38.51 | 21.88 | 67.31 |
| High Blood Pressure | 64.86 | 64.58 | 65.38 |
| Coronary Artery Disease | 35.14 | 27.08 | 50.00 |
| Diabetes Mellitus | 47.30 | 44.79 | 51.92 |
| Chronic Kidney Disease | 24.32 | 20.83 | 30.77 |
| Obesity | 38.51 | 40.62 | 34.62 |
| Dyslipidemia | 66.89 | 69.79 | 61.54 |
| Alcoholic | 17.57 | 17.71 | 17.31 |
| Smoker | 39.19 | 36.46 | 44.23 |
| Parameter | Time Point | Median (IQR) | Mean (SD) | Min–Max |
|---|---|---|---|---|
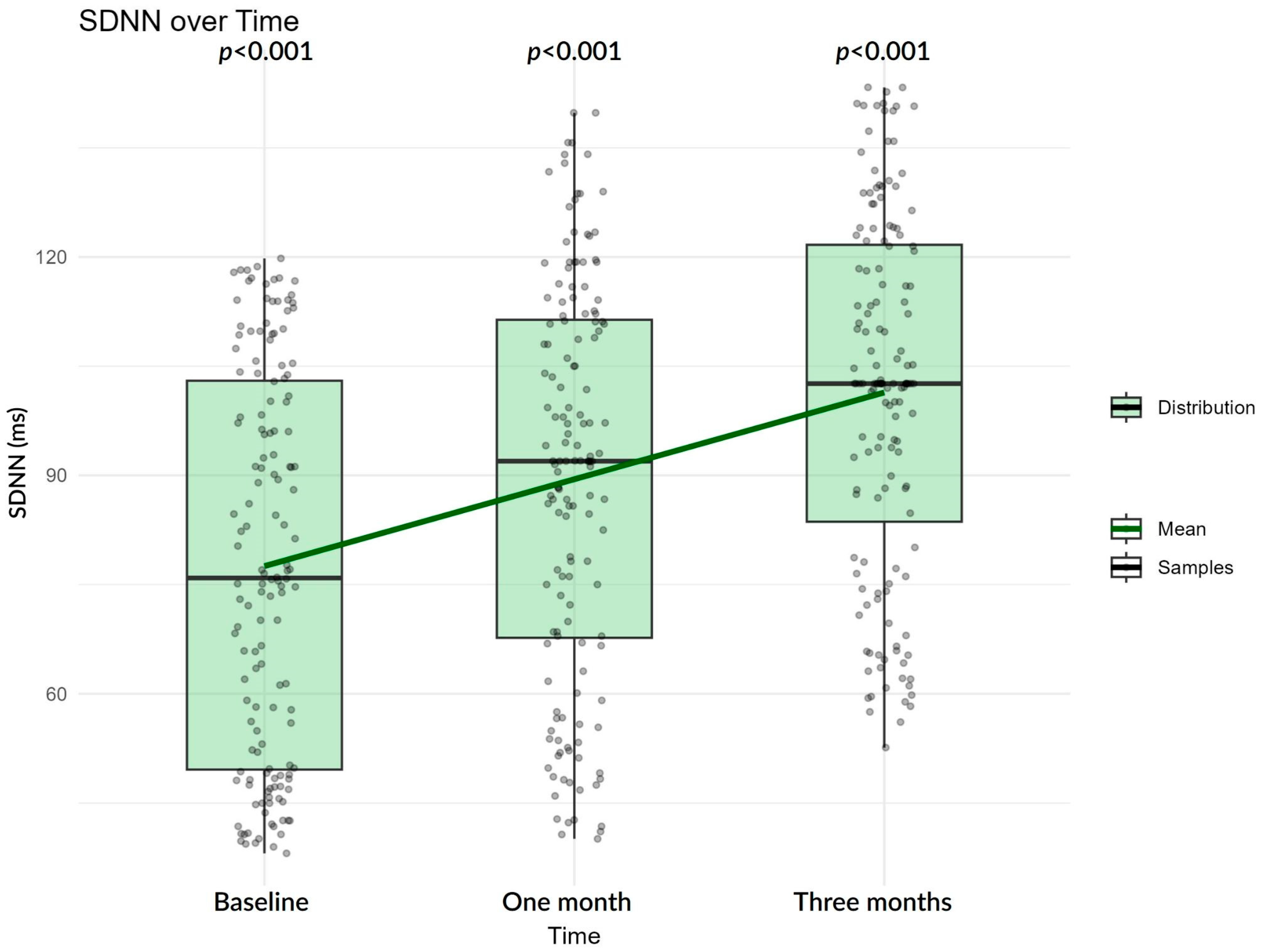
| SDNN | Baseline | 75.90 (49.60–103.00) | 77.53 (26.41) | 38.10–119.80 |
| One month | 91.95 (67.68–112.20) | 89.86 (27.70) | 40.10–139.80 | |
| Three months | 102.05 (77.03–122.40) | 100.7 (25.98) | 49.65–148.07 | |
| LF power | Baseline | 953.50 (864.00–1036.00) | 959.93 (127.95) | 735–1291 |
| One month | 889.00 (806.25–1023.00) | 912.42 (143.14) | 629–1293 | |
| Three months | 782.00 (670.25–897.50) | 772.80 (190.20) | 267–1245 | |
| HF power | Baseline | 282.41 ± 44.16 | 282.41 (44.16) | 189–401 |
| One month | 327.00 (295.00–355.25) | 326.04 (50.66) | 209–499 | |
| Three months | 336.50 (298.50–386.25) | 351.76 (96.41) | 130–930 | |
| LF/HF | Baseline | 3.29 (2.94–3.76) | 3.48 (0.87) | 1.00–6.79 |
| One month | 2.75 (2.40–3.17) | 2.88 (0.71) | 1.71–5.17 | |
| Three months | 2.30 (1.84–2.70) | 2.32 (0.71) | 0.81–4.67 | |
| mRS | Baseline | 2.00 (1.00–3.00) | 2.15 (1.11) | 0–4 |
| One month | 2.00 (1.00–3.00) | 1.85 (1.42) | 0–6 | |
| Three months | 1.00 (0.00–2.00) | 1.68 (1.70) | 0–6 |
| Parameter | Time | Estimate | 95% CI (Lower–Upper) | p | Change% |
|---|---|---|---|---|---|
| SDNN | Baseline | 72.81 | 69.10–76.71 | <0.001 | |
| Baseline to one month | 1.17 | 1.13–1.20 | <0.001 | 16.60 | |
| Baseline to three months | 1.35 | 1.31–1.39 | <0.001 | 34.84 | |
| LF power | Baseline | 951.67 | 922.05–982.24 | <0.001 | |
| Baseline to one month | 0.95 | 0.92–0.98 | <0.001 | −5.19 | |
| Baseline to three months | 0.78 | 0.76–0.81 | <0.001 | −21.61 | |
| HF power | Baseline | 278.82 | 270.65–287.25 | <0.001 | |
| Baseline to one month | 1.15 | 1.11–1.19 | <0.001 | 15.30 | |
| Baseline to three months | 1.22 | 1.18–1.27 | <0.001 | 22.26 | |
| LF/HF | Baseline | 3.38 | 3.24–3.53 | <0.001 | |
| Baseline to one month | 0.83 | 0.79–0.87 | <0.001 | −16.91 | |
| Baseline to three months | 0.65 | 0.62–0.68 | <0.001 | −35.41 |
| Parameter | Time | Estimate | SE | p | Change% |
|---|---|---|---|---|---|
| SDNN | Baseline to one month | 0.15 | 0.01 | <0.001 | 16.60 |
| Baseline to three months | 0.29 | 0.01 | <0.001 | 34.84 | |
| One month to three months | 0.14 | 0.01 | <0.001 | 15.65 | |
| LF power | Baseline to one month | −0.05 | 0.02 | <0.01 | −5.19 |
| Baseline to three months | −0.24 | 0.02 | <0.001 | −21.61 | |
| One month to three months | −0.19 | 0.02 | <0.001 | −17.32 | |
| HF power | Baseline to one month | 0.14 | 0.02 | <0.001 | 15.30 |
| Baseline to three months | 0.20 | 0.02 | <0.001 | 22.26 | |
| One month to three months | 0.05 | 0.02 | <0.001 | 6.03 | |
| LF/HF | Baseline to one month | −0.18 | 0.02 | <0.001 | −16.91 |
| Baseline to three months | −0.43 | 0.02 | <0.001 | −35.41 | |
| One month to three months | −0.25 | 0.02 | <0.001 | −22.27 |
| Parameter | Time | Good Outcome (mRS ≤ 2) | Poor Outcome (mRS > 2) | Good Outcome Median (IQR) | Poor Outcome Median (IQR) | p |
|---|---|---|---|---|---|---|
| SDNN | Baseline | N = 96 | N = 52 | 92.60 (33.85) | 48.20 (12.57) | <0.001 |
| LF power | 930.50 (183.00) | 987.00 (169.75) | <0.01 | |||
| HF power | 286.00 (60.75) | 279.50 (61.00) | 0.06 | |||
| LF/HF | 3.27 (0.76) | 3.41 (1.20) | 0.01 | |||
| SDNN | One month | N = 108 | N = 40 | 99.30 (29.30) | 56.65 (25.00) | <0.001 |
| LF power | 886.50 (218.25) | 1000.50 (175.50) | <0.01 | |||
| HF power | 333.00 (63.50) | 327.00 (44.25) | 0.18 | |||
| LF/HF | 2.72 (0.82) | 2.80 (0.68) | 0.16 | |||
| SDNN | Three months | N = 113 | N = 35 | 109.70 (34.40) | 65.30 (0.15) | <0.001 |
| LF power | 772.00 (222.00) | 267.00 (590.00) | <0.01 | |||
| HF power | 336.00 (87.00) | 355.00 (18.00) | 0.72 | |||
| LF/HF | 2.20 (0.99) | 2.08 (0.45) | 0.35 |
| Correlations Between HRV Parameters | |||
|---|---|---|---|
| Parameter | Time | Rho | p |
| SDNN | Baseline | −0.68 | <0.001 |
| LF power | 0.32 | <0.001 | |
| HF power | −0.12 | 0.12 | |
| LF/HF | 0.23 | <0.01 | |
| SDNN | One month | −0.60 | <0.001 |
| LF power | 0.30 | <0.001 | |
| HF power | −0.16 | 0.03 | |
| LF/HF | 0.32 | <0.001 | |
| SDNN | Three months | −0.48 | <0.001 |
| LF power | 0.26 | <0.001 | |
| HF power | −0.18 | 0.02 | |
| LF/HF | 0.34 | <0.001 | |
| mRS Distribution Spread | |||
| Time point | Distribution mRS: Count (%) | ||
| Baseline | 0: 8 (5.40%), 1: 36 (24.30%), 2: 52 (35.10%), 3: 30 (20.30%), 4: 22 (14.90%), 5: 0 (0%), 6: 0 (0%); Median 2 (IQR of 1–3); Range of 0–4 | ||
| One month | 0: 26 (17.60%), 1: 37 (25.00%), 2: 45 (30.40%), 3: 25 (16.90%), 4: 9 (6.10%), 5: 0 (0%), 6: 6 (4.10%); Median 2 (IQR of 1–3), Range of 0–6 | ||
| Three months | 0: 40 (27%), 1: 37 (25%), 2: 36 (24.30%), 3: 14 (9.50%), 4: 3 (2%), 5: 0 (0%), 6: 18 (12.20%); Median 1 (IQR of 0–2), Range of 0–6 | ||
| Baseline | One Month | Three Month | ||||
|---|---|---|---|---|---|---|
| Parameter | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| SDNN | 0.86 (0.80–0.91) | <0.001 | 0.91 (0.86–0.94) | <0.001 | 0.94 (0.90–0.97) | <0.001 |
| LF/HF | 2.89 (1.35–6.99) | 0.01 | 1.92 (0.73–5.70) | 0.21 | 3.27 (1.29–9.13) | 0.02 |
| Age | 1.02 (0.95–1.11) | 0.54 | 1 (0.93–1.08) | 0.99 | 1.02 (0.96–1.09) | 0.5 |
| Sex | 2.9 (0.67–14.87) | 0.17 | 1.05 (0.27–4.27) | 0.94 | 1.47 (0.48–4.8) | 0.51 |
| Alcohol | 1.24 (0.16–12.04) | 0.84 | 2.84 (0.39–24.14) | 0.31 | 2.84 (0.68–12.6) | 0.16 |
| Smoking | 2.82 (0.68–13.63) | 0.17 | 3.8 (0.88–19.64) | 0.09 | 1.41 (0.44–4.68) | 0.56 |
| Diabetes Mellitus | 0.75 (0.18–2.95) | 0.68 | 0.96 (0.27–3.43) | 0.96 | 0.71 (0.24–2.03) | 0.52 |
| Coronary Artery Disease | 2.24 (0.53–10.82) | 0.28 | 11.86 (2.85–66.67) | <0.001 | 2.65 (0.88–8.29) | 0.08 |
| Chronic Kidney Disease | 0.98 (0.22–4.43) | 0.98 | 3.66 (0.97–15.26) | 0.06 | 2.2 (0.70–7.14) | 0.18 |
| High Blood Pressure | 1.1 (0.29–4.44) | 0.89 | 0.46 (0.12–1.65) | 0.25 | 1.88 (0.62–5.97) | 0.27 |
| Heart Failure | 6.75 (1.70–33.07) | 0.01 | 3.25 (0.93–12.54) | 0.07 | 2.33 (0.80–7.15) | 0.13 |
| Obesity | 0.24 (0.03–1.24) | 0.11 | 0.16 (0.03–0.67) | 0.02 | 0.33 (0.09–1.09) | 0.08 |
| Dyslipidemia | 0.31 (0.06–1.4) | 0.14 | 0.23 (0.05–1.01) | 0.06 | 2.13 (0.63–7.74) | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandu, O.E.; Bogdan, C.; Apostol, A.; Simu, M.A.; Moga, V.-D.; Pecingina, R.-M.; Covaciu, A.; Ivan, V.M. Heart Rate Variability Dynamics as Predictors of Functional Recovery and Mortality After Acute Ischemic Stroke. Biomedicines 2025, 13, 2217. https://doi.org/10.3390/biomedicines13092217
Sandu OE, Bogdan C, Apostol A, Simu MA, Moga V-D, Pecingina R-M, Covaciu A, Ivan VM. Heart Rate Variability Dynamics as Predictors of Functional Recovery and Mortality After Acute Ischemic Stroke. Biomedicines. 2025; 13(9):2217. https://doi.org/10.3390/biomedicines13092217
Chicago/Turabian StyleSandu, Oana Elena, Carina Bogdan, Adrian Apostol, Mihaela Adriana Simu, Victor-Dan Moga, Radu-Mihai Pecingina, Alexandru Covaciu, and Viviana Mihaela Ivan. 2025. "Heart Rate Variability Dynamics as Predictors of Functional Recovery and Mortality After Acute Ischemic Stroke" Biomedicines 13, no. 9: 2217. https://doi.org/10.3390/biomedicines13092217
APA StyleSandu, O. E., Bogdan, C., Apostol, A., Simu, M. A., Moga, V.-D., Pecingina, R.-M., Covaciu, A., & Ivan, V. M. (2025). Heart Rate Variability Dynamics as Predictors of Functional Recovery and Mortality After Acute Ischemic Stroke. Biomedicines, 13(9), 2217. https://doi.org/10.3390/biomedicines13092217






