Prior Aerobic Exercise Training Fails to Confer Cardioprotection Under Varying Exercise Volumes in Early Post-Infarction Cardiac Remodeling in Female Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
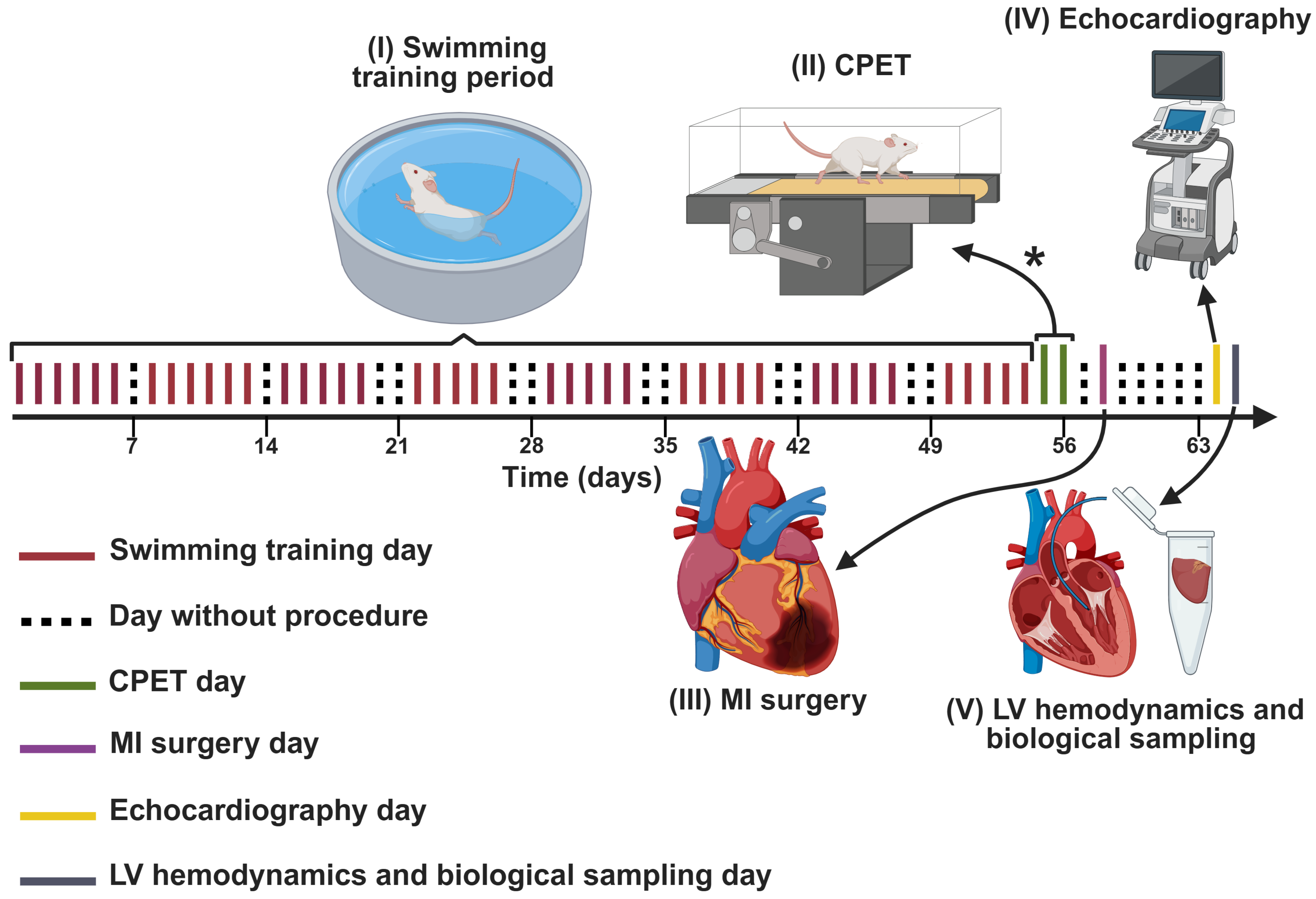
2.2. Experimental Design
2.3. Swimming Training Protocols
2.4. CPET
2.5. MI Model
2.6. Echocardiography
2.7. LV Hemodynamics
2.8. Euthanasia and Collection of Biological Materials
2.9. ELISA Assays
2.10. Antioxidant Enzyme Activity
2.11. Western Blotting
2.12. Statistical Analysis
3. Results
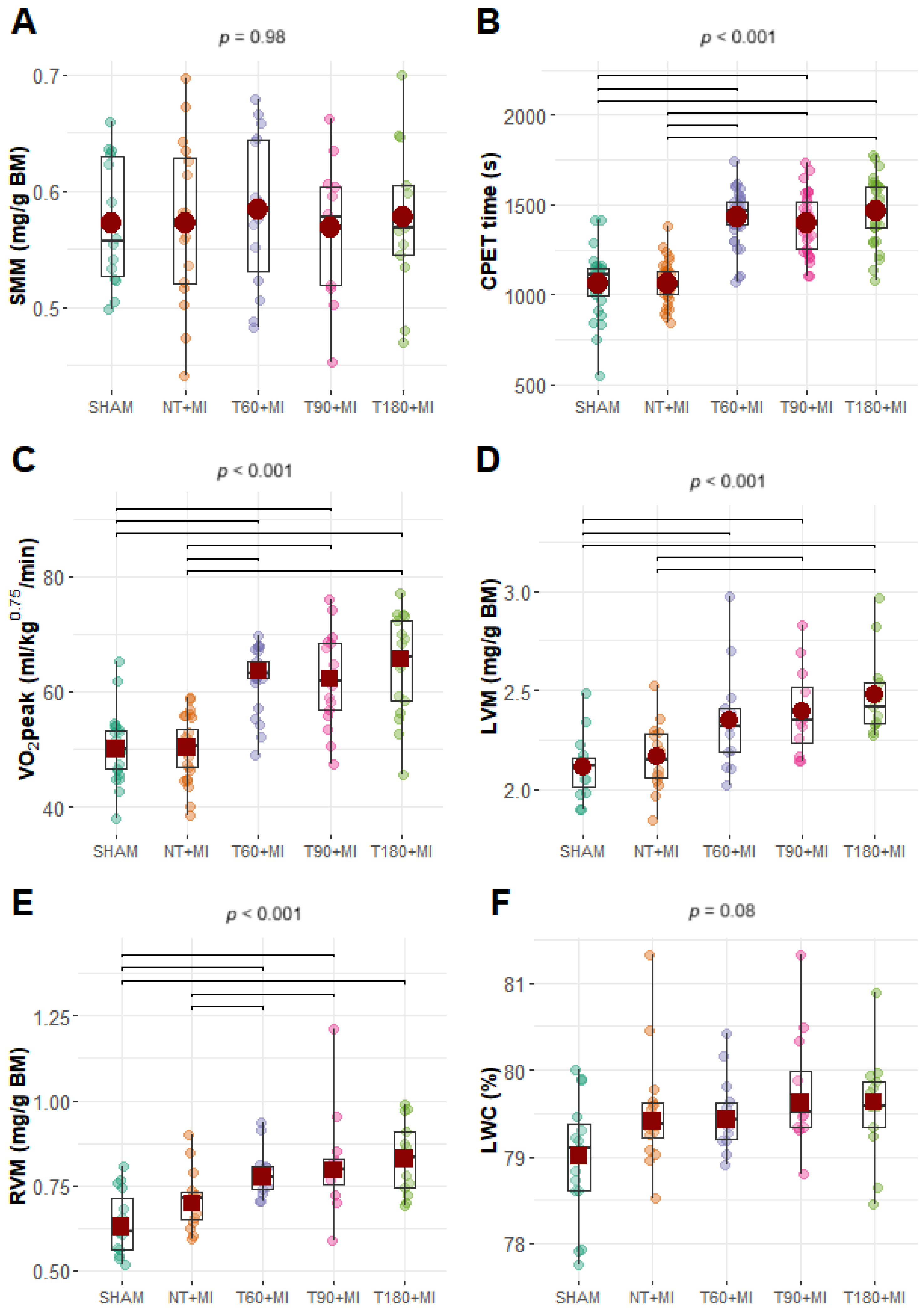
3.1. Post-MI Mortality
3.2. Biometric and CPET Data
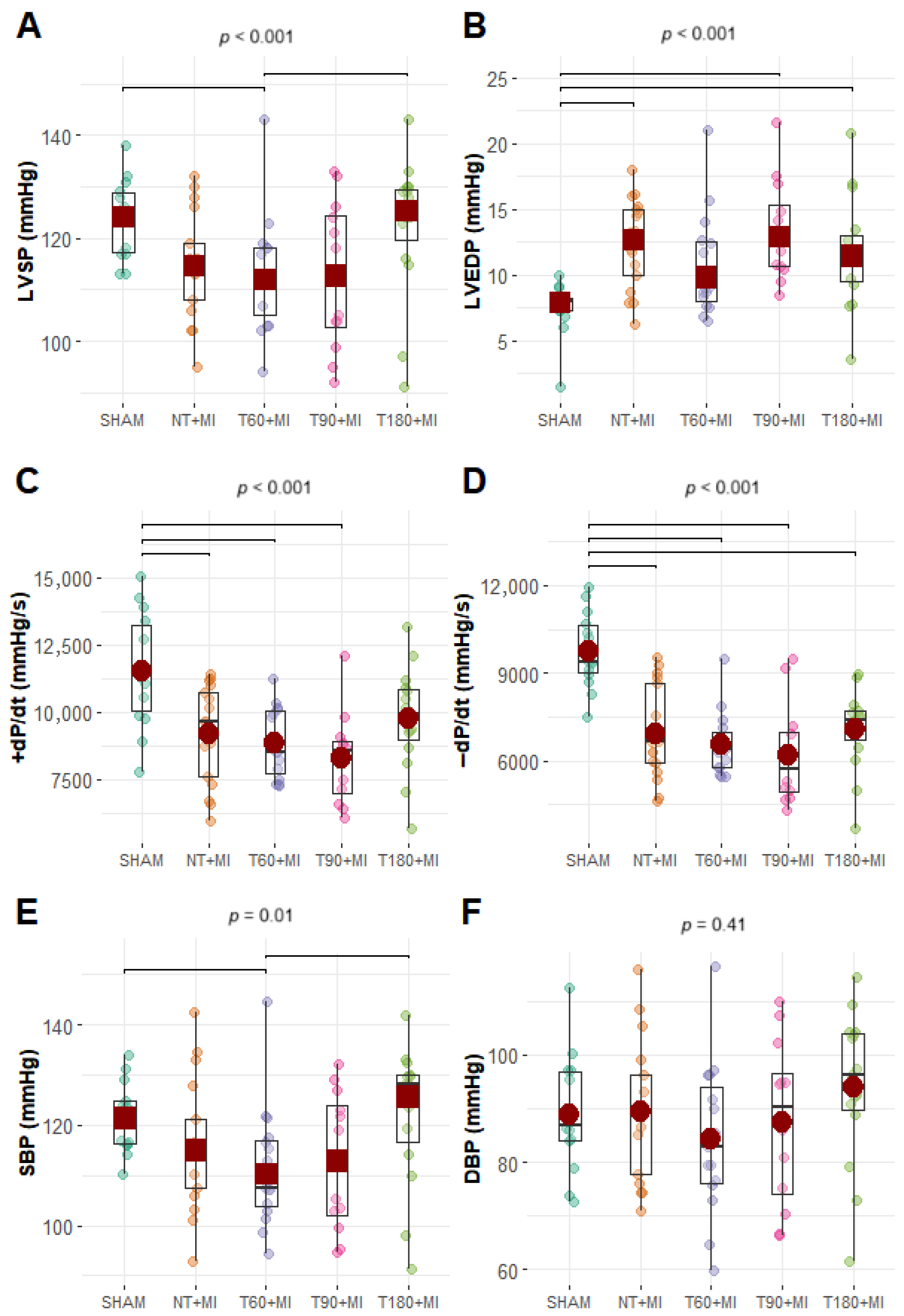
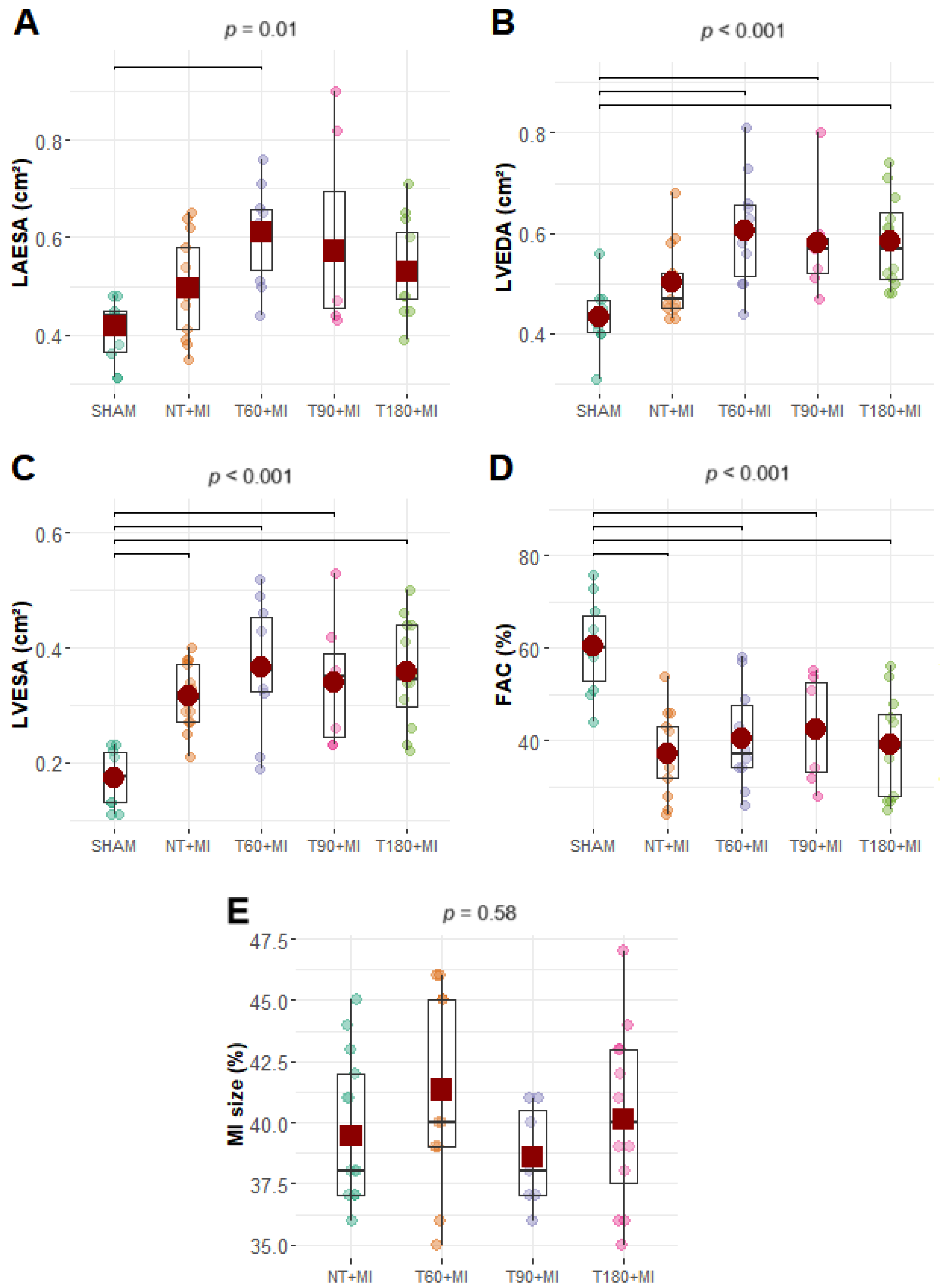
3.3. Echocardiographic and Hemodynamic Data
3.4. Cytokines, Oxidative Stress Antioxidant Enzymes, and Endothelial Markers
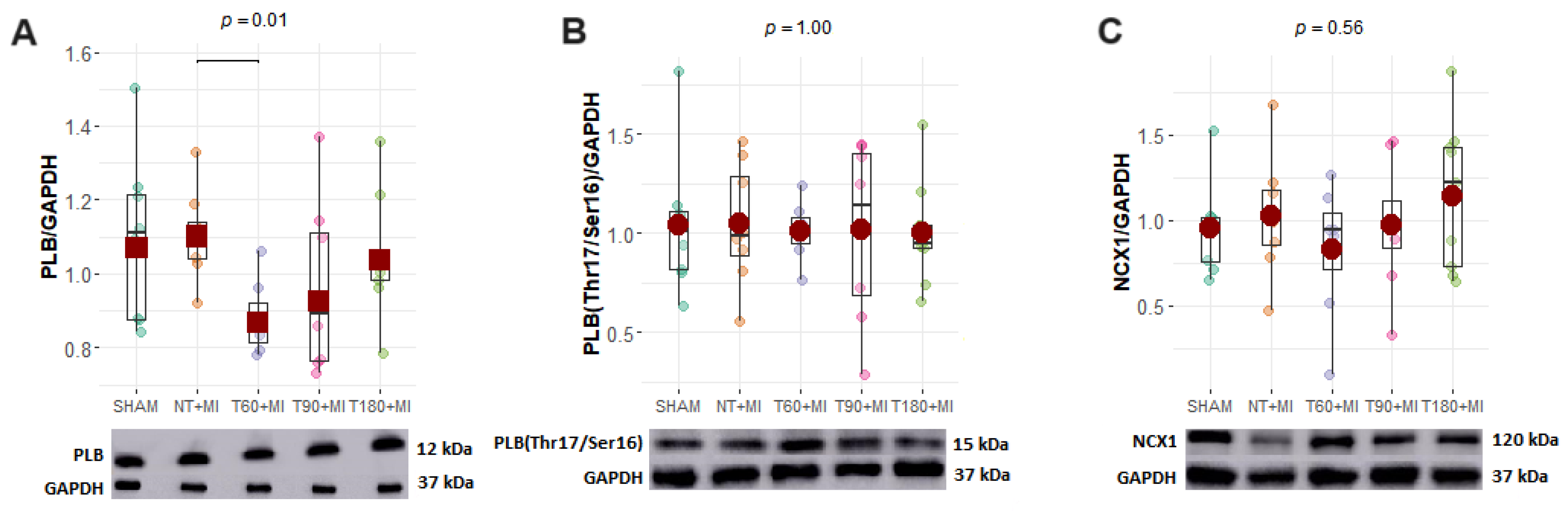
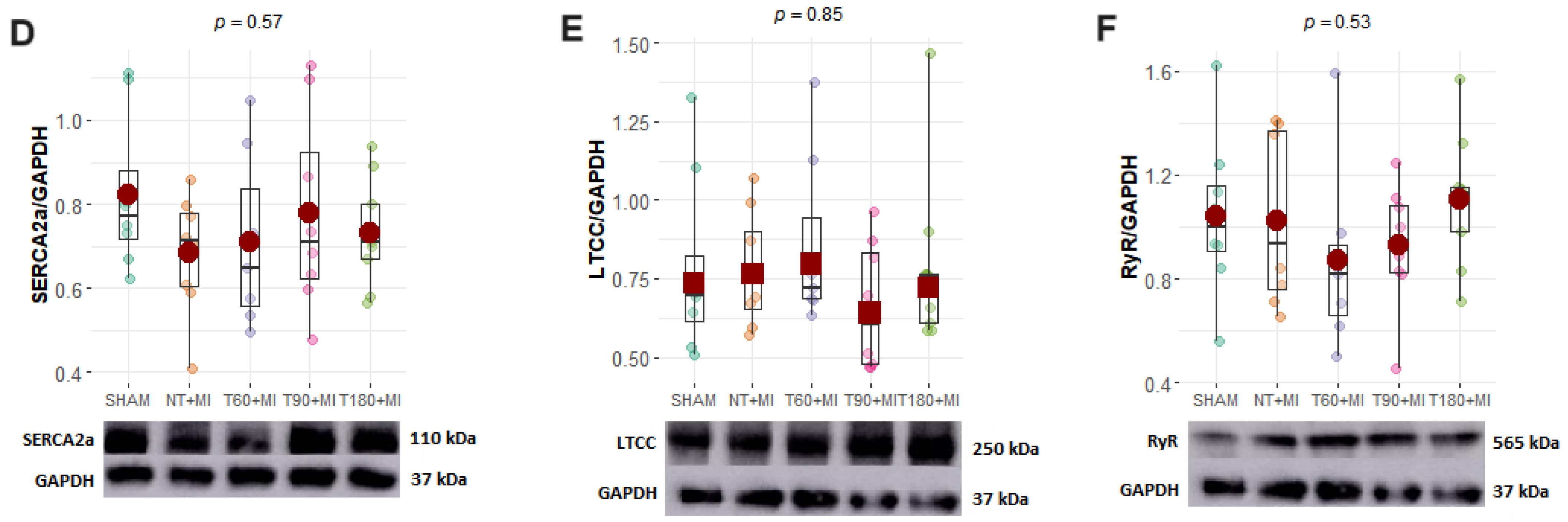
3.5. Calcium-Handling Proteins
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thijssen, D.H.J.; Redington, A.; George, K.P.; Hopman, M.T.E.; Jones, H. Association of exercise preconditioning with immediate cardioprotection: A review. JAMA Cardiol. 2018, 3, 169. [Google Scholar] [CrossRef]
- Ejlersen, H.; Andersen, Z.J.; Von Euler-Chelpin, M.C.; Johansen, P.P.; Schnohr, P.; Prescott, E. Prognostic impact of physical activity prior to myocardial infarction: Case fatality and subsequent risk of heart failure and death. Eur. J. Prev. Cardiol. 2017, 24, 1112–1119. [Google Scholar] [CrossRef]
- Peytz, N.C.; Jabbari, R.; Bojesen, S.E.; Nordestgaard, B.; Schnohr, P.; Prescott, E. Physical activity and risk of instant and 28-day case-fatality in myocardial infarction. PLoS ONE 2019, 14, e0217398. [Google Scholar] [CrossRef] [PubMed]
- Veiga, E.C.D.A.; Melo, B.L.D.; Vieira, S.D.S.; Simões, R.S.; Valenti, V.E.; Campos, M.F.; Rossetti do Vale, J.E.T.M.; Rica, R.L.; Soares-Júnior, J.M.; Baracat, E.C.; et al. Prior exercise training and experimental myocardial infarction: A systematic review and meta-analysis. Clinics 2020, 75, e1293. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, Y.; Tan, Y.; Zhang, Z.; Hou, Z.; Gao, C.; Feng, P.; Zhang, X.; Yi, W.; Gao, F. Short-duration swimming exercise after myocardial infarction attenuates cardiac dysfunction and regulates mitochondrial quality control in aged mice. Oxid. Med. Cell. Longev. 2018, 2018, 4079041. [Google Scholar] [CrossRef]
- Tan, Y.; Feng, P.; Feng, L.; Shi, L.; Song, Y.; Yang, J.; Duan, W.; Gao, E.; Liu, J.; Yi, D.; et al. Low-dose exercise protects the heart against established myocardial infarction via IGF-1-upregulated CTRP9 in male mice. MedComm 2023, 4, e411. [Google Scholar] [CrossRef]
- Wen, C.P.; Wai, J.P.M.; Tsai, M.K.; Yang, Y.C.; Cheng, T.Y.D.; Lee, M.-C.; Chan, H.T.; Tsao, C.K.; Tsai, S.P.; Wu, X. Minimum amount of physical activity for reduced mortality and extended life expectancy: A prospective cohort study. Lancet 2011, 378, 1244–1253. [Google Scholar] [CrossRef]
- Arem, H.; Moore, S.C.; Patel, A.; Hartge, P.; Berrington de Gonzalez, A.; Visvanathan, K.; Campbell, P.T.; Freedman, M.; Weiderpass, E.; Adami, H.O.; et al. Leisure time physical activity and mortality: A detailed pooled analysis of the dose-response relationship. JAMA Intern. Med. 2015, 175, 959. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Manek, N.; Nichols, M.; Kelly, P.; Foster, C.; Webster, P.; Kaur, A.; Friedemann Smith, C.; Wilkins, E.; Rayner, M.; et al. Quantifying the association between physical activity and cardiovascular disease and diabetes: A systematic review and meta-analysis. J. Am. Heart Assoc. 2016, 5, e002495. [Google Scholar] [CrossRef] [PubMed]
- Meza-Ramos, A.; Alcarraz, A.; Lazo-Rodriguez, M.; Sangüesa, G.; Banon-Maneus, E.; Rovira, J.; Ramirez-Bajo, M.J.; Sitges, M.; Mont, L.; Ventura-Aguiar, P.; et al. High-intensity exercise promotes deleterious cardiovascular remodeling in a high-cardiovascular-risk model: A role for oxidative stress. Antioxidants 2023, 12, 1462. [Google Scholar] [CrossRef]
- Eijsvogels, T.M.H.; Thompson, P.D.; Franklin, B.A. The “Extreme Exercise Hypothesis”: Recent findings and cardiovascular health implications. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 84. [Google Scholar] [CrossRef]
- Lakin, R.; Polidovitch, N.; Yang, S.; Guzman, C.; Gao, X.; Wauchop, M.; Burns, J.; Izaddoustdar, F.; Backx, P.H. Inhibition of soluble TNFα prevents adverse atrial remodeling and atrial arrhythmia susceptibility induced in mice by endurance exercise. J. Mol. Cell. Cardiol. 2019, 129, 165–173. [Google Scholar] [CrossRef]
- Gazdag, P.; Oravecz, K.; Acsai, K.; Demeter-Haludka, V.; Ördög, B.; Szlovák, J.; Kohajda, Z.; Polyák, A.; Barta, B.A.; Oláh, A.; et al. Increased Ca2+ content of the sarcoplasmic reticulum provides arrhythmogenic trigger source in swimming-induced rat athlete’s heart model. Sci. Rep. 2020, 10, 19596. [Google Scholar] [CrossRef]
- Polyák, A.; Topal, L.; Zombori-Tóth, N.; Tóth, N.; Prorok, J.; Kohajda, Z.; Déri, S.; Demeter-Haludka, V.; Hegyi, P.; Venglovecz, V.; et al. Cardiac electrophysiological remodeling associated with enhanced arrhythmia susceptibility in a canine model of elite exercise. eLife 2023, 12, e80710. [Google Scholar] [CrossRef]
- Barta, B.A.; Ruppert, M.; Fröhlich, K.E.; Cosenza-Contreras, M.; Oláh, A.; Sayour, A.A.; Kovács, K.; Karvaly, G.B.; Biniossek, M.; Merkely, B.; et al. Sex-related differences of early cardiac functional and proteomic alterations in a rat model of myocardial ischemia. J. Transl. Med. 2021, 19, 507. [Google Scholar] [CrossRef]
- Pullen, A.B.; Kain, V.; Serhan, C.N.; Halade, G.V. Molecular and cellular differences in cardiac repair of male and female mice. J. Am. Heart Assoc. 2020, 9, e015672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.-R.; Liu, H.-T.; Zhang, H.-F.; Zhang, Q.-J.; Li, Q.-X.; Yu, Q.-J.; Guo, W.-Y.; Wang, H.-C.; Gao, F. Long-term aerobic exercise protects the heart against ischemia/reperfusion injury via PI3 kinase-dependent and Akt-mediated mechanism. Apoptosis 2007, 12, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.; Mello, A.F.S.; Antonio, E.L.; Tucci, P.J.F. Determination of myocardial infarction size in rats by echocardiography and tetrazolium staining: Correlation, agreements, and simplifications. Braz. J. Med. Biol. Res. 2008, 41, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Patil, I. Visualizations with statistical details: The ‘ggstatsplot’ approach. J. Open Source Softw. 2012, 6, 3167. [Google Scholar] [CrossRef]
- Ozdemir, A.F.; Wilcox, R.; Yildiztepe, E. Comparing J independent groups with a method based on trimmed means. Commun. Stat. Simul. Comput. 2018, 47, 852–863. [Google Scholar] [CrossRef]
- Hashimoto, N.Y.; Fernandes, T.; Soci, U.P.R.; de Oliveira, E.M. Molecular determinants of cardiac hypertrophy induced by different amounts of aerobic exercise training. Rev. Bras. Cardiol. 2011, 24, 153–162. [Google Scholar]
- Rodrigues, L.F.; Pelozin, B.R.A.; Silva Junior NDda Soci, U.P.R.; Carmo ECdo Mota Gde FAda Cachofeiro, V.; Lahera, V.; Oliveira, E.M.; Fernandes, T. Angiotensin II promotes skeletal muscle angiogenesis induced by volume-dependent aerobic exercise training: Effects on miRNAs-27a/b and oxidant–antioxidant balance. Antioxidants 2022, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Pelozin, B.R.A.; Soci, U.P.R.; Gomes, J.L.P.; Oliveira, E.M.; Fernandes, T. mTOR signaling-related microRNAs as cardiac hypertrophy modulators in high-volume endurance training. J. Appl. Physiol. 2022, 132, 126–139. [Google Scholar] [CrossRef]
- Veiga, E.C.A.; Antônio, E.L.; Santos, A.A.; Lemes, B.; Bocalini, D.S.; Picollo, C.; Levy, R.F.; Martins, F.L.; Girardi, A.C.C.; Serra, A.J.; et al. Delayed reperfusion—Coronary artery reperfusion close to complete myocardial necrosis benefits remote myocardium and is enhanced by exercise. Front. Physiol. 2019, 10, 157. [Google Scholar] [CrossRef]
- Hoshida, S.; Yamashita, N.; Otsu, K.; Hori, M. Repeated physiologic stresses provide persistent cardioprotection against ischemia-reperfusion injury in rats. J. Am. Coll. Cardiol. 2002, 40, 826–831. [Google Scholar] [CrossRef]
- Ma, J.; Pang, X.; Wang, T.; Ning, M.; Liang, Y.; Li, X.; Tian, X.; Mo, Y.; Laher, I.; Li, S. Acute aerobic exercise regulation of myocardial calcium homeostasis involves CASQ1, CASQ2, and TRDN. J. Appl. Physiol. 2023, 135, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Freimann, S.; Scheinowitz, M.; Yekutieli, D.; Feinberg, M.S.; Eldar, M.; Kessler-Icekson, G. Prior exercise training improves the outcome of acute myocardial infarction in the rat. J. Am. Coll. Cardiol. 2005, 45, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-Y.; Hong, H.-S.; Chen, L.-L.; Lin, X.-H.; Lin, J.-H.; Lin, Z. Effects of exercise of different intensities on the angiogenesis, infarct healing, and function of the left ventricle in postmyocardial infarction rats. Coron. Artery Dis. 2011, 22, 497–506. [Google Scholar] [CrossRef]
- Bozi, L.H.M.; Santos Costa Maldonado IRdos Baldo, M.P.; Silva MFda Moreira, J.B.N.; Novaes, R.D.; Ramos, R.M.S.; Mill, J.G.; Brum, P.C.; Felix, L.B.; Gomes, T.N.P.; Natali, A.J. Exercise training prior to myocardial infarction attenuates cardiac deterioration and cardiomyocyte dysfunction in rats. Clinics 2013, 68, 549–556. [Google Scholar] [CrossRef]
- Veiga, E.C.A.; Antonio, E.L.; Bocalini, D.S.; Murad, N.; Abreu, L.C.; Tucci, P.J.F.; Sato, M.A. Prior exercise training does not prevent acute cardiac alterations after myocardial infarction in female rats. Clinics 2011, 66, 889–893. [Google Scholar] [CrossRef]
- Rodrigues, F.; Feriani, D.J.; Barboza, C.A.; Abssamra, M.E.V.; Rocha, L.Y.; Carrozi, N.M.; Mostarda, C.; Figueroa, D.; Souza, G.I.H.; De Angelis, K.; et al. Cardioprotection afforded by exercise training prior to myocardial infarction is associated with autonomic function improvement. BMC Cardiovasc. Disord. 2014, 14, 84. [Google Scholar] [CrossRef]
- Santos, M.H.H.; Higuchi Mde, L.; Tucci, P.J.; Garavelo, S.M.; Reis, M.M.; Antonio, E.L.; Serra, A.J.; Maranhão, R.C. Previous exercise training increases levels of PPAR-α in long-term post-myocardial infarction in rats, which is correlated with better inflammatory response. Clinics 2016, 71, 163–168. [Google Scholar] [CrossRef]
- Riehle, C.; Bauersachs, J. Key inflammatory mechanisms underlying heart failure. Herz 2019, 44, 96–106. [Google Scholar] [CrossRef]
- Rusinkevich, V.; Huang, Y.; Chen, Z.; Qiang, W.; Wang, Y.; Shi, Y.; Yang, H. Temporal dynamics of immune response following prolonged myocardial ischemia/reperfusion with and without cyclosporine A. Acta Pharmacol. Sin. 2019, 40, 1168–1183. [Google Scholar] [CrossRef]
- Deten, A. Cardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in rats. Cardiovasc. Res. 2002, 55, 329–340. [Google Scholar] [CrossRef]
- Hill, M.F.; Singal, P.K. Right and left myocardial antioxidant responses during heart failure subsequent to myocardial infarction. Circulation 1997, 96, 2414–2420. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lu, X.; Chen, T.; Yang, Y.; Zheng, J.; Chen, C.; Zhang, Y.; Lei, W. Resveratrol protects against myocardial ischemic injury via the inhibition of NF-κB-dependent inflammation and the enhancement of antioxidant defenses. Int. J. Mol. Med. 2021, 47, 29. [Google Scholar] [CrossRef] [PubMed]
- Morrison, B.N.; George, K.; Kreiter, E.; Dixon, D.; Rebello, L.; Massarotto, R.J.; Cote, A.T. Effects of endurance exercise training on left ventricular structure in healthy adults: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2023, 30, 772–793. [Google Scholar] [CrossRef]
- Dayan, A.; Feinberg, M.S.; Holbova, R.; Deshet, N.; Scheinowitz, M. Swimming exercise training prior to acute myocardial infarction attenuates left ventricular remodeling and improves left ventricular function in rats. Ann. Clin. Lab. Sci. 2005, 35, 73–78. [Google Scholar] [PubMed]
- Cingolani, H.E.; Pérez, N.G.; Cingolani, O.H.; Ennis, I.L. The Anrep effect: 100 years later. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H175–H182. [Google Scholar] [CrossRef]
- Kötter, S.; Kazmierowska, M.; Andresen, C.; Bottermann, K.; Grandoch, M.; Gorressen, S.; Heinen, A.; Moll, J.M.; Scheller, J.; Gödecke, A.; et al. Titin-based cardiac myocyte stiffening contributes to early adaptive ventricular remodeling after myocardial infarction. Circ. Res. 2016, 119, 1017. [Google Scholar] [CrossRef]
- Caporizzo, M.A.; Prosser, B.L. The microtubule cytoskeleton in cardiac mechanics and heart failure. Nat. Rev. Cardiol. 2022, 19, 364–378. [Google Scholar] [CrossRef]
- Qin, R.; Murakoshi, N.; Xu, D.; Tajiri, K.; Feng, D.; Stujanna, E.N.; Yonebayashi, S.; Nakagawa, Y.; Shimano, H.; Nogami, A.; et al. Exercise training reduces ventricular arrhythmias through restoring calcium handling and sympathetic tone in myocardial infarction mice. Physiol. Rep. 2019, 7, e13972. [Google Scholar] [CrossRef]
- Danielsen, T.K.; Sadredini, M.; Manotheepan, R.; Aronsen, J.M.; Frisk, M.; Hansen, M.H.; Andressen, K.W.; Hougen, K.; Levy, F.O.; Louch, W.E.; et al. Exercise training stabilizes RyR2-dependent Ca2+ release in post-infarction heart failure. Front. Cardiovasc. Med. 2021, 7, 623922. [Google Scholar] [CrossRef]
- Morissette, M.P.; Susser, S.E.; Stammers, A.N.; Moffatt, T.L.; Wigle, J.T.; Wigle, T.J.; Netticadan, T.; Premecz, S.; Jassal, D.S.; O’Hara, K.A.; et al. Exercise-induced increases in the expression and activity of cardiac sarcoplasmic reticulum calcium ATPase 2 is attenuated in AMPKα2 kinase-dead mice. Can. J. Physiol. Pharmacol. 2019, 97, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, W.-Z.; Joiner, M.; Zhang, R.; Oddis, C.V.; Hou, Y.; Yang, J.; Price, E.E.; Gleaves, L.; Eren, M.; et al. Calmodulin kinase II inhibition protects against myocardial cell apoptosis in vivo. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H3065–H3075. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, T.; Mishra, S.; Dalton, N.D.; Kranias, E.G.; Peterson, K.L.; Bers, D.M.; Brown, J.H. Phospholamban ablation rescues sarcoplasmic reticulum Ca2+ handling but exacerbates cardiac dysfunction in CaMKIIδC transgenic mice. Circ. Res. 2010, 106, 354–362. [Google Scholar] [CrossRef]
- Valverde, C.A.; Mazzocchi, G.; Di Carlo, M.N.; Ciocci Pardo, A.; Salas, N.; Ragone, M.I.; Felice, J.I.; Cely-Ortiz, A.; Consolini, A.E.; Portiansky, E.; et al. Ablation of phospholamban rescues reperfusion arrhythmias but exacerbates myocardium infarction in hearts with Ca2+/calmodulin kinase II constitutive phosphorylation of ryanodine receptors. Cardiovasc. Res. 2019, 115, 556–569. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, Y.; Zhu, M.; Zhang, X.; Zhang, X.; Lv, J.; Tang, W.; Weng, Q.; Lin, Y.; Tong, L.; et al. mROS-calcium feedback loop promotes lethal ventricular arrhythmias and sudden cardiac death in early myocardial ischemia. Int. J. Mol. Med. 2023, 53, 5. [Google Scholar] [CrossRef] [PubMed]
- McElroy, C.L.; Gissen, S.A.; Fishbein, M.C. Exercise-induced reduction in myocardial infarct size after coronary artery occlusion in the rat. Circulation 1978, 57, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Paroo, Z.; Haist, J.V.; Karmazyn, M.; Noble, E.G. Exercise improves postischemic cardiac function in males but not females: Consequences of a novel sex-specific heat shock protein 70 response. Circ. Res. 2002, 90, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Thorp, D.B.; Haist, J.V.; Leppard, J.; Milne, K.J.; Karmazyn, M.; Noble, E.G. Exercise training improves myocardial tolerance to ischemia in male but not in female rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R363–R371. [Google Scholar] [CrossRef] [PubMed]
- Vesterbekkmo, E.K.; Aksetøy, I.A.; Follestad, T.; Nilsen, H.O.; Hegbom, K.; Wisløff, U.; Wiseth, R.; Madssen, E. High-intensity interval training induces beneficial effects on coronary atheromatous plaques: A randomized trial. Eur. J. Prev. Cardiol. 2023, 30, 384–392. [Google Scholar] [CrossRef]
- Hambrecht, R.; Niebauer, J.; Marburger, C.; Grunze, M.; Kälberer, B.; Hauer, K.; Schlierf, G.; Kübler, W.; Schuler, G. Various intensities of leisure time physical activity in patients with coronary artery disease: Effects on cardiorespiratory fitness and progression of coronary atherosclerotic lesions. J. Am. Coll. Cardiol. 1993, 22, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K.; Beanlands, R.S.; Dekemp, R.A.; Lortie, M.; Morin, J.; Aung, M.; McKelvie, R.; Davies, R.F. Effect of exercise training on myocardial blood flow in patients with stable coronary artery disease. Am. Heart J. 2006, 151, 1324.e11–1324.e18. [Google Scholar] [CrossRef] [PubMed]
- Carneiro-Júnior, M.A.; Pelúzio, M.C.; Silva, C.H.; Amorim, P.R.; Silva, K.A.; Souza, M.O.; Castro, C.A.; Roman-Campos, D.; Prímola-Gomes, T.N.; Natali, A.J. Exercise training and detraining modify the morphological and mechanical properties of single cardiac myocytes obtained from spontaneously hypertensive rats. Braz. J. Med. Biol. Res. 2010, 43, 1042–1046. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Craig, B.W.; Martin, G.; Betts, J.; Lungren, M.; Lambret, V.; Kaiserauer, S. The influence of training-detraining upon the heart, muscle and adipose tissue of female rats. Mech. Ageing Dev. 1991, 57, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Massarotto, R.J.; Campbell, A.J.; Kreiter, E.; Claydon, V.E.; Cote, A.T. Effects of detraining on left ventricular mass in endurance-trained individuals: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2024, 31, 415–424. [Google Scholar] [CrossRef]
| SHAM | NT+MI | T60+MI | T90+MI | T180+MI | p | |
|---|---|---|---|---|---|---|
| E-wave (m/s) | 0.86 (0.77–0.94) | 0.74 (0.68–0.79) | 0.84 (0.76–0.93) | 0.86 (0.74–0.98) | 0.81 (0.73–0.89) | 0.10 |
| A-wave (m/s) | 0.23 (0.19–0.27) | 0.20 (0.16–0.25) | 0.19 (0.17–0.21) | 0.26 (0.14–0.39) | 0.24 (0.17–0.31) | 0.15 |
| E/A (a.u.) | 3.78 (3.2–4.37) | 3.93 (3.16–4.69) | 4.56 (4.11–5.02) | 3.56 (2.24–4.88) | 4.21 (2.81–5.60) | 0.17 |
| DT (ms) | 42.7 [25.8–59.5] | 38.9 [32–45.7] | 36.7 [29.1–44.2] | 39.4 [27–51.8] | 40.7 [37.9–43.6] | 0.79 |
| IVRT (ms) | 23.2 [19.3–27.1] | 24 [19.7–28.3] | 25.7 [19.9–31.5] | 25.2 [19.3–31.1] | 23.6 [18.5–28.8] | 0.90 |
| SHAM | NT+MI | T60+MI | T90+MI | T180+MI | p | |
|---|---|---|---|---|---|---|
| IL-10 (pg/mg protein) | 34.9 [29.7–40] | 28.4 [25.8–31.1] | 29.3 [25.5–33] | 32.3 [26.2–38.4] | 29.6 [24–35.2] | 0.12 |
| IL-6 (pg/mg protein) | 70.4 (64.1–76.7) | 70.8 (64.6–76.9) | 71.6 (67–76.3) | 73.5 (66.7–80.3) | 71.4 (64.6–78.3) | 0.96 |
| IL-1β (pg/mg protein) | 12.6 (9.4–15.7) | 11.2 (8.5–13.8) | 13.8 (11–16.6) | 10 (6.6–13.4) | 11.8 (8.9–14.6) | 0.37 |
| TNF-α (pg/mg protein) | 7.5 (5.4–9.5) | 5 (3.5–6.5) | 5.8 (3.8–7.8) | 6 (4.2–7.8) | 6.2 (4.9–7.6) | 0.30 |
| 4-HNE/GAPDH | 0.47 [0.36–0.59] | 0.57 [0.44–0.69] | 0.52 [0.41–0.62] | 0.47 [0.39–0.55] | 0.53 [0.41–0.64] | 0.38 |
| GPX (U/mg protein) | 1.4 (1–1.9) | 1.6 (1.1–2.1) | 1.5 (1–1.9) | 1.3 (0.6–1.9) | 1.5 (1–2) | 0.88 |
| CAT (nmol/min/mg protein) | 190 (158–222) | 215 (157–274) | 193 (156–230) | 183 (136–229) | 194 (147–241) | 0.83 |
| SOD (U/mg protein) | 38.9 [34.3–43.5] | 43.4 [36.7–50.2] | 40.2 [33.1–47.2] | 44.7 [34–55.5] | 35.3 [28.3–42.3] | 0.34 |
| VEGF (pg/mg protein) | 3.6 (1.6–5.6) | 2.5 (1.2–3.8) | 2.1 (0.9–3.3) | 2.2 (0.7–3.7) | 3.7 (1.7–5.7) | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, A.R.L.; Antonio, E.L.; de Oliveira, H.A.; Teixeira, I.L.A.; Seibt, L.E.; Serra, A.J. Prior Aerobic Exercise Training Fails to Confer Cardioprotection Under Varying Exercise Volumes in Early Post-Infarction Cardiac Remodeling in Female Rats. Biomedicines 2025, 13, 2221. https://doi.org/10.3390/biomedicines13092221
Dias ARL, Antonio EL, de Oliveira HA, Teixeira ILA, Seibt LE, Serra AJ. Prior Aerobic Exercise Training Fails to Confer Cardioprotection Under Varying Exercise Volumes in Early Post-Infarction Cardiac Remodeling in Female Rats. Biomedicines. 2025; 13(9):2221. https://doi.org/10.3390/biomedicines13092221
Chicago/Turabian StyleDias, André Rodrigues Lourenço, Ednei Luiz Antonio, Helenita Antonia de Oliveira, Ighor Luiz Azevedo Teixeira, Larissa Emília Seibt, and Andrey Jorge Serra. 2025. "Prior Aerobic Exercise Training Fails to Confer Cardioprotection Under Varying Exercise Volumes in Early Post-Infarction Cardiac Remodeling in Female Rats" Biomedicines 13, no. 9: 2221. https://doi.org/10.3390/biomedicines13092221
APA StyleDias, A. R. L., Antonio, E. L., de Oliveira, H. A., Teixeira, I. L. A., Seibt, L. E., & Serra, A. J. (2025). Prior Aerobic Exercise Training Fails to Confer Cardioprotection Under Varying Exercise Volumes in Early Post-Infarction Cardiac Remodeling in Female Rats. Biomedicines, 13(9), 2221. https://doi.org/10.3390/biomedicines13092221







