mRNA Vaccines in Modern Immunotherapy for Non-Small Cell Lung Cancer (NSCLC)—A Comprehensive Literature Review with Focus on Current Clinical Trials
Abstract
1. Introduction
2. Landscape of mRNA Vaccine Research in NSCLC
2.1. Antigen Identification and Selection
2.2. Design and Engineering of the mRNA Construct
2.3. Delivery Systems
2.3.1. Peptide-Assembled Systems
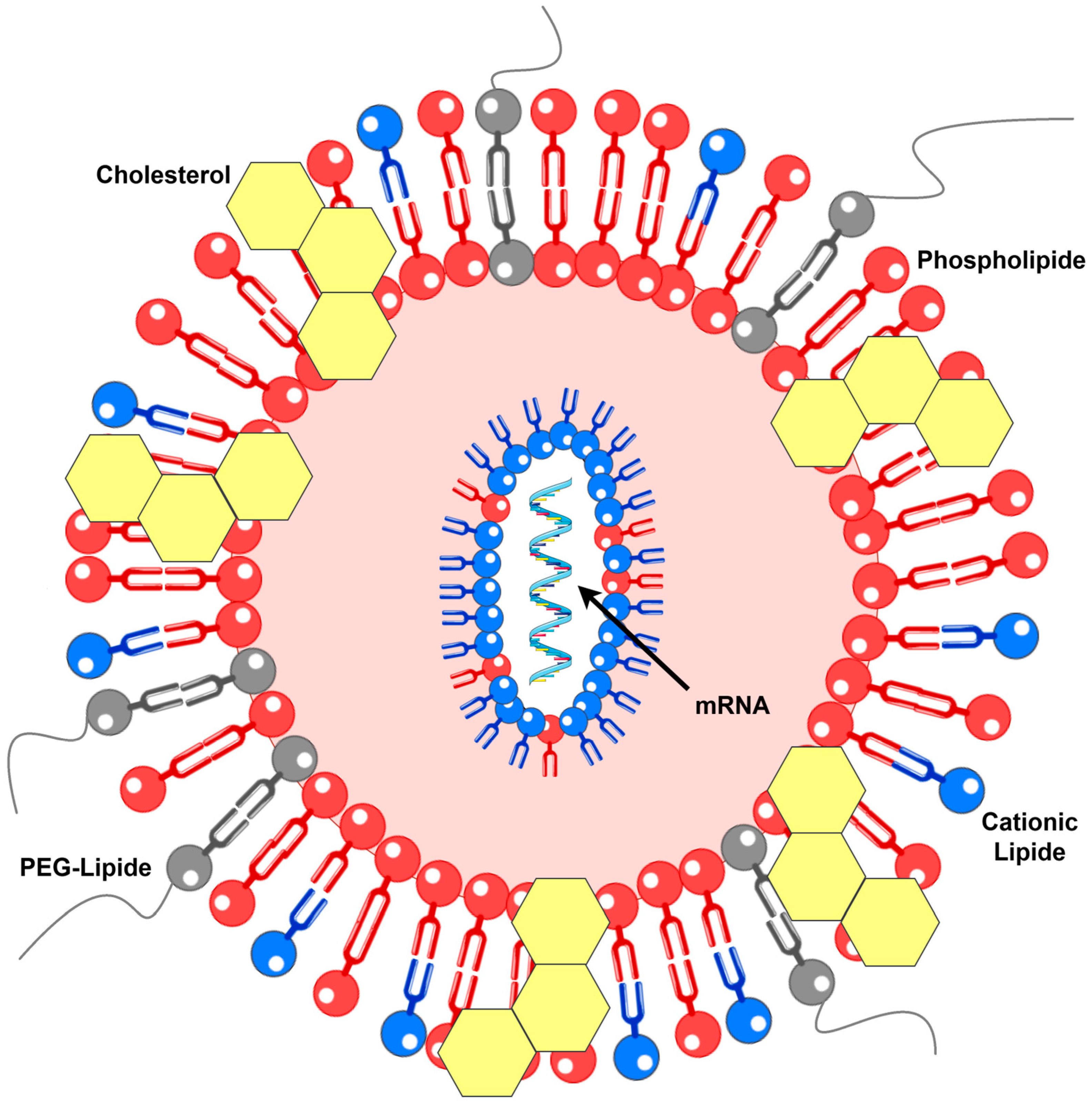
2.3.2. Lipid-Based Systems
2.4. Mechanism of Action
3. Current Evidence from mRNA Vaccine Research in NSCLC
3.1. Clinical Studies Completed
3.2. Clinical Studies Underway
3.3. In Silico Studies
4. Discussion
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, H.; Li, M.; Zhou, J.; Hu, L.; Lu, S.; Li, P. The Recent Research Progress of the Tumor mRNA Vaccine. Vaccines 2024, 12, 1167. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.J.; Xue, Y.; Chen, T.T.; Sun, X.K.; Shi, H.Y. Neoantigen-Based Immunotherapy in Lung Cancer: Advances, Challenges and Prospects. Cancers 2025, 17, 1953. [Google Scholar] [CrossRef]
- Alduais, Y.; Zhang, H.; Fan, F.; Chen, J.; Chen, B. Non-small cell lung cancer (NSCLC): A review of risk factors, diagnosis, and treatment. Medicine 2023, 102, e32899. [Google Scholar] [CrossRef]
- Tahayneh, K.; Idkedek, M.; Abu Akar, F. NSCLC: Current Evidence on Its Pathogenesis, Integrated Treatment, and Future Perspectives. J. Clin. Med. 2025, 14, 1025. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, A.; Maji, A.; Potdar, P.D.; Singh, N.; Parikh, P.; Bisht, B.; Mukherjee, A.; Paul, M.K. Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol. Cancer 2023, 22, 40. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, M.; Poznańska, J.; Fechner, F.; Michalska, N.; Paszkowska, S.; Napierała, A.; Mackiewicz, A. Cancer Vaccine Therapeutics: Limitations and Effectiveness—A Literature Review. Cells 2023, 12, 2159. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Chen, F.; Xu, M.; Liu, B.; Wang, L. Recent advances in neoantigen vaccines for treating non-small cell lung cancer. Thorac. Cancer 2023, 14, 3361–3368. [Google Scholar] [CrossRef]
- NCT03739931 Study Details|Dose Escalation Study of mRNA-2752 for Intratumoral Injection to Participants in Advanced Malignancies|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT03739931 (accessed on 30 August 2025).
- Sample, P.J.; Wang, B.; Reid, D.W.; Presnyak, V.; McFadyen, I.J.; Morris, D.R.; Seelig, G. Human 5′ UTR design and variant effect prediction from a massively parallel translation assay. Nat. Biotechnol. 2019, 37, 803–809. [Google Scholar] [CrossRef]
- Jin, L.; Zhou, Y.; Zhang, S.; Chen, S.J. mRNA vaccine sequence and structure design and optimization: Advances and challenges. J. Biol. Chem. 2025, 301, 108015. [Google Scholar] [CrossRef]
- Zarghampoor, F.; Azarpira, N.; Khatami, S.R.; Behzad-Behbahani, A.; Foroughmand, A.M. Improved translation efficiency of therapeutic mRNA. Gene 2019, 707, 231–238. [Google Scholar] [CrossRef]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug. Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef] [PubMed]
- Estapé Senti, M.; García Del Valle, L.; Schiffelers, R.M. mRNA delivery systems for cancer immunotherapy: Lipid nanoparticles and beyond. Adv. Drug. Deliv. Rev. 2024, 206, 115190. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, K.J.; Webber, M.J.; Anderson, D.G. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J. Control. Release 2016, 240, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Shoari, A.; Tooyserkani, R.; Tahmasebi, M.; Löwik, D.W.P.M. Delivery of Various Cargos into Cancer Cells and Tissues via Cell-Penetrating Peptides: A Review of the Last Decade. Pharmaceutics 2021, 13, 1391. [Google Scholar] [CrossRef]
- Mai, Y.; Guo, J.; Zhao, Y.; Ma, S.; Hou, Y.; Yang, J. Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cell. Immunol. 2020, 354, 104143. [Google Scholar] [CrossRef]
- Jacob, E.M.; Huang, J.; Chen, M. Lipid nanoparticle-based mRNA vaccines: A new frontier in precision oncology. Precis. Clin. Med. 2024, 7, pbae017. [Google Scholar] [CrossRef]
- Papachristofilou, A.; Hipp, M.M.; Klinkhardt, U.; Früh, M.; Sebastian, M.; Weiss, C.; Pless, M.; Cathomas, R.; Hilbe, W.; Pall, G.; et al. Phase Ib Evaluation of a Self-Adjuvanted Protamine Formulated mRNA-Based Active Cancer Immunotherapy, BI1361849 (CV9202), Combined with Local Radiation Treatment in Patients with Stage IV Non-Small Cell Lung Cancer. J. Immunother. Cancer 2019, 7, 38. [Google Scholar] [CrossRef]
- Saldanha, L.; Vale, N. The First Approved COVID-19 Vaccines: The Road to Cancer Vaccines. Int. J. Transl. Med. 2022, 2, 309–331. [Google Scholar] [CrossRef]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef]
- NCT06077760 Study Details|A Study of V940 Plus Pembrolizumab (MK-3475) Versus Placebo Plus Pembrolizumab in Participants with Non-Small Cell Lung Cancer (V940-002) (INTerpath-002)|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06077760 (accessed on 30 August 2025).
- Sebastian, M.; Schröder, A.; Scheel, B.; Hong, H.S.; Muth, A.; Von Boehmer, L.; Zippelius, A.; Mayer, F.; Reck, M.; Atanackovic, D.; et al. A Phase I/II a Study of the mRNA-Based Cancer Immunotherapy CV9201 in Patients with Stage IIIB/IV Non-Small Cell Lung Cancer. Cancer Immunol. Immunother. 2019, 68, 799–812. [Google Scholar] [CrossRef]
- Sebastian, M.; Papachristofilou, A.; Weiss, C.; Früh, M.; Cathomas, R.; Hilbe, W.; Wehler, T.; Rippin, G.; Koch, S.D.; Scheel, B.; et al. Phase Ib study evaluating a self-adjuvanted mRNA cancer vaccine (RNActive®) combined with local radiation as consolidation and maintenance treatment for patients with stage IV non-small cell lung cancer. BMC Cancer 2014, 14, 748. [Google Scholar] [CrossRef]
- Xu, S.; Yang, K.; Li, R.; Zhang, L. mRNA Vaccine Era—Mechanisms, Drug Platform and Clinical Prospection. Int. J. Mol. Sci. 2020, 21, 6582. [Google Scholar] [CrossRef]
- Shi, Y.; Lu, Y.; Qin, B.; Jiang, M.; Guo, X.; Li, X.; Liu, Y.; Huang, J.; Zhang, J.; Luo, Z.; et al. Antigen transfer from non-APCs to APCs impacts the efficacy and safety of protein- and mRNA- based vaccines. Nano Today 2021, 41, 101326. [Google Scholar] [CrossRef]
- Ni, L. Advances in mRNA-Based Cancer Vaccines. Vaccines 2023, 11, 1599. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X. Progress in mRNA cancer vaccine research, current limitations, application and future. Theor. Nat. Sci. 2023, 20, 141–147. [Google Scholar] [CrossRef]
- Galant, N.; Krzyżanowska, N.; Krawczyk, P. mRNA vaccines in the treatment of cancer. Oncol. Clin. Pract. 2024, 101582, Version of Record. [Google Scholar] [CrossRef]
- Rossin, A.; Miloro, G.; Hueber, A.O. TRAIL and FasL Functions in Cancer and Autoimmune Diseases: Towards an Increasing Complexity. Cancers 2019, 11, 639. [Google Scholar] [CrossRef]
- Zhong, Y.; Du, S.; Dong, Y. mRNA delivery in cancer immunotherapy. Acta Pharm. Sin. B 2023, 13, 1348–1357. [Google Scholar] [CrossRef]
- Morse, M.A.; Nair, S.K.; Mosca, P.J.; Hobeika, A.C.; Clay, T.M.; Deng, Y.; Boczkowski, D.; Proia, A.; Neidzwiecki, D.; Clavien, P.A.; et al. Immunotherapy with autologous, human dendritic cells transfected with carcinoembryonic antigen mRNA. Cancer Investig. 2003, 21, 341–349. [Google Scholar] [CrossRef]
- Gandhi, L.; Aufiero Ramirez, K.; Schwarzenberger, P.; Ricciardi, T.; Macri, M.J.; Ryan, A.; Venhaus, R.R. Phase 1/2 Study of mRNA Vaccine Therapy + Durvalumab (Durva) ± Tremelimumab (Treme) in Patients with Metastatic Non-Small Cell Lung Cancer (NSCLC). J. Clin. Oncol. 2018, 36, TPS9107. [Google Scholar] [CrossRef]
- Kiousi, E.; Lyraraki, V.; Mardiki, G.L.; Stachika, N.; Damianou, A.K.; Malainou, C.P.; Syrigos, N.; Gomatou, G.; Kotteas, E. Progress and Challenges of Messenger RNA Vaccines in the Therapeutics of NSCLC. Cancers 2023, 15, 5589. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, A.; Alt, J.; Baz, D.V.; Dziadziuszko, R.; Moreno, V.; Pose, V.; Juan-Vidal, O.; Munshi, N.; Markman, J.L.; Chen, V.; et al. 1486 Preliminary results from LuCa-MERIT-1, a phase I trial evaluating BNT116, a fixed antigen mRNA vaccine, plus cemiplimab in advanced non-small cell lung cancer after progression on PD-1 inhibition. J. Immunotherap. Cancer 2024, 12, A1716. [Google Scholar] [CrossRef]
- NCT02688686 Study Details|Safety and Efficacy of DC-CIK in Patients with Advanced Non-Small-Cell Lung Cancer with Bone Metastases|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT02688686 (accessed on 30 August 2025).
- NCT03948763 Study Details|A Study of mRNA-5671/V941 as Monotherapy and in Combination with Pembrolizumab (V941-001)|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT03948763 (accessed on 30 August 2025).
- NCT05202561 Study Details|A Study of RNA Tumor Vaccine in Patients with Advanced Solid Tumors|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05202561 (accessed on 30 August 2025).
- NCT03289962 Study Details|A Study of Autogene Cevumeran (RO7198457) as a Single Agent and in Combination with Atezolizumab in Participants with Locally Advanced or Metastatic Tumors|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT03289962 (accessed on 30 August 2025).
- Lopez, J.; Powles, T.; Braiteh, F.; Siu, L.L.; LoRusso, P.; Friedman, C.F.; Balmanoukian, A.S.; Gordon, M.; Yachnin, J.; Rottey, S.; et al. Autogene cevumeran with or without atezolizumab in advanced solid tumors: A phase 1 trial. Nat Med. 2025, 31, 152–164. [Google Scholar] [CrossRef]
- NCT04267237 Study Details|A Study of the Efficacy and Safety of RO7198457 in Combination with Atezolizumab Versus Atezolizumab Alone Following Adjuvant Platinum-Doublet Chemotherapy in Participants Who Are ctDNA Positive After Surgical Resection of Stage II–III Non-Small Cell Lung Cancer|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04267237 (accessed on 30 August 2025).
- NCT03908671 Study Details|Clinical Study of Personalized mRNA Vaccine Encoding Neoantigen in Patients with Advanced Esophageal Cancer and Non-small Cell Lung Cancer|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT03908671 (accessed on 30 August 2025).
- NCT06685653 Study Details|Personalized Neoantigen MRNA Vaccine Combined with Adebrelimab in Non-Small Cell Lung Cancer Patients|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06685653 (accessed on 30 August 2025).
- NCT06735508 Study Details|MRNA Neoantigen Vaccine in Non-Small Cell Lung Cancer|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06735508 (accessed on 30 August 2025).
- NCT05557591 Study Details|A Trial to Learn How the Cancer Vaccine BNT116 in Combination with Cemiplimab Works and How Safe the Combination Is in Adults with Advanced Non-Small Cell Lung Cancer (EMPOWERVAX Lung 1)|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05557591 (accessed on 30 August 2025).
- Zhao, J.; Xu, R.; Lu, T.; Wang, J.; Zhang, L. Identification of tumor antigens and immune subtypes in lung squamous cell carcinoma for mRNA vaccine development. J. Thorac. Dis. 2022, 14, 3517–3530. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, H.; Yu, T.; Chen, X.; Jing, F.; Shi, H. Potential Immune Biomarker Candidates and Immune Subtypes of Lung Adenocarcinoma for Developing mRNA Vaccines. Front. Immunol. 2021, 12, 755401. [Google Scholar] [CrossRef]
- Zhou, B.; Zang, R.; Zhang, M.; Song, P.; Liu, L.; Bie, F.; Peng, Y.; Bai, G.; Gao, S. Identifying novel tumor-related antigens and immune phenotypes for developing mRNA vaccines in lung adenocarcinoma. Int. Immunopharmacol. 2022, 109, 108816. [Google Scholar] [CrossRef]
- Xu, R.; Lu, T.; Zhao, J.; Wang, J.; Peng, B.; Zhang, L. Identification of Tumor Antigens and Immune Subtypes in Lung Adenocarcinoma for mRNA Vaccine Development. Front. Cell Dev. Biol. 2022, 10, 815596. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, X.; Shan, Y.; Li, J.; Cui, W.; Wang, J.; Jiang, J.; Xie, Q.; Zhang, C.; Duan, C. Recognition of immune-related tumor antigens and immune subtypes for mRNA vaccine development in lung adenocarcinoma. Comput. Struct. Biotechnol. J. 2022, 20, 5001–5013. [Google Scholar] [CrossRef]
- Ma, S.; Li, X.; Mai, Y.; Guo, J.; Zuo, W.; Yang, J. Immunotherapeutic treatment of lung cancer and bone metastasis with a mPLA/mRNA tumor vaccine. Acta Biomater. 2023, 169, 489–499. [Google Scholar] [CrossRef]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Jaiyesimi, I.A.; Leighl, N.B.; Ismaila, N.; Alluri, K.; Florez, N.; Gadgeel, S.; Masters, G.; Schenk, E.L.; Schneider, B.J.; Sequist, L.; et al. Therapy for Stage IV Non–Small Cell Lung Cancer Without Driver Alterations: ASCO Living Guideline, Version 2023.3. J. Clin. Oncol. 2024, 42, e23–e43. [Google Scholar] [CrossRef]
- Meyer, M.-L.; Fitzgerald, B.G.; Paz-Ares, L.; Cappuzzo, F.; Jänne, P.A.; Peters, S.; Hirsch, F.R. New Promises and Challenges in the Treatment of Advanced Non-Small-Cell Lung Cancer. Lancet 2024, 404, 803–822. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.S. Treatment Options after First-Line Immunotherapy in Metastatic NSCLC. Expert Rev. Anticancer. Ther. 2020, 20, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-Related Adverse Events with Immune Checkpoint Blockade: A Comprehensive Review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhao, X.; Hu, J.; Jiao, Y.; Yan, Y.; Pan, X.; Wang, X.; Jiao, F. mRNA Vaccines in the Context of Cancer Treatment: From Concept to Application. J. Transl. Med. 2025, 23, 12. [Google Scholar] [CrossRef]
- Wang, B.; Pei, J.; Xu, S.; Liu, J.; Yu, J. Recent Advances in mRNA Cancer Vaccines: Meeting Challenges and Embracing Opportunities. Front. Immunol. 2023, 14, 1246682. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Huang, L. mRNA Vaccine for Cancer Immunotherapy. Mol. Cancer. 2021, 20, 41. [Google Scholar] [CrossRef]
- Mao, Y.; Fan, W.; Hu, H.; Zhang, L.; Michel, J.; Wu, Y.; Wang, J.; Jia, L.; Tang, X.; Xu, L.; et al. MAGE-A1 in Lung Adenocarcinoma as a Promising Target of Chimeric Antigen Receptor T Cells. J. Hematol. Oncol. 2019, 12, 106. [Google Scholar] [CrossRef]
- Gadgeel, S.; Rodríguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Dómine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Vicente, D.; Tafreshi, A.; Robinson, A.; Soto Parra, H.; Mazières, J.; Hermes, B.; Cicin, I.; Medgyasszay, B.; Rodríguez-Cid, J.; et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J. Thorac. Oncol. 2020, 15, 1657–1669. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes with Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Xie, C.; Xia, X. Recent Progress in mRNA Cancer Vaccines. Hum. Vaccines Immunother. 2024, 20, 2307187. [Google Scholar] [CrossRef]
- Lin, M.J.; Svensson-Arvelund, J.; Lubitz, G.S.; Marabelle, A.; Melero, I.; Brown, B.D.; Brody, J.D. Cancer Vaccines: The next Immunotherapy Frontier. Nat. Cancer 2022, 3, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Razif, M.I.; Nizar, N.; Zainal Abidin, N.H.; Muhammad Ali, S.N.; Wan Zarimi, W.N.N.; Khotib, J.; Susanti, D.; Mohd Jailani, M.T.; Taher, M. Emergence of mRNA Vaccines in the Management of Cancer. Expert Rev. Vaccines 2023, 22, 629–642. [Google Scholar] [CrossRef]
- Duan, L.-J.; Wang, Q.; Zhang, C.; Yang, D.-X.; Zhang, X.-Y. Potentialities and Challenges of mRNA Vaccine in Cancer Immunotherapy. Front. Immunol. 2022, 13, 923647. [Google Scholar] [CrossRef]
- Yaremenko, A.V.; Khan, M.M.; Zhen, X.; Tang, Y.; Tao, W. Clinical Advances of mRNA Vaccines for Cancer Immunotherapy. Med 2025, 6, 100562. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Peng, X.; Yang, Y.; Chen, Q.; Liu, J.; She, Q.; Tan, J.; Lou, C.; Liao, Z.; et al. mRNA Vaccine in Cancer Therapy: Current Advance and Future Outlook. Clin. Transl. Med. 2023, 13, e1384. [Google Scholar] [CrossRef]
- He, Q.; Gao, H.; Tan, D.; Zhang, H.; Wang, J. mRNA Cancer Vaccines: Advances, Trends and Challenges. Acta Pharm. Sin. B 2022, 12, 2969–2989. [Google Scholar] [CrossRef] [PubMed]

| Vaccine Name | Delivery System | Main Mode of Action | Encoding Antigens | Administration Route |
|---|---|---|---|---|
| CV9201 | LNP/protamine | T-cell stimulation | NY-ESO-1, MAGE-C1, MAGE-C2, survivin, 5T4 | Intradermal |
| CV9202 | LNP/protamine | T-cell stimulation | NY-ESO-1, MAGE-C1, MAGE-C2, survivin, 5T4, MUC-1 | Intradermal |
| BNT116 | Lipoplex | T-cell stimulation | MAGE-A3, MAGE-A4, MAGE-C1, CLDN6, KK-LC-1, PRAME | Intravenous |
| mRNA-2752 | LNP | T-cell stimulation, pro-inflammatory cytokines induction | OX40L, IL-23, IL-36γ | Intratumoral |
| mRNA-4157/V940 | LNP | T-cell stimulation, inducing de novo T-cell responses | Personalized antigens (up to 34) | Intramuscular |
| mRNA-5671/V941 | LNP | T-cell stimulation | KRAS: G12D, G12V, G13D, G12C | Intramuscular |
| Study Name and Type | Vaccine Name | Vaccine Targets | No. of Enrolled Patients | Intervention | Key Findings |
|---|---|---|---|---|---|
| Morse et al. (Phase I/II) | CEA mRNA Vaccine | CEA | N = 29 (Phase I) N = 13 (Phase II) | CEA mRNA Vaccine | Phase I—18/24 evaluable patients had progression (75%) Phase II—median follow-up: 429 days, recurrence: 9/13 (69.2%) Median time to recurrence: 122 days; 3/13 remained NED > 500 days (23.1%); 3/13 died (23.1%) |
| Sebastian et al. (Phase I/IIa) | CV9201 | NY-ESO-1, MAGE-C1, MAGE-C2, survivin, 5T4 | N = 46 | CV9201 | Median PFS: 5.0 months (95% CI: 1.8–6.3); 6-month PFS: 38.9%; 12-month PFS: 16.7% Median OS: 10.8 months (95% CI: 8.1–16.7); 1-year OS: 44.4%; 2-year OS: 26.7%; 3-year OS: 20.7% |
| Papachristofilou et al. (Phase Ib) | CV9202 (BI 1361849) | NY-ESO-1, MAGE-C1, MAGE-C2, survivin, 5T4, MUC-1 | N = 26 | Stratum 1: CV9202 + local radiation + pemetrexed Stratum 2: CV9202 + local radiation Stratum 3: CV9202 + local radiation + gefitinib/erlotynib | Grade ≥ 3 CV9202- and/or radiation-related AEs: 15.4% No serious CV9202- related TEAEs SD as best response: 46.2% Median PFS: 2.87 months (95% CI: 1.43–4.27) Median OS: 13.95 months (95% CI: 8.93–20.87) |
| Gandhi et al. (Phase I/II) | CV9202 (BI 1361849) | NY-ESO-1, MAGE-C1, MAGE-C2, survivin, 5T4, MUC-1 | N = 61 | Arm A: CV9202 + durvalumab Arm B: CV9202 + durvalumab + tremelimumab | TRAEs: 56.5% (Arm A), 55.9% (Arm B) SAEs: 4.3% (Arm A), 8.8% (Arm B) PR: 26.3% (Arm A), 11.1% (Arm B) SD: 36.8% (Arm A), 29.6% (Arm B) PD: 36.8% (Arm A), 59.3% (Arm B) |
| Atmaca et al. (Phase I) | BNT116 | MAGE-A3, CLDN6, KK-LC-1, PRAME, MAGE-A4, MAGE-C1 | N = 20 | BNT116 + cemiplimab | TEAEs: 100% (45% grade 1/2; 55% grade 3) PR: 10% SD: 70% Median PFS: 5.5 months (95% CI: 2.9–9.5) |
| Trial Name and Type | Aim | Vaccine Targets | Key Findings | Completion |
|---|---|---|---|---|
| NCT02688686 (Phase I/II) | Evaluation of safety and efficacy of vaccine in combination with DCs and CIKs in patients with advanced NSCLC with bone metastasis | SOCS1, MUC-1, survivin | No published results (Unknown status) | – |
| NCT03948763 (Phase I) | Evaluation of safety and tolerability of mRNA-5671/V941 in monotherapy and in combination with pembrolizumab in patients with KRAS-mutated cancers | KRAS: G12D, G12V, G13D, G12C | No published results (Terminated) | – |
| NCT05202561 (Phase I) | Evaluation of safety and efficacy of RNA vaccine as monotherapy or in combination with PD-1 inhibitor in patients with KRAS mutation | KRAS: G12D, G12C, G12V | No published results (Unknown status) | January 2024 |
| NCT03289962 (Phase Ia/Ib) | Evaluation of the safety, tolerability, immune response, and pharmacokinetics of cevumeran (RO7198457) in monotherapy and with atezolizumab in patients with NSCLC | Personalized neoantigens |
| March 2025 |
| NCT04267237 (Phase I) | Evaluation of efficacy, safety, and pharmacokinetics of cevumeran (RO7198457) in combination with atezolizumab in patients with stage II–III NSCLC after surgery and chemotherapy | Personalized neoantigens | No published results (Withdrawn) | September 2025 |
| NCT03908671 (Phase I/II) | Evaluation of safety, tolerability, and efficacy of mRNA vaccines encoding neoantigens in patients with esophageal cancer and NSCLC after treatment failure | Personalized neoantigens | No published results (Recruiting) | December 2025 |
| NCT06685653 (Phase II) | Evaluation of safety and tolerability of personalized mRNA vaccine RGL-270 + adebrelimab in patients with operable NSCLC and recurrence after first-line treatment | Personalized neoantigens | No published results (Not yet recruiting) | November 2026 |
| NCT06735508 (Phase I/II) | Evaluation of safety, tolerability, immunogenicity, and efficacy of personalized mRNA neoantigen vaccine with adebelimab in patients with NSCLC | Personalized neoantigens | No published results (Not yet recruiting) | December 2026 |
| NCT06077760 (Phase III) | Comparison of efficacy of V940 + pembrolizumab vs. placebo + pembrolizumab in patients with completely resected stage II and IIIA/IIIB NSCLC | Personalized neoantigens | No published results (Recruiting) | December 2035 |
| NCT05557591 (Phase I/II) | Evaluation of safety, tolerability, and efficacy of BNT116 + cemiplimab in patients with advanced NSCLC | Fixed combination of shared TSAs (MAGE-A3, MAGE-A4, MAGE-C1, CLDN6, KK-LC-1, PRAME) | No published results (Active, not recruiting) | June 2027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabut, J.; Stępień, G.J.; Furgoł, T.; Miciak, M.; Nafalska, N.; Stopyra, M.; Jezierzański, M.; Feret, K.; Gisterek-Grocholska, I. mRNA Vaccines in Modern Immunotherapy for Non-Small Cell Lung Cancer (NSCLC)—A Comprehensive Literature Review with Focus on Current Clinical Trials. Biomedicines 2025, 13, 2187. https://doi.org/10.3390/biomedicines13092187
Kabut J, Stępień GJ, Furgoł T, Miciak M, Nafalska N, Stopyra M, Jezierzański M, Feret K, Gisterek-Grocholska I. mRNA Vaccines in Modern Immunotherapy for Non-Small Cell Lung Cancer (NSCLC)—A Comprehensive Literature Review with Focus on Current Clinical Trials. Biomedicines. 2025; 13(9):2187. https://doi.org/10.3390/biomedicines13092187
Chicago/Turabian StyleKabut, Jacek, Grzegorz J. Stępień, Tomasz Furgoł, Michał Miciak, Natalia Nafalska, Małgorzata Stopyra, Marcin Jezierzański, Krzysztof Feret, and Iwona Gisterek-Grocholska. 2025. "mRNA Vaccines in Modern Immunotherapy for Non-Small Cell Lung Cancer (NSCLC)—A Comprehensive Literature Review with Focus on Current Clinical Trials" Biomedicines 13, no. 9: 2187. https://doi.org/10.3390/biomedicines13092187
APA StyleKabut, J., Stępień, G. J., Furgoł, T., Miciak, M., Nafalska, N., Stopyra, M., Jezierzański, M., Feret, K., & Gisterek-Grocholska, I. (2025). mRNA Vaccines in Modern Immunotherapy for Non-Small Cell Lung Cancer (NSCLC)—A Comprehensive Literature Review with Focus on Current Clinical Trials. Biomedicines, 13(9), 2187. https://doi.org/10.3390/biomedicines13092187






