Abstract
Background/Objectives: Long-term cognitive outcomes after carotid surgery are influenced by diabetes and intraoperative changes. We aimed to analyze the postoperative cognitive changes in diabetic patients and nondiabetic patients after carotid endarterectomy (CEA). Additionally, major cardiovascular and cerebrovascular events (MACCEs) and the incidence of mortality at two years after surgery were assessed. Methods: We enrolled 37 diabetic and 67 nondiabetic patients undergoing elective carotid surgery. Intraoperatively, routine monitoring was completed with NIRS (near-infrared spectroscopy) and an Entropy monitor was used for neuromonitoring. The lowest cerebral tissue saturation levels during the cross-clamp period (rSO2lowestclamp) and the degree of desaturation were calculated. We used MMSE (Mini-Mental State Examination) and MoCA (Montreal Cognitive Assessment) to assess cognitive function. Cognitive change was defined as one standard deviation (SD) change from the preoperative test scores. Results: The MMSE and MoCA were available for 103 patients at three months and for 90 patients at 12 months after discharge. Compared with nondiabetic patients, diabetic patients exhibited greater decreases in MoCA scores (p = 0.028 and p = 0.042 at the 3rd and 12th months, respectively). Cognitive improvement was lower in the DM group than in the control group at the 12th month (18.75% vs. 42.86%, respectively; p = 0.029). The mean rSO2 in the pre-clamping period (67.4% vs. 74.6% in diabetic and in nondiabetic patients, respectively; p = 0.011) was lower in diabetic patients. Furthermore, MACCEs at the 24th month were observed at a higher rate in diabetic patients (p = 0.040). Conclusions: Diabetic patients demonstrated greater risks for cognitive decline, MACCEs, and mortality at two years after surgery.
1. Introduction
Diabetes mellitus is a chronic metabolic disorder that affects 537 million adults worldwide [1]. Among several long-term complications of this disease, there is a well-established association between diabetes and carotid atherosclerosis [2]. Carotid atherosclerosis (particularly extracranial internal carotid stenosis) is one of the leading causes of ischemic stroke, accounting for 8–15% of these events [3].
Given that diabetes is associated with an elevated risk for stroke and that the progression of neurologic injury represents an even worse outcome, the prevention of this development has demonstrated increased relevance [3,4,5]. Carotid endarterectomy is an effective method for preventing stroke; however, with respect to diabetic patients, there are conflicting results regarding the risks of complications overwhelming the benefits of surgery [3,6,7,8].
In addition to cardiovascular and cerebrovascular complications, a highly sensitive benchmark of the success of surgery is postoperative cognitive function, especially regarding changes in postoperative cognitive function. Postoperative cognitive decline is associated with increased mortality and morbidity and represents a significant financial burden on the healthcare system and families [9,10,11].
During carotid surgeries, alterations in cerebral perfusion play a substantial role in the postoperative change in cognitive function [12,13]. Preventing these major changes in cerebral perfusion may have a beneficial effect on cognition postoperatively. This underscores the relevance of neuromonitoring during this intervention.
According to the majority of clinical trials, diabetic patients have an elevated risk for cognitive impairment postoperatively [14]. The background of this phenomenon is complex. In addition to maintaining adequate glycemic control, the optimization of intraoperative hemodynamic parameters and the maintenance of adequate cerebral circulation during the cross-clamp period may be crucial in preventing complications and protecting the integrity of postoperative cognitive function in diabetic patients [15,16,17]. The monitoring of cerebral function is an important step in this management protocol [18]. NIRS (near-infrared spectroscopy) has been proven to be a reliable tool for monitoring cerebral perfusion [19]. A significant drop in cerebral tissue saturation may have a detrimental impact on postoperative cognition [20]. The use of an entropy monitor in tracking the depth of anesthesia has been demonstrated to reduce the risk of cognitive dysfunction [21]. The combination of these two techniques may increase patient outcomes with regard to cognitive safety.
For assessing cognitive function, several screening tools are available. Although the Mini-Mental State Examination (MMSE) is able to detect major changes in cognition, the Montreal Cognitive Assessment (MoCA) may be useful in the detection of mild cognitive changes [22,23].
The MoCA Memory Index Score (MoCA-MIS) has further enhanced of this detection ability and has been proven to be useful in distinguishing unimpaired cognition from mild impairment [24].
This study aimed to analyze the postoperative changes in cognitive function in diabetic and nondiabetic patients and to evaluate the connection between cognitive changes and cerebral tissue saturation values. The secondary aim of the study was to analyze the occurrence of two-year cerebral and cardiovascular events, as well as mortality.
2. Materials and Methods
Participants
This study was approved by the Ethical Committee of Semmelweis University (number: 17/2019, date: 15 February 2019) and registered on ClinicalTrials.gov (NCT03907943). Then, patients undergoing elective carotid endarterectomy at a cardiovascular center were enrolled consecutively in this study. After providing detailed information, all patients provided written informed consent to participate in the study.
All patients were asymptomatic with the severity of stenosis exceeding 70% based on the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria. The type of surgery (eversion endarterectomy (EEA) or thromboendarterectomy (TEA)) and the use of shunt insertion were utilized based on the discretion of the surgeons. The indication for shunt placement was incomplete circle of Willis.
The anatomy of the circle of Willis was classified as being complete (no component was hypoplastic or absent) or incomplete.
The exclusion criteria included patients with symptomatic carotid artery stenosis, atrial fibrillation, preoperative presence of dementia (preoperative MMSE score < 24), and lack of consent. Between May 2019 and November 2021 (13 May 2019–4 November 2021), 129 patients were enrolled in the study.
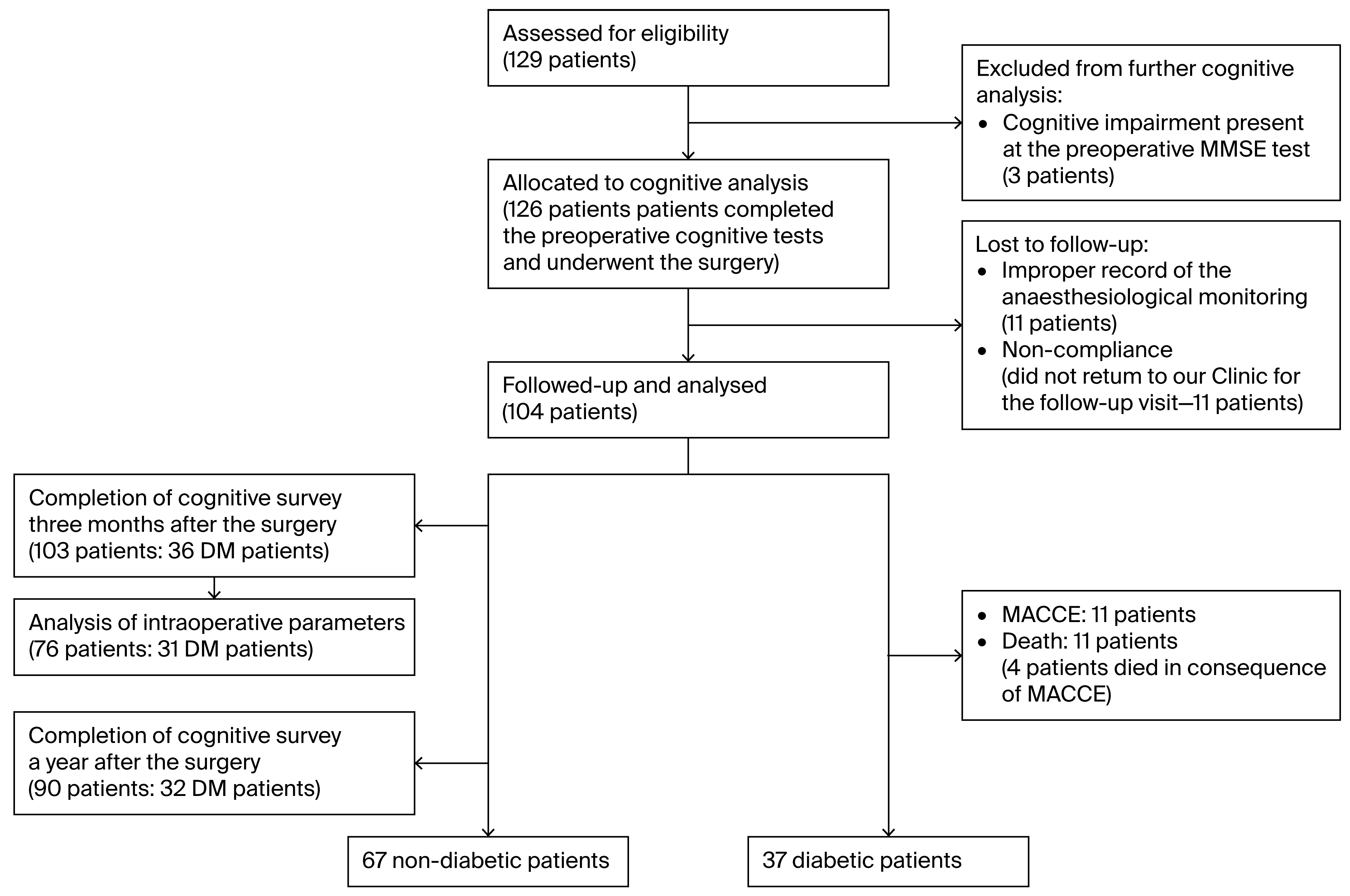
Three patients were not able to achieve 24 points on the MMSE. In eleven cases, accurate recording via anesthesiological monitoring techniques could not be interpreted due to technical reasons.
One patient died before the first control (due to SARS-CoV pneumonia). Eleven patients did not return for our follow-up visit. Since their data were missing at random, there was no selection bias, and they could be excluded from further analysis.
Thus, 103 patients completed three-month postoperative cognitive assessments. One year after surgery, cognitive data were available for 90 patients. For the 76 patients who completed the preoperative and 3-month cognitive surveys, complete intraoperative monitoring was appropriately recorded. The flow diagram of the enrollment and follow-up details of the study are shown in Figure 1. Cognitive follow-up ended in November 2023.
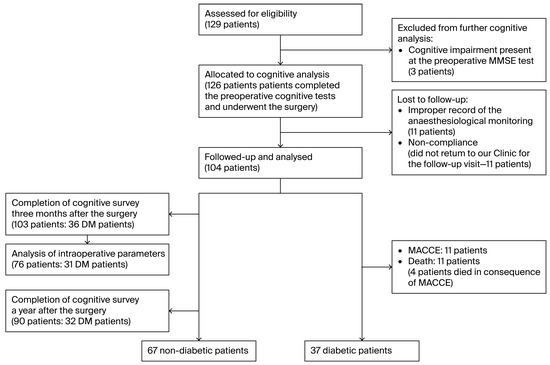
Figure 1.
Consort flow diagram of the enrollment and follow-up.
During data processing, patients were divided into two groups based on the presence of diabetes mellitus. The diabetes diagnosis was based on the definition established by the WHO [25]. Hypoglycemic medications and blood glucose levels were managed according to the guidelines of the Academy of Medical Royal Colleges [26].
The diabetes medications that our patients used are summarized in Supplementary Tables S1 and S2.
To predict survival, we used a recently published risk score [27]. This score was created based on the data of 24,713 patients with symptomatic carotid stenosis before carotid endarterectomy.
Parametric, nonparametric, and binary logistic regression statistical analyses were used to identify the determining factors. Factors that were significant at the 0.05 level in the multivariate regression analysis were included as predictors of the risk score.
The value of a factor’s beta coefficient indicates its weight on the score, which was calculated in the regression analysis. One point was added for every 0.25 interval.
The factors associated with a greater risk included female sex (1 point), diabetes (1 point), age (different age groups), malnutrition (BMI < 20) (2 points), a condition reaching a value of 4 on the American Society of Anesthesiology classification (2 points), and home status (1 point), as these factors had been shown to influence survival. When considering comorbidities, congestive heart failure (2 points), chronic obstructive pulmonary disease (2 points), intervention due to peripheral arterial disease (1 point), previous CEA or stent insertion (2 points), major lower extremity amputation indicated in the patients’ anamnesis (3 points), chronic renal insufficiency (2 points), the need for dialysis (4 points), and anemia (4 points) were identified as risk factors for impaired survival, in addition to the anamnesis of smoking (2 points). As the enrolled patients were symptomatic, the severity of the neurologic injury indicated by the Rankin score was emphasized in the score.
The efficiency of the score was also tested in symptomatic patients.
The score was strongly correlated with the mortality rate of symptomatic patients who underwent carotid artery stenting (AUC: 0.70; Hosmer–Lemeshow overall accuracy: 91.3%). This score proved to be reliable in predicting survival after the intervention.
Intraoperative management
All of our patients underwent general anesthesia. The anesthesia station was GE Aisys CS2 (GE Healthcare, Madison, WI, USA).
We used propofol (2–5 mg/kg or until loss of consciousness) and fentanyl (2–10 mcg/kg) for the induction, and sevoflurane (114 patients, 1.4–1.9%) or isoflurane (12 patients, 0.9–1.2%) for the maintenance of anesthesia with fentanyl (50–100 μg or as necessary to avoid signs of pain) as an analgesic agent. Atracurium or rocuronium was applied for muscle relaxation. Patients were ventilated with 40–50% oxygen, and end-tidal carbon dioxide was kept between 33 and 40 mmHg. Standard, routine monitoring (electrocardiogram, intra-arterial blood pressure measurement, pulse oximetry, and capnography) was completed using near-infrared spectroscopy (NIRS) and entropy monitoring. To detect regional cerebral oxygenation (rSO2), we used an INVOSTM 5100 C (Somanetics Co., Troy, MI, USA) cerebral oximeter, which continuously recorded the regional cerebral oxygen saturation throughout the entire procedure. The sampling frequency of the NIRS monitor was 0.166 Hz.
To calculate the degree of desaturation as a percentage during the cross-clamp period, we used the following formula: (rSO2preclamp − rSO2lowestclamp)/rSO2preclamp × 100.
As the baseline, we determined the median rSO2 values of the 2-min-long pre-clamping period, and the lowest rSO2 was defined as the median of 30 s of the lowest cerebral tissue saturation values during the cross-clamp period.
To monitor the depth of anesthesia, we used a GE entropy monitor to achieve and maintain the optimal level of anesthesia and avoid anesthetic overdose. The entropy values were maintained between 40 and 60 [28].
We paid special attention to maintaining hemodynamic stability. The mean arterial pressure (MAP) was maintained within the ±20% range of the preoperative level. Before and during the cross-clamp period, MAP was kept within the +0–20% range of the preoperative level. To prevent hypotension, we used intravenous norepinephrine at a dose of 0.03 µg/kg/min, which was modified by units of 0.02 µg/kg/min in cases of devi-ations from this range. Lidocaine was administered to the surgical area in cases of bradycardia. All of the patients received 2500 IU of heparin before the internal carotid artery (ICA) was clamped, which was reversed with the administration of protamine at the end of the procedure. To evaluate trends in the monitored vital parameters, the surgery was divided into three periods: pre-clamping, clamping, and post-clamping.
The pre-clamping period began with the completion of intubation and continued until the cross-clamping of the common carotid artery (CCA). The clamping period be-gan from the time of clamping of the CCA and internal carotid artery (ICA) and con-tinued until the complete removal of the clamp. Then, the post-clamping period began and continued until extubation. The data collected from the monitor were saved as .asc files and Excel files. The interventions performed by the surgeons and anesthesiologist were accurately indicated in the database.
Cognitive evaluation
For the assessment of cognitive function, we used MMSE and MoCA. The tests were scheduled to be administered one day before surgery and at 3 and 12 months after dis-charge. The tests were administered by the same physician (SA-HU710556625-01).
The assessment began with the administration of the MMSE in order to exclude those individuals who were suffering from dementia. The MMSE contains 11 major items and is useful for assessing the function of five cognition domains. Scores of 23 or lower are indicative of cognitive impairment. In our study, patients who were not able to achieve 24 points on the MMSE were excluded [29]. Cronbach’s alpha of the MMSE scores was 0.79.
The MOCA test was developed to detect mild cognitive impairment [22]. A score of 26 points or above indicates normal cognitive performance.
A subscore of the MoCA, the MoCA Memory Index Score, can reveal deficits in encoding memory. This score ranges from 0 to 15 and weighs recall based on the cor-rectly answered categories of the patients. The number of recalled words is multiplied by 3 if they are provided in free delayed recall, by 2 if they are provided in category-cued recall, and by 1 if they are provided in multiple-choice-cued recall. The cutoff value was demonstrated to be 7 points; however, these data should be interpreted in conjunction with the total score of the MoCA test [30].
Outcome variables
Cognitive changes represented the primary outcome of this study, and the sec-ondary outcomes were the occurrence of major adverse cardiovascular and cerebro-vascular events and mortality. Postoperative cognitive change was defined as the difference between the results of the postoperative tests and the preoperative scores by one standard deviation or more (MMSE: 1.79; MoCA: 2.28; MIS: 2.55) [31]. For each test and for the MIS, cognitive improvement was defined as one SD or greater change in the score compared with the baseline. Cognitive decline was defined as a decrease in the follow-up score by a value of one SD or more. If the change in the scores remained within the +/−1 SD limits, no change in cognitive function was detected.
The occurrences of MACCEs were registered during the follow-up period and were defined as myocardial infarction, new onset of arrhythmia, hemodynamic instability, cardiac failure, cardiac arrest, death due to cardiac causes, pulmonary embolism, in-tracerebral hemorrhage, transient ischemic attack (TIA) or stroke, and stroke-related death.
Statistical analysis
For statistical analysis, RStudio (R version 4.4.1, RStudio version 2024.12.0) and SPSS software (IBM SPSS Statistics Version 20) were used, and the figures were created via SPSS, RStudio (ggplot2 version 3.5.1) and GraphPAD Prism (GraphPAD Prism version 10.0.0., Boston, MA, USA).
The distribution of the data was determined using the Kolmogorov–Smirnov test or Shapiro–Wilk test. The data are presented as the means and standard deviations in cases with a normal distribution and as medians and interquartile ranges in cases with nonnormal distributions. Categorical variables are presented as numbers and percentages.
We used the Mann–Whitney U test and Student’s t test to compare groups in cases of nonnormally distributed data and normally distributed data, respectively. Discrete data were compared using Pearson’s chi-square test, whereas data containing a small number of cases were analyzed using Fisher’s exact test.
In the analysis of the intraoperative data, discrete signals were aligned to a common reference frame and normalized using third-degree spline interpolation to ensure comparability across operations and individuals. Time-alignment and Gaussian windowing were applied for NIRS signal preprocessing. Statistical analysis included hypothesis testing and LOESS regression, with appropriate tests selected based on variable distribution characteristics using R algorithms. A sliding window minimum was computed to identify the lowest sustained intervals, with the difference value calculated as the difference between the minimum and sustained minimum values.
Linear regression analyses were used to determine the associations between pre- and intraoperative factors and postoperative MOCA changes compared to the baseline. Uni- and multivariable Cox regression were used to determine the association between perioperative factors, post-discharge cognitive disorders, and 2-year MACCE and mortality. All significant risk factors were entered into the multivariable regression model.
A p value < 0.05 was considered statistically significant.
3. Results
3.1. Baseline Characteristics
The data of 104 patients were analyzed. The median follow-up time was 37 months (IQR: 30–40.75 months). In total, 37 patients (35.7% of the population) had diabetes, 25 (67.56%) of whom received oral hypoglycemic medications, and 12 (32.43%) patients received insulin alone or in combination with oral hypoglycemic medications.
Diabetic patients did not demonstrate significant differences in terms of coexisting diseases, with the exceptions of congestive heart failure and intervention due to peripheral arterial disease in the anamnesis. The preoperative creatinine level revealed that the occurrence of renal insufficiency was greater in the diabetic group. Preoperative cholesterol levels were lower in the diabetic group than in the control group (p = 0.009), whereas fasting blood glucose levels were higher in diabetic patients (p = 0.001). The baseline characteristics of the two groups are summarized in Table 1.

Table 1.
Baseline characteristics of the two groups.
In diabetic patients, the pathology of the cerebral arteries was characterized by more severe stenosis of the contralateral carotid artery. During the operation, shunt insertion and eversion endarterectomy were more frequently used in the diabetic group. These data are presented in Table 2.

Table 2.
Operative parameters.
3.2. Cognitive Evaluation
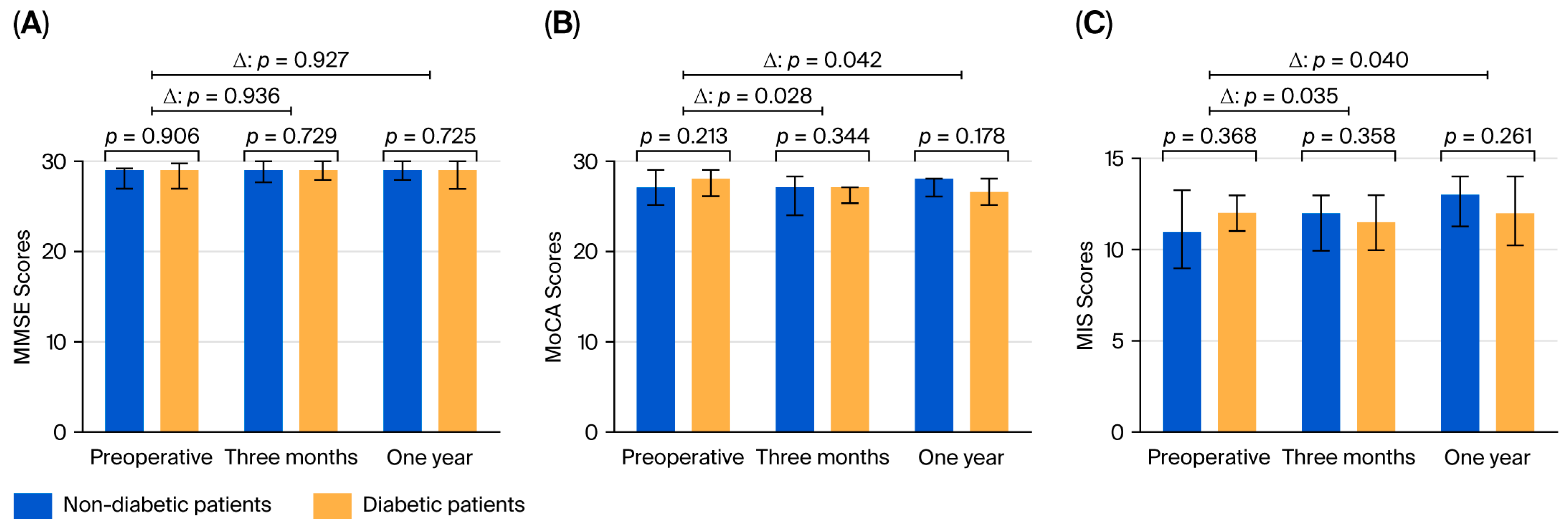
Three months after surgery, cognitive function was assessed in 103 patients. The preoperative MMSE and MoCA scores did not differ between the groups. Three months after surgery, the change in the MMSE score (p = 0.936) was not significant; however, the change in the MoCA score was significant between diabetic and nondiabetic patients (p = 0.028). The data are shown in Figure 2. Compared with the score at baseline, individual decline in the MIS score was significantly higher in the diabetic group than in the nondiabetic group at three months after surgery.
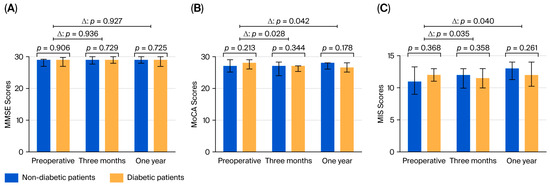
Figure 2.
Results of the cognitive tests: (A) MMSE, (B) MoCA, and (C) MIS, showing the statistical signifi-cance of the difference between the groups and of the change from the preoperative level. MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment; MIS: Memory Index Score.
One year after surgery, individual changes in the MMSE score were not significantly different between the groups (p = 0.921), whereas the decline in the MoCA score was greater in diabetic patients than in nondiabetic patients (p = 0.042). The MIS scores were significantly different in the 12th month between diabetic and nondiabetic patients (p = 0.042).
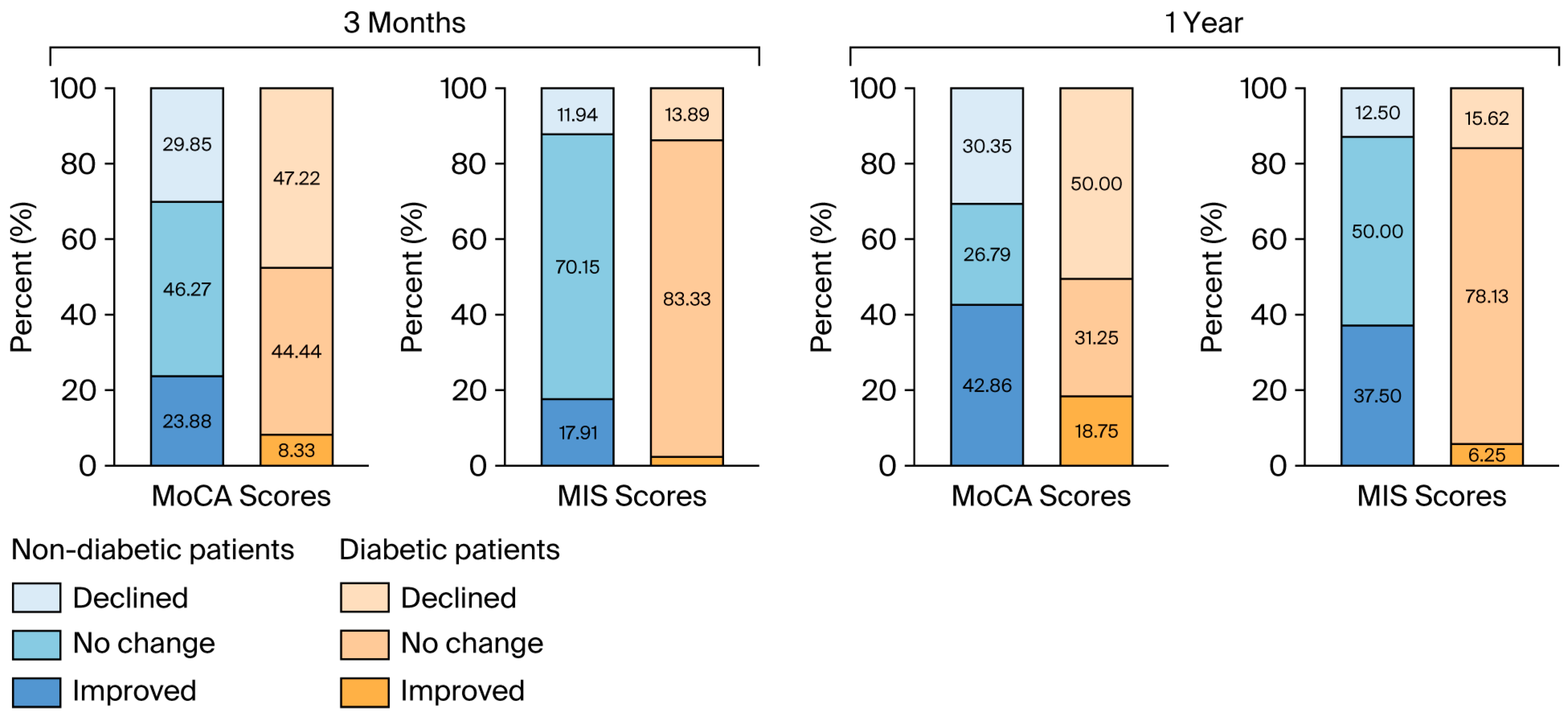
Cognitive improvement based on the MoCA test was less likely to occur in diabetic patients than in nondiabetic patients (p = 0.029) one year after surgery. Similarly, improvement based on the MIS scores was significantly greater in the nondiabetic group (p = 0.002) at the time of this assessment. The data are shown in Figure 3.
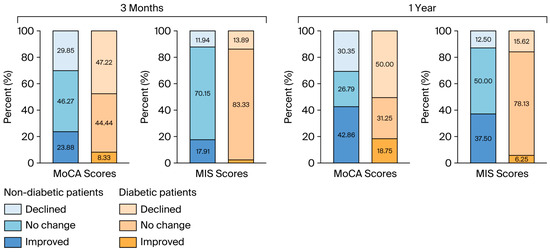
Figure 3.
The proportions of the directions of cognitive changes based on the MoCA and MIS scores at 3 months and a year after surgery. MoCA: Montreal Cognitive Assessment; MIS: Memory Index Score.
Univariable linear regression revealed that the change in the MoCA score at 12 months after surgery was positively associated with the median value of the lowest rSO2 values during the cross-clamp period and male sex. It was negatively associated with diabetes mellitus, the median value of the degree of cerebral tissue desaturation, and shunt use. The results are presented in Table S3 in the Supplementary Materials. Multiple linear regression revealed that diabetes mellitus, the degree of cerebral tissue desaturation, and male sex were independently associated with the 12-month MoCA change. The results of the analysis are shown in Table S4 in the Supplementary Materials.
3.3. Major Adverse Cerebral and Cardiovascular Events
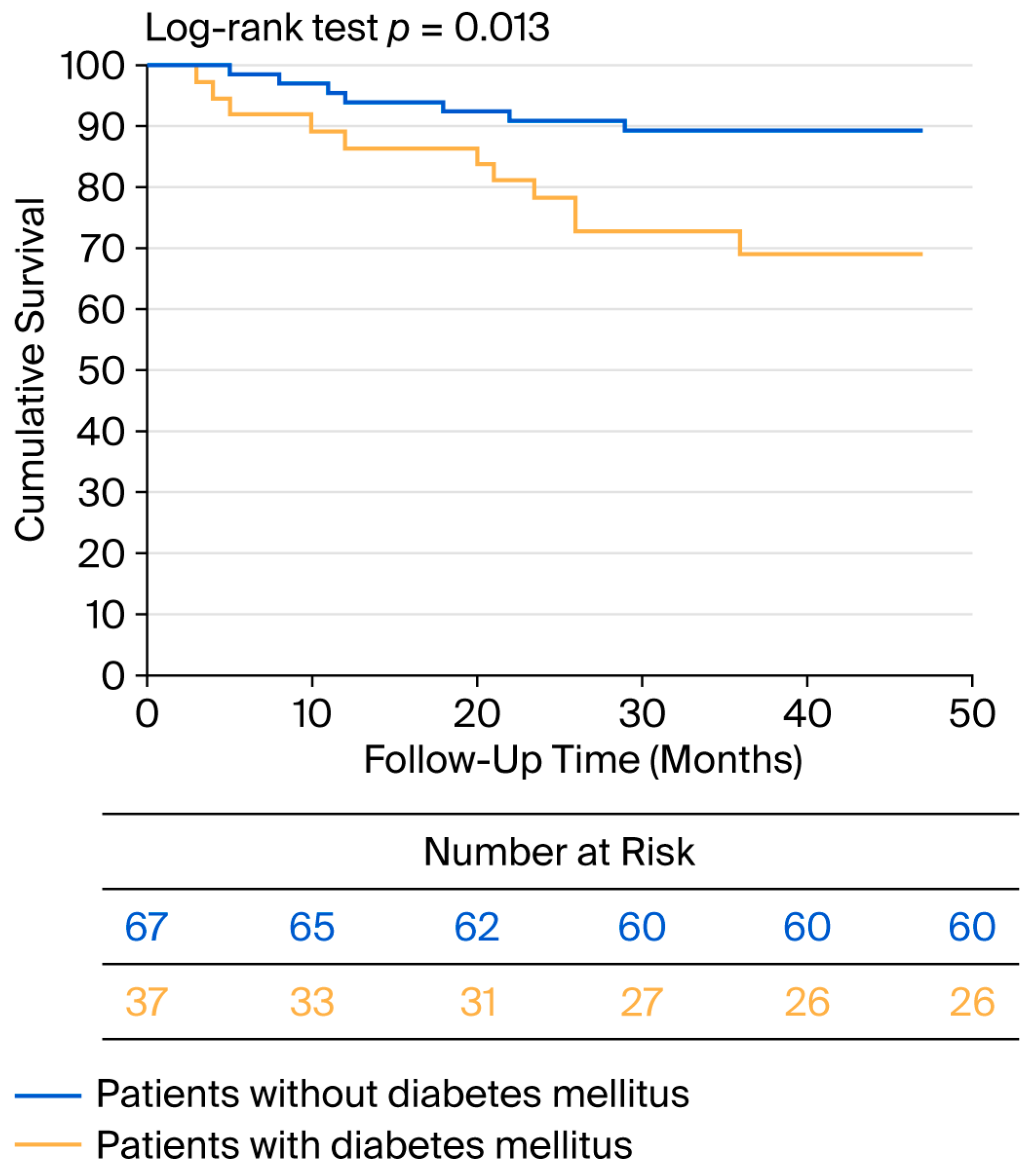
No patient died within the first 30 days after surgery. During the perioperative period, MACCEs occurred in four patients, three of whom had diabetes (p = 0.087). Additionally, two patients experienced TIA. During the 34-month average follow-up period, eleven patients died, four of whom experienced MACCE-related events. All-cause mortality was greater in the diabetic group compared with the nondiabetic group (p = 0.040). During the follow-up, eleven patients experienced MACCEs, seven of whom had diabetes (p = 0.040). A Kaplan–Meier analysis was performed for worse postoperative outcome (survival + major cardiovascular and cerebrovascular event), and the curve is shown in Figure 4.
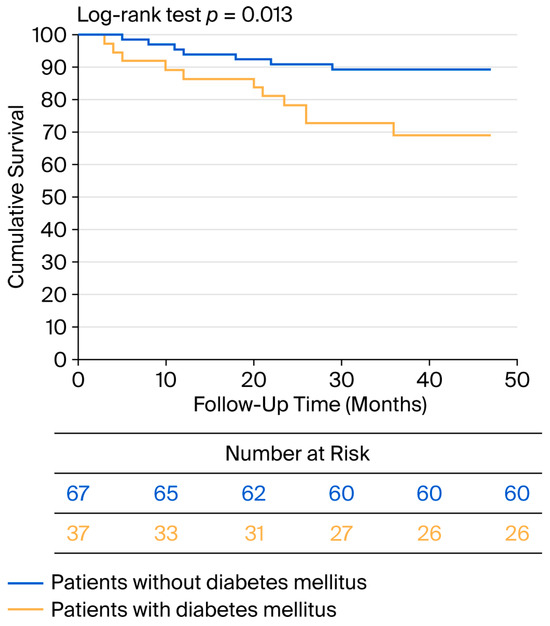
Figure 4.
Kaplan–Meier curve for negative postoperative outcome in diabetic and nondiabetic patients.
Using the risk score of Blecha et al., we identified 4.5 points as the threshold for survival, with a sensitivity of 72.7% and specificity of 74.2% (ROC analysis AUC: 0.848; p = 0.001).
In the multivariate Cox regression, postoperative cognitive decline, as measured with the MoCA (p = 0.018), and the risk score reported by Blecha et al. (p = 0.001) were independently associated with mortality and MACCEs. Stratification according to diabetes status did not affect these relationships. The results of the univariate Cox regression analysis are shown in Table S5 in the Supplementary Materials, and the results of the multivariate analysis are summarized in Table 3.

Table 3.
Multivariate Cox regression analysis for two-year mortality and major cardiovascular and cerebrovascular events.
3.4. Intraoperative Parameters
Seventy-six patients had complete intraoperative registration information. Systolic arterial blood pressure (SAP) values were significantly greater in the diabetic group than in the nondiabetic group across all three surgical periods: pre-clamping (p = 0.036), cross-clamping (p < 0.001), and post-clamping (p = 0.034). The mean arterial pressure (MAP) was also greater in diabetic patients during the cross-clamping period (p = 0.001), whereas the diastolic arterial pressure (DAP) was observed to be significantly different during the cross-clamping period (p = 0.037). The frequencies of vasopressor use at pre-clamping and post-clamping (p = 0.001 and p = 0.023, respectively) were significantly greater among diabetic patients, whereas the total applied doses were significantly greater in all three phases of the operation (pre-clamp.: p = 0.005; cross-clamp.: p = 0.003; post-clamp.: p = 0.033).
Significant differences were also observed in cerebral tissue oxygenation. Diabetic patients exhibited lower median ipsilateral rSO2 values in the pre-clamping period (67.4% vs. 74.6%, p = 0.011) and lower minimum values during the clamping (54.4% vs. 60.0%, p = 0.052) and pre-clamping (61.1% vs. 68.3%, p = 0.033) periods. Although the contralateral rSO2 values exhibited a similar trend, the differences were not statistically significant.
A categorical analysis revealed that during the pre-clamping period, a median rSO2 value of less than 65% was observed in 38.70% of diabetic patients and in only 15.55% of nondiabetic patients, presenting a significant difference (p = 0.022).
With respect to entropy, minimum state entropy (SE) was significantly lower in the post-clamping period among diabetic patients (29.1 vs. 37.6, p = 0.003), thereby suggesting a deeper anesthetic effect or altered brain activity. Additionally, the SE difference (mean–minimum) was significantly greater in diabetic patients than in nondiabetic patients in the post-clamping period (p = 0.009).
Heart rate (HR) was higher in diabetic patients during the post-clamping period than in nondiabetic patients (71.8 vs. 64.6, p = 0.035), which aligns with the overall observed increased hemodynamic lability.
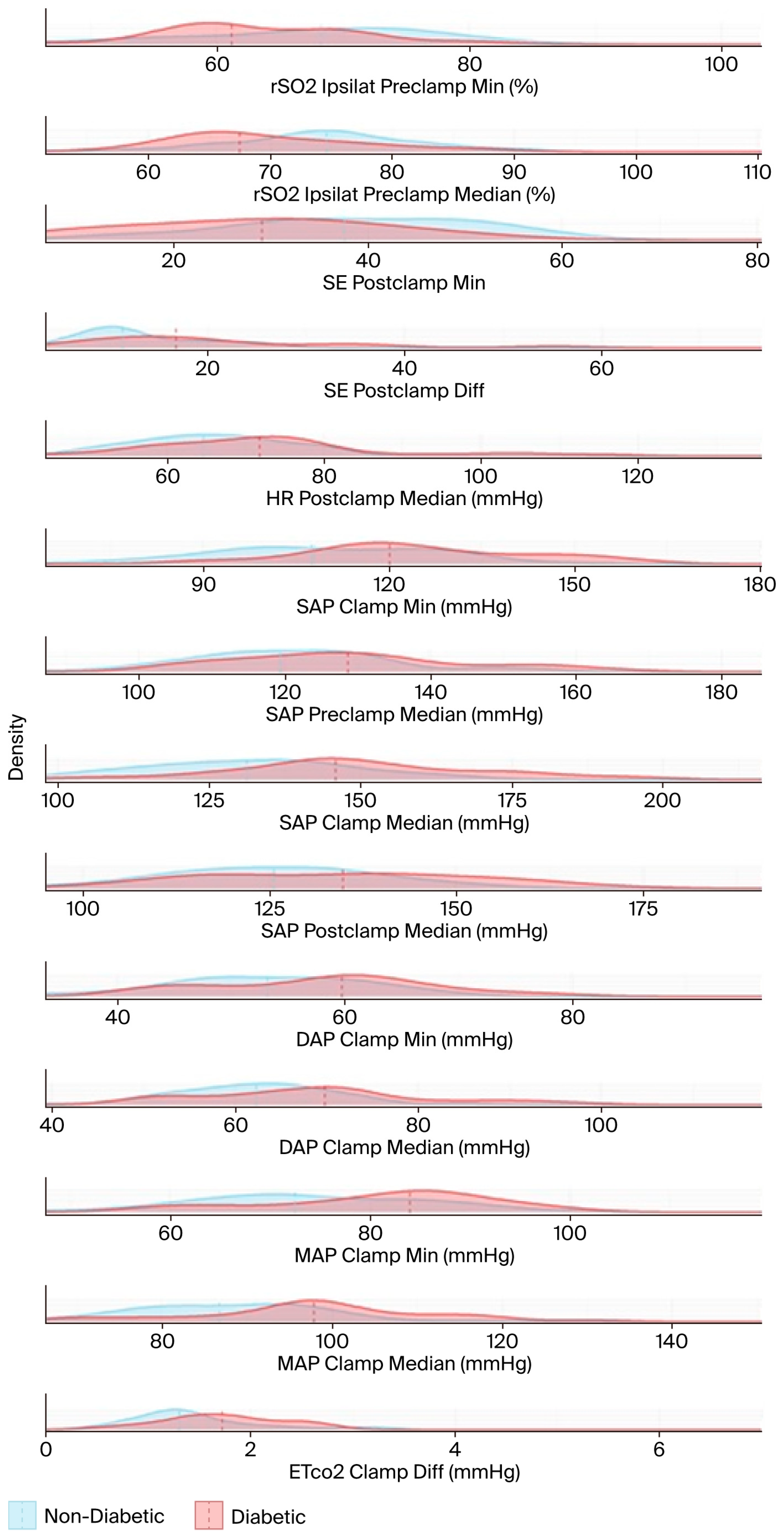
The results in Table A1 and Table A2 are based on hypothesis tests and are visualized using stacked density plots in Figure 5. Each plot shows the distribution of diabetic (in red) and non-diabetic (in blue) patient groups for the respective variable. The central tendency (in this case, the median) of each group is indicated by a dashed line. The X-axes are not aligned to enhance visual clarity. The median, the minimum (calculated using a sliding window of 10 timestamps), and the difference between the minimum and the median were computed for each variable.
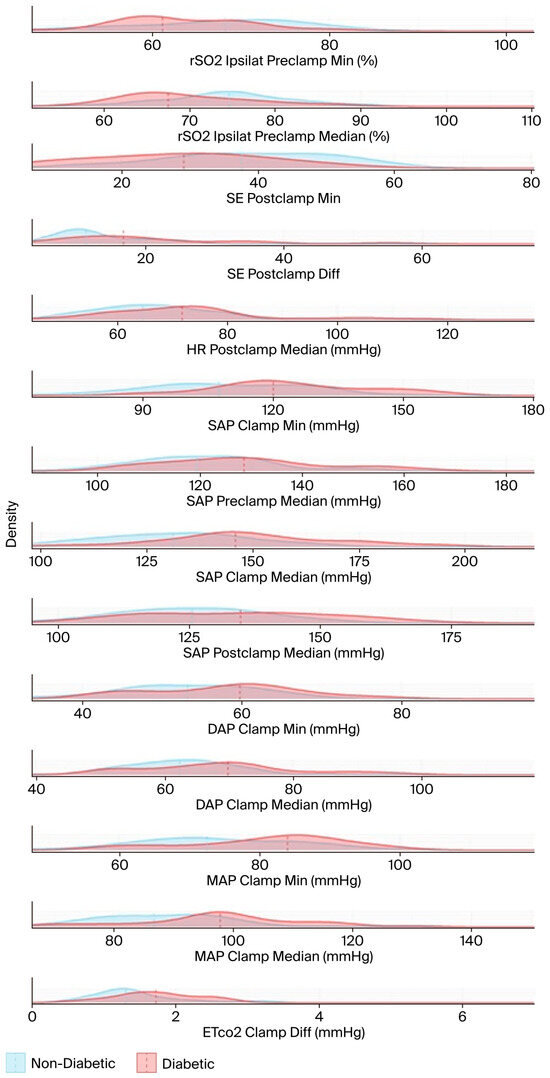
Figure 5.
The density functions of the test variables used in the hypothesis tests, and the medians (dashed lines). The name of each test variable and its unit of measurement are displayed to the right of the corresponding density plot. DAP: diastolic arterial pressure; EtCO2: end-tidal carbon dioxide; HR: heart rate; MAP: mean arterial pressure; rSO2: regional cerebral oxygen saturation; SAP: systolic arterial pressure; SE: state entropy.
4. Discussion
We observed that the performance of diabetic patients on postoperative cognitive assessments was worse than that of nondiabetic patients; specifically, diabetic patients exhibited a greater decline in preoperative scores on the MoCA test and a lower MIS score for both the 3- and 12-month assessments after discharge than nondiabetic patients. Poor outcome at the 24th months–both MACCE and mortality together– was independently associated with cognitive decline detected at one year after surgery and the risk score developed by Blecha et al.
Diabetic patients exhibited higher blood pressure values throughout the operation; however, they required more vasopressors. They exhibited lower rSO2 values in the preclamping period, and the lowest rSO2 values were also observed to be lower in diabetic patients than in nondiabetic patients.
Postoperative decline in cognitive function demonstrates many adverse consequences, both regarding perioperative circumstances, morbidity, and mortality, and in terms of enormous emotional and financial burdens on families [9,10,11,32].
Furthermore, impairment of memory involves both difficulties in everyday activities. New onset of a decreasing tendency in MIS results may increase the possibility of conversion from mild cognitive impairment to Alzheimer’s disease [33]. In our study population, diabetic patients achieved lower scores on both of the postoperative MoCA tests. In accordance with our results, a worse cognitive performance of diabetic patients has been demonstrated in several previous trials [32,34,35].
Preservation, or ideally, improvement of cognition, is one of the most desired outcomes after carotid surgery. Intact cognition is essential to an independent and healthy life with good quality. One year after the surgery, there was a significant difference in relation to cognitive improvement with diabetic patients falling behind nondiabetic patients. Beyond intraoperative factors, chronic hyperglycemia may worsen this outcome [36,37]. As glycated hemoglobin was observed to be elevated in a substantial proportion (70%) of our diabetic patients, this scenario may have exerted a negative influence on postoperative cognitive performance. This raises the question of whether preoperative optimization of metabolic state, tight perioperative gylcemic control, and accurate risk factor assessment and treatment could eliminate this difference, as these interventions have been proven beneficial in reducing postoperative complications [6,38].
Worse cognitive performance was only observed in relation to the MoCA and its subscore (the MIS), which is in accordance with the well-known feature of the MoCA test regarding the notion that it is more sensitive for detecting mild changes in cognitive function [22,39,40]. The MMSE cannot detect these changes and appears to be less effective for use in follow-ups.
Cerebral tissue saturation absolute values and relative changes have been linked to the postoperative modification of cognition [20,41,42]. A lower absolute rSO2 value has been demonstrated to be an independent predictor of postoperative delirium and complications in patients receiving valvular heart and thoracic surgery [43]. In another study, the threshold for elevated risk for postoperative morbidity was determined at rSO2 values of 65% or lower [44]. In our population, a median value of 65% or less in cerebral desaturation was observed in approximately 15% of the nondiabetic patients, whereas it was detected in approximately 39% of the diabetic patients. In a recent study, the normal value of average cerebral tissue saturation was observed to be 67.6%, with the lowest threshold of 56% being observed in healthy volunteers [45]. In our cohort, the rSO2 values measured during the preclamping period were demonstrated to be 67.4% in diabetic patients and 74.6% in nondiabetic patients. The lowest rSO2 value measured during the cross-clamping period was 54.4% in diabetic patients, whereas it was observed to be 60% in nondiabetic patients. Our diabetic patients exhibited lower saturation values compared to the determined “normal” values (despite higher blood pressure values being observed), which may indicate microvascular dysfunction [46]. This observation further strengthens the relevance of the use of neuromonitoring during carotid surgery, notedly in diabetic patients, as these pathological conditions may have exerted a negative impact on cognitive outcomes. Providing the most adequate level of anesthesia and keeping vital parameters in physiologic range may have highlighted the role in these patients of avoiding negative consequences.
We also aimed to explain the higher two-year mortality rate of diabetic patients. Elevated glucose levels are associated with oxidative stress and exert detrimental effects via numerous different cellular and biochemical pathways [47,48,49]. The greater degree of stenosis of the contralateral carotid artery observed in the diabetic group may offer less compensation for cerebral hemodynamics and may represent a sign of more severe atherosclerotic changes [50,51].
Diabetes mellitus was demonstrated to be significantly associated with the occurrence of long-term complications and mortality. In a multivariate model, only a carotid severity score by Blecha and the deterioration of cognitive function (as demonstrated by the MoCA test) were risk factors for this outcome. This result highlights and strengthens the importance of preserving cognitive function.
During the operation, diabetic patients required more vasopressors, and their blood pressure exhibited greater alterations and lower cerebral saturation values on both hemispheres, which may be influenced by cardiovascular autonomic neuropathy (CAN). Cardiovascular autonomic neuropathy is a microangiopathic complication of diabetes that affects the parasympathetic and sympathetic fibers that innervate the respiratory and cardiovascular systems [52,53]. The interaction of anesthesia and CAN may cause this hemodynamic instability. The interplay of them may explain the tendency toward lower cerebral saturation and a greater need for vasopressors.
Our results underscore the importance of neuromonitoring during carotid surgeries, especially in patients with diabetes mellitus, in favor of better postoperative outcomes.
As diabetic patients have a higher risk both for the occurrence of cognitive impairment and worse survival, accurate preoperative risk factor assessment and proper anesthesiologic care have a highlighted role in their management.
Limitations:
This was a single-center prospective study of patients who underwent surgery under general anesthesia. The small patient size limits our conclusions regarding MACCEs and mortality. Therefore, we used the score for adjustment in our cohort. Based on the preoperative and postoperative levels of HbA1c, the metabolic status of our diabetic patients was poorly controlled. As further limitation, we have to mention the lack of completeness analyzing those factors, which may have an impact on the alteration of cognition postoperatively.
5. Conclusions
In summary, diabetic patients are at increased risk for long-term cardiovascular and cerebrovascular complications and mortality, which are not demonstrated in the immediate perioperative period.
Cerebral tissue saturation values were significantly lower in the preclamping period among diabetic patients, and the lowest rSO2 values were also lower in this group. The observed hemodynamic instability and lower rSO2 values in diabetic patients may be linked to less cognitive improvement observed one year after the operation in the diabetic group.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines13092188/s1, Table S1: Summary of diabetic medications in the diabetic group, Table S2: The applied hypoglycemic agents and their frequencies, Table S3: Results of the linear regression analysis for all of the variables in relation to the change of the MoCA scores, Table S4: Results of the multiple linear regression analysis for all of the variables in relation to the change of the MoCA scores, Table S5: Results of Cox regression analysis for all of the variables in relation to mortality and long-term cardiovascular and cerebrovascular complications.
Author Contributions
Conceptualization, A.S. (Andrea Székely); methodology, A.S. (Andrea Székely), Á.D.S.; formal analysis, Á.D.S., P.M.S., T.K., G.V., F.K., C.M.; investigation, A.S. (Andrea Székely), Á.D.S.; resources, A.S. (Andrea Székely), P.S.; data curation, Á.D.S.; writing—original draft preparation, Á.D.S.; writing—review and editing, A.S. (Andrea Székely), P.S., Z.C., Z.M., A.S. (András Szabó); visualization, M.I.; supervision, A.S. (Andrea Székely); project administration, A.S. (Andrea Székely); funding acquisition, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research, Development and Innovation Office, grant number NKFI grant: 129277.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethical Committee of Semmelweis University (Semmelweis University Regional and Institutional Committee of Science and Research Ethics, 17/2019, 15 February 2019).
Informed Consent Statement
All of the patients provided written informed consent to participate in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [Andrea Székely], upon reasonable request.
Conflicts of Interest
The author Tibor Kézi is employed by Genotech Ltd. The remaining authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ASA score | American Society of Anesthesiologists physical status classification |
| BMI | body mass index |
| CCA | common carotid artery |
| CEA | carotid endarterectomy |
| C.I. | confidence interval |
| COPD | chronic obstructive pulmonary disease |
| CoW | Circle of Willis |
| DAP | diastolic arterial blood pressure |
| EEA | eversion endarterectomy |
| EtCO2 | end-tidal carbon dioxide |
| HR | heart rate |
| ICA | internal carotid artery |
| IQR | interquartile range |
| MAP | mean arterial pressure |
| MIS | Memory Index Score |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| NASCET | North American Symptomatic Carotid Endarterectomy Trial |
| NIRS | near-infrared spectroscopy |
| OR | odds ratio |
| PAD | peripheral arterial disease |
| PNCD | perioperative neurocognitive disorder |
| rSO2 | regional cerebral oxygen saturation |
| rSO2preclamp | median of regional cerebral oxygen saturation during the preclamping period |
| rSO2lowestclamp | lowest rSO2 values during the clamping period |
| SAP | systolic arterial blood pressure |
| SD | standard deviation |
| TEA | thromboendarterectomy |
| Vascular-POSSUM | Vascular-Physiological and Operative Severity Score for the enUmeration of Mortality and Morbidity |
Appendix A

Table A1.
Comparison of intraoperative hemodynamic parameters between the groups.
Table A1.
Comparison of intraoperative hemodynamic parameters between the groups.
| Characteristics | Pre-Clamp | Cross-Clamp | Post-Clamp | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 67.6 (7.8) | 64.6 (9.9) | 0.118 | 70.4 (18.2) | 62.4 (17.6) | 0.125 | 71.8 (14.2) | 64.6 (12.5) | 0.035 |
| ETCO2 difference | 2.4 (1.7) | 2.9 (2.1) | 0.425 | 1.7 (0.7) | 1.3 (0.7) | 0.018 | 4.5 (3.9) | 4.1 (2.4) | 0.315 |
| DAP mean | 59.7 (12.6) | 58.1 (10.7) | 0.173 | 69.7 (11.9) | 62.2 (10.6) | 0.021 | 60.5 (15.6) | 60.1 (10.4) | 0.258 |
| DAP minimum | 43.5 (10.1) | 41.4 (11.0) | 0.104 | 59.8 (12.7) | 53.2 (12.4) | 0.037 | 46.7 (11.1) | 45.9 (11.0) | 0.208 |
| SAP mean | 128.7 (19.2) | 119.5 (17.0) | 0.036 | 145.8 (20.8) | 131.3 (22.8) | 0.000 | 134.8 (28.5) | 125.6 (19.4) | 0.034 |
| SAP minimum | 91.8 (20.6) | 86.2 (16.9) | 0.123 | 120.0 (22.1) | 107.6 (28.5) | 0.002 | 93.8 (18.4) | 92.0 (17.2) | 0.272 |
| MAP mean | 85.9 (16.7) | 80.5 (10.4) | 0.099 | 97.9 (12.5) | 86.7 (15.4) | 0.001 | 85.9 (15.9) | 82.5 (11.7) | 0.089 |
| MAP minimum | 61.4 (11.9) | 56.0 (11.9) | 0.125 | 84.0 (13.5) | 72.4 (15.6) | 0.013 | 61.1 (13.8) | 61.7 (13.2) | 0.286 |
| vasopressor use (%) | 29 (78.37) | 26 (38.80) | 0.001 | 29 (78.37) | 36 (53.73) | 0.099 | 25 (67.56) | 25 (37.31) | 0.023 |
| vasopressor (ug) | 13.32 (5.24–25.45) | 5 (0–14.16) | 0.005 | 141.56 (42.44–222.93) | 38.08 (5–89.37) | 0.003 | 14.16 (2.18–29.16) | 8.33 (0–15.83) | 0.033 |
EtCO2: end-tidal carbon dioxide, DAP: diastolic blood pressure, HR: heart rate, MAP: mean arterial pressure, SAP: systolic arterial pressure.

Table A2.
Comparison of intraoperative neuromonitoring parameters between the groups.
Table A2.
Comparison of intraoperative neuromonitoring parameters between the groups.
| Characteristics | Pre-Clamp | Cross-Clamp | Post-Clamp | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Diabetic Group | Nondiabetic Group | p Value | Diabetic Group | Nondiabetic Group | p Value | Diabetic Group | Nondiabetic Group | p Value | |
| rSO2 ipsilateral mean | 67.4 (10.4) | 74.6 (7.9) | 0.011 | 59.6 (11.9) | 64.4 (12.8) | 0.133 | 67.4 (10.8) | 72.4 (14.9) | 0.169 |
| rSO2 contralateral mean | 71.1 (8.3) | 76.3 (13.8) | 0.076 | 70.1 (6.9) | 75.0 (14.9) | 0.186 | 70.2 (8.1) | 74.6 (12.5) | 0.205 |
| rSO2 ipsilateral minimum | 61.1 (9.9) | 68.3 (13.1) | 0.033 | 54.4 (14.5) | 60.0 (15.7) | 0.052 | 60.0 (10.1) | 64.5 (15.3) | 0.241 |
| SE difference | 10.4 (8.7) | 12.4 (7.3) | 0.648 | 6.0 (7.4) | 5.5 (5.1) | 0.442 | 16.7 (13.6) | 11.4 (8.3) | 0.009 |
| SE minimum | 37.5 (9.1) | 34.9 (12.8) | 0.901 | 31.8 (18.4) | 40.9 (20.1) | 0.292 | 29.1 (17.5) | 37.6 (17.9) | 0.003 |
rSO2: regional cerebral tissue saturation, SE: state entropy, SE: difference between the mean and minimum values.
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th Edition. 2021. Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 6 December 2021).
- Naylor, R.; Rantner, B.; Ancetti, S.; De Borst, G.J.; De Carlo, M.; Halliday, A.; Kakkos, S.K.; Markus, H.S.; McCabe, D.J.H.; Sillesen, H.; et al. European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 7–111. [Google Scholar] [CrossRef]
- Krawisz, A.K.; Carroll, B.J.; Secemsky, E.A. Risk Stratification and Management of Extracranial Carotid Artery Disease. Cardiol. Clin. 2021, 39, 539–549. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Rastenyte, D.; Jousilahti, P.; Sarti, C.; Vartiainen, E. Diabetes mellitus as a risk factor for death from stroke Prospective study of the middle-aged Finnish population. Stroke 1996, 27, 210–215. [Google Scholar] [CrossRef]
- Kurukulasuriya, L.R.; Govindarajan, G.; Sowers, J. Stroke prevention in diabetes and obesity. Expert Rev. Cardiovasc. Ther. 2006, 4, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Mizuhashi, S.; Kataoka, H.; Sano, N.; Ideguchi, M.; Higashi, M.; Miyamoto, Y.; Iihara, K. Impact of diabetes mellitus on characteristics of carotid plaques and outcomes after carotid endarterectomy. Acta Neurochir. 2014, 156, 927–933. [Google Scholar] [CrossRef]
- Dimic, A.; Markovic, M.; Vasic, D.; Dragas, M.; Zlatanovic, P.; Mitrovic, A.; Davidovic, L. Impact of diabetes mellitus on early outcome of carotid endarterectomy. Vasa 2019, 48, 148–156. [Google Scholar] [CrossRef]
- Parlani, G.; De Rango, P.; Cieri, E.; Verzini, F.; Giordano, G.; Simonte, G.; Isernia, G.; Cao, P. Diabetes is not a predictor of outcome for carotid revascularization with stenting as it may be for carotid endarterectomy. J. Vasc. Surg. 2012, 55, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Relander, K.; Hietanen, M.; Nuotio, K.; Ijäs, P.; Tikkala, I.; Saimanen, E.; Lindsberg, P.J.; Soinne, L. Cognitive Dysfunction and Mortality After Carotid Endarterectomy. Front. Neurol. 2021, 11, 593719. [Google Scholar] [CrossRef]
- Suraarunsumrit, P.; Pathonsmith, C.; Srinonprasert, V.; Sangarunakul, N.; Jiraphorncharas, C.; Siriussawakul, A. Postoperative cognitive dysfunction in older surgical patients associated with increased healthcare utilization: A prospective study from an upper-middle-income country. BMC Geriatr. 2022, 22, 213. [Google Scholar] [CrossRef] [PubMed]
- Ton, T.G.N.; DeLeire, T.; May, S.G.; Hou, N.; Tebeka, M.G.; Chen, E.; Chodosh, J. The financial burden and health care utilization patterns associated with amnestic mild cognitive impairment. Alzheimers Demen. 2017, 13, 217–224. [Google Scholar] [CrossRef]
- Piegza, M.; Wieckiewicz, G.; Wierzba, D.; Piegza, J. Cognitive Functions in Patients after Carotid Artery Revascularization—A Narrative Review. Brain Sci. 2021, 11, 1307. [Google Scholar] [CrossRef]
- Aceto, P.; Lai, C.; De Crescenzo, F.; Crea, M.A.; Di Franco, V.; Pellicano, G.R.; Perilli, V.; Lai, S.; Papanice, D.; Sollazzi, L. Cognitive decline after carotid endarterectomy: Systematic review and meta-analysis. Eur. J. Anaesthesiol. 2019, 37, 1066–1074. [Google Scholar] [CrossRef]
- Liu, H.; Chen, J.; Ling, J.; Wu, Y.; Yang, P.; Liu, X.; Liu, J.; Zhang, D.; Yin, X.; Yu, P.; et al. The association between diabetes mellitus and postoperative cognitive dysfunction: A systematic review and meta-analysis. Int. J. Surg. 2025, 111, 633–2650. [Google Scholar] [CrossRef]
- Long, C.A.; Fang, Z.B.; Hu, F.Y.; Arya, S.; Brewster, L.P.; Duggan, E.; Duwayri, Y. Poor glycemic control is a strong predictor of postoperative morbidity and mortality in patients undergoing vascular surgery. J. Vasc. Surg. 2019, 69, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Ogasawara, K.; Kuroda, H.; Suzuki, T.; Chida, K.; Kobayashi, M.; Yoshida, K.; Kubo, Y.; Ogawa, A. Combination of preoperative cerebral blood flow and 123I-iomazenil SPECT imaging predicts postoperative cognitive improvement in patients undergoing uncomplicated endarterectomy for unilateral carotid stenosis. Clin. Nucl. Med. 2012, 37, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Lazkani, A.; Lebuffe, G. Post-operative consequences of hemodynamic optimization. J. Visc. Surg. 2016, 153, S5–S9. [Google Scholar] [CrossRef] [PubMed]
- Vuurberg, N.E.; Post, I.C.J.H.; Keller, B.P.J.A.; Schaafsma, A.; Vos, C.G. A Systematic Review and Meta-Analysis on Perioperative Cerebral and Hemodynamic Monitoring Methods during Carotid Endarterectomy. Ann. Vasc. Surg. 2023, 88, 384–409. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wang, T.; Zhao, L.; Feng, H.; Wang, Q.; Fan, L.; Feng, X.; Xiao, W.; Feng, K. The Efficacy of Near-Infrared Spectroscopy Monitoring in Carotid Endarterectomy: A Prospective, Single-Center, Observational Study. Cell Transpl. 2019, 28, 170–175. [Google Scholar] [CrossRef]
- Ding, X.; Zha, T.; Abudurousuli, G.; Zhao, C.; Chen, Z.; Zhang, Y.; Gui, B. Effects of regional cerebral oxygen saturation monitoring on postoperative cognitive dysfunction in older patients: A systematic review and meta-analysis. BMC Geriatr. 2023, 23, 123. [Google Scholar] [CrossRef]
- Cotae, A.-M.; Ţigliş, M.; Cobilinschi, C.; Băetu, A.E.; Iacob, D.M.; Grinţescu, I.M. The Impact of Monitoring Depth of Anesthesia and Nociception on Postoperative Cognitive Function in Adult Multiple Trauma Patients. Medicina 2021, 57, 408. [Google Scholar] [CrossRef]
- Pinto, T.C.C.; Machado, L.; Bulgacov, T.M.; Rodrigues-Júnior, A.L.; Costa, M.L.G.; Ximenes, R.C.C.; Sougey, E.B. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int. Psychogeriatr. 2019, 31, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Fasnacht, J.S.; Wueest, A.S.; Berres, M.; Thomann, A.E.; Krumm, S.; Gutbrod, K.; Steiner, L.A.; Goettel, N.; Monsch, A.U. Conversion between the Montreal Cognitive Assessment and the Mini-Mental Status Examination. J. Am. Geriatr. Soc. 2023, 71, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.S.; Edland, D.; Peavy, G.M. The MoCA Memory Index Score: An Efficient Alternative to Paragraph Recall for the Detection of Amnestic Mild Cognitive Impairment. Alzheimer Dis. Assoc. Disord. 2018, 32, 120–124. [Google Scholar] [CrossRef]
- World Health Organization. Classification of Diabetes Mellitus. 2019. Available online: https://www.who.int/publications/i/item/classification-of-diabetes-mellitus (accessed on 21 April 2019).
- Centre for Perioperative Care; Academy of Medical Royal Colleges. Guideline for Perioperative Care for People with Diabetes Mellitus Undergoing Elective and Emergency Surgery. 2021. Available online: https://cpoc.org.uk/sites/cpoc/files/documents/2024-03/CPOC-DiabetesGuideline2023.pdf (accessed on 8 March 2021).
- Blecha, M.; Weise, L.; Liu, A.; Yuan, K.; Terry, T.; Paraskevas, K.I. Risk score for two-year mortality following carotid endarterectomy performed for symptomatic stenosis. J. Vasc. Surg. 2025, 81, 905–918. [Google Scholar] [CrossRef]
- Singh, S.; Bansal, S.; Kumar, G.; Gupta, I.; Thakur, J.R. Entropy as an Indicator to Measure Depth of Anaesthesia for Laryngeal Mask Airway (LMA) Insertion during Sevoflurane and Propofol Anaesthesia. J. Clin. Diagn. Res. 2017, 11, UC01–UC03. [Google Scholar] [CrossRef]
- Bikbov, M.M.; Kazakbaeva, G.M.; Iakupova, E.M.; Panda-Jonas, S.; Fakhretdinova, A.A.; Tuliakova, A.M.; Rusakova, I.A.; Jonas, J.B. Cognitive impairment in the population-based ural very old study. Front. Ageing Neurosci. 2022, 14, 912755. [Google Scholar] [CrossRef]
- Kessels, R.P.C.; De Vent, N.R.; Bruijnen, C.J.W.H.; Jansen, M.G.; de Jonghe, J.F.M.; Dijkstra, B.A.G.; Oosterman, J.M. Regression-Based Normative Data for the Montreal Cognitive Assessment (MoCA) and Its Memory Index Score (MoCA-MIS) for Individuals Aged 18–91. J. Clin. Med. 2022, 11, 4059. [Google Scholar] [CrossRef]
- Mahanna, E.P.; Blumenthal, J.A.; White, W.D.; Croughwell, N.D.; Clancy, C.P.; Smith, L.R.; Newman, M.F. Defining neuropsychological dysfunction after coronary artery bypass grafting. Ann. Thorac. Surg. 1996, 61, 1342–1347. [Google Scholar] [CrossRef]
- Varpaei, H.A.; Farhadi, K.; Mohammadi, M.; Khafaee pour khamseh, A.; Mokhtari, T. Postoperative cognitive dysfunction: A concept analysis. Aging Clin. Exp. Res. 2024, 36, 133. [Google Scholar] [CrossRef] [PubMed]
- Julayanont, P.; Brousseau, M.; Chertkow, H.; Phillips, N.; Nasreddine, Z.S. Montreal Cognitive Assessment Memory Index Score (MoCA-MIS) as a predictor of conversion from mild cognitive impairment to Alzheimer’s disease. J. Am. Geriatr. Soc. 2014, 62, 679–684. [Google Scholar] [CrossRef]
- Feinkohl, I.; Winterer, G.; Pischon, T. Diabetes is associated with risk of postoperative cognitive dysfunction: A meta-analysis. Diabetes Metab. Res. Rev. 2017, 33, e2884. [Google Scholar] [CrossRef]
- Seven, S.; Ceylan, Í.; Kaymak, D.; Kara, A.G.; Erden, V. The effect of type 2 diabetes mellitus on early postoperative cognitive functions. J. Surg. Med. 2022, 6, 552–556. [Google Scholar] [CrossRef]
- Gupta, M.; Pandey, S.; Rumman, M.; Singh, B.; Mahdi, A.A. Molecular mechanisms underlying hyperglycemia associated cognitive decline. IBRO Neurosci. Rep. 2023, 14, 57–63. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Wen, Z.; Yang, Y.; Bu, T.; Bu, X.; Ni, Q. Cognitive dysfunction in diabetes: Abnormal glucose metabolic regulation in the brain. Front. Endocrinol. 2023, 14, 1192602. [Google Scholar] [CrossRef]
- Kwon, S.; Thompson, R.; Dellinger, P.; Yanez, D.; Farrohki, E.; Flum, D. Importance of perioperative glycemic control in general surgery: A report from the Surgical Care and Outcomes Assessment Program. Ann. Surg. 2013, 257, 8–14. [Google Scholar] [CrossRef]
- Pinto, T.C.C.; Machado, L.; Costa, M.L.G.; Santos, M.S.P.; Bulgacov, T.M.; Rolim, A.P.P.; Silva, G.A.; Rodrigues-Júnior, A.L.; Sougey, E.B.; Ximenes, R.C.C. Accuracy and Psychometric Properties of the Brazilian Version of the Montreal Cognitive Assessment as a Brief Screening Tool for Mild Cognitive Impairment and Alzheimer’s Disease in the Initial Stages in the Elderly. Dement. Geriatr. Cogn. Disord. 2019, 47, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, N.; Sokolowski, R.; Mazur, E.; Podhorecka, M.; Polak-Szabela, A.; Kędziora-Kornatowska, K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr. Pol. 2016, 50, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.W.; Chen, X.L.; Yao, L. The value of intraoperative monitoring of cerebral oxygen saturation on postoperative cognitive function in elderly patients undergoing cardiac surgery. Zhonghua Yi Xue Za Zhi 2021, 101, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Uysal, S.; Lin, H.M.; Trinh, M.; Park, C.H.; Reich, D.L. Optimizing cerebral oxygenation in cardiac surgery: A randomized controlled trial examining neurocognitive and perioperative outcomes. J. Thorac. Cardiovasc. Surg. 2020, 159, 943–953. [Google Scholar] [CrossRef]
- Soh, S.; Shim, J.K.; Song, J.W.; Choi, N.; Kwak, Y.L. Preoperative transcranial Doppler and cerebral oximetry as predictors of delirium following valvular heart surgery: A case-control study. J. Clin. Monit. Comput. 2020, 34, 715–723. [Google Scholar] [CrossRef]
- Kazan, R.; Bracco, D.; Hemmerling, T.M. Reduced cerebral oxygen saturation measured by absolute cerebral oximetry during thoracic surgery correlates with postoperative complications. Br. J. Anaesth. 2009, 103, 811–816. [Google Scholar] [CrossRef]
- Eyeington, C.T.; Ancona, P.; Osawa, E.A.; Cutuli, S.L.; Eastwood, G.M.; Bellomo, R. Modern technology–derived normative values for cerebral tissue oxygen saturation in adults. Anaesth. Intensive Care 2019, 47, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Van Sloten, T.T.; Sedaghat, S.; Carnethon, M.R.; Launer, L.J.; Stehouwer, C.D. Cerebral microvascular complications of type 2 diabetes: Stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020, 8, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Lefèbvre, P.J.; Scheen, A.J. The postprandial state and risk of cardiovascular disease. Diabet. Med. 1998, 15, S63–S68. [Google Scholar] [CrossRef]
- Duncan, A.E. Hyperglycemia and Perioperative Glucose Management. Curr. Pharm. Des. 2012, 18, 6195–6203. [Google Scholar] [CrossRef]
- Bagdade, J.D.; Root, R.K.; Bulger, R.J. Impaired Leukocyte Function in Patients with Poorly Controlled Diabetes. Diabetes 1974, 23, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Stoneham, M.D.; Thompson, J.P. Arterial pressure management and carotid endarterectomy. Br. J. Anaesth. 2009, 102, 442–452. [Google Scholar] [CrossRef]
- Halm, E.A.; Hannan, E.L.; Rojas, M.; Tuhrim, S.; Riles, T.S.; Rockman, C.B.; Chassin, M.R. Clinical and operative predictors of outcomes of carotid endarterectomy. J. Vasc. Surg. 2005, 42, 420–428. [Google Scholar] [CrossRef]
- Serhiyenko, V.A.; Serhiyenko, A.A. Cardiac autonomic neuropathy: Risk factors, diagnosis and treatment. World J. Diabetes 2018, 15, 1–24. [Google Scholar] [CrossRef]
- Agashe, S.; Petak, S. Cardiac Autonomic Neuropathy in Diabetes Mellitus. Methodist Debakey Cardiovasc. J. 2018, 14, 251–256. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).