Abstract
Background: Ibrutinib has been associated with an increased risk of cardiovascular adverse events (CVAEs), including atrial fibrillation (AF), hypertension (HTN), heart failure (HF), and ventricular arrhythmias (VAs). However, baseline predictors of CVAEs remain poorly characterized. In this study, we sought to identify baseline patient characteristics associated with the occurrence of ibrutinib-related CVAEs, with particular emphasis on parameters linked to the renin–angiotensin system. Methods: We conducted a prospective, single-center cohort study of consecutive patients treated with ibrutinib for B-cell malignancy, with systematic assessment of a predefined panel of potential predictors of CVAEs at baseline (NCT03678337). These predictors included demographic and clinical variables, 16 circulating biomarkers related to inflammation, fibrosis, and neurohormonal activation, as well as nine echocardiographic parameters. The primary objective was to evaluate the association between baseline patient characteristics and the occurrence of CVAEs from ibrutinib initiation through the end of follow-up. The CVAE endpoint was defined as a composite of atrial fibrillation, new or worsening hypertension, new or worsening heart failure, and ventricular arrhythmias. Statistical analyses were performed using the Wilcoxon–Mann–Whitney test or Fisher’s exact test, with a p-value < 0.05 considered statistically significant. Results: Among the 25 patients included, 7 experienced a total of 9 CVAEs over a median follow-up of 672 days. Elevated baseline plasma renin levels (>1336.10 pg/mL) were significantly associated with CVAEs occurrence (57% vs. 11%, p = 0.032). Higher baseline plasma aldosterone levels (>488.95 pg/mL) were also observed in patients who developed CVAEs, although this association did not reach statistical significance (p = 0.058). Conclusions: Baseline plasma renin level was univariably associated with CVAEs occurrence, while plasma aldosterone levels were higher among patients with CVAEs but did not reach statistical significance. These findings provide preliminary insights into the mechanisms underlying ibrutinib-related cardiovascular toxicity, suggesting a potential role for the renin–angiotensin–aldosterone system. Confirmation of this hypothesis, however, will require larger, dedicated studies.
1. Introduction
Ibrutinib, a Bruton’s tyrosine kinase inhibitor (BTKi), is approved for the treatment of B-cell malignancies [1]. In chronic lymphocytic leukemia (CLL), ibrutinib has demonstrated a significant survival benefit, reducing the risk of progression or death by 84% compared with chlorambucil after a median follow-up of 18.4 months [2].
Nevertheless, it is now well-known that ibrutinib is associated with an increased risk of several cardiovascular adverse events (CVAEs), including atrial fibrillation (AF), new or worsening arterial hypertension (HTN), new or worsening heart failure (HF), and ventricular arrhythmia (VA) [3]. For instance, a safety meta-analysis found an increased risk of HTN with ibrutinib with a relative risk (RR) of 2.82 (95% CI: 1.52–5.23) and an increased risk of AF with an RR of 4.69 (95% CI: 2.17–7.64) [4]. These CVAEs are associated with significative morbimortality. In a retrospective cohort study of 298 CLL patients where 16% developed ibrutinib-related AF, patients with AF had lower progression-free survival (hazard ratio (HR) = 2.0, 95% CI: 1.1–3.8, p = 0.02) and shorter overall survival (HR = 3.2, 95% CI: 1.6–6.3, p = 0.001) [5].
Although ibrutinib-related CVAEs are now encountered daily in clinical practice, predictive factors are not known; thus, no predictive score exists to predict CVAEs in the ibrutinib-exposed population. ESC cardio-oncology guidelines recommend the evaluation of every patient with a cardio-oncologic examination before BTKi initiation to stratify the baseline risk of developing cardiovascular toxicity [3]; however, to date, the only characteristics that seem associated with ibrutinib-related CVAEs are age, history of HTN, and left atrium dilatation [6].
In the context of ibrutinib-related CVAEs, early identification of patients at risk is essential. The aim of this study was therefore to assess baseline clinical, echocardiographic, and biological characteristics associated with the development of CVAEs in a prospective, single-center cohort of patients treated with ibrutinib for B-cell malignancies.
2. Materials and Methods
2.1. Study Protocol and Population
This study was nested within the PICARO cohort (NCT03678337), a prospective observational study. We included adult patients with active CLL referred for a baseline cardio-oncology evaluation at Caen–Normandy University Hospital (France) either prior to, or within one month of, ibrutinib initiation between 6 December 2018 and 1 April 2021 [7]. Non-adult and adult protected patients, pregnant or nursing women, and patients under guardianship, curatorship, safeguard of justice, or legal protection were not included. The study protocol was compliant with the STROBE Statement [8].
All patients underwent a baseline cardio-oncology evaluation before or within 1 month after the introduction of ibrutinib according to the ESC cardio-oncology guidelines including a careful clinical history and physical examination, a 12-lead ECG, a blood examination including cardiac biomarkers, and a standard transthoracic echocardiography [3]. Blood peripheral collection tubes were collected at the end of the clinical evaluation, with the patients resting quietly in a semi-recumbent position. Blood samples were collected in EDTA2K tubes, rapidly centrifuged at 2300× g for 11 min, and then plasma was aliquoted and stored at −80 °C.
2.2. Measurement of Levels of Plasma Protein Biomarkers by ELISA
Commercially available enzyme-linked immunosorbent assays (ELISAs) were utilized to quantify the levels of C reactive protein (CRP) (R&D Systems, Abingdon, UK), Galectin-3 (R&D Systems), Myeloperoxydase (R&D Systems), renin (R&D Systems), aldosterone (ThermoFisher Scientific, Illkirch-Graffenstaden, France), Tumor Necrosis Factor alpha (TNF-α) (R&D Systems), interleukin (IL-6) (R&D Systems), angiotensin converting enzyme 2 (ACE-2) (ThermoFisher Scientific), and troponin (Abcam, Cambridge, UK) according to the manufacturer’s instruction. For routine analyses (serum electrolytes, creatinine…), commercially available kits were used.
2.3. Measurement of Expression of Serum microRNAs (miRNAs) by RT-qPCR
We selected six microRNAs (miR-9, miR-99, miR-199, miR-328, miR-22, and miR-150-5p) that have previously been associated with CVAEs or with cardiovascular disease in populations other than patients treated with ibrutinib [9,10,11,12,13,14]. MiRNAs were isolated from samples according to the manufacturers’ recommendations using the NucleoSpin miRNA Plasma kit (Macherey-Nagel, Hoerdt, France). As previously described, known quantities (200 attomoles in 5 µL) of three exogenous miRNAs, cel-miR-39-3p, cel-miR-54-3p, and cel-miR-238-3p, were added to the samples after the denaturing step [15]. The expression of each miRNA was determined by RT-qPCR. miRNAs were first retrotranscribed using an miRNA Reverse Transcription Kit (ThermoFisher Scientific). The ID references for stem-loop primers and Taqman hydrolysis probes (ThermoFisher Scientific) were as follows: cel-miR-39-3p (000200), cel-miR-54-3p (001361), cel miR 238-3p (000248), cel-miR-9-5p (000583), cel-miR-22-5p (002301), cel-miR-99b-5p (00436), cel-miR-150 5p (000473), cel-miR-199a 5p (00498), and cel-miR 328 3p (00543). Fluorescence and threshold baselines were measured using a Roche LightCycler® 480 system with the LightCycler® 480 Software version 1.5.1.62 SP3 (Roche Diagnostics, Meylan, France). Absolute standard curves were generated according to the MIQE guidelines by diluting synthetic cel-miRs and previous cited miRNAs at 2.105, 2.104, 2.103, 2.102, 2.101, and 2 zmol/mL prior to quantitative PCR with reverse transcription (RT-qPCR) steps as previously described [16]. They were used to convert quantitative cycles (Cq) into a log of quantities and then into miRNA concentrations expressed in zeptomoles per microliter of RNA extracts. Serum isolation yields of cel-miR-39, cel-miR-54-3p, and cel-miR-238-3p were calculated for each sample by dividing their recovered quantities by their added quantities. For each sample, serum miRNA concentrations were estimated in zeptomoles per microliter by dividing their recovered quantity by the geometric mean of the three cel-miR yields.
2.4. Follow-Up
2.4.1. Blood Pressure Monitoring
Blood pressure was measured every 6 months during the systematic consultation and out-of-the-office measurement was conducted systematically every week by the patient themselves during the follow-up.
2.4.2. ECG Monitoring
A 10-s 12-lead ECG was carried out during every semestrial consultation. Out-of-the-office 5-day ECG monitoring was systematically carried out every 6 months during the follow-up.
2.4.3. Echocardiography
Echocardiography was systematically performed every year during the follow-up. The echocardiographic assessment of left ventricular (LV) and left atrial (LA) functions was performed in accordance with current guidelines, using a commercially available echocardiographic system (Epiq 7 equipped with an X5-1 xMATRIX-array transducer, Philips, Netherlands, Amsterdam) [17]. All data were stored digitally, and offline analysis was conducted using the Philips IntelliSpace workstation. The LV ejection fraction (EF) was calculated using the Biplane Modified Simpson’s method. Diastolic function was evaluated from transmitral E and A velocities, E/A ratio, average of the septal and lateral annular Ea velocity, and E/Ea ratio [17]. Doppler parameters were obtained as the average value of three consecutive cardiac cycles. LA volume was estimated by the Biplane method of disks at end-systole from apical 4-chamber and 2-chamber views. LA dilatation was defined as an LA volume > 34 mL/m2 and if not available area > 20 cm2 by apical 4-cavity view [18]. Two-dimensional-speckle tracking measurements of LA phasic strains were performed following current practice recommendations [19]. The LA reservoir, conduit, and contractile strains were calculated with the first reference frameset at the onset of the QRS-wave of the surface ECG [19]. Values were obtained from a single apical 4-chamber view. Indexing LA reservoir strain to E/Ea was used as a surrogate for LA compliance, as previously described [20].
2.5. Primary Objective and Analysis
The primary objective of this study was to investigate the association between baseline patient characteristics (demographics, clinical, circulating biomarkers expression, echocardiography parameters) and CVAEs occurrence from ibrutinib introduction to 31 May 2023, the end of follow-up. CVAEs outcome was a composite of AF, new or worsening HTN, new or worsening HF, and ventricular arrhythmias [3]. AF was defined as supraventricular tachyarrhythmia with uncoordinated atrial electrical activation lasting at least 30 s or lasting an entire 12-lead ECG [21]. HTN was diagnosed if in-the-office blood pressure was superior or equal to 180 mmHg for systolic blood pressure and superior or equal to 110 mmHg for systolic blood pressure. Otherwise, for in-the-office systolic blood pressure measured between 140 mmHg and 179 mmHg and/or in-the-office diastolic blood pressure measure between 90 and 109 mmHg, diagnosis of HTN was performed with out-of-the-office blood pressure measures with means >135 mmHg for systolic blood pressure and/or >85 mmHg for diastolic blood pressure [22]. In patients with a history of HTN, the need of anti-hypertensive treatment up-titration for hypertension indication counted as an event. HF was defined as a first or recurrent unplanned hospitalization or urgent visit for HF or need to introduce/increase the dose of loop diuretic [23]. VA was defined as any sustained (lasting at least 30 s) ventricular arrhythmia documented on a 12-lead ECG or on a Holter-ECG [24].
2.6. Secondary Objectives and Analyses
Each of the components of the primary outcome was described as well as death from any cause and B-cell malignancy progression during follow-up.
Time to CVAE onset and severity by Common Terminology Criteria for Adverse Events (CTCAE) of CVAEs were described.
Description of treatment adjustments, involving ACE inhibitors, ARBs, and anticoagulant therapies, were described.
2.7. Statistical Analyses
A descriptive analysis of the cohort study was performed on the available data. Quantitative variables were expressed as median and interquartile range. Qualitative variables were expressed in effectives and percentages. Comparative analyses were performed between patients with and without CVAE occurrence during follow-up. The biological variables were dichotomized using the threshold that maximized Youden’s index, identifying the value with the greatest discriminative ability between groups (Table S1) [16]. The non-parametric Wilcoxon–Mann–Whitney test was used for quantitative variables, and Fisher exact test was used for qualitative variables. Kaplan–Meier curves and log-rank test were performed for significant qualitative variables. Variance inflation factors (VIFs) were calculated for variables that were significant in the univariate analysis to assess collinearity. Statistical significance was defined as a p-value of <0.05. Statistical analyses were performed with RStudio software V.4.2.1 for Windows using the following packages: data.table, dplyr, fst, ggplot2, kableExtra, lubridate, pROC, questionr, rlang, stringr, survival, survminer, tidyverse.
3. Results
3.1. The Study Population
From 6 December 2018 to 12 March 2021, a total of 25 consecutive patients referred for a baseline cardio-oncological evaluation were included before or within 1 month after the introduction of ibrutinib for B-cell malignancy. The median follow-up was 672 days (588–738) and nine patients stopped ibrutinib for non-cardiovascular causes during this follow-up. Baseline characteristics of the included patients are presented in Table 1. The median age was 72 (63–77) years old, 64% of patients were male, 36% had a prior history of hypertension, and only one patient had a prior history of atrial fibrillation. All included patients had a baseline LVEF > 60% and no patient had a previous history of HF. Ibrutinib was introduced mainly for chronic lymphocytic leukemia (68%). At baseline, 16% of the patients were being treated with beta-blockers, 24% with aspirin, 8% with anticoagulants, 12% with angiotensin-converting enzyme inhibitors, 16% with angiotensin-receptor blockers, 32% with dihydropyridine calcium-channel blockers, and 20% with statins.

Table 1.
Baseline characteristics of cohort. CVAE: cardiovascular adverse event; BMI: body mass index; HTN: hypertension; AF: atrial fibrillation; LBBB: left bundle branch block; RBBB: right bundle branch block; AV: atrio-ventricular; CCB: calcium-channel blocker, LVEF: left ventricular ejection fraction; LA: left atrium; CRP: C reactive protein; TNF: tumor necrosis factor; IL: interleukin; ACE-2: angiotensin-converting enzyme 2.
3.2. Primary Objective
During the follow-up, nine CVAEs occurred in 7 of the 25 patients. Among the 9 CVAEs observed, there were two cases of new-onset HTN and four cases of worsening HTN, along with two cases of new-onset AF and one recurrence of AF. No new or worsening HF or VA was reported.
The characteristics of the patients at baseline according to CVAEs occurrence are presented in Table 1. Patients who experienced CVAEs were significantly more at baseline on dihydropyridine calcium-channel blockers (71.4 vs. 16.7%), had non-significant higher body mass index (24.6 vs. 22.9 kg/m2), non-significantly higher HTN history (71.4% vs. 22.2%), and had a non-significantly higher E/Ea ratio (9.9 vs. 8.1; p = 0.083). Patients who experienced CVAEs had significant higher plasmatic renin (57% vs. 11% with renin > 1336.1 pg/mL; p = 0.032) and lower miR-150-5p (57% vs. 100% with miR-150-5p > 27.38 zmol/µL; p = 0.015) levels at baseline (Figure 1). Furthermore, creatinine levels (43% vs. 6% with creatinine > 100 µmol/L), aldosterone levels (71% vs. 22% with aldosterone > 488.95 pg/mL), and troponin levels (43% vs. 6% with troponin > 127.2 pg/mL) were higher in patients experiencing CVAEs without reaching statistical significance (Table 1).
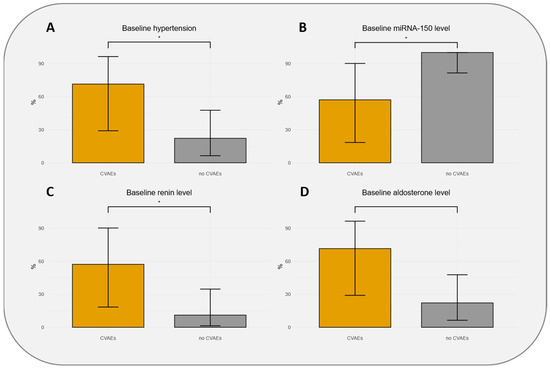
Figure 1.
Distributions of population with 95% confidence intervals according to hypertension baseline status (A), thresholds of baseline miR-150-5p level (B), baseline renin level (C), and baseline aldosterone level (D). CVAEs: cardiovascular adverse events. * indicates significant association (p-value < 0.05).
The survival analysis with Kaplan–Meier curves and log-rank test confirms the association between baseline HTN, baseline plasmatic renin level, and baseline plasmatic miR-150-5p level and CVAEs occurrence with respective p-values of 0.013, 0.024, and <0.0001 (Figure 2).
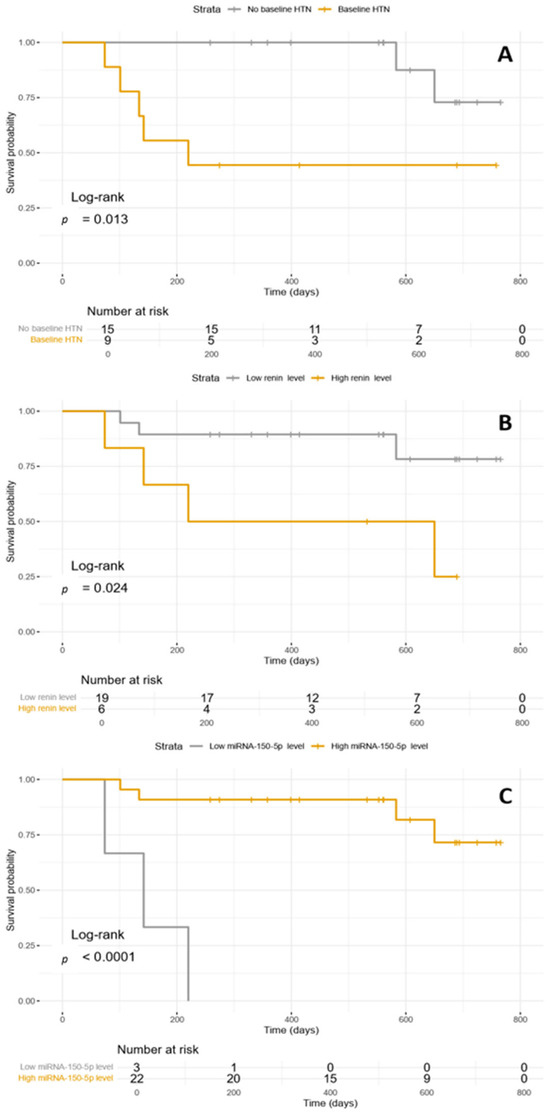
Figure 2.
(A) Kaplan–Meyer curve illustrating CVAEs occurrence according to baseline HTN; (B) Kaplan–Meyer curves illustrating CVAEs occurrence according to baseline plasmatic renin level; (C) Kaplan–Meyer curves illustrating CVAEs occurrence according to baseline miR-150-5p level. HTN: hypertension.
All VIF values were below 5, specifically 1.262, 1.262, and 1.000, respectively for baseline HTN, baseline plasmatic renin level, and baseline plasmatic miR-150-5p level.
3.3. Secondary Objectives
During the follow-up, five patients experienced B-cell malignancy progression and five patients died from non-cardiovascular causes. Among the 9 CVAEs, only one AF event was classified as severe with a CTCAE = 3 (Figure 3). The median time to onset of CVAEs was 181 (136–492) days.
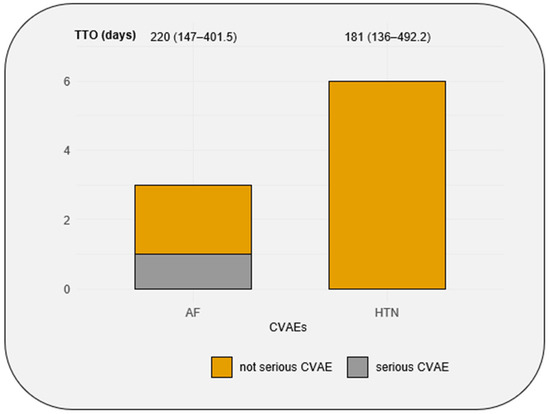
Figure 3.
Number of AF and HTN events in the cohort, the seriousness of AF and HTN events, and the median of time to onset of AF and HTN events. CVAEs: cardiovascular adverse events; TTO: time to onset; AF: atrial fibrillation; HTN: hypertension. Time to onset is expressed with median (quartile 1–quartile 3).
Among patients who experienced one or more CVAEs, direct oral anticoagulants were initiated in three patients for atrial fibrillation, and an ACE inhibitor was initiated in one patient. In the group without CVAEs, a direct oral anticoagulant was initiated in one patient for an indication other than atrial fibrillation, and the ACE inhibitor dosage was reduced in one patient.
4. Discussion
Ibrutinib, a first-in-class irreversible oral inhibitor of BTK, has proven highly effective in B-cell malignancies [2]. These malignancies are usually diagnosed in elderly patients in whom frequent cardiovascular comorbidities coexist at diagnosis that increase, independently of ibrutinib exposure, the risk of CVAEs [25]. Some baseline patient characteristics were previously associated with CVAEs occurrence in patients exposed to ibrutinib such as age > 65, history of HTN or AF, and left atrial dilatation or strain but these comorbidities are well-known risk factors for cardiovascular diseases in the general population [6,26,27]. Therefore, today there is no specific baseline predictive factor associated with ibrutinib-related CVAEs.
In this prospective cohort, although monocentric and including a small number of patients, we systematically assessed a large number of baseline patient characteristics including demographic, clinical, biological, and echocardiographic parameters. Particularly, we designed a large panel of biological variables to study inflammation, fibrosis, and neurohormonal pathways, which represent major signaling pathways in the development of HTN, AF, and HF [28]. Importantly, baseline renin levels were associated with CVAEs occurrence in our cohort and baseline aldosterone levels were higher in patients with CVAEs occurrence, although not statistically significant. It is now well established that the renin–angiotensin–aldosterone system (RAAS) and plasmatic aldosterone levels are associated with HTN, AF, and HF development [29]. Its role in hypertension is notably due to the vasopressive effect of angiotensin-2 and due to the sodium reabsorption effect in the renal tubule [30]. Aldosterone is also known to promote AF independently of HTN. Our group previously demonstrated that patients presenting with primary aldosteronism had a significantly higher rate of cardiovascular events than matched patients affected by essential hypertension [31]. Interestingly, AF was diagnosed in 7.3% of patients with primary aldosteronism and 0.6% of patients with essential hypertension; therefore, primary aldosteronism was associated with a 12-fold higher prevalence of AF compared with essential hypertension. Plasmatic aldosterone levels were previously associated with the development of AF, HTN, and HF in different settings and populations [32,33,34]. Moreover, there is evidence that aldosterone and the activation of its receptor, mineralocorticoid receptor (MR), promote atrial fibrosis and inflammation and modify cardiac action potential by interacting with ion channels and therefore can create a substrate for AF development [35]. A meta-analysis that included 7914 patients showed that mineralocorticoid receptor antagonists (MRAs) were associated with a significantly lower AF risk compared with no MRA treatment (15.0% versus 32.2%; odds ratio, 0.55; 95% CI, 0.44–0.70 [p < 0.00001]) [36]. MRAs are effective therapies for managing HTN (e.g., spironolactone) and HF with reduced ejection fraction (e.g., spironolactone and eplerenone), where they reduce overall mortality. More recently, MRAs such as finerenone have also shown beneficial effects on HF outcomes in patients with mildly reduced or preserved ejection fraction [22,23,37,38]. Interestingly, MRA efficacy does not seem confined to patients with HF [38].
There are several reasons to believe that aldosterone and the activation of its receptor, MR, might play an important role in the underlying pathophysiology of ibrutinib-related CVAEs occurrence [39]. The underlying mechanisms of ibrutinib-related CVAEs are not yet fully elucidated but it is believed that the binding of ibrutinib to off-target kinases (e.g., other than BTK) at therapeutic concentrations may potentially contribute to the development of CVAEs [40]. This hypothesis is strongly corroborated by observations in a mouse model where inhibition of the C-Terminal Src Kinase (CSK) by ibrutinib was identified as the causal mechanism of ibrutinib-related AF [41]. CSK has previously been linked to HTN, cardiac fibrosis, and inflammation in populations not exposed to ibrutinib. Furthermore, a cardiovascular magnetic resonance imaging study indicated that ibrutinib exposure induces fibrosis and inflammation of the left atrium, potentially increasing susceptibility to atrial fibrillation [42]. Moreover, inflammation, fibrosis, HTN, and AF induced by aldosterone and its receptor, MR, appeared to be mediated by the inhibition of CSK, resulting in the activation of Src in several experimental models; therefore, the CSK–Src–aldosterone–MR axis emerged as a potential axis involved in cardiovascular diseases (Figure 4). Indeed, in mice, reduced CSK activity has been shown to increase CYP11B2 (aldosterone synthase) expression in the zona glomerulosa of the adrenal gland, leading to elevated aldosterone levels [43]. Moreover, CSK inhibition results in enhanced Src activation, which not only amplifies angiotensin II production via the MAPK/ERK signaling pathway but may also contribute to increased renin release, potentially through the modulation of sympathetic signaling or juxtaglomerular cell function [44]. Taken together, these observations support a potential role for the CSK–Src–aldosterone–mineralocorticoid receptor (MR) axis in the development of ibrutinib-related CVAEs. Although hypothesis-generating and requiring confirmation in future studies, it is conceivable that MRAs could be effective in preventing or treating CVAEs in patients with B-cell malignancies receiving ibrutinib, particularly non-steroidal MRAs, which are emerging as safer and potentially more efficacious than steroidal MRAs [43,45,46,47].
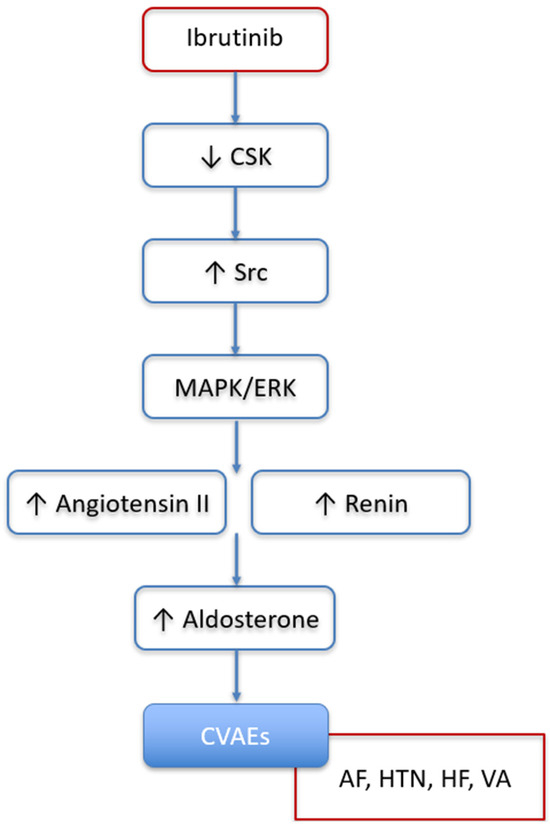
Figure 4.
Potential role of renin–angiotensin–aldosterone system, activated by CSK inhibition and subsequent Src activation, in promoting development of ibrutinib-related cardiovascular adverse events. CSK: C-Terminal Src Kinase; CVAEs: cardiovascular adverse events; AF: atrial fibrillation; HTN: hypertension; HF: heart failure; VA: ventricular arrythmias.
Our analysis highlighted that baseline plasmatic miR-150-5p was significantly lower in patients who experienced ibrutinib-related CVAEs. MiRNAs are small non-coding RNAs involved in genetic expression regulation at a post-transcriptional level [48]. MiR-150-5p is known to regulate cellular proliferation and cardiac fibrosis and was previously associated with AF in the general population [10]. In a prospective study comparing 112 AF patients with 99 controls without AF, miR-150-5p was associated with AF with a significantly greater expression in the control group after adjustment (p = 0.04) [10]. Kawaguchi et al. demonstrated in both mice and humans that miR-150-5p is downregulated during heart failure and acts as an inhibitor of the proapoptotic protein small proline-rich protein 1a, thereby reducing cardiac apoptosis and fibrosis [49].
In previous published studies, the age and history of CV diseases or the presence of CV risk factors were associated with ibrutinib-related CVAEs [6,26,27]. In our study, none of these parameters came out statistically significant. The absence of statistically significant parameters such as age, history of hypertension, and left atrial dilatation or strain is probably explained by the small number of patients included in our cohort. In a prospective cohort of 53 patients treated with ibrutinib, with an AF incidence of 38% over two years of follow-up, left atrial enlargement, defined as a left atrial area > 40 mL/m2, was associated with a markedly increased risk of AF with a HR = 8.68 (% CI: 2.86–26.26) [6]. In a retrospective study of 66 patients treated with ibrutinib, 22 developed AF, and several parameters exhibited a significant association with ibrutinib-related AF: E/Ea was significantly higher among the AF group (11.5 vs. 9.3, p = 0.04) and peak atrial longitudinal strain was significantly lower among the AF group (30.3% vs. 36.3%, p = 0.01) [26].
5. Study Limitations
The main limitation of this study is the small size of the cohort, which implies low statistical power and not enough events to undertake multivariate analysis. Indeed, our results are not adjusted on potential confounding factors. Furthermore, biological parameters were assessed only at baseline prior to ibrutinib initiation, as follow-up samples were not available. Additionally, the absence of cardiac tissue precluded direct measurement of CSK activity, limiting our ability to strengthen the evidence supporting the involvement of the CSK–Src–aldosterone–mineralocorticoid receptor axis in the pathophysiology of ibrutinib-related CVAEs. Moreover, baseline use of angiotensin-receptor blockers (ARBs) and ACE inhibitors can clearly influence plasma concentrations of renin and aldosterone. Therefore, these results should be interpreted with caution. This study has great potential to be prospective but this is a monocentric study, which can bias the external validity. Indeed, every patient included in this study is followed in our cardio-oncology program, which allows for specialized management of the specificities of these patients suffering from hematologic malignancies and at risk of CVAEs.
6. Conclusions
In this small prospective and monocentric cohort of 25 patients exposed to ibrutinib for B-cell malignancy, 7 patients experienced 9 CVAEs during a median follow-up of 672 days. The CSK–Src–aldosterone–mineralocorticoid receptor axis may represent a potential pathway involved in the development of ibrutinib-related CVAEs, although this remains to be further explored. Furthermore, history of HTN and miR-150-5p levels at baseline were univariably associated with CVAEs occurrence. These results need to be confirmed in larger multicenter cohort studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines13092184/s1, Table S1: Optimal Threshold for Cohort Stratification Determined by Youden’s Index.
Author Contributions
Conceptualization, J.A. and C.D. (Christophe Denoyelle); methodology, J.F., C.D. (Charles Dolladille), J.A. and C.D. (Christophe Denoyelle); software, J.F. and N.V.; validation, J.A., J.F. and C.D. (Christophe Denoyelle); formal analysis, J.F.; investigation, A.H., S.B., M.V. and J.F.; resources, J.A., A.H. and C.D. (Christophe Denoyelle); data curation, J.F.; writing—original draft preparation, J.F. and J.A.; writing—review and editing, C.D. (Christophe Denoyelle), A.H., P.M., A.N., A.-F.P., A.D.-S., B.D., G.D. and D.L.; visualization, J.F.; supervision, J.A.; project administration, J.A.; funding acquisition, J.A. and C.D. (Christophe Denoyelle). All authors have read and agreed to the published version of the manuscript.
Funding
This study received two internal grants from both the Caen-Normandy University Hospital and the INSERM U1086 ANTICIPE.
Institutional Review Board Statement
Ethical approval for this study was granted on 13 June 2018 by the Comité de Protection des Personnes Nord-Ouest IV, Lille, France (No. 2018-A00429-46), in accordance with the principles of the Declaration of Helsinki.
Informed Consent Statement
Written informed consent was obtained from all participants.
Data Availability Statement
Data are available for academic purposes upon reasonable request.
Acknowledgments
Authors thank CRB Innova BIO for the packaging and storage of the biologic samples.
Conflicts of Interest
Prof. Alexandre reports honoraria for presentations and consulting fees from Biotronik, Bioserenity, Amgen, BMS, Pfizer, Boerhinger, Bayer, Astra Zeneca, Janssen, Servier, and Novartis, outside of the submitted work. Dr. Legallois reports honoraria for presentations and consulting fees from Astrazeneca, Boehringer Ingelheim, Lilly, Pfizer, and Takeda, outside of the submitted work. Dr. Dolladille reports honoraria for presentations and consulting fees from Bioserenity and Pfizer, outside of the submitted work. The remaining authors have nothing to disclose.
References
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL Guidelines for Diagnosis, Indications for Treatment, Response Assessment, and Supportive Management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Bartlett, N.L.; Li, J.; et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on Cardio-Oncology Developed in Collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the Task Force on Cardio-Oncology of the European Society of Cardiology (ESC). Eur. Heart J. 2022, 43, ehac244. [Google Scholar] [CrossRef]
- Caldeira, D.; Alves, D.; Costa, J.; Ferreira, J.J.; Pinto, F.J. Ibrutinib Increases the Risk of Hypertension and Atrial Fibrillation: Systematic Review and Meta-Analysis. PLoS ONE 2019, 14, e0211228. [Google Scholar] [CrossRef] [PubMed]
- Archibald, W.J.; Rabe, K.G.; Kabat, B.F.; Herrmann, J.; Ding, W.; Kay, N.E.; Kenderian, S.S.; Muchtar, E.; Leis, J.F.; Wang, Y.; et al. Atrial Fibrillation in Patients with Chronic Lymphocytic Leukemia (CLL) Treated with Ibrutinib: Risk Prediction, Management, and Clinical Outcomes. Ann. Hematol. 2021, 100, 143–155. [Google Scholar] [CrossRef]
- Baptiste, F.; Cautela, J.; Ancedy, Y.; Resseguier, N.; Aurran, T.; Farnault, L.; Escudier, M.; Ammar, C.; Gaubert, M.; Dolladille, C.; et al. High Incidence of Atrial Fibrillation in Patients Treated with Ibrutinib. Open Heart 2019, 6, e001049. [Google Scholar] [CrossRef]
- Agnelli, G.; Becattini, C.; Meyer, G.; Muñoz, A.; Huisman, M.V.; Connors, J.M.; Cohen, A.; Bauersachs, R.; Brenner, B.; Torbicki, A.; et al. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N. Engl. J. Med. 2020, 382, 1599–1607. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Shen, N.-N.; Zhang, C.; Li, Z.; Kong, L.-C.; Wang, X.-H.; Gu, Z.-C.; Wang, J.-L. MicroRNA Expression Signatures of Atrial Fibrillation: The Critical Systematic Review and Bioinformatics Analysis. Exp. Biol. Med. 2020, 245, 42–53. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.D.; Tanriverdi, K.; Lin, H.; Esa, N.; Kinno, M.; Mandapati, D.; Tam, S.; Okike, O.N.; Ellinor, P.T.; Keaney, J.F.; et al. Plasma microRNAs Are Associated with Atrial Fibrillation and Change after Catheter Ablation (the miRhythm Study). Heart Rhythm 2015, 12, 3–10. [Google Scholar] [CrossRef]
- Ramasamy, S.; Velmurugan, G.; Rekha, B.; Anusha, S.; Shanmugha Rajan, K.; Shanmugarajan, S.; Ramprasath, T.; Gopal, P.; Tomar, D.; Karthik, K.V.; et al. Egr-1 Mediated Cardiac miR-99 Family Expression Diverges Physiological Hypertrophy from Pathological Hypertrophy. Exp. Cell Res. 2018, 365, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Wiedmann, F.; Kraft, M.; Kallenberger, S.; Büscher, A.; Paasche, A.; Blochberger, P.L.; Seeger, T.; Jávorszky, N.; Warnecke, G.; Arif, R.; et al. MicroRNAs Regulate TASK-1 and Are Linked to Myocardial Dilatation in Atrial Fibrillation. J. Am. Heart Assoc. 2022, 11, e023472. [Google Scholar] [CrossRef]
- Kura, B.; Szeiffova Bacova, B.; Kalocayova, B.; Sykora, M.; Slezak, J. Oxidative Stress-Responsive MicroRNAs in Heart Injury. Int. J. Mol. Sci. 2020, 21, 358. [Google Scholar] [CrossRef]
- Huang, H.; Chen, H.; Liang, X.; Chen, X.; Chen, X.; Chen, C. Upregulated miR-328-3p and Its High Risk in Atrial Fibrillation: A Systematic Review and Meta-Analysis with Meta-Regression. Medicine 2022, 101, e28980. [Google Scholar] [CrossRef]
- Vigneron, N.; Meryet-Figuière, M.; Guttin, A.; Issartel, J.-P.; Lambert, B.; Briand, M.; Louis, M.-H.; Vernon, M.; Lebailly, P.; Lecluse, Y.; et al. Towards a New Standardized Method for Circulating miRNAs Profiling in Clinical Studies: Interest of the Exogenous Normalization to Improve miRNA Signature Accuracy. Mol. Oncol. 2016, 10, 981–992. [Google Scholar] [CrossRef]
- Vigneron, N.; Vernon, M.; Meryet-Figuière, M.; Lambert, B.; Briand, M.; Louis, M.-H.; Krieger, S.; Joly, F.; Lheureux, S.; Blanc-Fournier, C.; et al. Predictive Relevance of Circulating miR-622 in Patients with Newly Diagnosed and Recurrent High-Grade Serous Ovarian Carcinoma. Clin. Chem. 2020, 66, 352–362. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of Left Atrial, Right Ventricular, and Right Atrial Deformation Imaging Using Two-Dimensional Speckle Tracking Echocardiography: A Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Obokata, M.; Egbe, A.; Yang, J.H.; Pislaru, S.; Lin, G.; Carter, R.; Borlaug, B.A. Left Atrial Strain and Compliance in the Diagnostic Evaluation of Heart Failure with Preserved Ejection Fraction. Eur. J. Heart Fail. 2019, 21, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, ehae176. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the Management of Elevated Blood Pressure and Hypertension: Developed by the Task Force on the Management of Elevated Blood Pressure and Hypertension of the European Society of Cardiology (ESC) and Endorsed by the European Society of Endocrinology (ESE) and the European Stroke Organisation (ESO). Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Developed by the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by the Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Dickerson, T.; Wiczer, T.; Waller, A.; Philippon, J.; Porter, K.; Haddad, D.; Guha, A.; Rogers, K.A.; Bhat, S.; Byrd, J.C.; et al. Hypertension and Incident Cardiovascular Events Following Ibrutinib Initiation. Blood 2019, 134, 1919–1928. [Google Scholar] [CrossRef]
- Singh, A.; El Hangouche, N.; McGee, K.; Gong, F.-F.; Lentz, R.; Feinglass, J.; Akhter, N. Utilizing Left Atrial Strain to Identify Patients at Risk for Atrial Fibrillation on Ibrutinib. Echocardiography 2021, 38, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Moslehi, J.; O’Brien, S.; Ghia, P.; Hillmen, P.; Cymbalista, F.; Shanafelt, T.D.; Fraser, G.; Rule, S.; Kipps, T.J.; et al. Characterization of Atrial Fibrillation Adverse Events Reported in Ibrutinib Randomized Controlled Registration Trials. Haematologica 2017, 102, 1796–1805. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice: Developed by the Task Force for Cardiovascular Disease Prevention in Clinical Practice with Representatives of the European Society of Cardiology and 12 Medical Societies With the Special Contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Buffolo, F.; Tetti, M.; Mulatero, P.; Monticone, S. Aldosterone as a Mediator of Cardiovascular Damage. Hypertension 2022, 79, 1899–1911. [Google Scholar] [CrossRef]
- Palmer, L.G.; Schnermann, J. Integrated Control of Na Transport along the Nephron. Clin. J. Am. Soc. Nephrol. 2015, 10, 676–687. [Google Scholar] [CrossRef]
- Milliez, P.; Girerd, X.; Plouin, P.-F.; Blacher, J.; Safar, M.E.; Mourad, J.-J. Evidence for an Increased Rate of Cardiovascular Events in Patients with Primary Aldosteronism. J. Am. Coll. Cardiol. 2005, 45, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.; Saloux, E.; Chequel, M.; Allouche, S.; Ollitrault, P.; Plane, A.-F.; Legallois, D.; Fischer, M.-O.; Saplacan, V.; Buklas, D.; et al. Preoperative Plasma Aldosterone and the Risk of Atrial Fibrillation after Coronary Artery Bypass Surgery: A Prospective Cohort Study. J. Hypertens. 2016, 34, 2449–2457. [Google Scholar] [CrossRef]
- Kim, S.K.; Pak, H.-N.; Park, J.H.; Ko, K.J.; Lee, J.S.; Choi, J.I.; Choi, D.H.; Kim, Y.-H. Clinical and Serological Predictors for the Recurrence of Atrial Fibrillation after Electrical Cardioversion. Europace 2009, 11, 1632–1638. [Google Scholar] [CrossRef]
- Swedberg, K.; Eneroth, P.; Kjekshus, J.; Wilhelmsen, L. Hormones Regulating Cardiovascular Function in Patients with Severe Congestive Heart Failure and Their Relation to Mortality. CONSENSUS Trial Study Group. Circulation 1990, 82, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Reil, J.-C.; Hohl, M.; Selejan, S.; Lipp, P.; Drautz, F.; Kazakow, A.; Münz, B.M.; Müller, P.; Steendijk, P.; Reil, G.-H.; et al. Aldosterone Promotes Atrial Fibrillation. Eur. Heart J. 2012, 33, 2098–2108. [Google Scholar] [CrossRef]
- Alexandre, J.; Dolladille, C.; Douesnel, L.; Font, J.; Dabrowski, R.; Shavit, L.; Legallois, D.; Funck-Brentano, C.; Champ-Rigot, L.; Ollitrault, P.; et al. Effects of Mineralocorticoid Receptor Antagonists on Atrial Fibrillation Occurrence: A Systematic Review, Meta-Analysis, and Meta-Regression to Identify Modifying Factors. J. Am. Heart Assoc. 2019, 8, e013267. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Vaduganathan, M.; Claggett, B.; Jhund, P.S.; Desai, A.S.; Henderson, A.D.; Lam, C.S.P.; Pitt, B.; Senni, M.; et al. Finerenone in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2024, 391, 1475–1485. [Google Scholar] [CrossRef]
- Oraii, A.; Healey, J.S.; Kowalik, K.; Pandey, A.K.; Benz, A.P.; Wong, J.A.; Conen, D.; McIntyre, W.F. Mineralocorticoid Receptor Antagonists and Atrial Fibrillation: A Meta-Analysis of Clinical Trials. Eur. Heart J. 2024, 45, 756–774. [Google Scholar] [CrossRef]
- Pawlonka, J.; Buchalska, B.; Buczma, K.; Borzuta, H.; Kamińska, K.; Cudnoch-Jędrzejewska, A. Targeting the Renin–Angiotensin–Aldosterone System (RAAS) for Cardiovascular Protection and Enhanced Oncological Outcomes: Review. Curr. Treat. Options Oncol. 2024, 25, 1406–1427. [Google Scholar] [CrossRef]
- Alexandre, J.; Moslehi, J.J.; Bersell, K.R.; Funck-Brentano, C.; Roden, D.M.; Salem, J.-E. Anticancer Drug-Induced Cardiac Rhythm Disorders: Current Knowledge and Basic Underlying Mechanisms. Pharmacol. Ther. 2018, 189, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Salem, J.-E.; Clauss, S.; Hanley, A.; Bapat, A.; Hulsmans, M.; Iwamoto, Y.; Wojtkiewicz, G.; Cetinbas, M.; Schloss, M.J.; et al. Ibrutinib-Mediated Atrial Fibrillation Attributable to Inhibition of C-Terminal Src Kinase. Circulation 2020, 142, 2443–2455. [Google Scholar] [CrossRef] [PubMed]
- Buck, B.; Chum, A.P.; Patel, M.; Carter, R.; Nawaz, H.; Yildiz, V.; Ruz, P.; Wiczer, T.; Rogers, K.A.; Awan, F.T.; et al. Cardiovascular Magnetic Resonance Imaging in Patients With Ibrutinib-Associated Cardiotoxicity. JAMA Oncol. 2023, 9, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Kang, J.-O.; Lim, J.E.; Hwang, S.-Y.; Oh, B. Csk Regulates Blood Pressure by Controlling the Synthetic Pathways of Aldosterone. Circ. J. 2017, 82, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Wu, X.-H.; He, G.; Salomon, S.; Schiffrin, E.L. Increased Angiotensin II-Mediated Src Signaling via Epidermal Growth Factor Receptor Transactivation Is Associated with Decreased C-Terminal Src Kinase Activity in Vascular Smooth Muscle Cells from Spontaneously Hypertensive Rats. Hypertension 2002, 39, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Xu, W.; Fang, N.; Li, L.; Yang, N.; Zhao, X.; Hao, H.; Zhang, Y.; Liang, Q.; Wang, Z.; et al. Ibrutinib-Induced Pulmonary Angiotensin-Converting Enzyme Activation Promotes Atrial Fibrillation in Rats. iScience 2024, 27, 108926. [Google Scholar] [CrossRef]
- Braun, S.; Lösel, R.; Wehling, M.; Boldyreff, B. Aldosterone Rapidly Activates Src Kinase in M-1 Cells Involving the Mineralocorticoid Receptor and HSP84. FEBS Lett. 2004, 570, 69–72. [Google Scholar] [CrossRef]
- Callera, G.E.; Yogi, A.; Briones, A.M.; Montezano, A.C.I.; He, Y.; Tostes, R.C.A.; Schiffrin, E.L.; Touyz, R.M. Vascular Proinflammatory Responses by Aldosterone Are Mediated via C-Src Trafficking to Cholesterol-Rich Microdomains: Role of PDGFR. Cardiovasc. Res. 2011, 91, 720–731. [Google Scholar] [CrossRef]
- Latronico, M.V.G.; Condorelli, G. MicroRNAs and Cardiac Pathology. Nat. Rev. Cardiol. 2009, 6, 418–429. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Moukette, B.; Sepúlveda, M.N.; Hayasaka, T.; Aonuma, T.; Haskell, A.K.; Mah, J.; Liangpunsakul, S.; Tang, Y.; Conway, S.J.; et al. SPRR1A Is a Key Downstream Effector of MiR-150 during Both Maladaptive Cardiac Remodeling in Mice and Human Cardiac Fibroblast Activation. Cell Death Dis. 2023, 14, 446. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).