Immunomodulatory Effects of RAAS Inhibitors: Beyond Hypertension and Heart Failure
Abstract
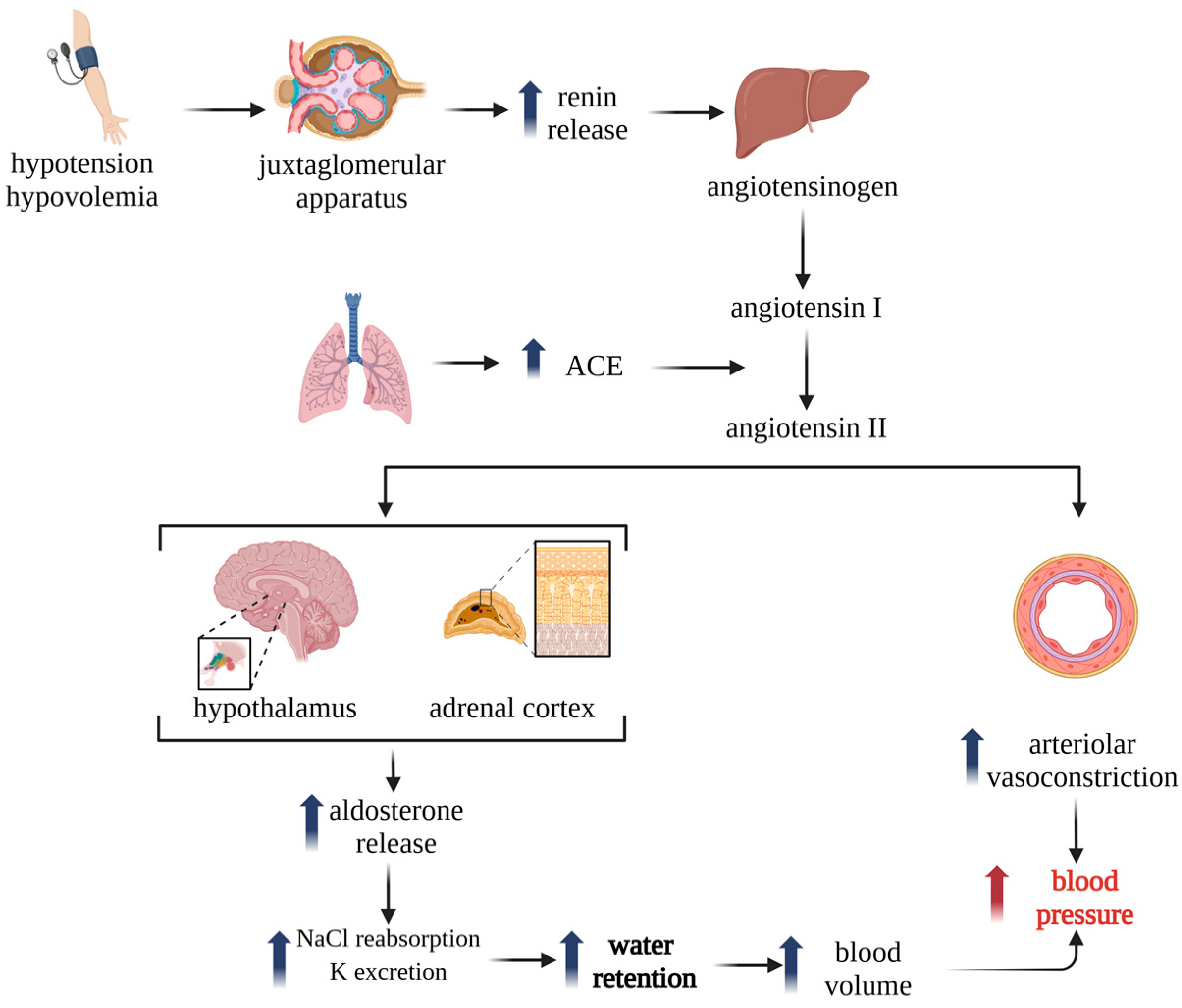
1. Introduction
2. Immunomodulatory Effects of ACEIs and ARBs
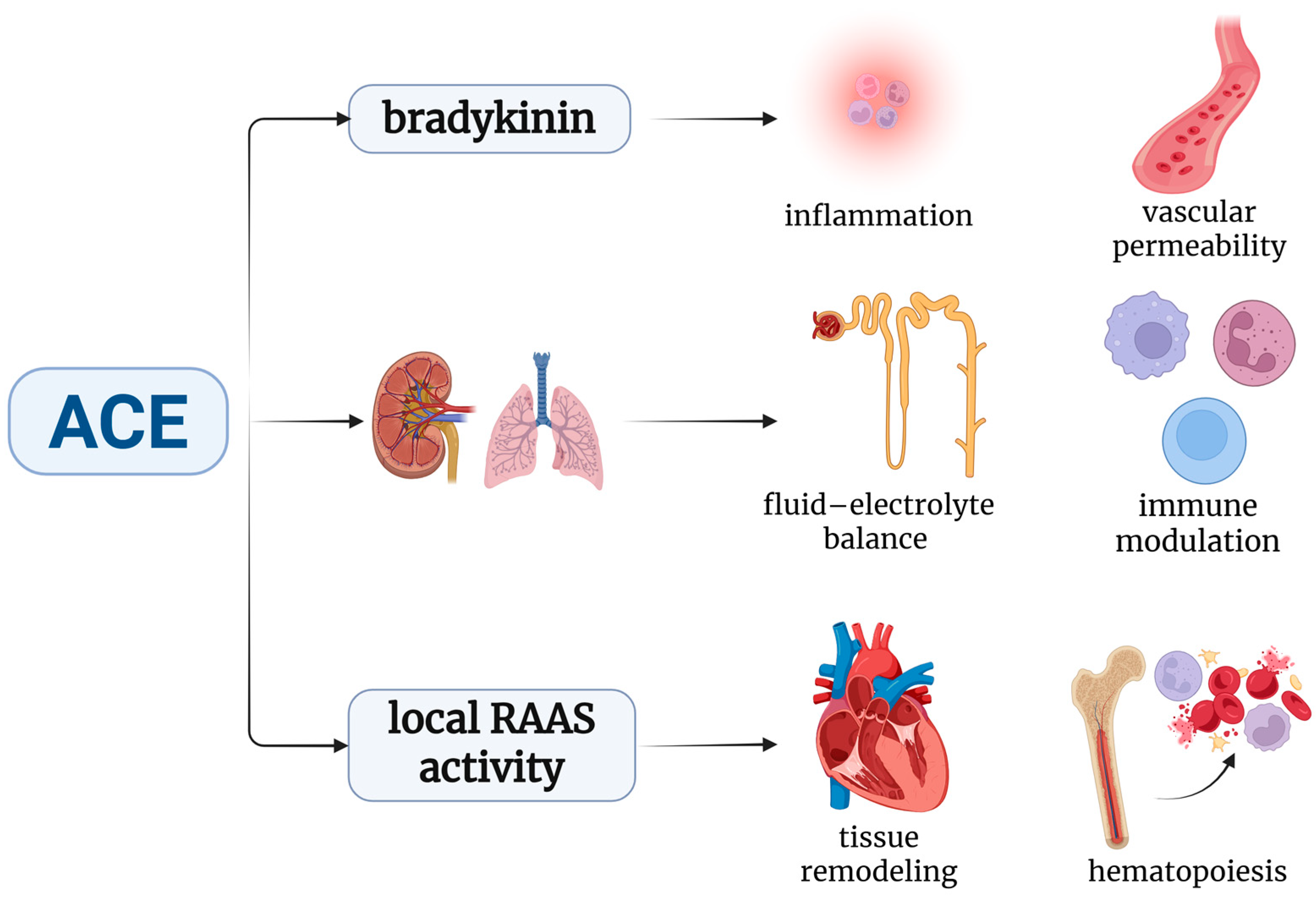
2.1. ACE and Immune Modulation: From Blood Pressure to Immunity
| Biological Effects of ACE | Mechanisms | Effects | Experimental Model | RAAS Inhibitor Used | References |
|---|---|---|---|---|---|
| Ang II-dependent | AT1R activation → NF-κB → increases IL-6, TNF-α | Promotes Th17 polarization; inflammatory cytokine production | DSS-induced colitis in mice | Telmisartan | [35] |
| Ang II-dependent | AT2R activation → NO release → reduces inflammation | Anti-inflammatory, vasodilatory, and antifibrotic effects | Endothelial cell culture under shear stress | Candesartan | [36] |
| Ang II-independent | ACE hydrolyzes Aβ1–42 → modulation of amyloid burden | Neuroprotective in AD models | APP/PS1 transgenic mice | Ramipril | [37] |
| Ang II-independent | MHC class I peptide trimming by ACE → augment antigen presentation | Enhanced CD8+ T-cell activation | ACE 10/10 mice | Captopril | [24,38] |
2.2. Effects of RAAS Inhibitors on Innate Immunity
2.2.1. Modulation of Macrophage and Dendritic Cell Function
2.2.2. Impact on Neutrophil Activation and Oxidative Stress
| Immune Function | Effects of ACEi | Effects of ARBs |
|---|---|---|
| Macrophage polarization | Inhibits M1 phenotype (pro-inflammatory); reduces IL-12 and NO; increases IL-10; suppresses pro-inflammatory activity (captopril) [34,38] | Context-dependent: may promote M1 (pro-inflammatory) phenotype via LOX-1 (losartan) or induce M2 (anti-inflammatory) phenotype (candesartan) [41,42] |
| Dendritic cell and CD8+ T-cell activation | Reduces activation (reverses ACE overexpression effects) [37] | No significant effect on activation seen in ACE 10/10 model |
| Tumor growth (ACEIs) and bacterial clearance | Suppresses enhanced resistance seen with ACE overexpression [39,40] | Decreased risk of cancer overall and several site-specific cancers [46] |
| Neutrophil function and ROS production | Reduces NADPHox-mediated ROS; impairs bacterial killing [39,43] | Reduce ROS via PPARγ; may compromise neutrophil function; intrinsic antimicrobial activity (e.g., candesartan) [44,45] |
2.3. Effects of RAAS Inhibitors on Adaptive Immunity
2.3.1. Influence on T-Cell Differentiation
2.3.2. Modulation of B-Cell Function and Antibody Production
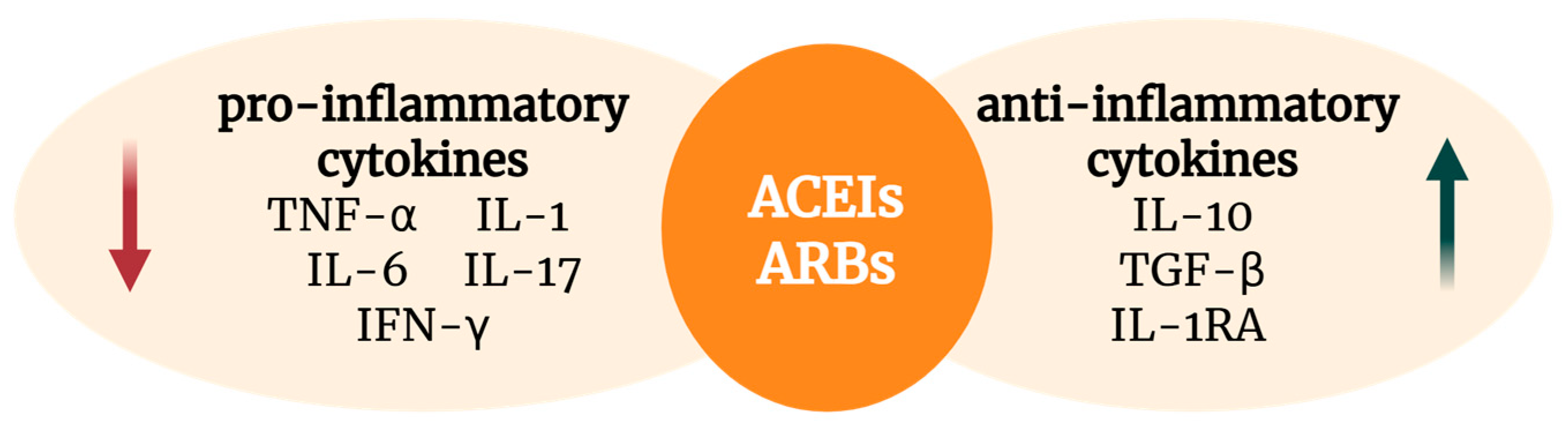
2.4. Cytokine Regulation and Inflammatory Pathways
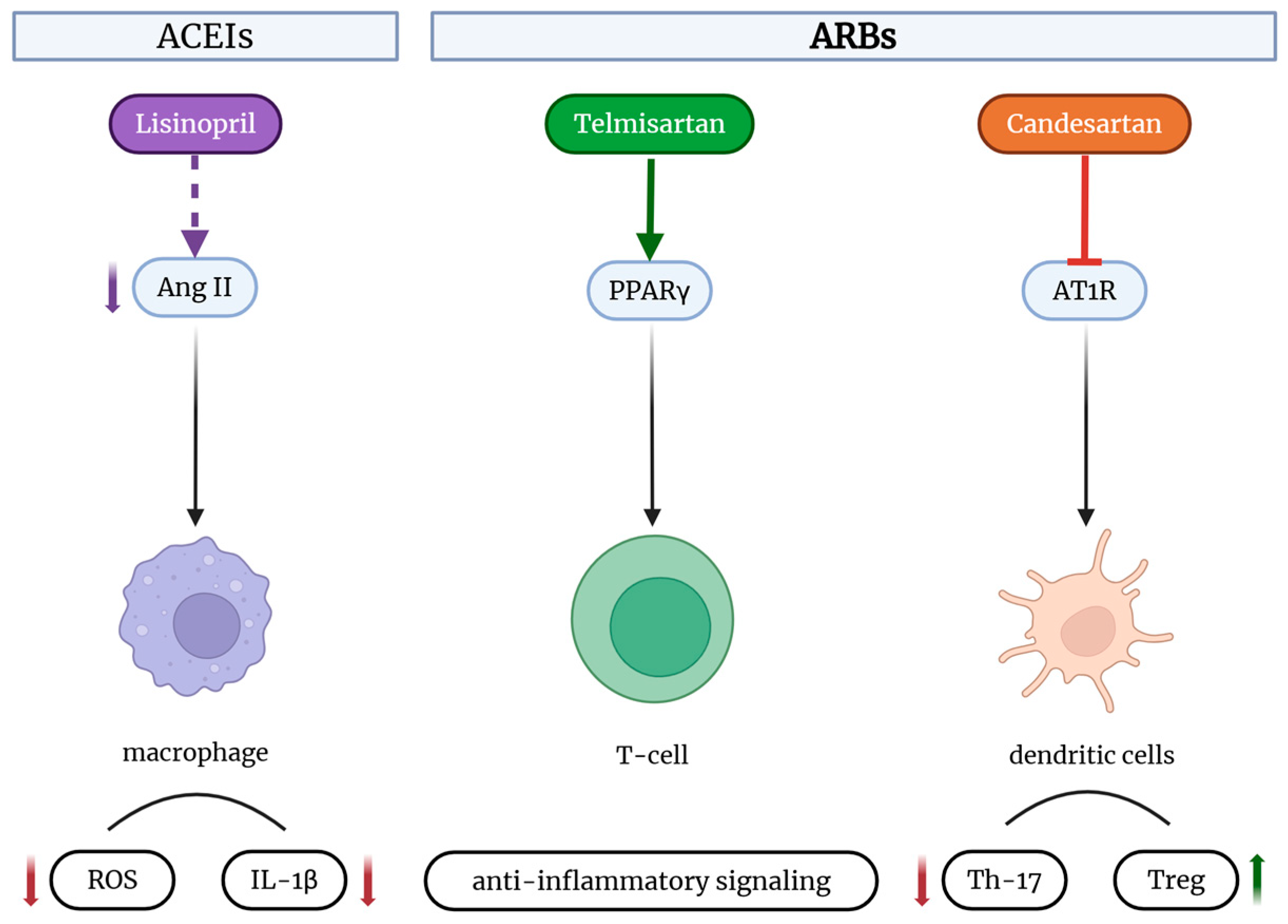
2.5. Key Signaling Pathways in RAAS–Immune System Crosstalk
2.6. Comparative Immunomodulatory Profile: ACEIs vs. ARBs
2.7. Adverse Effects and Safety Considerations
3. Clinical Implications, Emerging Applications, and Future Directions
3.1. Potential Benefits in Autoimmune Diseases (Multiple Sclerosis, Autoimmune Myocarditis)
3.2. Role in Infectious Diseases
3.3. Impact on Cancer Immunology and Tumor Microenvironment
3.4. Potential Benefits in Neurodegenerative Diseases
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ardila, D.L.V.; Walsh, K.A.; Fifis, T.; Paolini, R.; Kastrappis, G.; Christophi, C.; Perini, M.V. Immunomodulatory effects of renin–angiotensin system inhibitors on T lymphocytes in mice with colorectal liver metastases. J. Immunother. Cancer 2020, 8, e000487. [Google Scholar] [CrossRef]
- Bryniarski, P.; Nazimek, K.; Marcinkiewicz, J. Immunomodulatory Activity of the Most Commonly Used Antihypertensive Drugs—Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers. Int. J. Mol. Sci. 2022, 23, 1772. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, S. Renin–angiotensin–aldosterone pathway modulators in chronic kidney disease: A comparative review. Front. Pharmacol. 2023, 14, 1101068. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ksiazek, S.H.; Hu, L.; Andò, S.; Pirklbauer, M.; Säemann, M.D.; Ruotolo, C.; Zaza, G.; La Manna, G.; De Nicola, L.; Mayer, G.; et al. Renin–Angiotensin–Aldosterone System: From History to Practice of a Secular Topic. Int. J. Mol. Sci. 2024, 25, 4035. [Google Scholar] [CrossRef] [PubMed]
- Bavishi, C.; Bangalore, S.; Messerli, F.H. Renin Angiotensin Aldosterone System Inhibitors in Hypertension: Is There Evidence for Benefit Independent of Blood Pressure Reduction? Prog. Cardiovasc. Dis. 2016, 59, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Garcia, B.; Su, F.; Manicone, F.; Dewachter, L.; Favory, R.; Khaldi, A.; Moiroux-Sahroui, A.; Moreau, A.; Herpain, A.; Vincent, J.-L.; et al. Angiotensin 1–7 in an experimental septic shock model. Crit. Care 2023, 27, 106. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.K.; Kaur, G.; Buttar, H.S.; Bagabir, H.A.; Bagabir, R.A.; Bagabir, S.A.; Haque, S.; Tuli, H.S.; Telessy, I.G. Role of the renin–angiotensin system in the pathophysiology of coronary heart disease and heart failure: Diagnostic biomarkers and therapy with drugs and natural products. Front. Physiol. 2023, 14, 1034170. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Zaballos, S.; Martínez-Sellés, M. Angiotensin-Converting Enzyme and Heart Failure. Front. Biosci. (Landmark Ed.) 2023, 28, 150. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, C.; Barraclough, M.; Su, J.; Tanic, M.; Bingham, K.; Ruttan, L.; Beaton, D.; Wither, J.; Tartaglia, M.C.; Sano, M.; et al. Centrally acting ACE inhibitor (cACEi) and angiotensin receptor blocker (cARB) use and cognitive dysfunction in patients with SLE. Lupus Sci. Med. 2023, 10, e000923. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Májer, R.; Boczán, J.; Sipka, S., Jr.; Szabó, A.; Enyedi, E.E.; Tatai, O.; Fagyas, M.; Papp, Z.; Csiba, L.; et al. Enalapril Is Superior to Lisinopril in Improving Endothelial Function without a Difference in Blood–Pressure–Lowering Effects in Newly Diagnosed Hypertensives. Biomedicines 2023, 11, 3323. [Google Scholar] [CrossRef] [PubMed]
- Ouk, M.; Wu, C.-Y.; Rabin, J.S.; Jackson, A.; Edwards, J.D.; Ramirez, J.; Masellis, M.; Swartz, R.H.; Herrmann, N.; Lanctôt, K.L.; et al. The use of angiotensin-converting enzyme inhibitors vs. angiotensin receptor blockers and cognitive decline in Alzheimer’s disease: The importance of blood-brain barrier penetration and APOE ε4 carrier status. Alzheimer’s Res. Ther. 2021, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Kim, S.; Jo, Y.; Kim, Y.; Ye, B.S.; Yu, Y.M. Neuroprotective effect of angiotensin II receptor blockers on the risk of incident Alzheimer’s disease: A nationwide population-based cohort study. Front. Aging Neurosci. 2023, 15, 1137197. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wei, G.; Wang, Y.; Li, X.; Zhao, Q.; Zhu, L.; Xiao, Q.; Xiong, X. Efficacy and safety of nonsteroidal mineralocorticoid receptor antagonists for renal and cardiovascular outcomes in patients with chronic kidney disease: A meta-analysis of randomized clinical trials. Front. Pharmacol. 2024, 15, 1338044. [Google Scholar] [CrossRef] [PubMed]
- Nocito, C.; Lubinsky, C.; Hand, M.; Khan, S.; Patel, T.; Seliga, A.; Winfield, M.; Zuluaga-Ramirez, V.; Fernandes, N.; Shi, X.; et al. Centrally Acting Angiotensin-Converting Enzyme Inhibitor Suppresses Type I Interferon Responses and Decreases Inflammation in the Periphery and the CNS in Lupus-Prone Mice. Front. Immunol. 2020, 11, 573677. [Google Scholar] [CrossRef] [PubMed]
- Maejima, Y.; Okada, H.; Haraguchi, G.; Onai, Y.; Kosuge, H.; Suzuki, J.-I.; Isobe, M. Telmisartan, a unique ARB, improves left ventricular remodeling of infarcted heart by activating PPAR gamma. Lab. Investig. 2011, 91, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Schmerbach, K.; Pfab, T.; Zhao, Y.; Culman, J.; Mueller, S.; Villringer, A.; Muller, D.N.; Hocher, B.; Unger, T.; Thoene-Reineke, C. Effects of aliskiren on stroke in rats expressing human renin and angiotensinogen genes. PLoS ONE 2010, 5, e15052. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J. Elevation of serum angiotension-converting-enzyme (ACE) level in sarcoidosis. Am. J. Med. 1975, 59, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, K.E.; Khan, Z.; Giani, J.F.; Cao, D.-Y.; Bernstein, E.A.; Shen, X.Z. Angiotensin-converting enzyme in innate and adaptive immunity. Nat. Rev. Nephrol. 2018, 14, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.M.; Xue, Y.; Brewer, S.M.; Bernstein, K.E.; Quake, S.R.; Monack, D.M. Single-cell profiling identifies ACE+ granuloma macrophages as a nonpermissive niche for intracellular bacteria during persistent Salmonella infection. Sci. Adv. 2023, 9, eadd4333. [Google Scholar] [CrossRef] [PubMed]
- Leong, M.; Li, X.; Chaum, M. Pocket ACEs: Discovering new function within an old player. Front. Physiol. 2023, 14, 1151908. [Google Scholar] [CrossRef] [PubMed]
- Rudi, W.-S.; Molitor, M.; Garlapati, V.; Finger, S.; Wild, J.; Münzel, T.; Karbach, S.H.; Wenzel, P. ACE Inhibition Modulates Myeloid Hematopoiesis after Acute Myocardial Infarction and Reduces Cardiac and Vascular Inflammation in Ischemic Heart Failure. Antioxidants 2021, 10, 396. [Google Scholar] [CrossRef] [PubMed]
- Oosthuizen, D.; Sturrock, E.D. Exploring the Impact of ACE Inhibition in Immunity and Disease. J. Renin-Angiotensin-Aldosterone Syst. 2022, 2022, 9028969. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.Z.; Li, P.; Weiss, D.; Fuchs, S.; Xiao, H.D.; Adams, J.A.; Williams, I.R.; Capecchi, M.R.; Taylor, W.R.; Bernstein, K.E. Mice with enhanced macrophage angiotensin-converting enzyme are resistant to melanoma. Am. J. Pathol. 2007, 170, 2122–2134. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Du, J.; Chen, Y.; Liu, C.; Zhou, M.; Adhikari, S.; Rubin, D.T.; Pekow, J.; Li, Y.C. Renin-angiotensin system promotes colonic inflammation by inducing TH17 activation via JAK2/STAT pathway. Am. J. Physiol. Gastrointest Liver Physiol. 2019, 316, G774–G784. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hou, X.; Zhang, M.; Zheng, Y.; Zheng, X.; Yang, Q.; Li, J.; Gu, N.; Zhang, M.; Sun, Y.; et al. MicroRNA-223-3p modulates dendritic cell function and ameliorates experimental autoimmune myocarditis by targeting the NLRP3 inflammasome. Mol. Immunol. 2020, 117, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, H.; Liu, Q.; Cheng, P.; Zhao, T.; Yang, T.; Zhao, Y.; Sha, W.; Zhao, Y.; Qu, H. Targeting regulatory T cells for cardiovascular diseases. Front. Immunol. 2023, 14, 1126761. [Google Scholar] [CrossRef] [PubMed]
- Skillbäck, T.; Farahmand, B.Y.; Rosén, C.; Mattsson, N.; Nägga, K.; Kilander, L.; Religa, D.; Wimo, A.; Winblad, B.; Schott, J.M.; et al. Cerebrospinal fluid tau and amyloid-β1-42 in patients with dementia. Brain 2015, 138 Pt 9, 2716–2731. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.Z.; Billet, S.; Lin, C.; Okwan-Duodu, D.; Chen, X.; Lukacher, A.E.; Bernstein, K.E. The carboxypeptidase ACE shapes the MHC class I peptide repertoire. Nat. Immunol. 2011, 12, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kaplan, N.; Wysocki, J.; Yang, W.; Lu, K.; Peng, H.; Batlle, D.; Lavker, R.M. The ACE2-deficient mouse: A model for a cytokine storm-driven inflammation. FASEB J. 2020, 34, 10505–10515. [Google Scholar] [CrossRef] [PubMed]
- Felkle, D.; Jarczyński, M.; Kaleta, K.; Zięba, K.; Nazimek, K. The immunomodulatory effects of antihypertensive therapy: A review. Biomed. Pharmacother. 2022, 153, 113287. [Google Scholar] [CrossRef] [PubMed]
- Ziaja, M.; Urbanek, K.A.; Kowalska, K.; Piastowska-Ciesielska, A.W. Angiotensin II and Angiotensin Receptors 1 and 2—Multifunctional System in Cells Biology, What Do We Know? Cells 2021, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.Z.; Okwan-Duodu, D.; Blackwell, W.-L.; Ong, F.S.; Janjulia, T.; Bernstein, E.A.; Fuchs, S.; Alkan, S.; Bernstein, K.E. Myeloid expression of angiotensin-converting enzyme facilitates myeloid maturation and inhibits the development of myeloid-derived suppressor cells. Lab. Investig. 2014, 94, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Okwan-Duodu, D.; Weiss, D.; Peng, Z.; Veiras, L.C.; Cao, D.-Y.; Saito, S.; Khan, Z.; Bernstein, E.A.; Giani, J.F.; Taylor, W.R.; et al. Overexpression of myeloid angiotensin-converting enzyme (ACE) reduces atherosclerosis. Biochem. Biophys. Res. Commun. 2019, 520, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Sreedhar, R.; Thandavarayan, R.A.; Giridharan, V.V.; Karuppagounder, V.; Pitchaimani, V.; Afrin, M.R.; Miyashita, S.; Nomoto, M.; Harima, M.; et al. Telmisartan treatment targets inflammatory cytokines to suppress the pathogenesis of acute colitis induced by dextran sulphate sodium. Cytokine 2015, 74, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Barauna, V.G.; Mantuan, P.R.; Magalhães, F.C.; Campos, L.C.G.; Krieger, J.E. AT1 receptor blocker potentiates shear-stress induced nitric oxide production via modulation of eNOS phosphorylation of residues Thr495 and Ser1177. Biochem. Biophys. Res. Commun. 2013, 441, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, K.E.; Koronyo, Y.; Salumbides, B.C.; Sheyn, J.; Pelissier, L.; Lopes, D.H.; Shah, K.H.; Bernstein, E.A.; Fuchs, D.-T.; Yu, J.J.-Y.; et al. Angiotensin-converting enzyme overexpression in myelomonocytes prevents Alzheimer′s-like cognitive decline. J. Clin. Investig. 2014, 124, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Bryniarski, P.; Nazimek, K.; Marcinkiewicz, J. Anti-Inflammatory Activities of Captopril and Diuretics on Macrophage Activity in Mouse Humoral Immune Response. Int. J. Mol. Sci. 2021, 22, 11374. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Shen, X.Z.; Bernstein, E.A.; Giani, J.F.; Eriguchi, M.; Zhao, T.V.; Gonzalez-Villalobos, R.A.; Fuchs, S.; Liu, G.Y.; Bernstein, K.E. Angiotensin-converting enzyme enhances the oxidative response and bactericidal activity of neutrophils. Blood 2017, 130, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Okwan-Duodu, D.; Datta, V.; Shen, X.Z.; Goodridge, H.S.; Bernstein, E.A.; Fuchs, S.; Liu, G.Y.; Bernstein, K.E. Angiotensin-converting enzyme overexpression in mouse myelomonocytic cells augments resistance to Listeria and methicillin-resistant Staphylococcus aureus. J. Biol. Chem. 2010, 285, 39051–39060. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Lu, H.; Liu, H.; Yao, K.; Sun, A.; Zou, Y.; Ge, J. Losartan attenuates human monocyte-derived dendritic cell immune maturation via downregulation of lectin-like oxidized low-density lipoprotein receptor-1. J. Cardiovasc. Pharmacol. 2012, 60, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Torika, N.; Asraf, K.; Apte, R.N.; Fleisher-Berkovich, S. Candesartan ameliorates brain inflammation associated with Alzheimer’s disease. CNS Neurosci. Ther. 2018, 24, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Trikha, R.; Greig, D.; Kelley, B.V.; Mamouei, Z.; Sekimura, T.; Cevallos, N.; Olson, T.; Chaudry, A.; Magyar, C.; Leisman, D.; et al. Inhibition of Angiotensin Converting Enzyme Impairs Anti-staphylococcal Immune Function in a Preclinical Model of Implant Infection. Front. Immunol. 2020, 11, 1919. [Google Scholar] [CrossRef] [PubMed]
- El-Ganiny, A.M.; Gad, A.I.; El-Sayed, M.A.; Shaldam, M.A.; Abbas, H.A. The promising anti-virulence activity of candesartan, domperidone, and miconazole on Staphylococcus aureus. Braz. J. Microbiol. 2022, 53, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.A.; Gad, A.I.; El-Sayed, M.A.; El-Ganiny, A.M. Impeding Virulence of Candida albicans by Candesartan and Domperidone. Curr. Microbiol. 2021, 78, 3957–3967. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.-H.; Lee, J.-H.; Lee, C.J.; Shin, J.-H.; Kang, S.H.; Kwon, C.H.; Kim, D.-H.; Kim, W.-h.; Kim, H.L.; Kim, H.M.; et al. Effect of angiotensin receptor blockers on the development of cancer: A nationwide cohort study in korea. J. Clin. Hypertens. 2021, 23, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Boskabadi, J.; Askari, V.R.; Hosseini, M.; Boskabady, M.H. Immunomodulatory properties of captopril, an ACE inhibitor, on LPS-induced lung inflammation and fibrosis as well as oxidative stress. Inflammopharmacology 2019, 27, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.-H.; Liu, D.; Xu, M.; Liu, H.; Wu, M.; Tang, R.-N.; Lv, L.-L.; Ma, K.-L.; Liu, B.-C. Enalapril inhibits tubulointerstitial inflammation and NLRP3 inflammasome expression in BSA-overload nephropathy of rats. Acta Pharmacol. Sin. 2014, 35, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Stocker, N.; Radzikowska, U.; Wawrzyniak, P.; Tan, G.; Huang, M.; Ding, M.; Akdis, C.A.; Sokolowska, M. Regulation of angiotensin-converting enzyme 2 isoforms by type 2 inflammation and viral infection in human airway epithelium. Mucosal Immunol. 2023, 16, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Ogulur, I.; Mitamura, Y.; Yazici, D.; Pat, Y.; Ardicli, S.; Li, M.; D’aVino, P.; Beha, C.; Babayev, H.; Zhao, B.; et al. Type 2 immunity in allergic diseases. Cell. Mol. Immunol. 2025, 22, 211–242. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Im, D.-S. Suppressive effects of type I angiotensin receptor antagonists, candesartan and irbesartan on allergic asthma. Eur. J. Pharmacol. 2019, 852, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Cucak, H.; Fink, L.N.; Pedersen, M.H.; Rosendahl, A. Enalapril treatment increases T cell number and promotes polarization towards M1-like macrophages locally in diabetic nephropathy. Int. Immunopharmacol. 2015, 25, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Tawinwung, S.; Petpiroon, N.; Chanvorachote, P. Blocking of Type 1 Angiotensin II Receptor Inhibits T-lymphocyte Activation and IL-2 Production. Vivo 2018, 32, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Trebak, M.; Kinet, J.-P. Calcium signalling in T cells. Nat. Rev. Immunol. 2019, 19, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, H.; Wang, Y.; Xue, M.; Li, X.; Cheng, W.; Xuan, Y.; Yin, J.; Yang, N.; Yan, S. Valsartan Upregulates Kir2.1 in Rats Suffering from Myocardial Infarction via Casein Kinase 2. Cardiovasc. Drugs Ther. 2015, 29, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Lim, I.-R.; Joo, H.J.; Park, C.-Y.; Choi, S.-C.; Jeong, H.S.; Hong, S.J. Fimasartan reduces neointimal formation and inflammation after carotid arterial injury in apolipoprotein E knockout mice. Mol. Med. 2019, 25, 33. [Google Scholar] [CrossRef] [PubMed]
- Silva-Filho, J.L.; Souza, M.C.; Ferreira-DaSilva, C.T.; Silva, L.S.; Costa, M.F.S.; Padua, T.A.; Henriques, M.d.G.; Morrot, A.; Savino, W.; Caruso-Neves, C.; et al. Angiotensin II Is a New Component Involved in Splenic T Lymphocyte Responses during Plasmodium berghei ANKA Infection. PLoS ONE 2013, 8, e62999. [Google Scholar] [CrossRef] [PubMed]
- Waltman, T.J.; Harris, T.J.; Cesario, D.; Ziegler, M.; Maisel, A.S. Effects of enalapril on T and B cell function in rats after myocardial infarction. J. Card. Fail. 1995, 1, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Andersson, P.; Cederholm, T.; Johansson, A.-S.; Palmblad, J. Captopril-impaired production of tumor necrosis factor-α–induced interleukin-1β in human monocytes is associated with altered intracellular distribution of nuclear factor-κB. J. Lab. Clin. Med. 2002, 140, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-S.; Han, A.; Yeo, H.-L.; Park, M.-J.; You, M.-J.; Choi, H.J.; Hong, C.-W.; Lee, S.-H.; Kim, S.H.; Kim, B.; et al. Chronic high dose of captopril induces depressive-like behaviors in mice: Possible mechanism of regulatory T cell in depression. Oncotarget 2017, 8, 72528–72543. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-P.; Xie, X.-M. Captopril inhibits the production of tumor necrosis factor-alpha by human mononuclear cells in patients with congestive heart failure. Clin. Chim. Acta 2001, 304, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Carrasco, J.L.; Zambrano, S.; Blanca, A.J.; Mate, A.; Vázquez, C.M. Captopril reduces cardiac inflammatory markers in spontaneously hypertensive rats by inactivation of NF-kB. J. Inflamm. 2010, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Shalkami, A.-G.S.; Hassan, M.I.A.; El-Ghany, A.A.A. Perindopril regulates the inflammatory mediators, NF-κB/TNF-α/IL-6, and apoptosis in cisplatin-induced renal dysfunction. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.; Kane, A.; Heinze-Milne, S.; Grandy, S.A.; Howlett, S.E. Chronic Treatment With the ACE Inhibitor Enalapril Attenuates the Development of Frailty and Differentially Modifies Pro- and Anti-inflammatory Cytokines in Aging Male and Female C57BL/6 Mice. J. Gerontol. Ser. A 2019, 74, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Amirshahrokhi, K.; Ghazi-khansari, M.; Mohammadi-Farani, A.; Karimian, G. Effect of Captopril on TNF-α and IL-10 in the Livers of Bile Duct Ligated Rats. Iran. J. Immunol. 2010, 7, 247–251. [Google Scholar] [PubMed]
- Haas, M.J.; Jurado-Flores, M.; Hammoud, R.; Feng, V.; Gonzales, K.; Onstead-Haas, L.; Mooradian, A.D. The Effects of Known Cardioprotective Drugs on Proinflammatory Cytokine Secretion From Human Coronary Artery Endothelial Cells. Am. J. Ther. 2019, 26, e321–e332. [Google Scholar] [CrossRef] [PubMed]
- Salmenkari, H.; Pasanen, L.; Linden, J.; Korpela, R.; Vapaatalo, H. Beneficial Anti-Inflammatory Effect of Angiotensin-Converting Enzyme Inhibitor and Angiotensin Receptor Blocker in the Treatment of Dextran Sulfate Sodium-Induced Colitis in Mice. J. Physiol. Pharmacol. 2018, 69, 4. [Google Scholar]
- Wang, S.-L.; Yuan, B.; Dan, Q.-Q.; Yang, X.-Y.; Meng, B.-L.; Zhang, Y.-H. Effects of enalapril on IL-1beta, IL-6 expression in rat lung exposure to acrolein. Sichuan Da Xue Xue Bao Yi Xue Ban 2010, 41, 1003–1007, 1038. [Google Scholar] [PubMed]
- Navarro, J.J.; Milena, F.F.; Mora, C.; León, C.; Claverie, F.; Flores, C.; García, J. Tumor necrosis factor-alpha gene expression in diabetic nephropathy: Relationship with urinary albumin excretion and effect of angiotensin-converting enzyme inhibition. Kidney Int. 2005, 68, S98–S102. [Google Scholar] [CrossRef] [PubMed]
- Takamura, C.; Suzuki, J.-I.; Ogawa, M.; Watanabe, R.; Tada, Y.; Maejima, Y.; Akazawa, H.; Komuro, I.; Isobe, M. Suppression of murine autoimmune myocarditis achieved with direct renin inhibition. J. Cardiol. 2016, 68, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Gentile, A.; Musella, A.; De Vito, F.; Rizzo, F.R.; Fresegna, D.; Bullitta, S.; Vanni, V.; Guadalupi, L.; Bassi, M.S.; Buttari, F.; et al. Immunomodulatory Effects of Exercise in Experimental Multiple Sclerosis. Front. Immunol. 2019, 10, 2197. [Google Scholar] [CrossRef] [PubMed]
- Guerra, G.C.B.; Araújo, A.A.; Lira, G.A.; Melo, M.N.; Souto, K.K.O.; Fernandes, D.; Silva, A.L.; Araújo Júnior, R.F. Telmisartan decreases inflammation by modulating TNF-α, IL-10, and RANK/RANKL in a rat model of ulcerative colitis. Pharmacol. Rep. 2015, 67, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.-Y.; Giani, J.F.; Veiras, L.C.; Bernstein, E.A.; Okwan-Duodu, D.; Ahmed, F.; Bresee, C.; Tourtellotte, W.G.; Karumanchi, S.A.; Bernstein, K.E.; et al. An ACE inhibitor reduces bactericidal activity of human neutrophils in vitro and impairs mouse neutrophil activity in vivo. Sci. Transl. Med. 2021, 13, eabj2138. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, K.E.; Cao, D.Y.; Shibata, T.; Saito, S.; Bernstein, E.A.; Nishi, E.; Yamashita, M.; Tourtellotte, W.G.; Zhao, T.V.; Khan, Z. Classical and nonclassical effects of angiotensin-converting enzyme: How increased ACE enhances myeloid immune function. J. Biol. Chem. 2024, 300, 107388. [Google Scholar] [CrossRef] [PubMed]
- Godsel, L.M.; Leon, J.S.; Wang, K.; Fornek, J.L.; Molteni, A.; Engman, D.M. Captopril Prevents Experimental Autoimmune Myocarditis. J. Immunol. 2003, 171, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Benicky, J.; Sánchez-Lemus, E.; Honda, M.; Pang, T.; Orecna, M.; Wang, J.; Leng, Y.; Chuang, D.-M.; Saavedra, J.M. Angiotensin II AT1 Receptor Blockade Ameliorates Brain Inflammation. Neuropsychopharmacology 2011, 36, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-S.; He, S.-L.; Zhang, Y.-M. The effects of telmisartan on the nuclear factor of activated T lymphocytes signalling pathway in hypertensive patients. J. Renin-Angiotensin-Aldosterone Syst. 2016, 17, 1470320316655005. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liang, L.; Liu, S.; Kung, J.Y.; Banh, H.L. Angiotensin-converting enzyme inhibitor induced cough compared with placebo, and other antihypertensives: A systematic review, and network meta-analysis. J. Clin. Hypertens. 2023, 25, 661–688. [Google Scholar] [CrossRef] [PubMed]
- Hundemer, G.L.; Sood, M.M. Hyperkalemia with RAAS inhibition: Mechanism, clinical significance, and management. Pharmacol. Res. 2021, 172, 105835. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef] [PubMed]
- Stegbauer, J.; Lee, D.-H.; Seubert, S.; Ellrichmann, G.; Manzel, A.; Kvakan, H.; Muller, D.N.; Gaupp, S.; Rump, L.C.; Gold, R.; et al. Role of the renin-angiotensin system in autoimmune inflammation of the central nervous system. Proc. Natl. Acad. Sci. USA 2009, 106, 14942–14947. [Google Scholar] [CrossRef] [PubMed]
- Bahk, T.J.; Daniels, M.D.; Leon, J.S.; Wang, K.; Engman, D.M. Comparison of angiotensin converting enzyme inhibition and angiotensin II receptor blockade for the prevention of experimental autoimmune myocarditis. Int. J. Cardiol. 2008, 125, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Leisman, D.E.; Privratsky, J.R.; Lehman, J.R.; Abraham, M.N.; Yaipan, O.Y.; Brewer, M.R.; Nedeljkovic-Kurepa, A.; Capone, C.C.; Fernandes, T.D.; Griffiths, R.; et al. Angiotensin II enhances bacterial clearance via myeloid signaling in a murine sepsis model. Proc. Natl. Acad. Sci. USA 2022, 119, e2211370119. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, J.; Witkowski, J.M.; Olszanecki, R. The dual role of the immune system in the course of COVID-19. The fatal impact of the aging immune system. Cent. Eur. J. Immunol. 2021, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, Z.; Ni, L.; Chen, L.; Zhou, C.; Gao, C.; Wu, X.; Hua, L.; Huang, X.; Cui, X.; et al. Impact of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers on the Inflammatory Response and Viral Clearance in COVID-19 Patients. Front. Cardiovasc. Med. 2021, 8, 710946. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.; de Oliveira, S.C. The Impact of Angiotensin-Converting Enzyme 2 (ACE2) Expression Levels in Patients with Comorbidities on COVID-19 Severity: A Comprehensive Review. Microorganisms 2021, 9, 1692. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, S.H.; Wehbe, Z.; Abdelhady, S.; Kobeissy, F.; Eid, A.H.; El-Yazbi, A.F. Dysregulation of Angiotensin Converting Enzyme 2 Expression and Function in Comorbid Disease Conditions Possibly Contributes to Coronavirus Infectious Disease 2019 Complication Severity. Mol. Pharmacol. 2021, 99, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Amraei, R.; Rahimi, N. COVID-19, Renin-Angiotensin System and Endothelial Dysfunction. Cells 2020, 9, 1652. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, P.; Gu, W.; Yang, C.; Yang, X.; Deng, X.; Xu, J.; Jiang, J.; Jiang, C. Potential of angiotensin II receptor blocker telmisartan in reducing mortality among hospitalized patients with COVID-19 compared with recommended drugs. Cell Discov. 2022, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- García-Álvarez, R.M.; Zapata-Cachafeiro, M.; Visos-Varela, I.; Rodríguez-Fernández, A.; Pintos-Rodríguez, S.; Piñeiro-Lamas, M.; Herdeiro, T.M.; Figueiras, A.; Salgado-Barreira, A.; COVID-Drug Group. Impact of prior antihypertensive treatment on COVID-19 outcomes, by active ingredient. Inflammopharmacology 2024, 32, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shi, X.; Yu, J.; Lv, F.; Wu, J.; Sheng, X.; Pan, Q.; Yang, J.; Cao, H.; Li, L. Association of ACEi/ARB Use and Clinical Outcomes of COVID-19 Patients With Hypertension. Front. Cardiovasc. Med. 2021, 8, 577398. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.Y.; Kondo, T.; Campbell, R.T.; Petrie, M.C.; Sattar, N.; Solomon, S.D.; Vaduganathan, M.; Jhund, P.S.; McMurray, J.J.V. Effects of renin–angiotensin system blockers on outcomes from COVID-19: A systematic review and meta-analysis of randomized controlled trials. Eur. Heart J.-Cardiovasc. Pharmacother. 2024, 10, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Nandave, M. Role of ACE Inhibitors and Angiotensin Receptor Blockers in Covid19 Patients. In Angiotensin-Converting Enzyme Inhibitors vs. Angiotensin Receptor Blockers; Nandave, M., Ed.; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Mancia, G.; Rea, F.; Ludergnani, M.; Apolone, G.; Corrao, G. Renin–Angiotensin–Aldosterone System Blockers and the Risk of COVID-19. N. Engl. J. Med. 2020, 382, 2431–2440. [Google Scholar] [CrossRef] [PubMed]
- Fosbøl, E.L.; Butt, J.H.; Østergaard, L.; Andersson, C.; Selmer, C.; Kragholm, K.; Schou, M.; Phelps, M.; Gislason, G.H.; Gerds, T.A.; et al. Association of Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Use With COVID-19 Diagnosis and Mortality. JAMA 2020, 324, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Zhu, B.; Ren, J.; Song, X.; Fan, B.; Ding, H.; Shang, J.; Wu, H.; Li, J.; Wang, H.; et al. Ang-(1–7)/MasR axis promotes functional recovery after spinal cord injury by regulating microglia/macrophage polarization. Cell Biosci. 2023, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Laghlam, D.; Jozwiak, M.; Nguyen, L.S. Renin–Angiotensin–Aldosterone System and Immunomodulation: A State-of-the-Art Review. Cells 2021, 10, 1767. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.K.; Gibson, W.; Giri, S.; Nath, N.; Donald, C.D. Angiotensin II up-regulates PAX2 oncogene expression and activity in prostate cancer via the angiotensin II type I receptor. Prostate 2009, 69, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, J.; Fushida, S.; Harada, S.; Yagi, Y.; Fujita, H.; Kinami, S.; Ninomiya, I.; Fujimura, T.; Kayahara, M.; Yashiro, M.; et al. Local angiotensin II-generation in human gastric cancer: Correlation with tumor progression through the activation of ERK1/2, NF-κB and survivin. Int. J. Oncol. 2009, 34, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, P.; Wang, M.; Zheng, Y.; Tian, T.; Yang, S.; Deng, Y.; Wu, Y.; Zhai, Z.; Hao, Q.; et al. Antihypertensive medications are associated with the risk of kidney and bladder cancer: A systematic review and meta-analysis. Aging 2020, 12, 1545–1562. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yi, C.-H.; Ya, K.-G. Renin–angiotensin system inhibitor use and colorectal cancer risk and mortality: A dose-response meta analysis. J. Renin-Angiotensin-Aldosterone Syst. 2020, 21, 1470320319895646. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Park, J.; Park, R.W.; Shin, S.J.; Kim, J.; Sung, J.D.; Kim, D.J.; Yang, K. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Cancer: A Population-Based Cohort Study Using a Common Data Model. Diagnostics 2022, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Hicks, B.M.; Filion, K.B.; Yin, H.; Sakr, L.; Udell, J.A.; Azoulay, L. Angiotensin converting enzyme inhibitors and risk of lung cancer: Population based cohort study. BMJ 2018, 363, k4209. [Google Scholar] [CrossRef] [PubMed]
- Sever, N.; Yunusov, E.; Çelebi, A.; Yaşar, A.; Majidova, N.; Kocaaslan, E.; Erel, P.; Ağyol, Y.; Güren, A.K.; Işık, S.; et al. Impact of renin angiotensinsystem inhibitors on survival of patients with metastatic non-small cell lung cancer. Ann. Saudi Med. 2025, 45, 18–24. [Google Scholar]
- Santala, E.E.E.; Murto, M.O.; Artama, M.; Pukkala, E.; Visvanathan, K.; Murtola, T.J. Angiotensin Receptor Blockers Associated with Improved Breast Cancer Survival—A Nationwide Cohort Study from Finland. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2376–2382. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Choi, C.H.; Kim, M.K.; Kim, M.L.; Yun, B.S.; Seong, S.J. The effect of angiotensin system inhibitors (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) on cancer recurrence and survival: A meta-analysis. Eur. J. Cancer Prev. 2017, 26, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Sun, J.F.; Hu, S.Q. The renin-angiotensin system blockers as adjunctive therapy for cancer: A meta-analysis of survival outcome. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1375–1383. [Google Scholar] [PubMed]
- Riddiough, G.E.; Fifis, T.; Walsh, K.A.; Muralidharan, V.; Christophi, C.; Tran, B.M.; Vincan, E.; Perini, M.V. Captopril, a Renin-Angiotensin System Inhibitor, Attenuates Features of Tumor Invasion and Down-Regulates C-Myc Expression in a Mouse Model of Colorectal Cancer Liver Metastasis. Cancers 2021, 13, 2734. [Google Scholar] [CrossRef] [PubMed]
- Regan, D.P.; Coy, J.W.; Chahal, K.K.; Chow, L.; Kurihara, J.N.; Guth, A.M.; Kufareva, I.; Dow, S.W. The Angiotensin Receptor Blocker Losartan Suppresses Growth of Pulmonary Metastases via AT1R-Independent Inhibition of CCR2 Signaling and Monocyte Recruitment. J. Immunol. 2019, 202, 3087–3102. [Google Scholar] [CrossRef] [PubMed]
- Gulati, G.; Heck, S.L.; Ree, A.H.; Hoffmann, P.; Schulz-Menger, J.; Fagerland, M.W.; Gravdehaug, B.; von Knobelsdorff-Brenkenhoff, F.; Bratland, Å.; Storås, T.H.; et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): A 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J. 2016, 37, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Guglin, M.; Krischer, J.; Tamura, R.; Fink, A.; Bello-Matricaria, L.; McCaskill-Stevens, W.; Munster, P.N. Randomized Trial of Lisinopril Versus Carvedilol to Prevent Trastuzumab Cardiotoxicity in Patients With Breast Cancer. J. Am. Coll. Cardiol. 2019, 73, 2859–2868. [Google Scholar] [CrossRef] [PubMed]
- Wittayanukorn, S.; Qian, J.; Westrick, S.C.; Billor, N.; Johnson, B.; Hansen, R.A. Prevention of Trastuzumab and Anthracycline-induced Cardiotoxicity Using Angiotensin-converting Enzyme Inhibitors or β-blockers in Older Adults With Breast Cancer. Am. J. Clin. Oncol. 2018, 41, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Fazal, K.; Perera, G.; Khondoker, M.; Howard, R.; Stewart, R. Associations of centrally acting ACE inhibitors with cognitive decline and survival in Alzheimer’s disease. BJPsych Open 2017, 3, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; O’CAoimh, R.; Healy, L.; Kerins, D.M.; Eustace, J.; Guyatt, G.; Sammon, D.; Molloy, D.W. Effects of centrally acting ACE inhibitors on the rate of cognitive decline in dementia. BMJ Open 2013, 3, e002881. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Healy, L.; Gao, Y.; Svendrovski, A.; Kerins, D.M.; Eustace, J.; Kehoe, P.G.; Guyatt, G.; Molloy, D.W. Effects of centrally acting angiotensin converting enzyme inhibitors on functional decline in patients with Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 40, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Adesuyan, M.; Brauer, R.; Jani, Y. Central-acting vs. Peripheral-acting Angiotensin Converting Enzyme-inhibitors and the risk of Alzheimer’s disease: A cohort study. Alzheimer′s Dement. 2023, 19 (Suppl. 22), e071074. [Google Scholar] [CrossRef]
- Ho, J.K.; Moriarty, F.; Manly, J.J.; Larson, E.B.; Evans, D.A.; Rajan, K.B.; Hudak, E.M.; Hassan, L.; Liu, E.; Sato, N.; et al. Blood-Brain Barrier Crossing Renin-Angiotensin Drugs and Cognition in the Elderly: A Meta-Analysis. Hypertension 2021, 78, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Jiang, J.; Wang, J.; Pan, D.; Zhu, Y.; Li, H.; Zhang, X.; Liu, X.; Xu, Y.; Li, Y.; et al. Angiotensin Receptor Blockers Are Associated With a Lower Risk of Progression From Mild Cognitive Impairment to Dementia. Hypertension 2022, 79, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Wang, H.-F.; Wang, X.; Li, J.; Xing, C.-M. The association of renin-angiotensin system blockade use with the risks of cognitive impairment of aging and Alzheimer’s disease: A meta-analysis. J. Clin. Neurosci. 2016, 33, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Wang, S.Y.; Wei, B.; Deng, Y.; Fu, X.X.; Gong, P.Y.; Sun, X.J.; Cao, H.M.; Shi, J.Q.; Jiang, T.; et al. Angiotensin-(1–7) Analogue AVE0991 Modulates Astrocyte-Mediated Neuroinflammation via lncRNA SNHG14/miR-223-3p/NLRP3 Pathway and Offers Neuroprotection in a Transgenic Mouse Model of Alzheimer′s Disease. J. Inflamm. Res. 2021, 14, 7007–7019. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-F.; Kataoka, K.; Tokutomi, Y.; Nako, H.; Nakamura, T.; Toyama, K.; Sueta, D.; Koibuchi, N.; Yamamoto, E.; Ogawa, H.; et al. Perindopril, a centrally active angiotensin-converting enzyme inhibitor, prevents cognitive impairment in mouse models of Alzheimer’s disease. FASEB J. 2011, 25, 2911–2920. [Google Scholar] [CrossRef] [PubMed]
- Rygiel, K. Can angiotensin-converting enzyme inhibitors impact cognitive decline in early stages of Alzheimer’s disease? An overview of research evidence in the elderly patient population. J. Postgrad. Med. 2016, 62, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Ababei, D.-C.; Bild, V.; Macadan, I.; Vasincu, A.; Rusu, R.-N.; Blaj, M.; Stanciu, G.D.; Lefter, R.-M.; Bild, W. Therapeutic Implications of Renin–Angiotensin System Modulators in Alzheimer’s Dementia. Pharmaceutics 2023, 15, 2290. [Google Scholar] [CrossRef] [PubMed]
| Class | Examples | Mechanism of Action |
|---|---|---|
| ACE inhibitors (centrally acting—C-ACEIs) | Perindopril, ramipril, trandolapril | Cross the blood–brain barrier (lipophilic); may affect the central RAAS and neuroinflammation [10,15] |
| ACE inhibitors (non-centrally acting—NC-ACEIs) | Enalapril, lisinopril, benazepril | Do not significantly penetrate the CNS; act primarily peripherally [11,12] |
| Angiotensin II receptor blockers (ARBs) | Losartan, telmisartan, valsartan, candesartan | Block AT1 receptors; some (e.g., telmisartan) also activate PPARγ [13,16] |
| Direct renin inhibitors | Aliskiren | Inhibit conversion of angiotensinogen to Ang I; rarely used alone [17] |
| Mineralocorticoid receptor antagonists (MRAs) | Spironolactone, eplerenone | Block aldosterone receptors; reduce sodium retention and fibrosis [14] |
| Domain | ACEIs | ARBs |
|---|---|---|
| Tumor cell biology | Reduce proliferation and metastasis; induce apoptosis (e.g., captopril in colorectal cancer) [108] | Suppress tumor growth and angiogenesis [109] |
| Angiogenesis | Indirect inhibition via decreased Ang II levels [108] | Direct inhibition via AT1R blockade; suppress VEGF-A expression [98] |
| Immune modulation | Modify tumor microenvironment: ↑CD4−CD8−, ↓CD4+ T-cells; complex immunoregulatory effects [108] | Less clearly defined; potential anti-inflammatory role |
| Cancer risk | Increased risk of lung, bladder, and kidney cancers (long-term use) [100,103] | Decreased risk of colorectal, keratinocyte, and prostate cancers [101,102] |
| Cancer survival | Improve survival, particularly when combined with chemotherapy [104,107] | Associated with reduced cancer-specific mortality; dose–response effect seen in breast cancer [105] |
| Cardiotoxicity protection | Mitigate cardiotoxicity from anthracyclines and trastuzumab [110,111,112] | Similar cardioprotective effects in chemotherapy settings [110,111,112] |
| Domain | ACEIs | ARBs |
|---|---|---|
| Amyloid beta clearance | Inhibit ACE-mediated Aβ1–42 degradation; potential reduction in clearance [37] | Not directly involved in Aβ degradation |
| Cognitive outcomes | C-ACEIs (e.g., perindopril, ramipril) shown to provide short-term improvement; NC-ACEIs less effective [113] | Lower dementia risk versus that with ACEIs in hypertensive patients with mild cognitive impairment [118] |
| Neuroinflammation | Reduce pro-inflammatory cytokines; potential neuroprotective effect [120] | May exert anti-inflammatory effects via AT1R blockade [120] |
| Cerebral perfusion | Improve cerebral blood flow by inhibiting Ang II-mediated vasoconstriction [121] | Reduce vasoconstriction via AT1R blockade [118,120] |
| Cholinergic function | Enhance acetylcholine release; may synergize with cholinesterase inhibitors [113] | Effect on cholinergic transmission not well established |
| Clinical implications | Potential early-stage benefit in AD; recommended in hypertensive older adults [113] | Lower dementia risk in hypertensive patients with mild cognitive impairment [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haliga, R.E.; Cojocaru, E.; Sîrbu, O.; Hrițcu, I.; Alexa, R.E.; Haliga, I.B.; Șorodoc, V.; Coman, A.E. Immunomodulatory Effects of RAAS Inhibitors: Beyond Hypertension and Heart Failure. Biomedicines 2025, 13, 1779. https://doi.org/10.3390/biomedicines13071779
Haliga RE, Cojocaru E, Sîrbu O, Hrițcu I, Alexa RE, Haliga IB, Șorodoc V, Coman AE. Immunomodulatory Effects of RAAS Inhibitors: Beyond Hypertension and Heart Failure. Biomedicines. 2025; 13(7):1779. https://doi.org/10.3390/biomedicines13071779
Chicago/Turabian StyleHaliga, Raluca Ecaterina, Elena Cojocaru, Oana Sîrbu, Ilinca Hrițcu, Raluca Elena Alexa, Ioana Bianca Haliga, Victorița Șorodoc, and Adorata Elena Coman. 2025. "Immunomodulatory Effects of RAAS Inhibitors: Beyond Hypertension and Heart Failure" Biomedicines 13, no. 7: 1779. https://doi.org/10.3390/biomedicines13071779
APA StyleHaliga, R. E., Cojocaru, E., Sîrbu, O., Hrițcu, I., Alexa, R. E., Haliga, I. B., Șorodoc, V., & Coman, A. E. (2025). Immunomodulatory Effects of RAAS Inhibitors: Beyond Hypertension and Heart Failure. Biomedicines, 13(7), 1779. https://doi.org/10.3390/biomedicines13071779









