A Biological-Driven Approach to Explore Dose-Escalated Ultra-Hypofractionation in Breast Cancer Radiotherapy
Abstract
1. Introduction
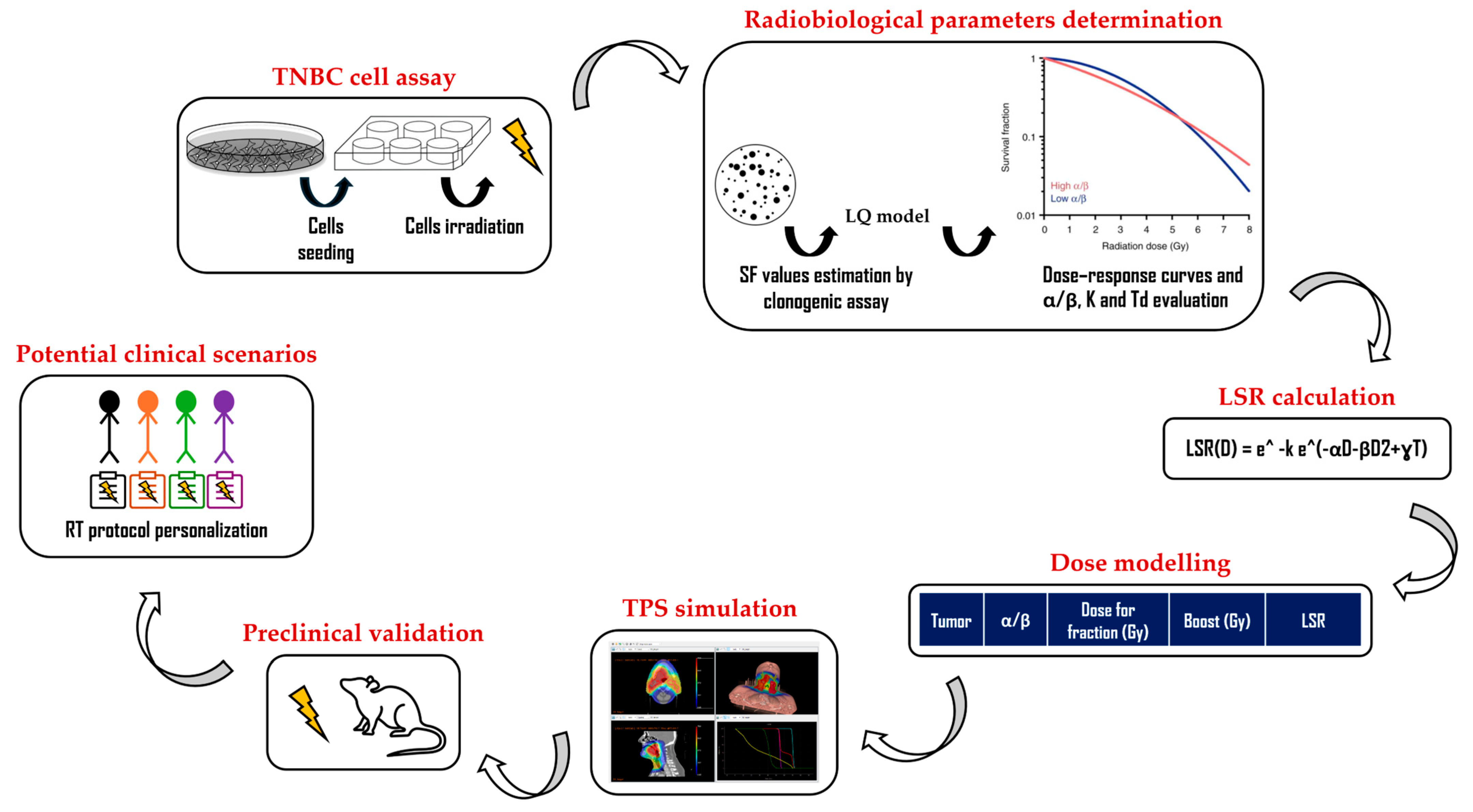
2. Materials and Methods
2.1. Cell Cultures and Radiation Treatments
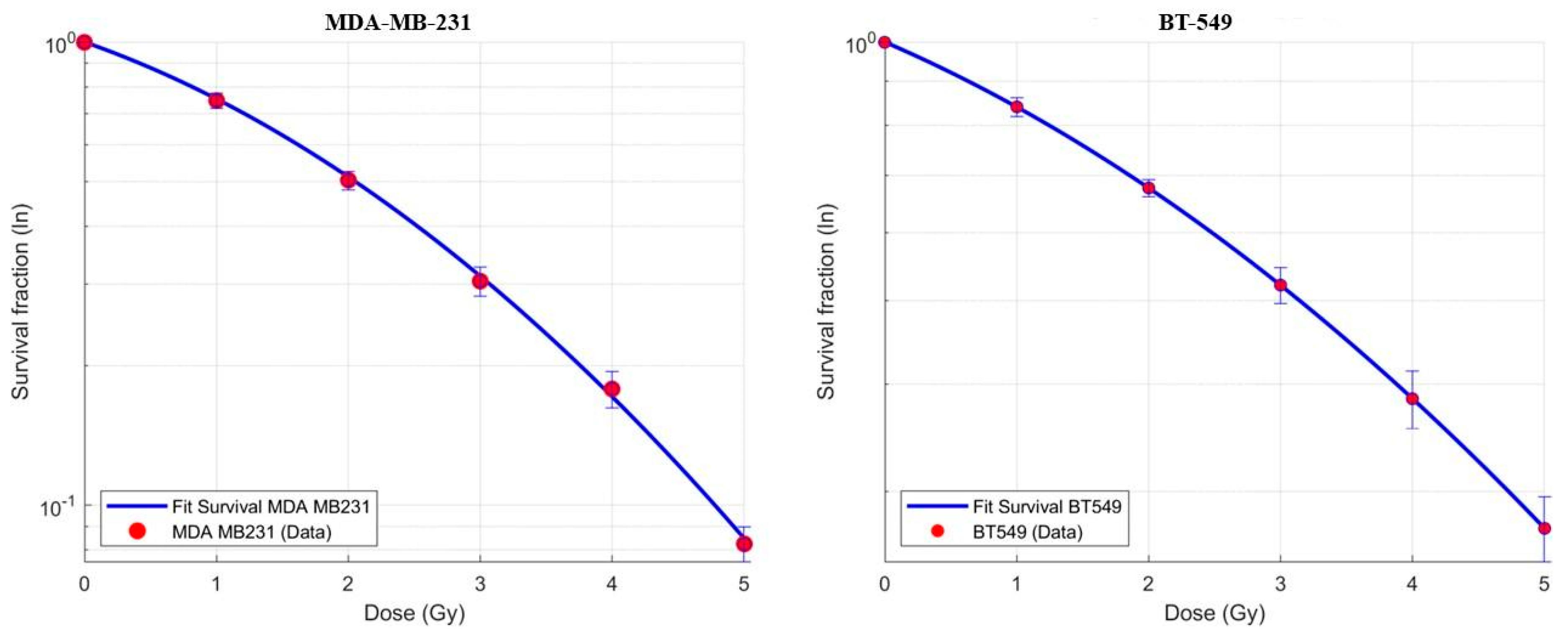
2.2. Clonogenic Assay, Dose–Response Curves, and α and β Parameter Determination
2.3. Local Disease-Free Survival-Rate Model Application
2.4. Treatment-Planning System Simulation
3. Results
3.1. Dose–Response Curves and (α) and (β) Parameter Determination
3.2. Application of the Local Disease-Free Survival-Rate Model
3.3. Treatment-Planning System Simulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TNBC | Triple-negative breast cancer |
| LSR | Local disease-free survival |
| RT | Radiotherapy |
| IMRT | Intensity-modulated radiation therapy |
| IGRT | Image-guided radiation therapy |
| SBRT | Stereotactic body radiation therapy |
| SF | Surviving fraction |
| PTV | Planning target volume |
| CTV | Clinical target volume |
| pCR | Pathological complete response |
| SOC | Standard of care |
| OAR | Organ at risk |
References
- Zhu, L.; Lu, X.; Wang, A.; Xiang, Z. Comparison of VMAT and IMRT plans for SBRT treatment of multiple liver metastases using a single isocenter. Int. J. Radiat. Res. 2023, 21, 189–194. [Google Scholar]
- de Mey, S.; Dufait, I.; De Ridder, M. Radioresistance of Human Cancers: Clinical Implications of Genetic Expression Signatures. Front. Oncol. 2021, 11, 761901. [Google Scholar] [CrossRef] [PubMed]
- Giardino, A.; Gupta, S.; Olson, E.; Sepulveda, K.; Lenchik, L.; Ivanidze, J.; Rakow-Penner, R.; Patel, M.J.; Subramaniam, R.M.; Ganeshan, D. Role of Imaging in the Era of Precision Medicine. Acad. Radiol. 2017, 24, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Alluri, P.; Newman, L.A. Basal-like and triple-negative breast cancers: Searching for positives among many negatives. Surg. Oncol. Clin. N. Am. 2014, 23, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Savoca, G.; Calvaruso, M.; Minafra, L.; Bravatà, V.; Cammarata, F.P.; Iacoviello, G.; Abbate, B.; Evangelista, G.; Spada, M.; Forte, G.I.; et al. Local Disease-Free Survival Rate (LSR) Application to Personalize Radiation Therapy Treatments in Breast Cancer Models. J. Pers. Med. 2020, 10, 177. [Google Scholar] [CrossRef]
- START Trialists’ Group; Bentzen, S.M.; Agrawal, R.K.; Aird, E.G.; Barrett, J.M.; Barrett-Lee, P.J.; Bliss, J.M.; Brown, J.; Dewar, J.A.; Dobbs, H.J.; et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet Oncol. 2008, 9, 331–341. [Google Scholar] [CrossRef]
- START Trialists’ Group; Bentzen, S.M.; Agrawal, R.K.; Aird, E.G.; Barrett, J.M.; Barrett-Lee, P.J.; Bentzen, S.M.; Bliss, J.M.; Brown, J.; Dewar, J.A.; et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet 2008, 371, 1098–1107. [Google Scholar] [CrossRef]
- Murray Brunt, A.; Haviland, J.S.; Wheatley, D.A.; Sydenham, M.A.; Alhasso, A.; Bloomfield, D.J.; Chan, C.; Churn, M.; Cleator, S.; Coles, C.E.; et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020, 395, 1613–1626. [Google Scholar] [CrossRef]
- Minafra, L.; Porcino, N.; Bravatà, V.; Gaglio, D.; Bonanomi, M.; Amore, E.; Cammarata, F.P.; Russo, G.; Militello, C.; Savoca, G.; et al. Radiosensitizing effect of curcumin-loaded lipid nanoparticles in breast cancer cells. Sci. Rep. 2019, 9, 11134. [Google Scholar] [CrossRef]
- Lee, K.J.; Mann, E.; Wright, G.; Piett, C.G.; Nagel, Z.D.; Gassman, N.R. Exploiting DNA repair defects in triple negative breast cancer to improve cell killing. Ther. Adv. Med. Oncol. 2020, 12, 1758835920958354. [Google Scholar] [CrossRef]
- Grosche, S.; Bogdanova, N.V.; Ramachandran, D.; Lüdeking, M.; Stemwedel, K.; Christiansen, H.; Henkenberens, C.; Merten, R. Effectiveness of hypofractionated and normofractionated radiotherapy in a triple-negative breast cancer model. Front. Oncol. 2022, 12, 852694. [Google Scholar] [CrossRef]
- Hawkins, R.B. Effect of heterogeneous radio sensitivity on the survival, alpha beta ratio and biologic effective dose calculation of irradiated mammalian cell populations. Clin. Transl. Radiat. Oncol. 2017, 4, 32–38. [Google Scholar] [CrossRef][Green Version]
- Kaplan, H.G.; Malmgren, J.A. Impact of Triple Negative Phenotype on Breast Cancer Prognosis. Breast J. 2008, 14, 456–463. [Google Scholar] [CrossRef]
- Mayer, I.A.; Dent, R.; Tan, T.; Savas, P.; Loi, S. Novel Targeted Agents and Immunotherapy in Breast Cancer. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; De Angelis, C.; Licata, L.; Gianni, L. Treatment landscape of triple-negative breast cancer—Expanded options, evolving needs. Nat. Rev. Clin. Oncol. 2022, 19, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Korde, L.A.; Somerfield, M.R.; Hershman, D.L.; Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer Guideline Expert Panel. Use of Immune Checkpoint Inhibitor Pembrolizumab in the Treatment of High-Risk, Early-Stage Triple-Negative Breast Cancer: ASCO Guideline Rapid Recommendation Update. J. Clin. Oncol. 2022, 40, 1696–1698. [Google Scholar] [CrossRef] [PubMed]
- Ciammella, P.; Podgornii, A.; Galeandro, M.; Micera, R.; Ramundo, D.; Palmieri, T.; Cagni, E.; Iotti, C. Toxicity and cosmetic outcome of hypofractionated whole-breast radiotherapy: Predictive clinical and dosimetric factors. Radiat. Oncol. 2014, 9, 97. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lazzari, G.; Terlizzi, A.; Della Vittoria Scarpati, G.; Perri, F.; De Chiara, V.; Turi, B.; Silvano, G. Predictive parameters in hypofractionated whole-breast 3D conformal radiotherapy according to the Ontario Canadian trial. Onco. Targets Ther. 2017, 10, 1835–1842. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gage, I.; Recht, A.; Gelman, R.; Nixon, A.J.; Silver, B.; Bornstein, B.A.; Harris, J.R. Long-term outcome following breast-conserving surgery and radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Vicini, F.A.; Kestin, L.L.; Goldstein, N.S. Defining the clinical target volume for patients with early-stage breast cancer treated with lumpectomy and accelerated partial breast irradiation: A pathologic analysis. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Bartelink, H.; Horiot, J.C.; Poortmans, P.M.; Struikmans, H.; Van den Bogaert, W.; Fourquet, A.; Jager, J.J.; Hoogenraad, W.J.; Oei, S.B.; Wárlám-Rodenhuis, C.C.; et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J. Clin. Oncol. 2007, 25, 3259–3265. [Google Scholar] [CrossRef] [PubMed]
- Coles, C.E.; Haviland, J.S.; Kirby, A.M.; Griffin, C.L.; Sydenham, M.A.; Titley, J.C.; Bhattacharya, I.; Brunt, A.M.; Chan, H.Y.C.; Donovan, E.M.; et al. Dose-escalated simultaneous integrated boost radiotherapy in early breast cancer (IMPORT HIGH): A multicentre, phase 3, non-inferiority, open-label, randomised controlled trial. Lancet 2023, 401, 2124–2137. [Google Scholar] [CrossRef] [PubMed]
- Livi, L.; Meattini, I.; Franceschini, D.; Saieva, C.; Meacci, F.; Marrazzo, L.; Gerlain, E.; Desideri, I.; Scotti, V.; Nori, J.; et al. Radiotherapy boost dose-escalation for invasive breast cancer after breast-conserving surgery: 2093 patients treated with a prospective margin-directed policy. Radiother. Oncol. 2013, 108, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; Moulder-Thompson, S.L. Neoadjuvant treatment of breast cancer. Ann. Oncol. 2012, 23 (Suppl. 10), x231–x236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spring, L.M.; Fell, G.; Arfe, A.; Sharma, C.; Greenup, R.; Reynolds, K.L.; Smith, B.L.; Alexander, B.; Moy, B.; Isakoff, S.J.; et al. Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis. Clin. Cancer Res. 2020, 26, 2838–2848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soares, R.F.; Garcia, A.R.; Monteiro, A.R.; Macedo, F.; Pereira, T.C.; Carvalho, J.C.; Pêgo, A.; Mariano, M.; Madeira, P.; Póvoa, S.; et al. Prognostic factors for early relapse in non-metastatic triple negative breast cancer—Real world data. Rep. Pract. Oncol Radiother. 2021, 26, 563–572. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colciago, R.R.; De Santis, M.C.; Giandini, C.; Carnevale, M.G.; Di Cosimo, S. Treatment of oligometastatic breast cancer: The role of patient selection. Breast 2025, 79, 103839. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chmura, S.J.; Winter, K.A.; Woodward, W.A.; Borges, V.F.; Salama, J.K.; Al-Hallaq, H.A.; Matuszak, M.; Milano, M.T.; Jaskowiak, N.T.; Bandos, H.; et al. NRG-BR002: A phase IIR/III trial of standard of care systemic therapy with or without stereotactic body radiotherapy (SBRT) and/or surgical resection (SR) for newly oligometastatic breast cancer (NCT02364557). J. Clin. Oncol. 2022, 40, 1007. [Google Scholar] [CrossRef]
- Tsai, C.J.; Yang, J.T.; Shaverdian, N.; Patel, J.; Shepherd, A.F.; Guttmann, D.; Yeh, R.; Gelblum, D.Y.; Namakydoust, A.; Preeshagul, I.; et al. Standard-of-care systemic therapy with or without stereotactic body radiotherapy in patients with oligoprogressive breast cancer or non-small-cell lung cancer (Consolidative Use of Radiotherapy to Block [CURB] oligoprogression): An open-label, randomised, controlled, phase 2 study. Lancet 2024, 403, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Minafra, L.; Cammarata, F.P.; Calvaruso, M. The Role of Radiation in Cancer Treatment: New Insights towards Personalized Therapies. J. Pers. Med. 2022, 12, 312. [Google Scholar] [CrossRef]
- Calvaruso, M.; Pucci, G.; Alberghina, C.; Minafra, L. Radiation Therapy Personalization in Cancer Treatment: Strategies and Perspectives. Int. J. Mol. Sci. 2025, 26, 6375. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh, A.; Rasti, R.; Tavakoli, M.B. Artificial intelligence for radiotherapy dose prediction: A comprehensive review. Cancer Radiother. 2025, 29, 104630. [Google Scholar] [CrossRef] [PubMed]
| Cell Line | Alpha/Beta | Dose for Fraction (Gy) | Boost (Gy) | LSR |
|---|---|---|---|---|
| BT549 | 16.51 | 5.2 | - | 82.0% |
| BT549 | 5.95 | 5.2 | - | 84.0% |
| BT549 | 22.93 | 5.2 | - | 75.0% |
| BT549 | 16.51 | 5.2 | 2 | 96.5% |
| BT549 | 5.95 | 5.2 | 2 | 97.4% |
| BT549 | 22.93 | 5.2 | 2 | 94.6% |
| MDA | 7 | 5.2 | - | 100% |
| MDA | 3.79 | 5.2 | - | 93.7% |
| MDA | 15 | 5.2 | - | 99.9% |
| MDA | 7 | 5.2 | 2 | 100% |
| MDA | 3.79 | 5.2 | 2 | 98.5% |
| MDA | 15 | 5.2 | 2 | 100% |
| Cell Line | Alpha/Beta | Dose for Fraction (Gy) | Boost (Gy) | LSR |
|---|---|---|---|---|
| BT549 | 16.51 | 9.1 | - | 100% |
| BT549 | 5.95 | 8 | - | 100% |
| BT549 | 22.93 | 9.9 | - | 100% |
| BT549 | 16.51 | 5.2 | 5.6 | 100% |
| BT549 | 5.95 | 5.2 | 5 | 100% |
| BT549 | 22.93 | 5.2 | 6.2 | 100% |
| MDA | 7 | 5.2 | - | 100% |
| MDA | 3.79 | 7.3 | - | 100% |
| MDA | 15 | 5.3 | - | 100% |
| MDA | 7 | 5.2 | 2 | 100% |
| MDA | 3.79 | 5.2 | 4.4 | 100% |
| MDA | 15 | 5.2 | 2 | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvaruso, M.; Panizza, D.; Colciago, R.R.; Faccenda, V.; Pucci, G.; Ponti, E.D.; Forte, G.I.; Russo, G.; Minafra, L.; Arcangeli, S. A Biological-Driven Approach to Explore Dose-Escalated Ultra-Hypofractionation in Breast Cancer Radiotherapy. Biomedicines 2025, 13, 2154. https://doi.org/10.3390/biomedicines13092154
Calvaruso M, Panizza D, Colciago RR, Faccenda V, Pucci G, Ponti ED, Forte GI, Russo G, Minafra L, Arcangeli S. A Biological-Driven Approach to Explore Dose-Escalated Ultra-Hypofractionation in Breast Cancer Radiotherapy. Biomedicines. 2025; 13(9):2154. https://doi.org/10.3390/biomedicines13092154
Chicago/Turabian StyleCalvaruso, Marco, Denis Panizza, Riccardo Ray Colciago, Valeria Faccenda, Gaia Pucci, Elena De Ponti, Giusi Irma Forte, Giorgio Russo, Luigi Minafra, and Stefano Arcangeli. 2025. "A Biological-Driven Approach to Explore Dose-Escalated Ultra-Hypofractionation in Breast Cancer Radiotherapy" Biomedicines 13, no. 9: 2154. https://doi.org/10.3390/biomedicines13092154
APA StyleCalvaruso, M., Panizza, D., Colciago, R. R., Faccenda, V., Pucci, G., Ponti, E. D., Forte, G. I., Russo, G., Minafra, L., & Arcangeli, S. (2025). A Biological-Driven Approach to Explore Dose-Escalated Ultra-Hypofractionation in Breast Cancer Radiotherapy. Biomedicines, 13(9), 2154. https://doi.org/10.3390/biomedicines13092154











