Abstract
Background/Objectives: Radiobiology has shown heterogeneity in the sensitivity of cells to ionizing radiation, depending on a variety of conditions. The presence of an extracellular matrix (ECM) appears to confer a radioprotective effect on cells and can influence the cellular microenvironment by modulating the availability of oxygen and nutrients, which can affect cellular metabolism and stress responses. A three-dimensional cell culture allows the synergistic effect on cell survival to be obtained based not only on the radioprotective properties of the extracellular matrix but also on the stress-resistant endogenous properties of the cell culture. The aim of this study was to investigate the survival of chondrocytes in a 3D cell culture during high-dose ionizing irradiation. Methods: The properties of nasal chondrocytes were evaluated using a pellet culture model in which the cells were surrounded by a de novo synthesized extracellular matrix. Tissue cultures were exposed by gamma radiation at doses of 10, 100, and 1300 Gy. Cell viability was assessed after 2 days of irradiation by live/dead staining using confocal scanning laser microscopy. Results: Tissue-cultured chondrocytes survive after gamma-irradiation of low (10 Gy), medium (100 Gy), and high (1300 Gy) dosages; however, after irradiation of 1300 Gy, the percentage of surviving cells was lower. The average percentages of viable cells were evaluated as 82%, 79%, and 63% in low-, medium-, and high-dose groups, respectively. Conclusions: Under determined conditions, human cells are able to survive at doses of ionizing radiation that are significantly higher than the current limits.
1. Introduction
Recently, it has been observed that cell cultures exhibit significant mortality following exposure to ionizing radiation at doses ranging from 20 to 40 Gy [1,2,3,4]. However, these results were primarily obtained from experiments performed on cell culture monolayers. Interestingly, the presence of an extracellular matrix (ECM) appears to confer a protective effect on the cells, thereby enhancing their viability [5,6]. Despite this, most studies investigating cellular resistance to high doses of ionizing radiation are predominantly focused on therapeutic doses, often overlooking the potential implications of higher, non-therapeutic exposures.
The ability of living organisms and tissues to survive exposure to high doses of ionizing radiation is still not fully understood. Recent studies have investigated organisms with inherent radioresistant properties and the mechanisms underlying radioprotection development. Previous studies have shown that Deinococcus radiodurans can survive doses of γ-irradiation up to 2000 Gy due to endogenous mechanisms of archaeal radioresistance [7,8,9]. In relation to human cells, the potential pathways for inducing high-dosage radioprotection involve interactions between cells and symbiotic organisms [10], as well as the modification of ECM properties [11,12]. Chondrocytes are highly radioresistant because they have a low mitotic rate, a dense extracellular matrix, and efficient DNA repair mechanisms [13,14,15], and historical studies and in vitro work report that tumor chondrocytes survive even above 80 Gy [16]. Using 3D cell culture models instead of tissue explants has introduced a paradigm shift in our understanding of cellular responses to ionizing radiation. Unlike traditional 2D monolayers, 3D cultures more accurately mimic the in vivo cellular microenvironment, including cell–cell and cell–matrix interactions, nutrient gradients, and the presence of ECM [17]. These factors collectively contribute to a more physiologically relevant model for studying cellular behavior under stress conditions, such as exposure to ionizing radiation [18].
In 3D cell cultures, the ECM not only provides mechanical support but also plays a critical role in modulating cellular signaling pathways involved in survival, proliferation, and DNA repair mechanisms. This protective effect of the ECM may result in increased cellular resistance to ionizing radiation, potentially altering the threshold doses required to achieve similar levels of cell death observed in 2D cultures [19,20]. The 3D cultures may exhibit differential gene expression profiles and activation of survival pathways that are not present in 2D monolayers. For example, hypoxia-inducible factors and other stress response elements may be upregulated in 3D environments, contributing to increased radioresistance [21]. In addition, the spatial organization of cells in 3D cultures may influence the diffusion and potency of radiation-induced reactive oxygen species (ROS), further enhancing cell viability [22,23].
Previous studies have shown that triple freeze–thaw cycles are commonly used to create controlled cell death models in tissue samples and generate samples with predictable levels of cell death for experimental controls. These studies have also shown that triple freeze–thaw cycles are an effective method for inducing heat-shock stress [24,25,26]. The time-dependent period of cell observation depended on viability evaluations, including metabolic and proliferative activity, as well as delayed complications. Two days of irradiation is sufficient for developing measurable endpoints, such as DNA damage, oxidative stress, and apoptosis, for in vitro studies [27,28] and in vivo investigations [15,29,30]. Additionally, this period enabled us to avoid the secondary effects associated with prolonged cell growth or stress unrelated to radiation exposure evaluation. Mitotically dead cells undergo apoptosis as a secondary response, often referred to as delayed apoptosis, which is observed one to two days after irradiation [31,32,33,34].
We hypothesized that exposure of tissue cultures to high doses of ionizing radiation may lead to cell surveillance, especially cells with low metabolic activity like chondrocytes.
2. Materials and Methods
2.1. Study Design
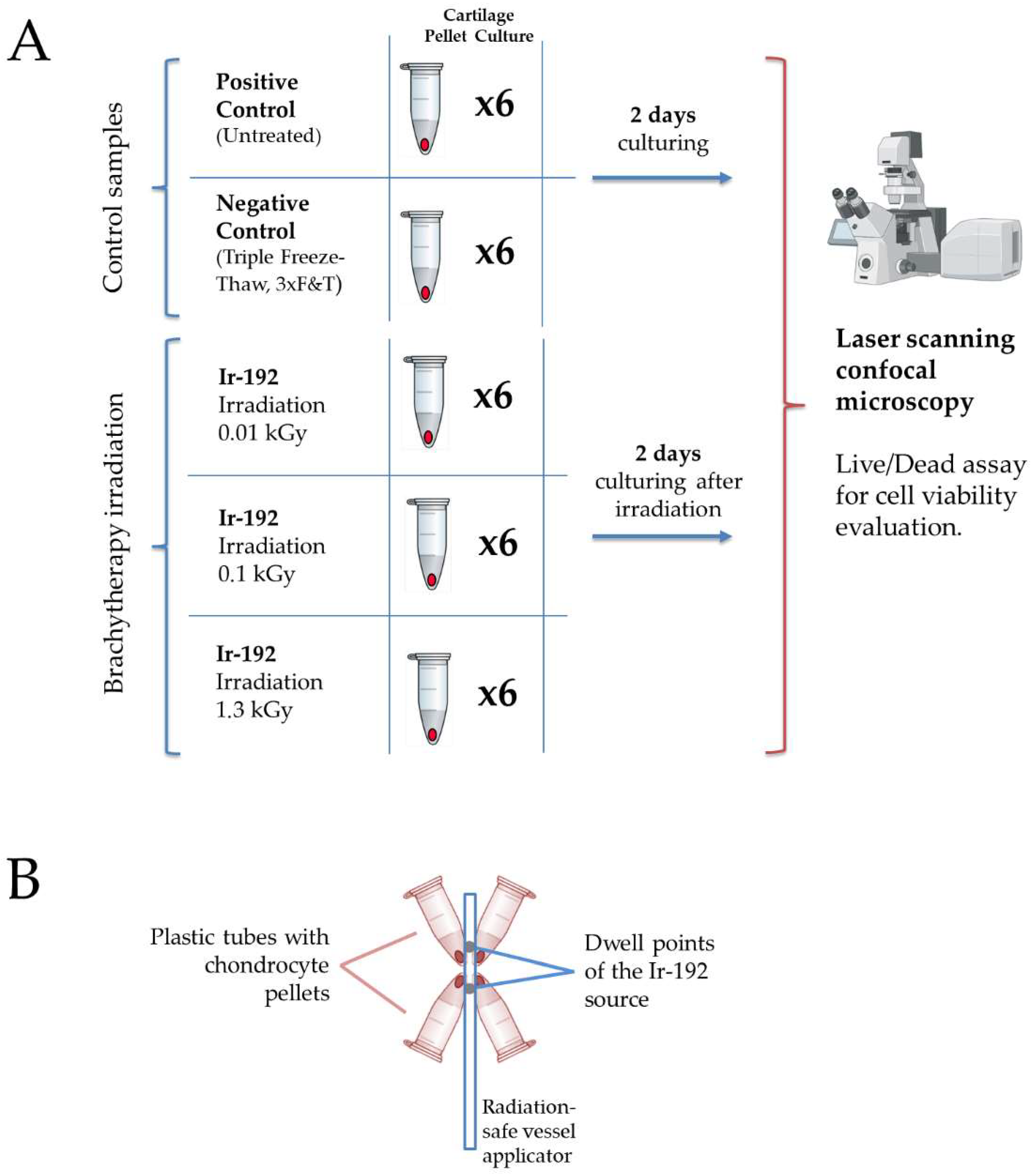
The study design of the radiobiological experiment is presented in Figure 1A. Schematic set-up for tube positioning is presented in Figure 1B.
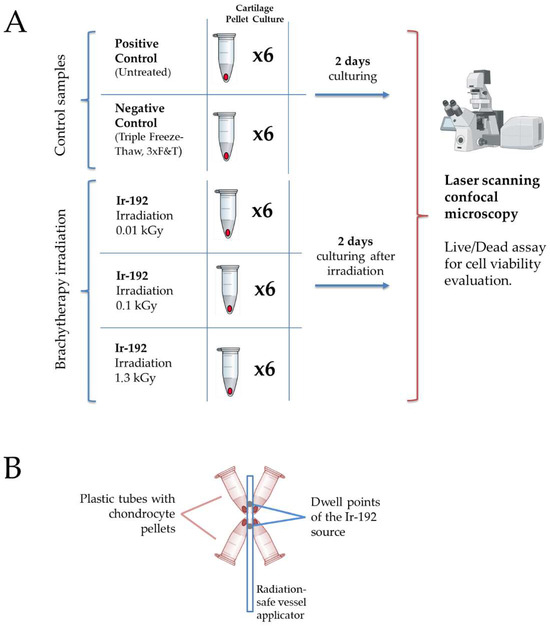
Figure 1.
(A) The study design. Created with Biorender.com. (B) Schematic set-up for tube positioning around the Ir−192 brachytherapy source.
2.2. Ionizing Irradiation
Gamma irradiation was performed on chondrocyte pellets derived from a patient at the University Hospital of Basel, with written informed consent and approval from the local ethics committee. Brachytherapy was carried out using gamma radiation from a sealed Iridium−192 (Ir−192) source. The Ir−192 source was calibrated and maintained according to institutional protocols to ensure consistent and accurate dosimetry.
The Ir−192 source, encapsulated in stainless steel and measuring 0.9 mm in diameter and 4.5 mm in length, was integrated into an afterloader system commonly used in brachytherapy. The Ir−192 source was stored in a radiation-shielded container and positioned at the treatment site using an applicator.
The administered dose during brachytherapy sessions was calculated based on a treatment plan tailored to the requirements of the experimental design. The irradiation doses were 10, 100, or 1300 Gy, depending on the experimental group. Chondrocyte pellets were mounted at the tips of Eppendorf plastic tubes (Eppendorf AG, Hamburg, Germany), with each sample positioned as close as possible to the optimized dwell positions of the Ir−192 source to minimize beam-on time. Sample locations were confirmed using computed tomography (SOMATOM Syngo CT, Siemens, Germany), which also served for dose calculations.
The small size of the pellet samples allowed for a relatively homogeneous dose distribution and efficient irradiation. Source positioning and dose optimization were performed using a commercial treatment-planning software (Oncentra Brachy, Version 4.5.2, Nucletron B.V., Netherlands). A schematic representation of the set-up, with four plastic tubes positioned around the applicator, is shown in Figure 1B.
2.3. Chondrocyte Sample Derivation
Nasal chondrocyte samples were used after obtaining written informed consent from the individuals and/or their relatives. The protocols of sample derivation were approved by the Local Ethical Commission (Switzerland; EKNZs; Ref.# 78/07) and the Local Ethics Committee of Sechenov University (Russia; Sechenov University; Ref.# 07–15).
2.4. Pellet Culture Chondrocytes
A pellet culture model of nasal chondrocytes was established according to a previously described protocol [35]. The pellets were used to evaluate the effect of different sources of ionizing radiation.
2.5. Cell Viability
Images, derived in the Biozentrum, University of Basel, were taken under a confocal laser scanning microscope Leica TCS SP8 (Leica Microsystems GmbH, Wetzlar, Germany), objective 20×, Z-stack step size 1 µm. Cell viability was assessed using confocal microscopy following the pre-staining of samples with calcein AM and ethidium homodimer−1, components of the Live/Dead Viability/Cytotoxicity Kit (Invitrogen, Carlsbad, CA, USA). Briefly, cells were cultured under the experimental conditions specified and then incubated with a staining solution containing 2 µM calcein AM and 4 µM ethidium homodimer−1 for 30 min at 37 °C in a humidified atmosphere with 5% CO2. Images were acquired from multiple random fields per sample to ensure representative sampling. Quantitative analysis of cell viability was performed using dedicated ImageJ (version 1.52a) software by counting live and dead cells.
2.6. Statistical Analysis
The data were obtained using six biological replicates per point. Data are presented as mean ± standard deviation (SD). Statistical analysis was performed using specific statistical software GraphPad PRISM v.8, applying one-way ANOVA with the significance level set at a p-value < 0.05.
3. Results
The pellet culture model of nasal chondrocytes shows significant differences from native cartilage, primarily characterized by a significantly higher and standardized number of cells per unit tissue volume. In this model, the newly formed tissue consists of chondrocytes and the cartilage ECM components synthesized by these cells.
Cultured pellets are compact, spherical aggregates of cells with a dense and uniform structure formed by the synthesized ECM. These pellets are typically rich in glycosaminoglycans and collagen, key components of the cartilage extracellular matrix, which contribute to their mechanical properties and functional similarities to native cartilage. The high cell density within these pellets facilitates robust matrix production, resulting in the formation of a tissue that, while not identical to native cartilage, closely mimics its biochemical and biomechanical properties.
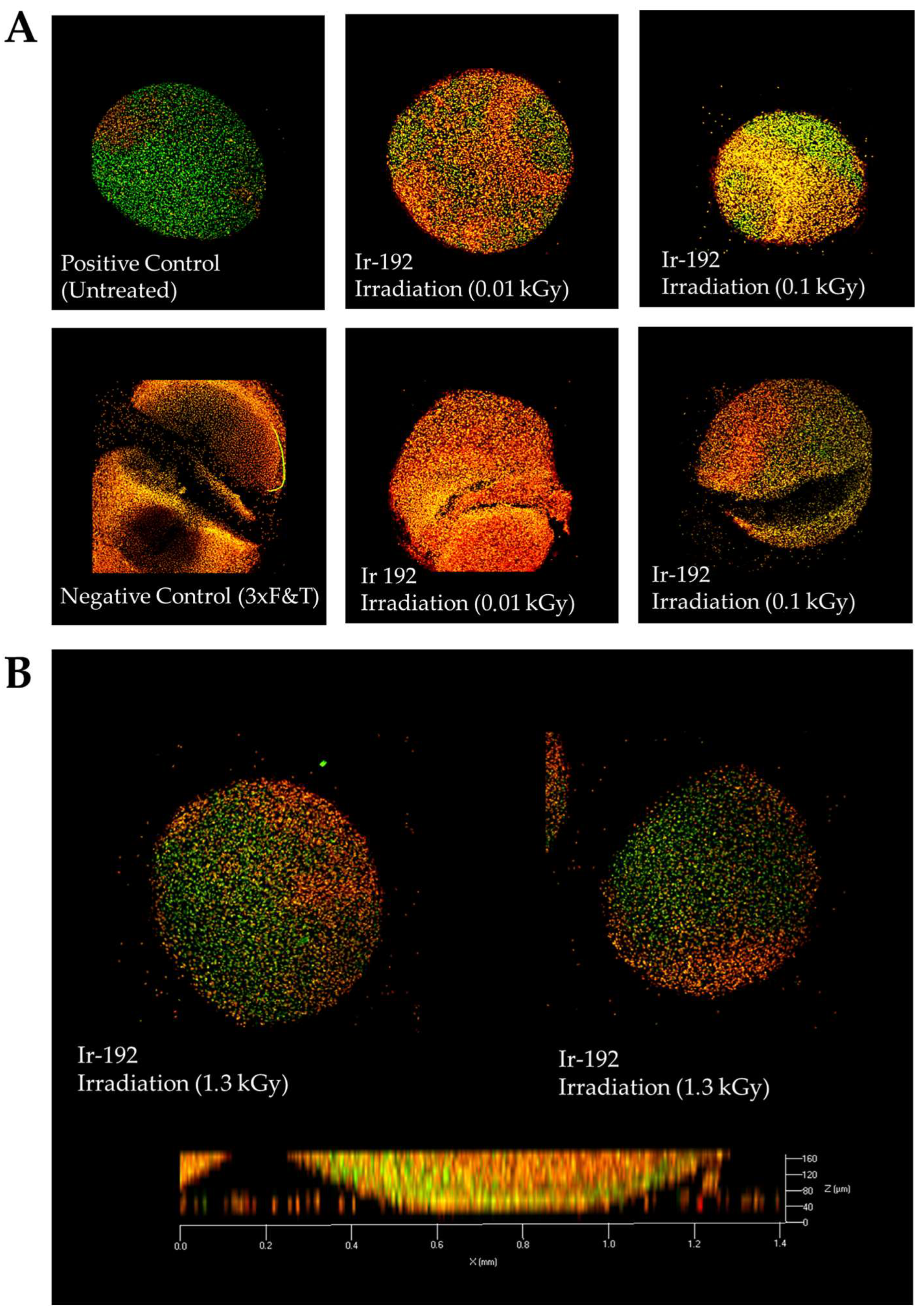
The irradiation of the pellet cultures with both low (Figure 2A) and high doses (Figure 2B) of gamma radiation was not responsible for all observed cell death, which can be explained by the low metabolic and proliferative activity of chondrocytes surrounded by de novo synthesized intercellular substances.
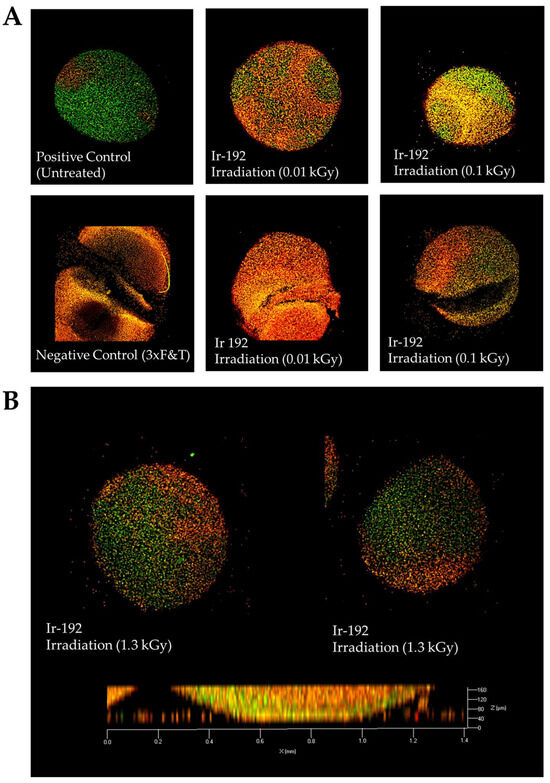
Figure 2.
(A) Viability of nasal chondrocytes after exposure to different doses of gamma radiation; (B) Viability of nasal chondrocytes after exposure to gamma radiation at a dose of 1.3 kGy. Live/dead staining, confocal microscopy, 63× magnification. Dead cells are stained orange; live cells are stained green.
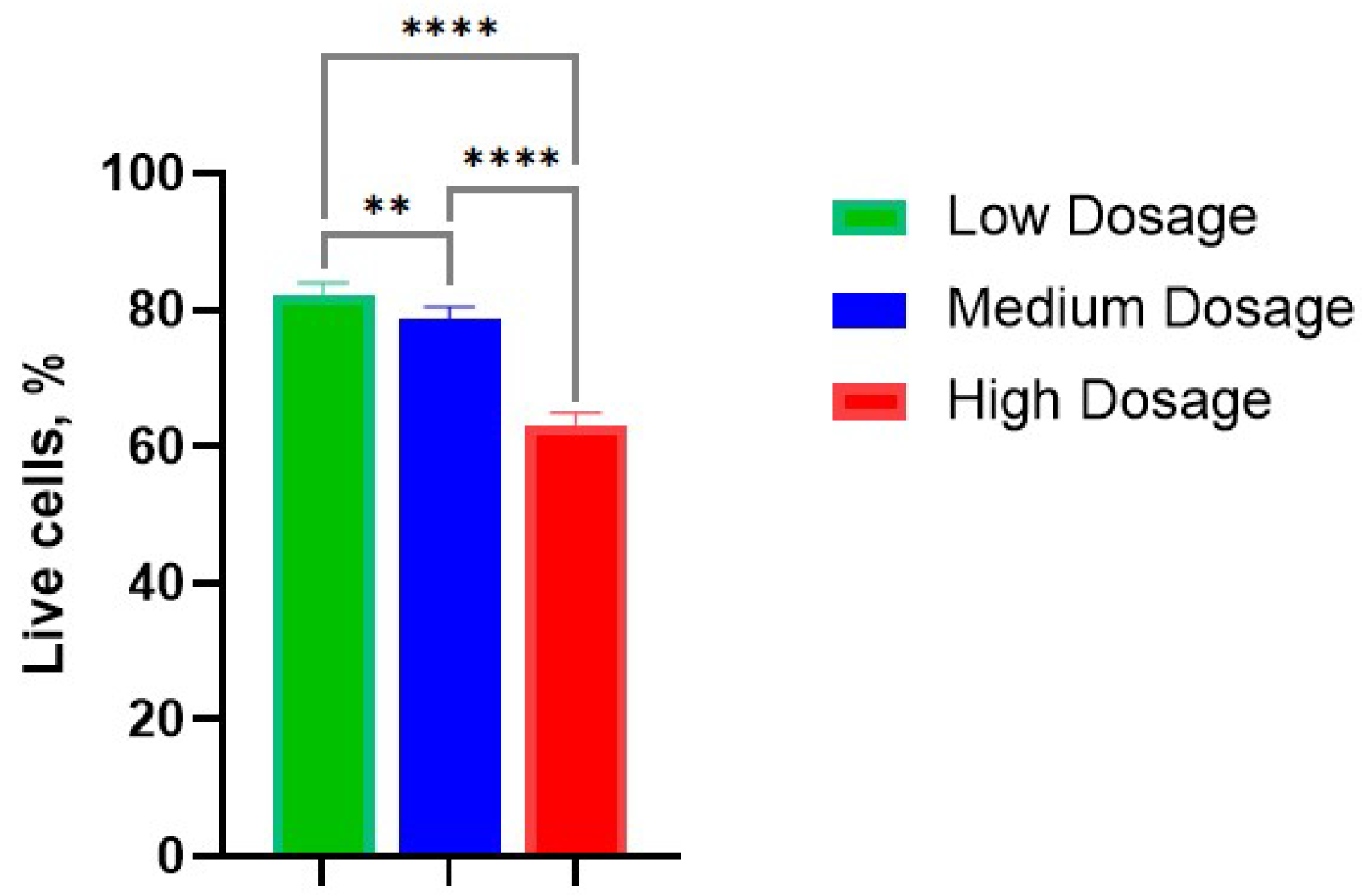
The average percentages of viable cells were evaluated as 82%, 79%, and 63% in low-, medium-, and high- dosage groups (Figure 3). It is not surprising that the percentage of living cells decreased with dosage rate; however, some cells remained alive and retained cellular respiration.
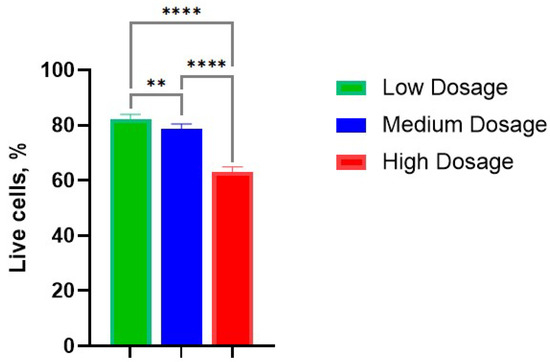
Figure 3.
Survival rate of cells in the chondrocyte pellet culture in various dosages of gamma irradiation: Low—10 Gy; Medium—100 Gy; High—1300 Gy. **, p-value < 0.01; ****, p-value < 0.0001.
A similar effect was observed during the electron beam irradiation of the chondrocyte pellet culture in the same conditions (data presented in Supplementary Files S1 and S2).
4. Discussion
The interaction of cells with the ECM modulates many important processes such as proliferation, differentiation, migration, and survival [36]. The ECM could assist cell viability and improve cell resistance to ionizing radiation, chemicals, hypoxia, and other external factors, but these effects seemed to be minor. Extreme cases of cell viability were observed in some animals, but there were no translations to humans or vertebrate animals [37,38].
The nature of induced cell radioresistance following residence in the ECM is multifaceted, influenced by several key factors. The stiffness and elasticity of the ECM can alter cellular behavior and enhance a cell’s ability to withstand radiation damage [39]. The reorganization and stabilization of the cytoskeleton in response to ECM mechanical signals can enhance cellular resilience by improving structural integrity and facilitating efficient repair mechanisms [40]. The biochemical composition of the ECM, including the presence of growth factors, cytokines, and other signaling molecules, can activate survival pathways and enhance DNA repair processes. The interaction between cell surface receptors and ECM components can trigger intracellular signaling cascades that promote cell survival and reduce apoptosis in response to radiation [41]. Additionally, the ECM can influence gene expression profiles, leading to the upregulation of genes associated with DNA repair, antioxidant defense, and anti-apoptotic pathways [42]. Chondrocyte regulation differs between cellular subpopulations and can affect viability and survival through intrinsic genomic and epigenetic mechanisms [43].
Moreover, the ECM can impact the cellular microenvironment by modulating the availability of oxygen and nutrients, which can affect cellular metabolism and stress responses. Hypoxic conditions within the ECM, for instance, can activate hypoxia-inducible factors (HIFs) that promote cell survival under stress conditions, including radiation exposure [44].
The mechanisms of cell death during exposure to ionizing irradiation have been widely investigated; however, cell death, viability, and anastasis in tissue-engineered constructs after ionizing radiation exposure have not been examined in detail [45,46,47], and the range of resistance of cells of different tissues to ionizing radiation and the specific mechanisms of cell death remain insufficiently studied. Therefore, the heterogeneity of the types of cell death [48], coupled with the phenomenon of cellular anastasis, can obscure the extent of damage following irradiation and highlight the intracellular mechanisms of enhanced radioresistance, as well as highlighting the need for studies aimed at reducing these mechanisms for the stimulation of radiosensibilization in tumor cells [11,49]. Last but not least is the fact that chondrocytes which are highly resistant to irradiation could be used in long-term space exploration [50]. There is no doubt that these findings could be used to investigate ways to increase the radioresistance of human and animal cells for long-term space exploration. In addition, the differences between ECM properties in tumor and normal tissues could be used in advanced protocols for radiotherapy with ECM-targeted adjuvants [51].
A notable limitation of the present study was the lack of a comprehensive assessment of cell proliferative activity. This could have been achieved by using Ki−67 expression immunohistochemistry assays, which are widely recognized for their ability to indicate active cell proliferation. In addition, evaluation of the colony-forming capacity of the cells would have provided direct insight into their proliferative potential. The use of chondrocyte pellets allowed us to obtain the synergetic effect on cell survival based not only on the radioprotective properties of the ECM but also the stress-resistance endogenic properties of the chondrocyte cell culture.
5. Conclusions
Tissue-cultured chondrocytes are capable of survival after irradiation at doses of at least 1300 Gy. This remarkable radioresistance demonstrates the resilience of these specialized cells and suggests that certain differentiated cell types may have unique structural or metabolic adaptations that confer exceptional tolerance to DNA damage and oxidative stress. This observed effect could be applied to space exploration and to developing advanced techniques for chondrosarcoma radiotherapy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines13092153/s1, File S1: Electron beam irradiation experiments; File S2: LAS raw data file.
Author Contributions
Conceptualization, D.B. and I.K.; methodology, D.B. and I.K.; investigation, D.B., A.Y., A.S. and I.K.; writing—original draft preparation, D.B. and I.K.; writing—review and editing, A.K., S.K., D.A., M.I., M.P., Y.S., V.S., S.B., V.N.S., R.C., B.A., P.S. and A.D.K.; funding acquisition, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
Part of this study was supported by the Russian Science Foundation through grant #24–14−00393.
Institutional Review Board Statement
The protocols of sample derivation were approved by the Ethikkommission Nordwest- und Zentralschweiz Ethical Commission (Switzerland; EKNZs; Ref.# 78/07, 3 August 2017) and the Local Ethics Committee of Sechenov University (Russia; Sechenov University; Ref.# 07–15, 15 July 2015).
Informed Consent Statement
Nasal chondrocyte samples were used after obtaining written informed consent from the individuals and/or their relatives.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DNA | Deoxyribonucleic acid |
| ECM | Extracellular matrix |
References
- Datta, R.; Saha, S. Quantitative determination of tolerance doses for preoperative and postoperative radiotherapy of bones. Med. Phys. 1983, 10, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Minafra, L.; Bravatà, V. Cell and molecular response to IORT treatment. Transl. Cancer Res. 2014, 3, 32–47. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, X.; Peng, Y.; Eichenbaum, J.V.; Ren, L.; Liu, Y. Effects of different radiation sources on the performance of collagen-based corneal repair materials and macrophage polarization. ACS Omega 2022, 7, 22559–22566. [Google Scholar] [CrossRef]
- Dombrowsky, A.C.; Schauer, J.; Sammer, M.; Blutke, A.; Walsh, D.W.M.; Schwarz, B.; Bartzsch, S.; Feuchtinger, A.; Reindl, J.; Combs, S.E.; et al. Acute Skin Damage and Late Radiation-Induced Fibrosis and Inflammation in Murine Ears after High-Dose Irradiation. Cancers 2019, 11, 727. [Google Scholar] [CrossRef]
- Acheva, A.; Aerts, A.; Rombouts, C.; Baatout, S.; Salomaa, S.; Manda, K.; Kämäräinen, M. Human 3-D tissue models in radiation biology: Current status and future perspectives. Int. J. Radiat. Res. 2014, 12, 81–98. [Google Scholar]
- Jones, G.D.; Symonds, P. Molecular, cellular and tissue effects of radiotherapy. In Walter and Miller’s Textbook of Radiotherapy: Radiation Physics, Therapy and Oncology; Churchill Livingstone-Elsevier: London, UK, 2012; pp. 279–292. [Google Scholar]
- Daly, M.J. A new perspective on radiation resistance based on Deinococcus radiodurans. Nat. Rev. Microbiol. 2009, 7, 237–245. [Google Scholar] [CrossRef]
- Slade, D.; Radman, M. Oxidative stress resistance in Deinococcus radiodurans. Microbiol. Mol. Biol. Rev. 2011, 75, 133–191. [Google Scholar] [CrossRef]
- Zivanovic, Y.; Armengaud, J.; Lagorce, A.; Leplat, C.; Guérin, P.; Dutertre, M.; Anthouard, V.; Forterre, P.; Wincker, P.; Confalonieri, F. Genome analysis and genome-wide proteomics of Thermococcus gammatolerans, the most radioresistant organism known amongst the Archaea. Genome Biol. 2009, 10, R70. [Google Scholar] [CrossRef]
- Yakimova, A.O.; Nikolaeva, A.; Galanova, O.; Shestakova, V.A.; Smirnova, E.I.; Levushkina, A.; Baranovskii, D.S.; Smirnova, A.N.; Stepanenko, V.N.; Kudlay, D.A.; et al. Microbiota-Induced Radioprotection: A Novel Approach to Enhance Human Radioresistance with In-Situ Genetically Engineered Gut Bacteria. Appl. Microbiol. 2025, 5, 1. [Google Scholar] [CrossRef]
- Busato, F.; Khouzai, B.E.; Mognato, M. Biological mechanisms to reduce radioresistance and increase the efficacy of radiotherapy: State of the art. Int. J. Mol. Sci. 2022, 23, 10211. [Google Scholar] [CrossRef] [PubMed]
- White, L.J.; Keane, T.J.; Smoulder, A.; Zhang, L.; Castleton, A.A.; Reing, J.E.; Turner, N.J.; Dearth, C.L.; Badylak, S.F. The impact of sterilization upon extracellular matrix hydrogel structure and function. J. Immunol. Regen. Med. 2018, 2, 11–20. [Google Scholar] [CrossRef]
- Matsumoto, T.; Iwasaki, K.; Sugihara, H. Effects of radiation on chondrocytes in culture. Bone 1994, 15, 97–100. [Google Scholar] [CrossRef]
- Sugrue, T.; Lowndes, N.F.; Ceredig, R. Mesenchymal stromal cells: Radio-resistant members of the bone marrow. Immunol. Cell Biol. 2013, 91, 5–11. [Google Scholar] [CrossRef]
- Saintigny, Y.; Cruet-Hennequart, S.; Hamdi, D.H.; Chevalier, F.; Lefaix, J.L. Impact of therapeutic irradiation on healthy articular cartilage. Radiat. Res. 2015, 183, 135–146. [Google Scholar] [CrossRef]
- Gilbert, A.; Tudor, M.; Montanari, J.; Commenchail, K.; Savu, D.I.; Lesueur, P.; Chevalier, F. Chondrosarcoma resistance to radiation therapy: Origins and potential therapeutic solutions. Cancers 2023, 15, 1962. [Google Scholar] [CrossRef]
- Eke, I.; Cordes, N. Radiobiology goes 3D: How ECM and cell morphology impact on cell survival after irradiation. Radiother. Oncol. 2011, 99, 271–278. [Google Scholar] [CrossRef]
- Shestakova, V.; Smirnova, A.; Yakimova, A.; Koryakin, S.; Baranovskii, D.; Saburov, V.; Yatsenko, E.; Moiseev, A.; Ivanov, S.; Smirnova, E.; et al. Biological Efficacy of Ionizing Radiation Sources on 3D Organotypic Tissue Slices Assessed by Fluorescence Microscopy. Curr. Radiopharm. 2025, 18, 64–72. [Google Scholar] [CrossRef]
- Zschenker, O.; Streichert, T.; Hehlgans, S.; Cordes, N. Genome-wide gene expression analysis in cancer cells reveals 3D growth to affect ECM and processes associated with cell adhesion but not DNA repair. PLoS ONE 2012, 7, e34279. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Sharma, D. Therapeutic response differences between 2D and 3D tumor models of magnetic hyperthermia. Nanoscale Adv. 2021, 3, 3663–3680. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhou, P.K. HIF-1 signaling: A key orchestrator of cancer radioresistance. Radiat. Med. Prot. 2020, 1, 7–14. [Google Scholar] [CrossRef]
- Babu, B.; Pawar, S.; Mittal, A.; Kolanthai, E.; Neal, C.J.; Coathup, M.; Seal, S. Nanotechnology enabled radioprotectants to reduce space radiation-induced reactive oxidative species. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1896. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Roman, N.; Chong, M.Y.; Chahal, S.K.; Caragher, S.P.; Jackson, M.R.; Stevenson, K.H.; Dongre, S.A.; Chalmers, A.J. Radiation responses of 2D and 3D glioblastoma cells: A novel, 3D-specific radioprotective role of VEGF/Akt signaling through functional activation of NHEJ. Mol. Cancer Ther. 2020, 19, 575–589. [Google Scholar] [CrossRef]
- Kheir, E.; Stapleton, T.; Shaw, D.; Jin, Z.; Fisher, J.; Ingham, E. Development and characterization of an acellular porcine cartilage bone matrix for use in tissue engineering. J. Biomed. Mater. Res. Part A 2011, 99, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Suto, K.; Urabe, K.; Naruse, K.; Uchida, K.; Matsuura, T.; Mikuni-Takagaki, Y.; Itoman, M.; Suto, M.; Nemoto, N.; Kamiya, K.; et al. Repeated freeze–thaw cycles reduce the survival rate of osteocytes in bone-tendon constructs without affecting the mechanical properties of tendons. Cell Tissue Bank. 2012, 13, 71–80. [Google Scholar] [CrossRef]
- Roth, S.P.; Glauche, S.M.; Plenge, A.; Erbe, I.; Heller, S.; Burk, J. Automated freeze-thaw cycles for decellularization of tendon tissue-a pilot study. BMC Biotechnol. 2017, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, Y.; Cui, X.; Yasuda, T.; Wang, B.; Yu, D.; Sekine-Suzuki, E.; Nenoi, M. Arsenite induces premature senescence via p53/p21 pathway as a result of DNA damage in human malignant glioblastoma cells. BMB Rep. 2014, 47, 575–580. [Google Scholar] [CrossRef]
- Suckert, T.; Rassamegevanon, T.; Müller, J.; Dietrich, A.; Graja, A.; Reiche, M.; Löck, S.; Krause, M.; Beyreuther, E.; von Neubeck, C. Applying Tissue Slice Culture in Cancer Research—Insights from Preclinical Proton Radiotherapy. Cancers 2020, 12, 1589. [Google Scholar] [CrossRef]
- Drayson, O.G.G.; Melemenidis, S.; Katila, N.; Viswanathan, V.; Kramár, E.A.; Zhang, R.; Kim, R.; Ru, N.; Petit, B.; Dutt, S.; et al. A Multi-Institutional Study to Investigate the Sparing Effect after Whole Brain Electron FLASH in Mice: Reproducibility and Temporal Evolution of Functional, Electrophysiological, and Neurogenic Endpoints. Radiother. Oncol. 2024, 201, 110534. [Google Scholar] [CrossRef]
- Essers, J.; van Steeg, H.; de Wit, J.; Swagemakers, S.M.; Vermeij, M.; Hoeijmakers, J.H.; Kanaar, R. Homologous and non-homologous recombination differentially affect DNA damage repair in mice. EMBO J. 2000, 19, 1703–1710. [Google Scholar] [CrossRef]
- Maity, A.; Kao, G.D.; Muschel, R.J.; McKenna, W.G. Potential molecular targets for manipulating the radiation response. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 639–653. [Google Scholar] [CrossRef]
- Fuks, Z.; Persauld, R.S.; Alfieri, A.; McLoughlin, M.; Ehleiter, C.; Schwartz, J.L.; Seddon, A.P.; Cordon-Cardo, C.; Haimovitz-Friedman, A. Basic fibroblast growth factor growth factor protects endothelial cells against radiation induced programmed cell death in vitro and in vivo. Cancer Res. 1994, 54, 2582–2590. [Google Scholar] [PubMed]
- Russell, J.; Wheldon, T.W.; Stanton, P.A. Radio resistant variant derived from a human neuroblastoma cell line is less prone to radiation-induced apoptosis. Cancer Res. 1995, 55, 4915–4921. [Google Scholar]
- Shrivastava, V.; Mishra, A.K.; Dwarakanath, B.S.; Ravindranath, T. Enhancement of radionuclide induced cytotoxicity by 2-deoxy-D-glucose in human tumor cell lines. J. Cancer Res. Ther. 2006, 2, 57–64. [Google Scholar]
- Barbero, A.; Grogan, S.; Schäfer, D.; Heberer, M.; Mainil-Varlet, P.; Martin, I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthr. Cartil. 2004, 12, 476–484. [Google Scholar] [CrossRef]
- Babel, L.; Grunewald, M.; Lehn, R.; Langhans, M.; Meckel, T. Direct evidence for cell adhesion-mediated radioresistance (CAM-RR) on the level of individual integrin β1 clusters. Sci. Rep. 2017, 7, 3393. [Google Scholar] [CrossRef]
- Swamy, C.S.U.S.; Boothby, T.C. Surviving extreme radiation. eLife 2024, 13, e100219. [Google Scholar] [CrossRef] [PubMed]
- Clark-Hachtel, C.M.; Hibshman, J.D.; De Buysscher, T.; Stair, E.R.; Hicks, L.M.; Goldstein, B. The tardigrade Hypsibius exemplaris dramatically upregulates DNA repair pathway genes in response to ionizing radiation. Curr. Biol. 2024, 34, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Liang, S.; Gao, J.; Wang, Z.; Li, C.; Wang, R.; Yu, W. Extracellular matrix stiffness mediates radiosensitivity in a 3D nasopharyngeal carcinoma model. Cancer Cell Int. 2022, 22, 364. [Google Scholar] [CrossRef]
- La Verde, G.; Artiola, V.; Panzetta, V.; Pugliese, M.; Netti, P.A.; Fusco, S. Cytoskeleton response to ionizing radiation: A brief review on adhesion and migration effects. Biomedicines 2021, 9, 1102. [Google Scholar] [CrossRef]
- Hehlgans, S.; Haase, M.; Cordes, N. Signalling via integrins: Implications for cell survival and anticancer strategies. Biochim. Biophys. Acta Rev. Cancer 2007, 1775, 163–180. [Google Scholar] [CrossRef]
- Mao, X.W.; Mekonnen, T.; Kennedy, A.R.; Gridley, D.S. Differential expression of oxidative stress and extracellular matrix remodeling genes in low- or high-dose-rate photon-irradiated skin. Radiat. Res. 2011, 176, 187–197. [Google Scholar] [CrossRef]
- Shestakova, V.A.; Smirnova, E.I.; Rao, L.; Kolobaev, I.V.; Atiakshin, D.A.; Ignatyuk, M.A.; Krasheninnikov, M.E.; Ahmedov, B.G.; Ivanov, S.A.; Baranovskii, D.S.; et al. Nasal Chondrocytes Intensively Invade and Repair Pathologically Altered Cartilage Through Intrinsic Genomic Mechanisms: A Narrative Review. Curr. Rheumatol. Rev. 2025. [Google Scholar] [CrossRef] [PubMed]
- Kabakov, A.E.; Yakimova, A.O. Hypoxia-induced cancer cell responses driving radioresistance of hypoxic tumors: Approaches to targeting and radiosensitizing. Cancers 2021, 13, 1102. [Google Scholar] [CrossRef] [PubMed]
- Prise, K.M.; Schettino, G.; Folkard, M.; Held, K.D. New insights on cell death from radiation exposure. Lancet Oncol. 2005, 6, 520–528. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Instructive Surfaces and Scaffolds for Tissue Engineering Using Radiation Technology; IAEA CRP F23030-E31007; International Atomic Energy Agency: Vienna, Austria, 2014; Available online: https://www.iaea.org/projects/crp/f23030 (accessed on 20 February 2025).
- Bustamante, P.; Anessi, C.; Santoro, N.; Ciavaro, N.; Horak, C. Synthesis and characterization of hydrogels cross-linked with gamma radiation for use as wound dressings. In VIII Latin American Conference on Biomedical Engineering and XLII National Conference on Biomedical Engineering, Proceedings of the CLAIB-CNIB 2019, Cancún, México, 2–5 October 2019; Springer International Publishing: Cham, Switzerland, 2020; pp. 643–651. [Google Scholar] [CrossRef]
- Yan, G.E.; Elbadawi, M.; Efferth, T. Multiple cell death modalities and their key features. World Acad. Sci. J. 2020, 2, 39–48. [Google Scholar] [CrossRef]
- Kudryavtsev, V.A.; Khokhlova, A.V.; Mosina, V.A.; Selivanova, E.I.; Kabakov, A.E. Induction of Hsp70 in tumor cells treated with inhibitors of the Hsp90 activity: A predictive marker and promising target for radiosensitization. PLoS ONE 2017, 12, e0173640. [Google Scholar] [CrossRef]
- Cortese, F.; Klokov, D.; Osipov, A.; Stefaniak, J.; Moskalev, A.; Schastnaya, J.; Cantor, C.; Aliper, A.; Mamoshina, P.; Ushakov, I.; et al. Vive la radiorésistance!: Converging research in radiobiology and biogerontology to enhance human radioresistance for deep space exploration and colonization. Oncotarget 2018, 9, 14692–14722. [Google Scholar] [CrossRef]
- Klabukov, I.; Smirnova, A.; Yakimova, A.; Kabakov, A.E.; Atiakshin, D.; Petrenko, D.; Shestakova, V.A.; Sulina, Y.; Yatsenko, E.; Stepanenko, V.N.; et al. Oncomatrix: Molecular Composition and Biomechanical Properties of the Extracellular Matrix in Human Tumors. J. Mol. Pathol. 2024, 5, 437–453. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).