Antimicrobial Activity of Natural Extracts Against Catheter-Colonizing Methicillin-Resistant Staphylococcus aureus Clinical Isolates
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Clinical Isolates
2.2. Food-Grade Natural Extracts
2.3. Antimicrobial Susceptibility to Natural Extracts
2.4. Statistical Analysis
3. Results
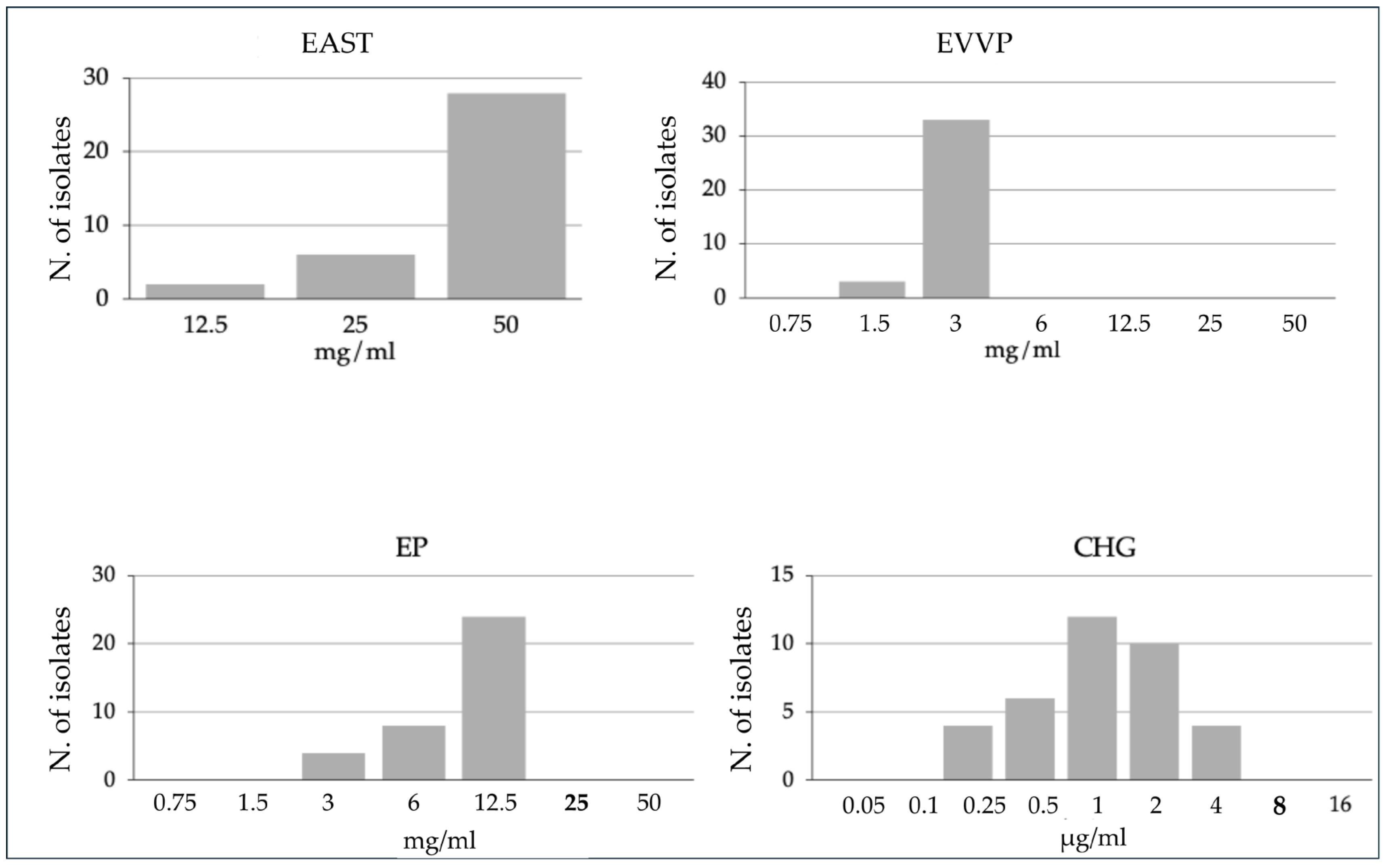
3.1. Bacteriostactic Effects of the Natural Extracts
3.2. Bactericidal Activity of the Natural Extracts
3.3. MICs of the Natural Extracts Do Not Exhibit Positive Correlations Between Them
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CHG | Chlorhexidine Gluconate |
| EAST | Extract of Allium sativum enriched in Thiosulfinates |
| EP | Propolis Extract |
| EVVP | Extract of Vitis vinifera enriched in Proanthocyanidins |
| IC | Intravascular Catheter |
| ICU | Intensive Care Unit |
| MBC | Minimum Bactericidal Concentration |
| MIC | Minimum Inhibitory Concentration |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
References
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef]
- Lena, P.; Ishak, A.; Karageorgos, S.A.; Tsioutis, C. Presence of Methicillin-Resistant Staphylococcus aureus (MRSA) on Healthcare Workers’ Attire: A Systematic Review. Trop. Med. Infect. Dis. 2021, 6, 42. [Google Scholar] [CrossRef]
- Genc, O.; Arikan, I. The relationship between hand hygiene practices and nasal Staphylococcus aureus carriage in healthcare workers. Med. Lav. 2020, 111, 54–62. [Google Scholar] [CrossRef]
- Hayden, M.K.; Lolans, K.; Haffenreffer, K.; Avery, T.R.; Kleinman, K.; Li, H.; Kaganov, R.E.; Lankiewicz, J.; Moody, J.; Septimus, E.; et al. Chlorhexidine and Mupirocin Susceptibility of Methicillin-Resistant Staphylococcus aureus Isolates in the REDUCE-MRSA Trial. J. Clin. Microbiol. 2016, 54, 2735–2742. [Google Scholar] [CrossRef]
- Edgeworth, J.D. Has decolonization played a central role in the decline in UK methicillin-resistant Staphylococcus aureus transmission? A focus on evidence from intensive care. J. Antimicrob. Chemother. 2011, 66 (Suppl. S2), ii41–ii47. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pérez, A.; Medina, S.; Sánchez-Bravo, P.; Domínguez-Perles, R.; García-Viguera, C. The (Poly)phenolic Profile of Separate Winery By-Products Reveals Potential Antioxidant Synergies. Molecules 2023, 28, 2081. [Google Scholar] [CrossRef] [PubMed]
- Barbu, I.A.; Ciorîță, A.; Carpa, R.; Moț, A.C.; Butiuc-Keul, A.; Pârvu, M. Phytochemical Characterization and Antimicrobial Activity of Several Allium Extracts. Molecules 2023, 28, 3980. [Google Scholar] [CrossRef] [PubMed]
- De Meneses Costa Ferreira, L.M.; de Souza, P.D.Q.; Pereira, R.R.; da Silva, E.O.; Barbosa, W.L.R.; Silva-Júnior, J.O.C.; Converti, A.; Ribeiro-Costa, R.M. Preliminary Study on the Chemical and Biological Properties of Propolis Extract from Stingless Bees from the Northern Region of Brazil. Processes 2024, 12, 700. [Google Scholar] [CrossRef]
- Avendaño-Ortiz, J.; Redondo-Calvo, F.J.; Lozano-Rodríguez, R.; Terrón-Arcos, V.; Bergón-Gutiérrez, M.; Rodríguez-Jiménez, C.; Rodríguez, J.F.; del Campo, R.; Gómez, L.A.; Bejarano-Ramírez, N.; et al. Thiosulfinate-Enriched Allium sativum Extract Exhibits Differential Effects between Healthy and Sepsis Patients: The Implication of HIF-1α. Int. J. Mol. Sci. 2023, 24, 6234. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, X.; Wang, Q.; Jiang, Y.; Shang, H.; Cui, L.; Cao, Y. Allicin enhances host pro-inflammatory immune responses and protects against acute murine malaria infection. Malar. J. 2012, 11, 268. [Google Scholar] [CrossRef]
- Deng, X.; Yang, P.; Gao, T.; Liu, M.; Li, X. Allicin attenuates myocardial apoptosis, inflammation and mitochondrial injury during hypoxia-reoxygenation: An in vitro study. BMC Cardiovasc. Disord. 2021, 21, 200. [Google Scholar] [CrossRef]
- Nakamoto, M.; Kunimura, K.; Suzuki, J.-I.; Kodera, Y. Antimicrobial properties of hydrophobic compounds in garlic: Allicin, vinyldithiin, ajoene and diallyl polysulfides. Exp. Ther. Med. 2020, 19, 1550–1553. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadi, M.; Chorianopoulos, N.G.; Nychas, G.-J.E.; Haroutounian, S.A. Antilisterial Activities of Polyphenol-Rich Extracts of Grapes and Vinification Byproducts. J. Agric. Food Chem. 2009, 57, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Serrano, H.D.A.; Mariezcurrena-Berasain, M.A.; Del Carmen Gutiérrez Castillo, A.; Del Carmen Gutiérrez Castillo, B.; Pliego, A.B.; Rojas, M.T.; Anele, U.Y.; Salem, A.Z.M.; Rivas-Caceres, R.R. Antimicrobial resistance of three common molecularly identified pathogenic bacteria to Allium aqueous extracts. Microb. Pathog. 2020, 142, 104028. [Google Scholar] [CrossRef]
- Liu, M.; Pan, Y.; Feng, M.; Guo, W.; Fan, X.; Feng, L.; Huang, J.; Cao, Y. Garlic essential oil in water nanoemulsion prepared by high-power ultrasound: Properties, stability and its antibacterial mechanism against MRSA isolated from pork. Ultrason. Sonochem. 2022, 90, 106201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Margarita, G.E.; Wu, D.; Yuan, W.; Yan, S.; Qi, S.; Xue, X.; Wang, K.; Wu, L. Antibacterial Activity of Chinese Red Propolis against Staphylococcus aureus and MRSA. Molecules 2022, 27, 1693. [Google Scholar] [CrossRef]
- Leal, C.; Santos, R.A.; Pinto, R.; Queiroz, M.; Rodrigues, M.; Saavedra, M.J.; Barros, A.; Gouvinhas, I. Recovery of bioactive compounds from white grape (Vitis vinifera L.) stems as potential antimicrobial agents for human health. Saudi J. Biol. Sci. 2020, 27, 1009–1015. [Google Scholar] [CrossRef]
- Kulikova, V.V.; Chernukha, M.Y.; Morozova, E.A.; Revtovich, S.V.; Rodionov, A.N.; Koval, V.S.; Avetisyan, L.R.; Kuliastova, D.G.; Shanginyan, I.A.; Demidkina, T.V. Antibacterial Effect of Thiosulfinates on Multiresistant Strains of Bacteria Isolated from Patients with Cystic Fibrosis. Acta Naturae 2018, 10, 77–80. [Google Scholar] [CrossRef]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Harris, J.C.; Cottrell, S.L.; Plummer, S.; Lloyd, D. Antimicrobial properties of Allium sativum (garlic). Appl. Microbiol. Biotechnol. 2001, 57, 282–286. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef]
- Yoshida, H.; Katsuzaki, H.; Ohta, R.; Ishikawa, K.; Fukuda, H.; Fujino, T.; Suzuki, A. Antimicrobial activity of the thiosulfinates isolated from oil-macerated garlic extract. Biosci. Biotechnol. Biochem. 1999, 63, 591–594. [Google Scholar] [CrossRef]
- Al-Habib, A.; Al-Saleh, E.; Safer, A.-M.; Afzal, M. Bactericidal effect of grape seed extract on methicillin-resistant Staphylococcus aureus (MRSA). J. Toxicol. Sci. 2010, 35, 357–364. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef]
- Chedea, V.S.; Braicu, C.; Chirilă, F.; Ogola, H.J.O.; Pelmuş, R.Ş.; Călin, L.G.; Socaciu, C. Antioxidant/Prooxidant and antibacterial/probacterial effects of a grape seed extract in complex with lipoxygenase. BioMed Res. Int. 2014, 2014, 313684. [Google Scholar] [CrossRef] [PubMed]
- Al-Mousawi, A.H.; Al-Kaabi, S.J.; Albaghdadi, A.J.H.; Almulla, A.F.; Raheem, A.; Algon, A.A.A. Effect of Black Grape Seed Extract (Vitis vinifera) on Biofilm Formation of Methicillin-Resistant Staphylococcus aureus and Staphylococcus haemolyticus. Curr. Microbiol. 2020, 77, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Zulhendri, F.; Chandrasekaran, K.; Kowacz, M.; Ravalia, M.; Kripal, K.; Fearnley, J.; Perera, C.O. Antiviral, Antibacterial, Antifungal, and Antiparasitic Properties of Propolis: A Review. Foods 2021, 10, 1360. [Google Scholar] [CrossRef] [PubMed]
| Compound | Concentration (mg/kg) |
|---|---|
| Total polyphenols | 13,910 |
| Total flavonoids | 3220 |
| Diallyl thiosulfinates (allicin) | 7030 |
| S-allyl-L-cysteine | 80 |
| Leucine | 586 |
| Isoleucine | 500 |
| Valine | 477 |
| Methionine | 316 |
| Cysteine | 811 |
| Phenylalanine | 556 |
| Tyrosine | 4499 |
| Aspartic acid | 901 |
| Glutamic acid | 2866 |
| Arginine | 4090 |
| Lysine | 617 |
| Histidine | 891 |
| Threonine | 812 |
| Serine | 385 |
| Glycine | 215 |
| Alanine | 897 |
| Thiamine (B1) | 552 |
| Riboflavin (B2) | 2 |
| Niacin (B3) | 26 |
| Pantothenic acid (B5) | 1556 |
| Biotin (B7) | 251 |
| Cobalamin (B12) | 898 |
| Ascorbic acid (C) | 3347 |
| Linoleic acid (F) | 276 |
| Tocopherol (E) | 7 |
| Menadione (K3) | 7 |
| Compound | Concentration (mg/kg) | Concentration (%) |
|---|---|---|
| Procyanidin B1 | 58.32 | 1.17 |
| Catechin | 105.77 | 2.12 |
| Procyanidin B2 | 48.15 | 0.96 |
| Epicatechin | 117.74 | 2.35 |
| Procyanidin C1 | 30.52 | 0.61 |
| Proanthocyanidins | 4624.18 | 92.48 |
| Total flavonoids and polyphenols | 4984.67 | 99.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avendaño-Ortiz, J.; Tribaldo, A.; Ballestero, L.; Gómez, L.A.; Gracia, I.; Rodríguez, J.F.; Ramírez, N.B.; Bodoque-Villar, R.; Vaz-Salgado, M.Á.; del Campo, R.; et al. Antimicrobial Activity of Natural Extracts Against Catheter-Colonizing Methicillin-Resistant Staphylococcus aureus Clinical Isolates. Biomedicines 2025, 13, 2150. https://doi.org/10.3390/biomedicines13092150
Avendaño-Ortiz J, Tribaldo A, Ballestero L, Gómez LA, Gracia I, Rodríguez JF, Ramírez NB, Bodoque-Villar R, Vaz-Salgado MÁ, del Campo R, et al. Antimicrobial Activity of Natural Extracts Against Catheter-Colonizing Methicillin-Resistant Staphylococcus aureus Clinical Isolates. Biomedicines. 2025; 13(9):2150. https://doi.org/10.3390/biomedicines13092150
Chicago/Turabian StyleAvendaño-Ortiz, José, Alba Tribaldo, Luna Ballestero, Luis Antonio Gómez, Ignacio Gracia, Juan Francisco Rodríguez, Natalia Bejarano Ramírez, Raquel Bodoque-Villar, María Ángeles Vaz-Salgado, Rosa del Campo, and et al. 2025. "Antimicrobial Activity of Natural Extracts Against Catheter-Colonizing Methicillin-Resistant Staphylococcus aureus Clinical Isolates" Biomedicines 13, no. 9: 2150. https://doi.org/10.3390/biomedicines13092150
APA StyleAvendaño-Ortiz, J., Tribaldo, A., Ballestero, L., Gómez, L. A., Gracia, I., Rodríguez, J. F., Ramírez, N. B., Bodoque-Villar, R., Vaz-Salgado, M. Á., del Campo, R., & Redondo-Calvo, F. J. (2025). Antimicrobial Activity of Natural Extracts Against Catheter-Colonizing Methicillin-Resistant Staphylococcus aureus Clinical Isolates. Biomedicines, 13(9), 2150. https://doi.org/10.3390/biomedicines13092150







