The Evolving Role of Cardiac Imaging in Hypertrophic Cardiomyopathy: Diagnosis, Prognosis, and Clinical Practice
Abstract
1. Introduction
2. Materials and Methods
3. Overview
4. Echocardiography
4.1. Confirming the Presence of Left Ventricular Hypertrophy
4.2. Assessment of Latent Left Ventricular Outflow Tract Obstruction
4.3. Systolic Anterior Motion and Mitral Valve Abnormalities
4.4. Left Atrial Enlargement
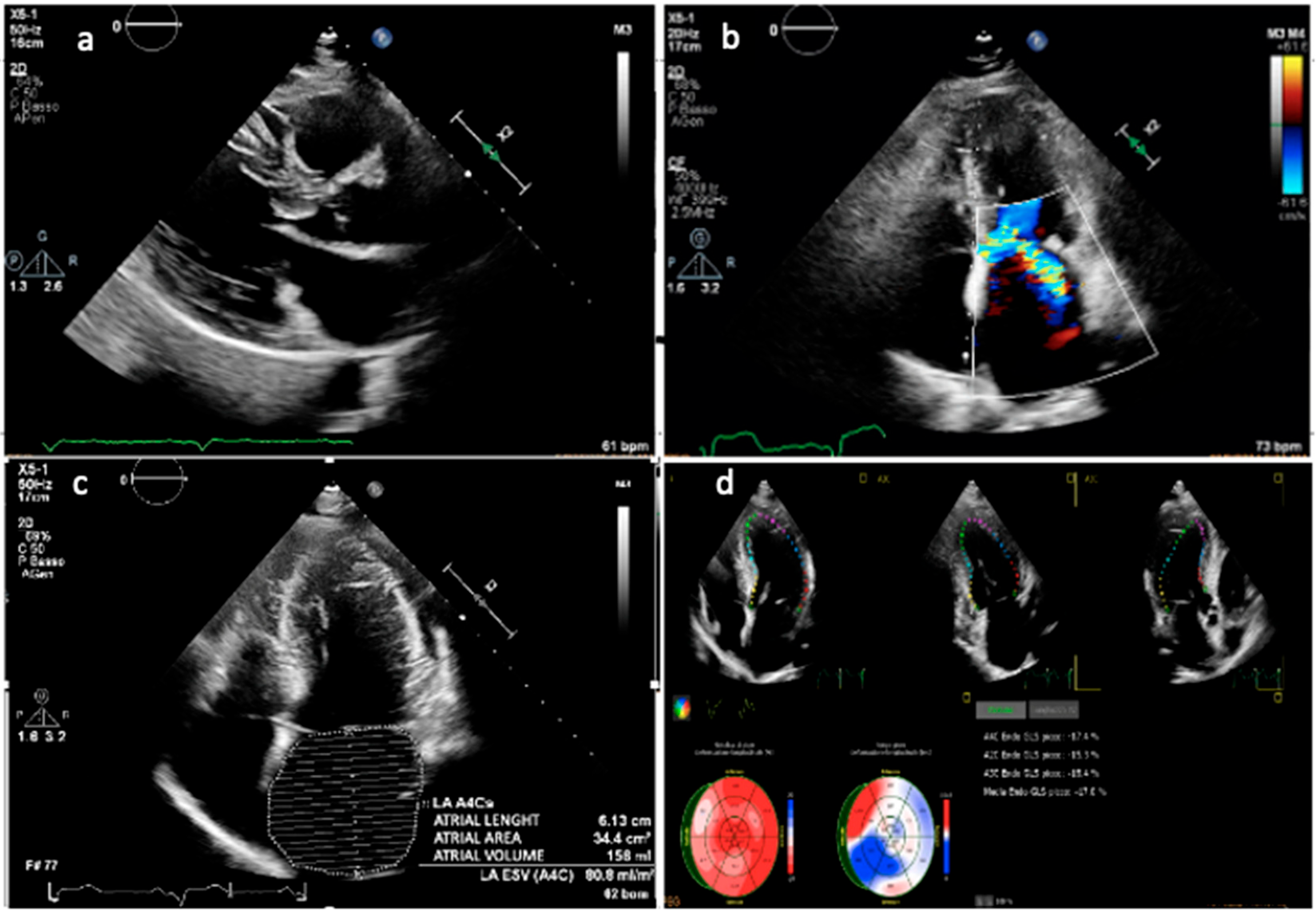
4.5. Diastolic Function
4.6. Strain Imaging
5. Cardiac Magnetic Resonance
5.1. Cine-MR and Phase-Contrast Velocity Mapping Sequences
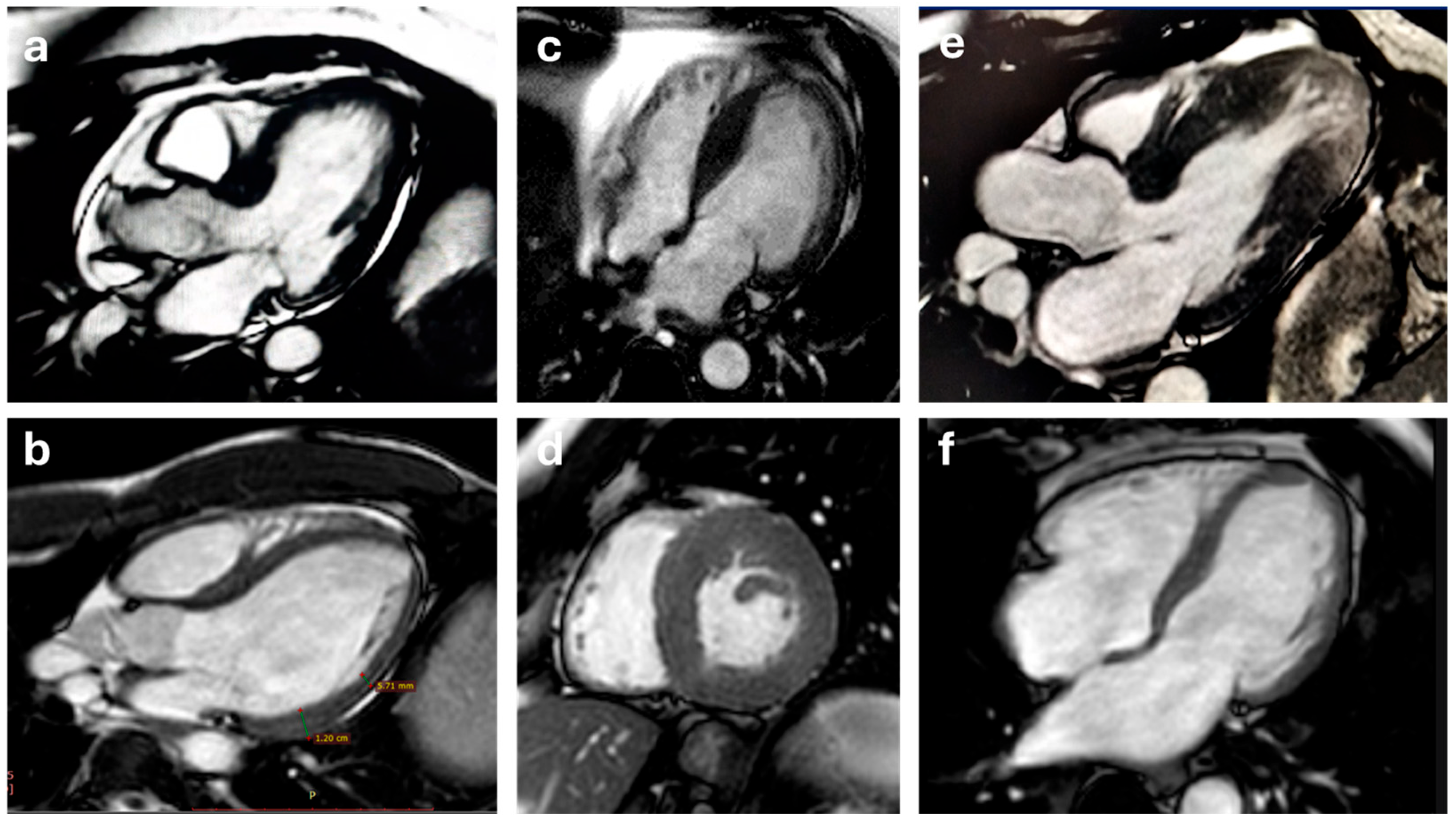
5.2. T1 and T2 Mapping
5.3. Late Gadolinium Enhancement
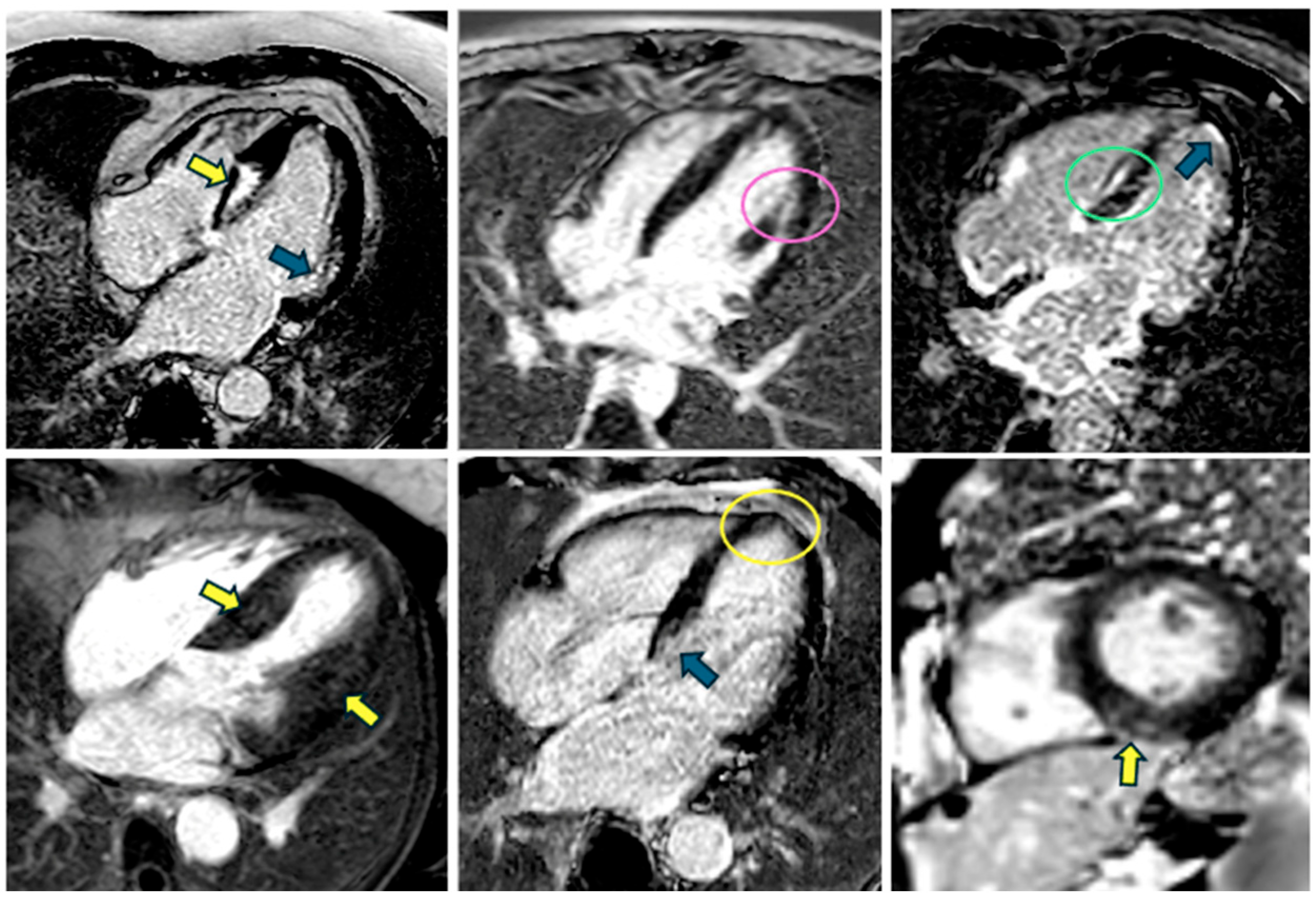
5.4. Perfusion
5.5. Evaluation of Intraventricular Flow Dynamics in HCM
6. Computed Tomography
7. Recent Advances and Emerging Techniques
7.1. Advanced Myocardial Strain Imaging
7.2. Artificial Intelligence in Cardiac Imaging for HCM
7.3. Emerging Molecular Imaging Techniques
8. The Differential Diagnosis from Athlete’s Heart: An Ongoing Challenge
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Wolf, C.M. Hypertrophic cardiomyopathy: Genetics and clinical perspectives. Cardiovasc Diagn Ther 2019, 9, S388–S415. [Google Scholar] [CrossRef]
- Viola, H.M.; Hool, L.C. Impaired calcium handling and mitochondrial metabolic dysfunction as early markers of hypertrophic cardiomyopathy. Arch. Biochem. Biophys. 2019, 665, 166–174. [Google Scholar] [CrossRef]
- Toepfer, C.N.; Garfinkel, A.C.; Venturini, G.; Wakimoto, H.; Repetti, G.; Alamo, L.; Sharma, A.; Agarwal, R.; Ewoldt, J.K.; Cloonan, P.; et al. Myosin Sequestration Regulates Sarcomere Function, Cardiomyocyte Energetics, and Metabolism, Informing the Pathogenesis of Hypertrophic Cardiomyopathy. Circulation 2020, 141, 828–842. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Nixon, B.R.; Glennon, M.S.; Shridhar, P.; Satterfield, S.L.; Su, Y.R.; Becker, J.R. Replication Stress Response Modifies Sarcomeric Cardiomyopathy Remodeling. J. Am. Heart Assoc. 2021, 10, e021768. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.C.; Owens, A.P., 3rd; Sadayappan, S. Tissue-level inflammation and ventricular remodeling in hypertrophic cardiomyopathy. J. Thromb. Thrombolysis 2020, 49, 177–183. [Google Scholar] [CrossRef]
- Briasoulis, A.; Mallikethi-Reddy, S.; Palla, M.; Alesh, I.; Afonso, L. Myocardial fibrosis on cardiac magnetic resonance and cardiac outcomes in hypertrophic cardiomyopathy: A meta-analysis. Heart 2015, 101, 1406–1411. [Google Scholar] [CrossRef]
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Diagnosis and Evaluation of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 372–389. [Google Scholar] [CrossRef] [PubMed]
- Canepa, M.; Fumagalli, C.; Tini, G.; Vincent-Tompkins, J.; Day, S.M.; Ashley, E.A.; Mazzarotto, F.; Ware, J.S.; Michels, M.; Jacoby, D.; et al. Temporal Trend of Age at Diagnosis in Hypertrophic Cardiomyopathy: An Analysis of the International Sarcomeric Human Cardiomyopathy Registry. Circ. Heart Fail. 2020, 13, e007230. [Google Scholar] [CrossRef]
- Nabeshima, Y.; Namisaki, H.; Teshima, T.; Kurashige, Y.; Kakio, A.; Fukumitsu, A.; Otsuji, Y.; Takeuchi, M. Impact of a training program incorporating cardiac magnetic resonance imaging on the accuracy and reproducibility of two-dimensional echocardiographic measurements of left ventricular volumes and ejection fraction. Cardiovasc. Ultrasound 2019, 17, 23. [Google Scholar] [CrossRef]
- Leo, I.; Dellegrottaglie, S.; Scatteia, A.; Torella, D.; Abete, R.; Aquaro, G.D.; Baggiano, A.; Barison, A.; Bogaert, J.; Calo, L.; et al. CarDiac magnEtic Resonance for prophylactic Implantable-cardioVerter defibrillAtor ThErapy in Non-Dilated Left Ventricular Cardiomyopathy: A sub-study from the DERIVATE Registry. Eur. Heart J. Cardiovasc. Imaging 2025, 26, 1233–1241. [Google Scholar] [CrossRef]
- Perone, F.; Dentamaro, I.; La Mura, L.; Alifragki, A.; Marketou, M.; Cavarretta, E.; Papadakis, M.; Androulakis, E. Current Insights and Novel Cardiovascular Magnetic Resonance-Based Techniques in the Prognosis of Non-Ischemic Dilated Cardiomyopathy. J. Clin. Med. 2024, 13, 1017. [Google Scholar] [CrossRef] [PubMed]
- Baggiano, A.; Fusini, L.; Del Torto, A.; Vivona, P.; Guglielmo, M.; Muscogiuri, G.; Soldi, M.; Martini, C.; Fraschini, E.; Rabbat, M.G.; et al. Sequential Strategy Including FFR(CT) Plus Stress-CTP Impacts on Management of Patients with Stable Chest Pain: The Stress-CTP RIPCORD Study. J. Clin. Med. 2020, 9, 2147. [Google Scholar] [CrossRef] [PubMed]
- Vetter, N.; Meder, B. Precision medicine in the diagnostics and treatment of cardiomyopathies: State of the art. Herz 2025, 50, 96–102. [Google Scholar] [CrossRef]
- Dicorato, M.M.; Citarelli, G.; Mangini, F.; Alemanni, R.; Albanese, M.; Cicco, S.; Greco, C.A.; Forleo, C.; Basile, P.; Carella, M.C.; et al. Integrative Approaches in the Management of Hypertrophic Cardiomyopathy: A Comprehensive Review of Current Therapeutic Modalities. Biomedicines 2025, 13, 1256. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Van Assen, M.; Tesche, C.; De Cecco, C.N.; Chiesa, M.; Scafuri, S.; Guglielmo, M.; Baggiano, A.; Fusini, L.; Guaricci, A.I.; et al. Artificial Intelligence in Coronary Computed Tomography Angiography: From Anatomy to Prognosis. Biomed Res. Int. 2020, 2020, 6649410. [Google Scholar] [CrossRef]
- Ghanbari, F.; Joyce, T.; Lorenzoni, V.; Guaricci, A.I.; Pavon, A.G.; Fusini, L.; Andreini, D.; Rabbat, M.G.; Aquaro, G.D.; Abete, R.; et al. AI Cardiac MRI Scar Analysis Aids Prediction of Major Arrhythmic Events in the Multicenter DERIVATE Registry. Radiology 2023, 307, e222239. [Google Scholar] [CrossRef]
- Gaibazzi, N.; Porter, T.; Lorenzoni, V.; Pontone, G.; De Santis, D.; De Rosa, A.; Guaricci, A.I. Effect of Coronary Revascularization on the Prognostic Value of Stress Myocardial Contrast Wall Motion and Perfusion Imaging. J. Am. Heart Assoc. 2017, 6, e006202. [Google Scholar] [CrossRef]
- Norrish, G.; Ding, T.; Field, E.; Ziolkowska, L.; Olivotto, I.; Limongelli, G.; Anastasakis, A.; Weintraub, R.; Biagini, E.; Ragni, L.; et al. Development of a Novel Risk Prediction Model for Sudden Cardiac Death in Childhood Hypertrophic Cardiomyopathy (HCM Risk-Kids). JAMA Cardiol. 2019, 4, 918–927. [Google Scholar] [CrossRef]
- Ommen, S.R.; Ho, C.Y.; Asif, I.M.; Balaji, S.; Burke, M.A.; Day, S.M.; Dearani, J.A.; Epps, K.C.; Evanovich, L.; Ferrari, V.A.; et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1239–e1311. [Google Scholar] [CrossRef]
- Muresan, I.D.; Agoston-Coldea, L. Phenotypes of hypertrophic cardiomyopathy: Genetics, clinics, and modular imaging. Heart Fail. Rev. 2021, 26, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Carella, M.C.; Forleo, C.; Caretto, P.; Naccarati, M.L.; Dentamaro, I.; Dicorato, M.M.; Basile, P.; Carulli, E.; Latorre, M.D.; Baggiano, A.; et al. Overcoming Resistance in Anderson-Fabry Disease: Current Therapeutic Challenges and Future Perspectives. J. Clin. Med. 2024, 13, 7195. [Google Scholar] [CrossRef]
- Dicorato, M.M.; Basile, P.; Muscogiuri, G.; Carella, M.C.; Naccarati, M.L.; Dentamaro, I.; Guglielmo, M.; Baggiano, A.; Mushtaq, S.; Fusini, L.; et al. Novel Insights into Non-Invasive Diagnostic Techniques for Cardiac Amyloidosis: A Critical Review. Diagnostics 2024, 14, 2249. [Google Scholar] [CrossRef]
- Gersh, B.J.; Maron, B.J.; Bonow, R.O.; Dearani, J.A.; Fifer, M.A.; Link, M.S.; Naidu, S.S.; Nishimura, R.A.; Ommen, S.R.; Rakowski, H.; et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2011, 58, e212–e260. [Google Scholar]
- Fry, E.T.A.; Wood, M.J.; Walsh, M.N. Maternal Health: The Heart of the Matter. J. Am. Coll. Cardiol. 2022, 80, 1107–1109. [Google Scholar] [CrossRef]
- Guigui, S.A.; Torres, C.; Escolar, E.; Mihos, C.G. Systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy: A narrative review. J. Thorac. Dis. 2022, 14, 2309–2325. [Google Scholar] [CrossRef]
- Vilcant, V.; Hai, O. Left Ventricular Outflow Tract Obstruction; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Pezel, T.; Lacotte, J.; Toupin, S.; Salerno, F.; Said, M.A.; Manenti, V.; Fiorina, L.; Garot, P.; Hovasse, T.; Unterseeh, T.; et al. Diagnostic Accuracy of Stress Perfusion CMR for Risk Stratification in Patients With MR-Conditional Pacemakers. JACC Cardiovasc. Imaging 2021, 14, 2053–2054. [Google Scholar] [CrossRef]
- Jain, C.C.; Newman, D.B.; Geske, J.B. Mitral Valve Disease in Hypertrophic Cardiomyopathy:Evaluation and Management. Curr. Cardiol. Rep. 2019, 21, 136. [Google Scholar] [CrossRef] [PubMed]
- Malcolmson, J.; Shipolini, A.; Mohiddin, S.; Savvatis, K. The mitral valve in hypertrophic cardiomyopathy. Curr. Opin. Cardiol. 2023, 38, 415–423. [Google Scholar] [CrossRef]
- Zhou, N.; Liu, A.; Weng, H.; Liu, W.; Tian, F.; Zhao, W.; Ma, J.; Guo, W.; Chen, H.; Pan, C.; et al. Three-dimensional echocardiography reveals early mitral valve alterations in hypertrophic cardiomyopathy genetic mutation carriers. Int. J. Cardiol. 2024, 395, 131576. [Google Scholar] [CrossRef] [PubMed]
- Groarke, J.D.; Galazka, P.Z.; Cirino, A.L.; Lakdawala, N.K.; Thune, J.J.; Bundgaard, H.; Orav, E.J.; Levine, R.A.; Ho, C.Y. Intrinsic mitral valve alterations in hypertrophic cardiomyopathy sarcomere mutation carriers. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39 e14. [Google Scholar] [CrossRef] [PubMed]
- Dentamaro, I.; Vestito, D.; Michelotto, E.; De Santis, D.; Ostuni, V.; Cadeddu, C.; Colonna, P. Evaluation of left atrial appendage function and thrombi in patients with atrial fibrillation: From transthoracic to real time 3D transesophageal echocardiography. Int. J. Cardiovasc. Imaging 2017, 33, 491–498. [Google Scholar] [CrossRef]
- Dicorato, M.M.; Basile, P.; Naccarati, M.L.; Carella, M.C.; Dentamaro, I.; Falagario, A.; Cicco, S.; Forleo, C.; Guaricci, A.I.; Ciccone, M.M.; et al. Predicting New-Onset Atrial Fibrillation in Hypertrophic Cardiomyopathy: A Review. J. Clin. Med. 2025, 14, 2018. [Google Scholar] [CrossRef]
- Saito, C.; Minami, Y.; Haruki, S.; Arai, K.; Ashihara, K.; Hagiwara, N. Prognostic Relevance of a Score for Identifying Diastolic Dysfunction according to the 2016 American Society of Echocardiography/European Association of Cardiovascular Imaging Recommendations in Patients with Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2022, 35, 469–476. [Google Scholar] [CrossRef]
- Liu, W.; Sun, D.; Yang, J. Diastolic Dysfunction of Hypertrophic Cardiomyopathy Genotype-Positive Subjects Without Hypertrophy Is Detected by Tissue Doppler Imaging: A Systematic Review and Meta-analysis. J. Ultrasound Med. 2017, 36, 2093–2103. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Lakkis, N.M.; Middleton, K.J.; Spencer, W.H., 3rd; Zoghbi, W.A.; Quinones, M.A. Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation 1999, 99, 254–261. [Google Scholar] [CrossRef]
- Haland, T.F.; Almaas, V.M.; Hasselberg, N.E.; Saberniak, J.; Leren, I.S.; Hopp, E.; Edvardsen, T.; Haugaa, K.H. Strain echocardiography is related to fibrosis and ventricular arrhythmias in hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 613–621. [Google Scholar] [CrossRef]
- Wazzan, A.A.; Taconne, M.; Rolle, V.L.; Forsaa, M.I.; Haugaa, K.H.; Galli, E.; Hernandez, A.; Edvardsen, T.; Donal, E. Risk profiles for ventricular arrhythmias in hypertrophic cardiomyopathy through clustering analysis including left ventricular strain. Int. J. Cardiol. 2024, 409, 132167. [Google Scholar] [CrossRef]
- Negri, F.; Muser, D.; Driussi, M.; Sanna, G.D.; Masè, M.; Cittar, M.; Poli, S.; De Bellis, A.; Fabris, E.; Puppato, M.; et al. Prognostic role of global longitudinal strain by feature tracking in patients with hypertrophic cardiomyopathy: The STRAIN-HCM study. Int. J. Cardiol. 2021, 345, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Tower-Rader, A.; Mohananey, D.; To, A.; Lever, H.M.; Popovic, Z.B.; Desai, M.Y. Prognostic Value of Global Longitudinal Strain in Hypertrophic Cardiomyopathy: A Systematic Review of Existing Literature. JACC Cardiovasc. Imaging 2019, 12, 1930–1942. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Chiesa, M.; Novelli, V.; Sommariva, E.; Biondi, M.L.; Manzoni, M.; Florio, A.; Lampus, M.L.; Avallone, C.; Zocchi, C.; et al. Role of advanced CMR features in identifying a positive genotype of hypertrophic cardiomyopathy. Int. J. Cardiol. 2024, 417, 132554. [Google Scholar] [CrossRef] [PubMed]
- Forleo, C.; Carella, M.C.; Basile, P.; Mandunzio, D.; Greco, G.; Napoli, G.; Carulli, E.; Dicorato, M.M.; Dentamaro, I.; Santobuono, V.E.; et al. The Role of Magnetic Resonance Imaging in Cardiomyopathies in the Light of New Guidelines: A Focus on Tissue Mapping. J. Clin. Med. 2024, 13, 2621. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, S.; Yu, S.; Wu, G.; Wang, D.; Liu, L.; Song, J.; Zhu, Y.; Kang, L.; Wang, J.; et al. Patterns of Replacement Fibrosis in Hypertrophic Cardiomyopathy. Radiology 2022, 302, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Soler, R.; Mendez, C.; Rodriguez, E.; Barriales, R.; Ochoa, J.P.; Monserrat, L. Phenotypes of hypertrophic cardiomyopathy. An illustrative review of MRI findings. Insights Imaging 2018, 9, 1007–1020. [Google Scholar] [CrossRef]
- Goyal, N.; Keir, G.; Esterson, Y.B.; Saba, S.G.; Cohen, S.; Rowin, E.; Romashko, M.; Chusid, J. Hypertrophic cardiomyopathy—Phenotypic variations beyond wall thickness. Clin. Imaging 2023, 95, 80–89. [Google Scholar] [CrossRef]
- White, R.D.; Obuchowski, N.A.; Gunawardena, S.; Lipchik, E.O.; Lever, H.M.; Van Dyke, C.W.; Lytle, B.W. Left ventricular outflow tract obstruction in hypertrophic cardiomyopathy: Presurgical and postsurgical evaluation by computed tomography magnetic resonance imaging. Am. J. Card. Imaging 1996, 10, 1–13. [Google Scholar]
- Chu, L.C.; Porter, K.K.; Corona-Villalobos, C.P.; Gulsun, M.A.; Shea, S.M.; Markl, M.; Abraham, T.P.; Bluemke, D.A.; Kamel, I.R.; Zimmerman, S.L. Evaluation of Left Ventricular Outflow Tract Obstruction With Four-Dimensional Phase Contrast Magnetic Resonance Imaging in Patients with Hypertrophic Cardiomyopathy-A Pilot Study. J. Comput. Assist. Tomogr. 2016, 40, 937–940. [Google Scholar] [CrossRef]
- Mangini, F.; Casavecchia, G.; Gravina, M.; Brunetti, N.D.; Di Monaco, A.; Dellegrottaglie, S.; Guglielmo, M.; Sgarra, L.; Milo, M.; Lucarelli, K.; et al. Before and Beyond Tissue Characterization: Cardiac Magnetic Resonance Imaging in the Morphological, Volumetric, and Functional Evaluation of the Right Ventricle in Arrhythmogenic Right Ventricular Cardiomyopathy, a Narrative Review. Echocardiography 2025, 42, e70167. [Google Scholar] [CrossRef]
- Cavus, E.; Muellerleile, K.; Schellert, S.; Schneider, J.; Tahir, E.; Chevalier, C.; Jahnke, C.; Radunski, U.K.; Adam, G.; Kirchhof, P.; et al. CMR feature tracking strain patterns and their association with circulating cardiac biomarkers in patients with hypertrophic cardiomyopathy. Clin. Res. Cardiol. 2021, 110, 1757–1769. [Google Scholar] [CrossRef]
- Li, Z.L.; He, S.; Xia, C.C.; Peng, W.L.; Li, L.; Liu, K.L.; Zhang, J.G.; Pu, J.; Guo, Y.K. Global longitudinal diastolic strain rate as a novel marker for predicting adverse outcomes in hypertrophic cardiomyopathy by cardiac magnetic resonance tissue tracking. Clin. Radiol. 2021, 76, 78.e19–78.e25. [Google Scholar] [CrossRef]
- Pu, C.; Fei, J.; Lv, S.; Wu, Y.; He, C.; Guo, D.; Mabombo, P.U.; Chooah, O.; Hu, H. Global Circumferential Strain by Cardiac Magnetic Resonance Tissue Tracking Associated With Ventricular Arrhythmias in Hypertrophic Cardiomyopathy Patients. Front. Cardiovasc. Med. 2021, 8, 670361. [Google Scholar] [CrossRef] [PubMed]
- Sharifian, M.; Rezaeian, N.; Asadian, S.; Mohammadzadeh, A.; Nahardani, A.; Kasani, K.; Toloueitabar, Y.; Farahmand, A.M.; Hosseini, L. Efficacy of Novel Noncontrast Cardiac Magnetic Resonance Methods in Indicating Fibrosis in Hypertrophic Cardiomyopathy. Cardiol. Res. Pract. 2021, 2021, 9931136. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Nakamori, S.; Dohi, K.; Ishida, M.; Goto, Y.; Imanaka-Yoshida, K.; Omori, T.; Goto, I.; Kumagai, N.; Fujimoto, N.; Ichikawa, Y.; et al. Native T1 Mapping and Extracellular Volume Mapping for the Assessment of Diffuse Myocardial Fibrosis in Dilated Cardiomyopathy. JACC Cardiovasc. Imaging 2018, 11, 48–59. [Google Scholar] [CrossRef]
- Weingartner, S.; Akcakaya, M.; Roujol, S.; Basha, T.; Tschabrunn, C.; Berg, S.; Anter, E.; Nezafat, R. Free-breathing combined three-dimensional phase sensitive late gadolinium enhancement and T1 mapping for myocardial tissue characterization. Magn. Reson. Med. 2015, 74, 1032–1041. [Google Scholar] [CrossRef]
- Gravina, M.; Casavecchia, G.; Mangini, F.; Mautone, F.; Ruggeri, D.; Guglielmi, G.; Macarini, L.; Brunetti, N.D. Magnetic resonance mapping for the assessment of cardiomyopathies and myocardial disease. Int. J. Cardiol. 2024, 415, 132440. [Google Scholar] [CrossRef]
- Duca, F.; Kammerlander, A.A.; Panzenbock, A.; Binder, C.; Aschauer, S.; Loewe, C.; Agis, H.; Kain, R.; Hengstenberg, C.; Bonderman, D.; et al. Cardiac Magnetic Resonance T(1) Mapping in Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2018, 11, 1924–1926. [Google Scholar] [CrossRef]
- Huang, L.; Ran, L.; Zhao, P.; Tang, D.; Han, R.; Ai, T.; Xia, L.; Tao, Q. MRI native T1 and T2 mapping of myocardial segments in hypertrophic cardiomyopathy: Tissue remodeling manifested prior to structure changes. Br. J. Radiol. 2019, 92, 20190634. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Valbuena, S.; Hinojar, R.; Petersen, S.E.; Greenwood, J.P.; Kramer, C.M.; Kwong, R.Y.; McCann, G.P.; Berry, C.; Nagel, E. Society for Cardiovascular Magnetic Resonance (SCMR) expert consensus for CMR imaging endpoints in clinical research: Part I—analytical validation and clinical qualification. J. Cardiovasc. Magn. Reson. 2018, 20, 67. [Google Scholar] [CrossRef]
- Kellman, P.; Arai, A.E.; McVeigh, E.R.; Aletras, A.H. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn. Reson. Med. 2002, 47, 372–383. [Google Scholar] [CrossRef]
- Mantilla, J.; Paredes, J.L.; Bellanger, J.-J.; Betancur, J.; Schnell, F.; Leclercq, C.; Garreau, M. Detection of fibrosis in late gadolinium enhancement cardiac MRI using kernel dictionary learning-based clustering. In Proceedings of the 2015 Computing in Cardiology Conference (CinC), Nice, France, 6–9 September 2015; pp. 357–360. [Google Scholar]
- Klues, H.G.; Schiffers, A.; Maron, B.J. Phenotypic spectrum and patterns of left ventricular hypertrophy in hypertrophic cardiomyopathy: Morphologic observations and significance as assessed by two-dimensional echocardiography in 600 patients. J. Am. Coll. Cardiol. 1995, 26, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Forleo, C.; Carella, M.C.; Basile, P.; Carulli, E.; Dadamo, M.L.; Amati, F.; Loizzi, F.; Sorrentino, S.; Dentamaro, I.; Dicorato, M.M.; et al. Missense and Non-Missense Lamin A/C Gene Mutations Are Similarly Associated with Major Arrhythmic Cardiac Events: A 20-Year Single-Centre Experience. Biomedicines 2024, 12, 1293. [Google Scholar] [CrossRef] [PubMed]
- Goldie, F.C.; Lee, M.M.Y.; Coats, C.J.; Nordin, S. Advances in Multi-Modality Imaging in Hypertrophic Cardiomyopathy. J. Clin. Med. 2024, 13, 842. [Google Scholar] [CrossRef]
- Basile, P.; Soldato, N.; Pedio, E.; Siena, P.; Carella, M.C.; Dentamaro, I.; Khan, Y.; Baggiano, A.; Mushtaq, S.; Forleo, C.; et al. Cardiac magnetic resonance reveals concealed structural heart disease in patients with frequent premature ventricular contractions and normal echocardiography: A systematic review. Int. J. Cardiol. 2024, 412, 132306. [Google Scholar] [CrossRef]
- Sivalokanathan, S. The Role of Cardiovascular Magnetic Resonance Imaging in the Evaluation of Hypertrophic Cardiomyopathy. Diagnostics 2022, 12, 314. [Google Scholar] [CrossRef]
- Kim, E.K.; Lee, S.C.; Chang, S.A.; Jang, S.Y.; Kim, S.M.; Park, S.J.; Choi, J.O.; Park, S.W.; Jeon, E.S.; Choe, Y.H. Prevalence and clinical significance of cardiovascular magnetic resonance adenosine stress-induced myocardial perfusion defect in hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2020, 22, 30. [Google Scholar] [CrossRef]
- Camaioni, C.; Knott, K.D.; Augusto, J.B.; Seraphim, A.; Rosmini, S.; Ricci, F.; Boubertakh, R.; Xue, H.; Hughes, R.; Captur, G.; et al. Inline perfusion mapping provides insights into the disease mechanism in hypertrophic cardiomyopathy. Heart 2020, 106, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sun, X.Y.; Zhong, M.; Li, L.; Zhang, M.; Lin, M.J.; Zhang, Y.K.; Jiang, G.H.; Zhang, W.; Shang, Y.Y. Evaluation of hemodynamics in patients with hypertrophic cardiomyopathy by vector flow mapping: Comparison with healthy subjects. Exp. Ther. Med. 2019, 17, 4379–4388. [Google Scholar] [CrossRef] [PubMed]
- Pruijssen, J.T.; Allen, B.D.; Barker, A.J.; Bonow, R.O.; Choudhury, L.; Carr, J.C.; Markl, M.; van Ooij, P. Hypertrophic Cardiomyopathy Is Associated with Altered Left Ventricular 3D Blood Flow Dynamics. Radiol. Cardiothorac. Imaging 2020, 2, e190038. [Google Scholar] [CrossRef] [PubMed]
- Conte, E.; Mushtaq, S.; Muscogiuri, G.; Formenti, A.; Annoni, A.; Mancini, E.; Ricci, F.; Melotti, E.; Gigante, C.; Lorenza, Z.; et al. The Potential Role of Cardiac CT in the Evaluation of Patients With Known or Suspected Cardiomyopathy: From Traditional Indications to Novel Clinical Applications. Front. Cardiovasc. Med. 2021, 8, 709124. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.; Emoto, T.; Nakaura, T.; Kidoh, M.; Utsunomiya, D.; Funama, Y.; Nagayama, Y.; Takashio, S.; Ueda, M.; Yamashita, T.; et al. Myocardial Late Iodine Enhancement and Extracellular Volume Quantification with Dual-Layer Spectral Detector Dual-Energy Cardiac CT. Radiol. Cardiothorac. Imaging 2019, 1, e180003. [Google Scholar] [CrossRef] [PubMed]
- Bytyci, I.; Dini, F.L.; Bajraktari, A.; Pugliese, N.R.; D’Agostino, A.; Bajraktari, G.; Lindqvist, P.; Henein, M.Y. Speckle Tracking-Derived Left Atrial Stiffness Predicts Clinical Outcome in Heart Failure Patients with Reduced to Mid-Range Ejection Fraction. J. Clin. Med. 2020, 9, 1244. [Google Scholar] [CrossRef]
- Dentamaro, I.; Guaricci, A.I.; Belahnech, Y.; Basile, P.; Pontone, G.; Rodriguez Palomares, J.F. Feature tracking cardiovascular magnetic resonance to predict major adverse cardiovascular events in patients with cardiac amyloidosis. Rev. Esp. Cardiol. (Engl. Ed.) 2025, in press. [Google Scholar] [CrossRef]
- Narula, S.; Shameer, K.; Salem Omar, A.M.; Dudley, J.T.; Sengupta, P.P. Machine-Learning Algorithms to Automate Morphological and Functional Assessments in 2D Echocardiography. J. Am. Coll. Cardiol. 2016, 68, 2287–2295. [Google Scholar] [CrossRef]
- Zhang, J.; Gajjala, S.; Agrawal, P.; Tison, G.H.; Hallock, L.A.; Beussink-Nelson, L.; Lassen, M.H.; Fan, E.; Aras, M.A.; Jordan, C.; et al. Fully Automated Echocardiogram Interpretation in Clinical Practice. Circulation 2018, 138, 1623–1635. [Google Scholar] [CrossRef]
- Ouyang, D.; He, B.; Ghorbani, A.; Yuan, N.; Ebinger, J.; Langlotz, C.P.; Heidenreich, P.A.; Harrington, R.A.; Liang, D.H.; Ashley, E.A.; et al. Video-based AI for beat-to-beat assessment of cardiac function. Nature 2020, 580, 252–256. [Google Scholar] [CrossRef]
- Liang, G.; Peng, Z.; Hu, J.; Liang, L.; Xie, D.; Chen, X.; Zhi, R.; Guan, X.; Deng, Y. Echocardiographic Texture Analysis Using Machine Learning for Predicting Myocardial Fibrosis in Hypertrophic Cardiomyopathy. Ultrasound Med. Biol. 2025, in press. [Google Scholar] [CrossRef]
- Puyol-Anton, E.; Ruijsink, B.; Baumgartner, C.F.; Masci, P.G.; Sinclair, M.; Konukoglu, E.; Razavi, R.; King, A.P. Automated quantification of myocardial tissue characteristics from native T(1) mapping using neural networks with uncertainty-based quality-control. J. Cardiovasc. Magn. Reson. 2020, 22, 60. [Google Scholar] [CrossRef]
- Dyverfeldt, P.; Bissell, M.; Barker, A.J.; Bolger, A.F.; Carlhall, C.J.; Ebbers, T.; Francios, C.J.; Frydrychowicz, A.; Geiger, J.; Giese, D.; et al. 4D flow cardiovascular magnetic resonance consensus statement. J. Cardiovasc. Magn. Reson. 2015, 17, 72. [Google Scholar] [CrossRef]
- Morita, S.X.; Kusunose, K.; Haga, A.; Sata, M.; Hasegawa, K.; Raita, Y.; Reilly, M.P.; Fifer, M.A.; Maurer, M.S.; Shimada, Y.J. Deep Learning Analysis of Echocardiographic Images to Predict Positive Genotype in Patients With Hypertrophic Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 669860. [Google Scholar] [CrossRef]
- Yang, Q.; Xue, S.; Ren, C.; Lin, X.; Hacker, M.; Chen, W.; Niu, N.; Li, X.; Huo, L. Fibroblast activation protein-targeted PET/CT in multiple non-ischemic cardiomyopathies. Eur. J. Nucl. Med. Mol. Imaging 2025. [Google Scholar] [CrossRef]
- Bagnall, R.D.; Weintraub, R.G.; Ingles, J.; Duflou, J.; Yeates, L.; Lam, L.; Davis, A.M.; Thompson, T.; Connell, V.; Wallace, J.; et al. A Prospective Study of Sudden Cardiac Death among Children and Young Adults. N. Engl. J. Med. 2016, 374, 2441–2452. [Google Scholar] [CrossRef] [PubMed]
- Alpert, C.; Day, S.M.; Saberi, S. Sports and Exercise in Athletes with Hypertrophic Cardiomyopathy. Clin. Sports Med. 2015, 34, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Rowin, E.J.; Casey, S.A.; Garberich, R.F.; Maron, M.S. What Do Patients With Hypertrophic Cardiomyopathy Die from? Am. J. Cardiol. 2016, 117, 434–435. [Google Scholar] [CrossRef]
- Lampert, R.; Ackerman, M.J.; Marino, B.S.; Burg, M.; Ainsworth, B.; Salberg, L.; Tome Esteban, M.T.; Ho, C.Y.; Abraham, R.; Balaji, S.; et al. Vigorous Exercise in Patients With Hypertrophic Cardiomyopathy. JAMA Cardiol. 2023, 8, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Lampert, R.; Olshansky, B.; Heidbuchel, H.; Lawless, C.; Saarel, E.; Ackerman, M.; Calkins, H.; Estes, N.A.M.; Link, M.S.; Maron, B.J.; et al. Safety of Sports for Athletes With Implantable Cardioverter-Defibrillators: Long-Term Results of a Prospective Multinational Registry. Circulation 2017, 135, 2310–2312. [Google Scholar] [CrossRef]
- Leyva, F.; Zegard, A.; Acquaye, E.; Gubran, C.; Taylor, R.; Foley, P.W.X.; Umar, F.; Patel, K.; Panting, J.; Marshall, H.; et al. Outcomes of Cardiac Resynchronization Therapy With or Without Defibrillation in Patients With Nonischemic Cardiomyopathy. J. Am. Coll. Cardiol. 2017, 70, 1216–1227. [Google Scholar] [CrossRef]
- Zeppilli, P.; Biffi, A.; Cammarano, M.; Castelletti, S.; Cavarretta, E.; Cecchi, F.; Colivicchi, F.; Contursi, M.; Corrado, D.; D’Andrea, A.; et al. Italian Cardiological Guidelines (COCIS) for Competitive Sport Eligibility in athletes with heart disease: Update 2024. Minerva Medica 2024, 115, 533–564. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Back, M.; Borjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Mills, H.; Espersen, K.; Jurlander, R.; Iversen, K.; Bundgaard, H.; Raja, A.A. Prevention of sudden cardiac death in hypertrophic cardiomyopathy: Risk assessment using left atrial diameter predicted from left atrial volume. Clin. Cardiol. 2020, 43, 581–586. [Google Scholar] [CrossRef]
- Saberi, S.; Wheeler, M.; Bragg-Gresham, J.; Hornsby, W.; Agarwal, P.P.; Attili, A.; Concannon, M.; Dries, A.M.; Shmargad, Y.; Salisbury, H.; et al. Effect of Moderate-Intensity Exercise Training on Peak Oxygen Consumption in Patients With Hypertrophic Cardiomyopathy: A Randomized Clinical Trial. JAMA 2017, 317, 1349–1357. [Google Scholar] [CrossRef]
- Pay, L.; Çetin, T.; Dereli, Ş.; Kadı, H.; Yumurtaş, A.Ç.; Çınar, T.; Hayıroğlu, M.İ. Validation of the HCM Risk-SCD model in patients with hypertrophic cardiomyopathy and future perspectives. Pacing Clin. Electrophysiol. 2023, 46, 1519–1525. [Google Scholar] [CrossRef]
- Kalenderoğlu, K. The Long-Term Mortality Predictors in Hypertrophic Cardiomyopathy Patients with Low Risk of Sudden Cardiac Death. Turk Kardiyol. Dern. Ars.-Arch. Turk. Soc. Cardiol. 2025, 32, 312–318. [Google Scholar] [CrossRef]
- Begg, A.; Dahiya, G.; Kyvernitakis, A.; Biederman, R.W.W. The use of contrast-enhanced transthoracic echocardiography for spiral-variant hypertrophic cardiomyopathy. Echocardiography 2020, 37, 1873–1876. [Google Scholar] [CrossRef]
- Augusto, J.B.; Davies, R.H.; Bhuva, A.N.; Knott, K.D.; Seraphim, A.; Alfarih, M.; Lau, C.; Hughes, R.K.; Lopes, L.R.; Shiwani, H.; et al. Diagnosis and risk stratification in hypertrophic cardiomyopathy using machine learning wall thickness measurement: A comparison with human test-retest performance. Lancet Digit. Health 2021, 3, e20–e28. [Google Scholar] [CrossRef] [PubMed]
- Ganame, J.; Mertens, L.; Eidem, B.W.; Claus, P.; D’Hooge, J.; Havemann, L.M.; McMahon, C.J.; Elayda, M.A.; Vaughn, W.K.; Towbin, J.A.; et al. Regional myocardial deformation in children with hypertrophic cardiomyopathy: Morphological and clinical correlations. Eur. Heart J. 2007, 28, 2886–2894. [Google Scholar] [CrossRef]
- Hartlage, G.R.; Kim, J.H.; Strickland, P.T.; Cheng, A.C.; Ghasemzadeh, N.; Pernetz, M.A.; Clements, S.D.; Williams, B.R., 3rd. The prognostic value of standardized reference values for speckle-tracking global longitudinal strain in hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging 2015, 31, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Luijkx, T.; Cramer, M.J.; Buckens, C.F.; Zaidi, A.; Rienks, R.; Mosterd, A.; Prakken, N.H.; Dijkman, B.; Mali, W.P.; Velthuis, B.K. Unravelling the grey zone: Cardiac MRI volume to wall mass ratio to differentiate hypertrophic cardiomyopathy and the athlete’s heart. Br. J. Sports Med. 2015, 49, 1404–1409. [Google Scholar] [CrossRef] [PubMed]

| Modality | Main Advantages | Limitations | Clinical Role |
|---|---|---|---|
| Conventional Echocardiography (2D + Doppler) |
|
| Initial diagnosis, follow-up, family screening |
| Strain Imaging (2D-STE) |
|
| Early dysfunction detection; prognosis |
| 3D Strain Imaging/CMR Feature Tracking |
|
| Advanced phenotyping, risk stratification |
| Cardiac MRI (cine + LGE) |
|
| Accurate diagnosis, prognosis, ICD indication |
| T1/T2 Mapping—CMR |
|
| Early phenotyping, therapy monitoring |
| 4D Flow CMR |
|
| Research, functional stratification |
| Cardiac CT |
|
| CAD exclusion, morphological evaluation |
| AI-based Imaging (Echo, CMR, CT) |
|
| Diagnostic support, workflow automation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dentamaro, I.; Dicorato, M.M.; Falagario, A.; Cicco, S.; Dentamaro, S.; Correale, M.; Manuppelli, V.; Citarelli, G.; Mangini, F.; Fiore, C.; et al. The Evolving Role of Cardiac Imaging in Hypertrophic Cardiomyopathy: Diagnosis, Prognosis, and Clinical Practice. Biomedicines 2025, 13, 2138. https://doi.org/10.3390/biomedicines13092138
Dentamaro I, Dicorato MM, Falagario A, Cicco S, Dentamaro S, Correale M, Manuppelli V, Citarelli G, Mangini F, Fiore C, et al. The Evolving Role of Cardiac Imaging in Hypertrophic Cardiomyopathy: Diagnosis, Prognosis, and Clinical Practice. Biomedicines. 2025; 13(9):2138. https://doi.org/10.3390/biomedicines13092138
Chicago/Turabian StyleDentamaro, Ilaria, Marco Maria Dicorato, Alessio Falagario, Sebastiano Cicco, Sergio Dentamaro, Michele Correale, Vincenzo Manuppelli, Gaetano Citarelli, Francesco Mangini, Corrado Fiore, and et al. 2025. "The Evolving Role of Cardiac Imaging in Hypertrophic Cardiomyopathy: Diagnosis, Prognosis, and Clinical Practice" Biomedicines 13, no. 9: 2138. https://doi.org/10.3390/biomedicines13092138
APA StyleDentamaro, I., Dicorato, M. M., Falagario, A., Cicco, S., Dentamaro, S., Correale, M., Manuppelli, V., Citarelli, G., Mangini, F., Fiore, C., Colonna, P., Petruccelli, E., Piscitelli, L., Giovannetti, G., Latorre, M. D., Forleo, C., Basile, P., Carella, M. C., Santobuono, V. E., ... Guaricci, A. I. (2025). The Evolving Role of Cardiac Imaging in Hypertrophic Cardiomyopathy: Diagnosis, Prognosis, and Clinical Practice. Biomedicines, 13(9), 2138. https://doi.org/10.3390/biomedicines13092138












