17βH-Neriifolin Improves Cardiac Remodeling Through Modulation of Calcium Handling Proteins in the Heart Failure Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Compound Isolation
2.2. Animal
2.3. Blood Pressure and ECG Measurement
2.4. Langendorff Analysis
2.5. Analysis of Heart Oxidative Stress Markers
2.6. Cardiac Injury Marker
2.7. Molecular Analyses
2.8. Histology Studies
2.9. Statistical Analysis
3. Results
3.1. Heart and Left Ventricle Weight Were Not Altered by SNA Treatment
3.2. Cellular Injury Markers Were Reduced After the SNA Treatment
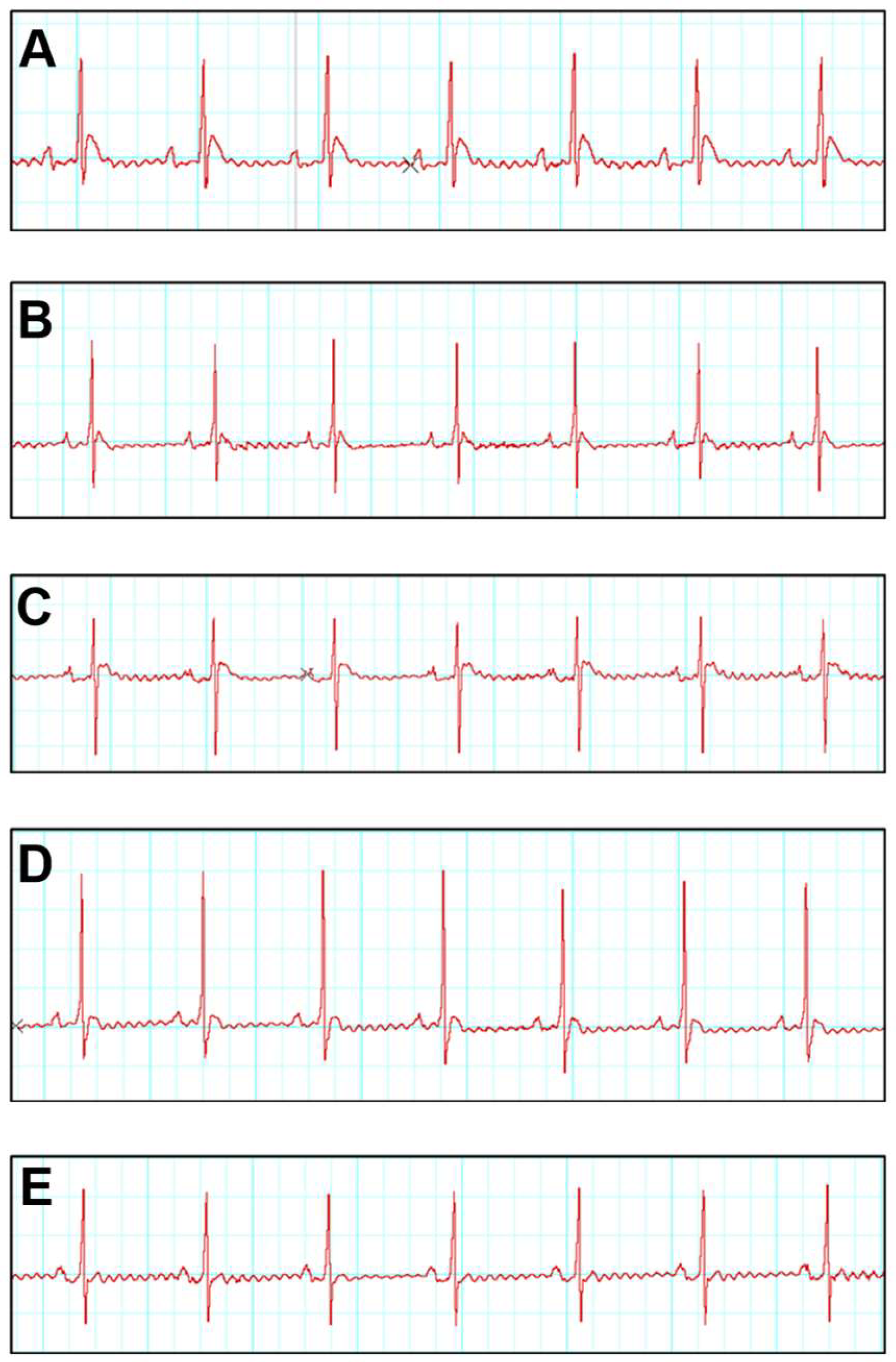
3.3. SNA Treatment Is Able to Improve Electrocardiogram (ECG) and Blood Pressure Parameters
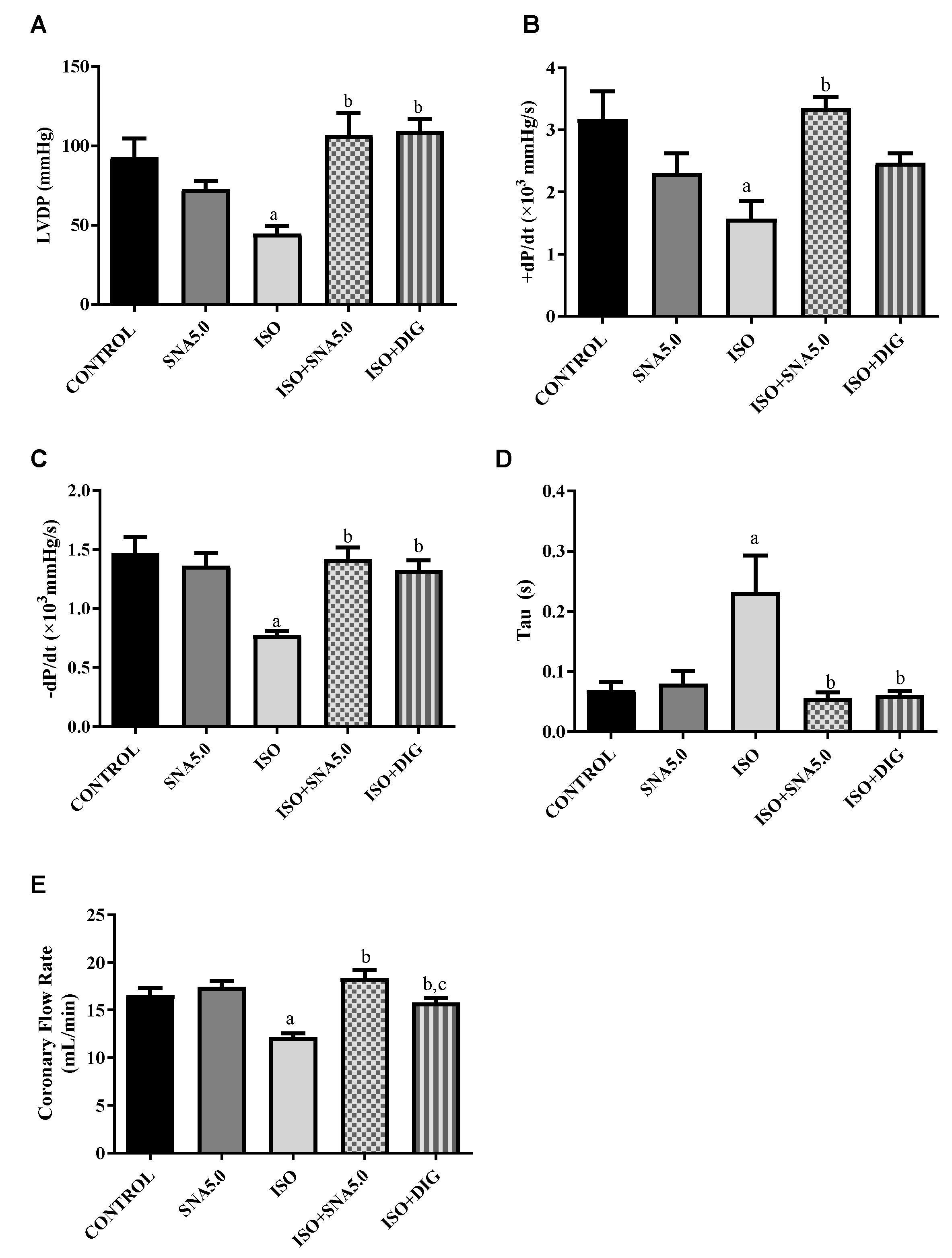
3.4. SNA Enhances the Contractility in Isolated Perfused Rat Hearts
3.5. SNA Is Able to Mitigate Oxidative Stress
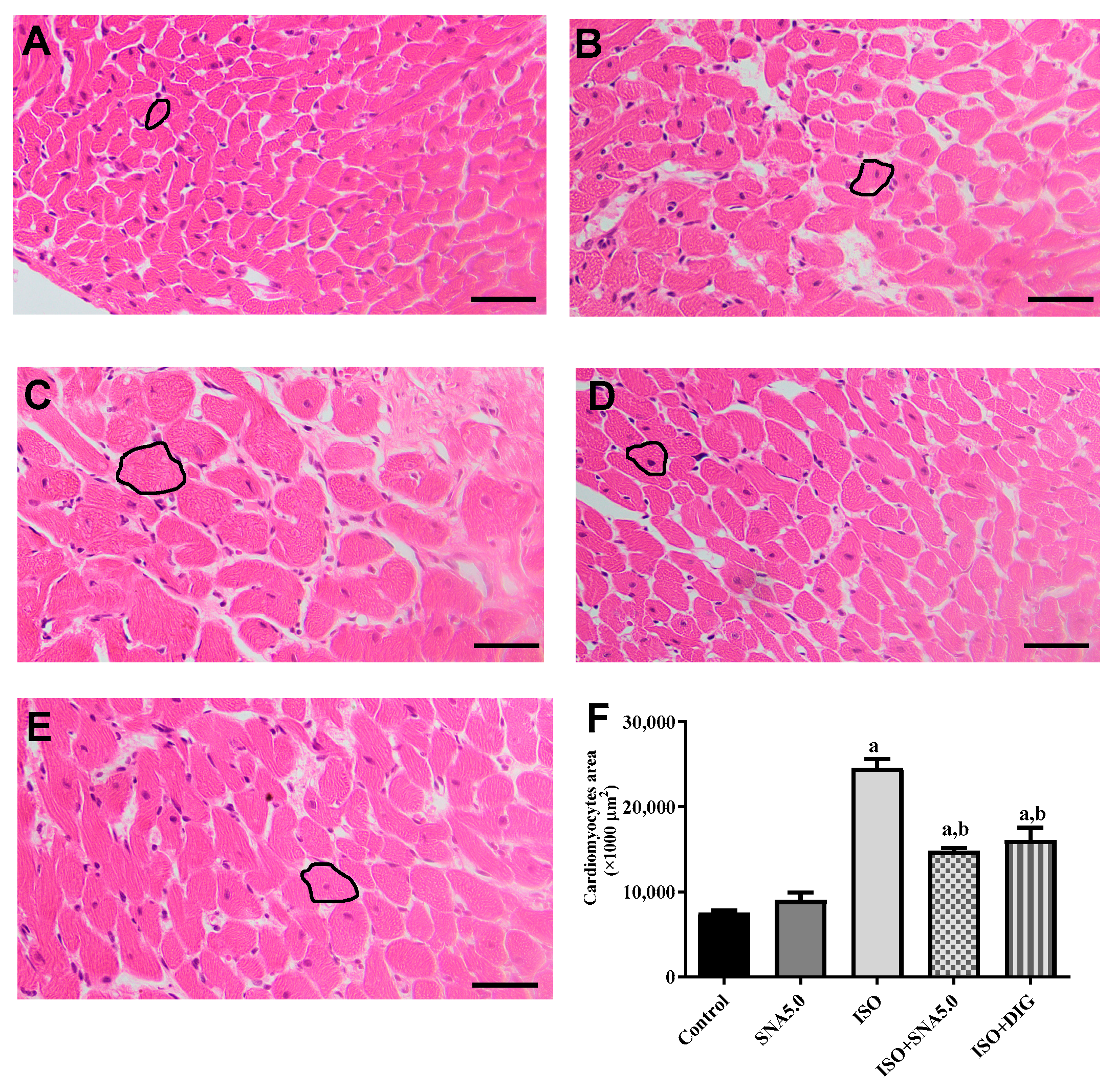
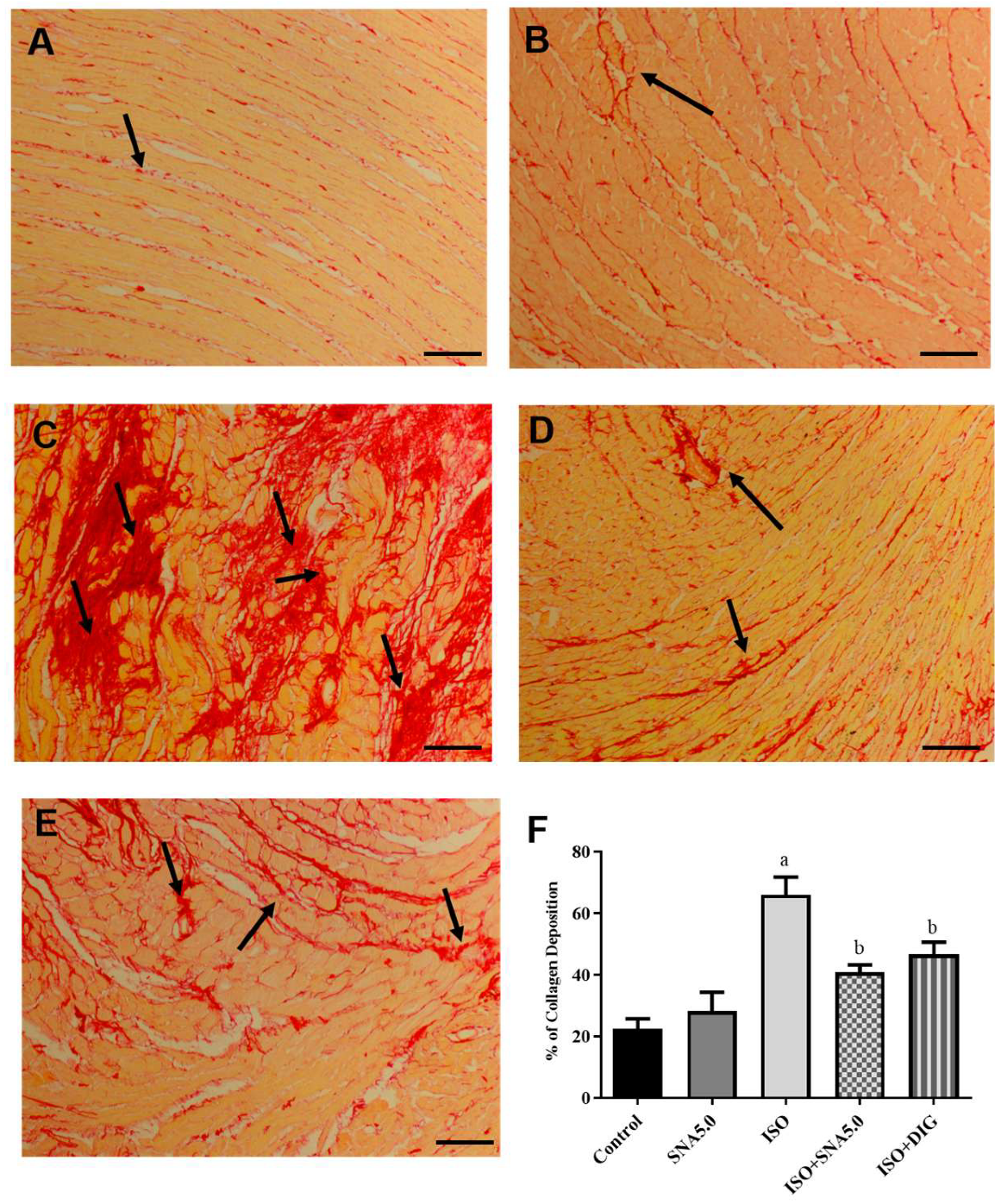
3.6. SNA Improves Cardiac Hypertrophy and Fibrosis in Rats
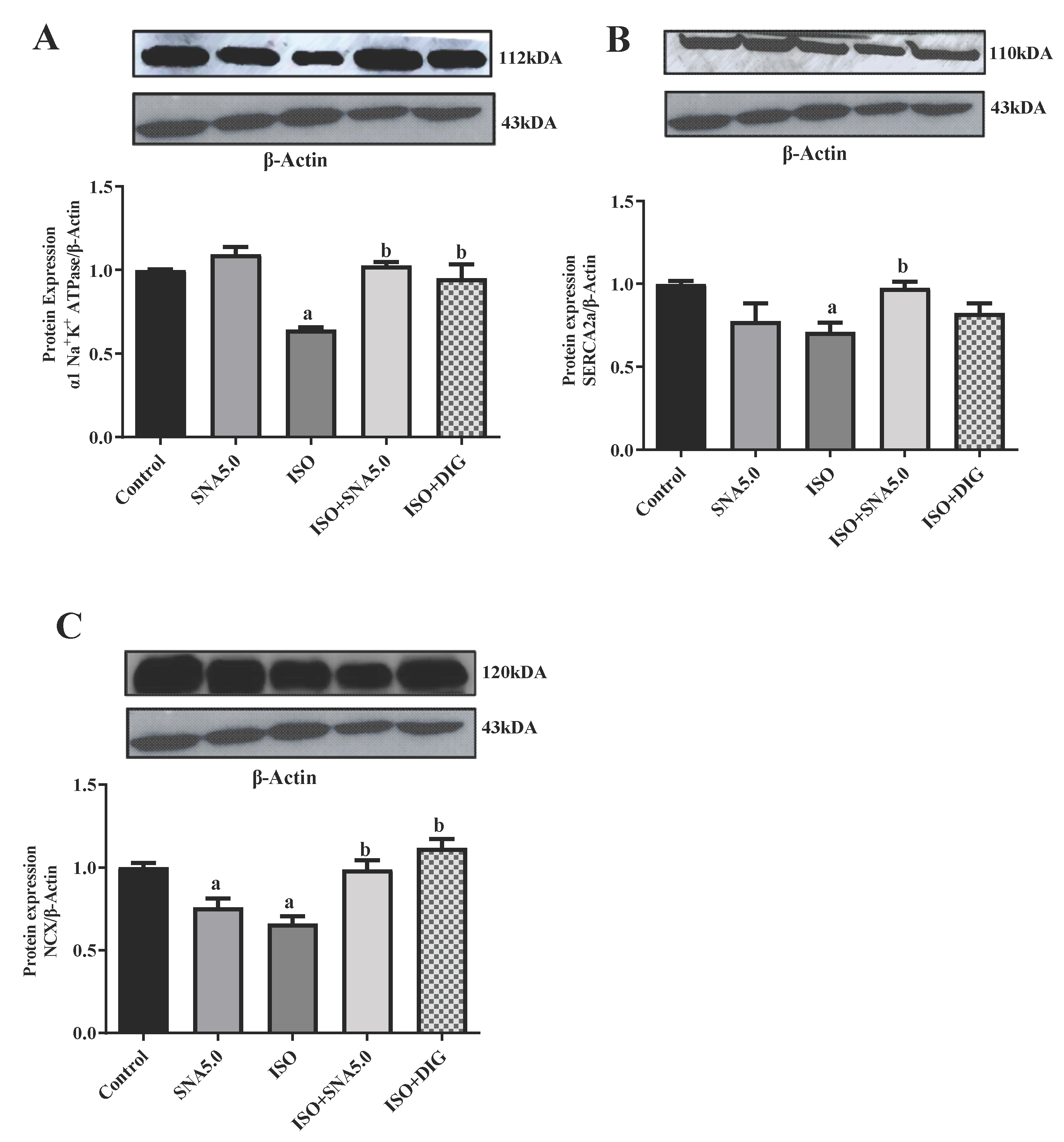
3.7. SNA Increases the Protein Expression Related to Contractility in Rats
4. Discussion
5. Conclusions
6. Future Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | One-way and repeated measures two-way analysis of variance |
| BP | Blood pressure |
| Ca2+ | Calcium ion |
| CaCl2 | Calcium chloride |
| CNS | Central nervous system |
| CO2 | Carbon dioxide |
| DBP | Diastolic blood pressure |
| DMSO | Dimethyl sulfoxide |
| DTNB | 5,5′-dithiobis-2-nitrobenzoic acid |
| ECG | Electrocardiography |
| GSH | Glutathione |
| H&E | Hematoxylin and eosin |
| HR | Heart rate |
| ISO | Isoprenaline |
| KCl | Potassium chloride |
| KH2PO4 | Monopotassium phosphate |
| LV | Left ventricular |
| LVDP | Left ventricular developed pressure |
| MAP | Mean arterial pressure |
| MgSO4 | Magnesium sulphate |
| Na+K+ | Sodium potassium |
| NaCl | Sodium chloride |
| NaHCO3 | Sodium bicarbonate |
| NCX | Na+Ca2+ exchanger |
| NT-ProBNP | NT-pro-B-type natriuretic peptide |
| O2 | Oxygen |
| PVDF | Polyvinylidene difluoride |
| rpm | Rate per minute |
| RPP | Rate of pressure product |
| SBP | Systolic blood pressure |
| SERCA | Sarcoplasmic reticulum ATPase |
| TBARS | Thiobarbituric acid-reactive substance |
| Tris-HCl | Aminomethane hydrochloride |
References
- Emmons-Bell, S.; Johnson, C.; Roth, G. Prevalence, incidence and survival of heart failure: A systematic review. Heart 2022, 108, 1351–1360. [Google Scholar] [CrossRef]
- Goldhaber, S.Z. Venous thromboembolism in heart failure patients: Pathophysiology, predictability, prevention. J. Am. Coll. Cardiol. 2020, 75, 159–162. [Google Scholar] [CrossRef]
- Patocka, J.; Nepovimova, E.; Wu, W.; Kuca, K. Digoxin: Pharmacology and toxicology—A review. Environ. Toxicol. Pharmacol. 2020, 79, 103400. [Google Scholar] [CrossRef]
- Hossan, M.S.; Chan, Z.-Y.; Collins, H.M.; Shipton, F.N.; Butler, M.S.; Rahmatullah, M.; Lee, J.B.; Gershkovich, P.; Kagan, L.; Bradshaw, T.D.; et al. Cardiac glycoside cerberin exerts anticancer activity through PI3K/AKT/mTOR signal transduction inhibition. Cancer Lett. 2019, 453, 57–73. [Google Scholar] [CrossRef]
- Saxena, M.; Jadhav, E.B.; Sankhla, M.S.; Singhal, M.; Parihar, K.; Awasthi, K.K.; Awasthi, G. Bintaro (Cerbera odollam and Cerbera manghas): An overview of its eco-friendly use, pharmacology, and toxicology. Environ. Sci. Pollut. Res. 2023, 30, 71970–71983. [Google Scholar] [CrossRef] [PubMed]
- Yunos, N.M.; Osman, A.; Jauri, M.H.; Sallehudin, N.J.; Mutalip, S.S. The in vitro anti-cancer activities of 17βH-neriifolin isolated from Cerbera odollam and its binding activity on Na+, K+-ATPase. Curr. Pharm. Biotechnol. 2020, 21, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Siti Syarifah, M.M.; Nurhanan, M.Y.; Muhd Haffiz, J.; Mohd Ilham, A.; Getha, K.; Asiah, O.; Norhayati, I.; Lili Sahira, H.; Anee Suryani, S. Potential anticancer compound from Cerbera odollam. J. Trop. For. Sci. 2011, 23, 89–96. [Google Scholar]
- Anamalley, R.; Rajassageran, L.; Apparoo, Y.A.; Jauri, M.H.; Kamisah, Y.; Yunos, N.M.; Zainalabidin, S. Repeated administration of low dose isoprenaline on the rat’s cardiovascular system. Sains Malays. 2022, 51, 2147–2157. [Google Scholar] [CrossRef]
- Zainalabidin, S.; Aziz, N.F.; Murugan, D.D.; Mahadi, M.K. S-Allylcysteine limits cardiac structural changes via antioxidant status in ovariectomized rats with induced myocardial injury. Eur. Heart J. 2023, 44 (Suppl. S1), ehac779.135. [Google Scholar] [CrossRef]
- Mohammed Yusof, N.L.; Zainalabidin, S.; Mohd Fauzi NBudin, S.B. Hibiscus sabdariffa (Roselle) polyphenolrich extract averts cardiac functional and structural abnormalities in type 1 diabetic rats. Appl. Physiol. Nutr. Metab. 2018, 43, 1224–1232. [Google Scholar] [CrossRef]
- Balasubramaniam, S.L.; Gopalakrishnapillai, A.; Gangadharan, V.; Duncan, R.L.; Barwe, S.P. Sodium-calcium exchanger 1 regulates epithelial cell migration via calcium-dependent extracellular signal-regulated kinase signaling. J. Biol. Chem. 2015, 290, 12463–12473. [Google Scholar] [CrossRef]
- Cellini, A.; Höfler, D.; Arias-Loza, P.A.; Bandleon, S.; Langsenlehner, T.; Kohlhaas, M.; Maack, C.; Bauer, W.R.; Eder-Negrin, P. The α2-isoform of the Na+/K+-ATPase protects against pathological remodeling and β-adrenergic desensitization after myocardial infarction. Am. J. Physiol.-Heart Circ. Physiol. 2021, 321, H650–H662. [Google Scholar] [CrossRef]
- Ali, S.S.; Mohamed, S.F.A.; Rozalei, N.H.; Boon, Y.W.; Zainalabidin, S. Anti-fibrotic actions of Roselle extract in rat model of myocardial infarction. Cardiovasc. Toxicol. 2019, 19, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, M.; Jessup, M.; Mullens, W.; Reza, N.; Shah, A.M.; Sliwa, K.; Mebazaa, A. Acute heart failure. Nat. Rev. Dis. Primers 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Schwinger, R.H. Pathophysiology of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 263. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Y.; Chen, Y.; Zhang, F.; Zhang, X.; Zhu, L.; Yao, X. Dissection of mechanisms of Chinese medicinal formula Si-Miao-Yong-an decoction protects against cardiac hypertrophy and fibrosis in isoprenaline-induced heart failure. J. Ethnopharmacol. 2020, 248, 112050. [Google Scholar] [CrossRef]
- Che, Y.; Shen, D.F.; Wang, Z.P.; Jin, Y.G.; Wu, Q.Q.; Wang, S.S.; Yuan, Y. Protective role of berberine in isoprenaline-induced cardiac fibrosis in rats. BMC Cardiovasc. Disord. 2019, 19, 219. [Google Scholar] [CrossRef]
- Liu, M.; Feng, J.; Du, Q.; Ai, J.; Lv, Z. Paeoniflorin attenuates myocardial fibrosis in isoprenaline-induced chronic heart failure rats via inhibiting P38 MAPK pathway. Curr. Med. Sci. 2020, 40, 307–312. [Google Scholar] [CrossRef]
- Li, Y.; He, B.; Zhang, C.; He, Y.; Xia, T.; Zeng, C. Naringenin attenuates isoprenaline-induced cardiac hypertrophy by suppressing oxidative stress through the AMPK/NOX2/MAPK signaling pathway. Nutrients 2023, 15, 1340. [Google Scholar] [CrossRef]
- Sharma, S.; Iqubal, A.; Khan, V.; Sharma, K.; Najmi, A.K.; Haque, S.E. Icariin ameliorates oxidative stress-induced inflammation, apoptosis, and heart failure in isoproterenol-challenged Wistar rats. Iran. J. Basic Med. Sci. 2023, 26, 517. [Google Scholar] [CrossRef]
- Yousry, S.M.; Taha, R.A.M.; El-Banna, H.A.; Emam, S.R. Curative and protective effect of salvia officinalis oil on isoprenaline-induced congestive heart failure in rats. Adv. Anim. Vet. Sci. 2021, 9, 1895–1907. [Google Scholar] [CrossRef]
- Khandelwal, R.; Vagha, J.D.; Meshram, R.J.; Patel, A.; Khandelwal, R., Jr. A comprehensive review on unveiling the journey of digoxin: Past, present, and future perspectives. Cureus 2024, 16, e56755. [Google Scholar] [CrossRef]
- Picollo, C.T.; Santos, A.A.D.; Antonio, E.L.; Silva, J.M.; Bocalini, D.; Serra, A.J.; Ihara, S.S.M.; Tucci, P.J. Digitoxin attenuates heart failure, reduces myocardial hypertrophy, and preserves the calcium-binding proteins in infarcted rats. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 265–272. [Google Scholar] [CrossRef]
- Shah, A.K.; Bhullar, S.K.; Elimban, V.; Dhalla, N.S. Oxidative stress as a mechanism for functional alterations in cardiac hypertrophy and heart failure. Antioxidants 2021, 10, 931. [Google Scholar] [CrossRef]
- Keihanian, F.; Moohebati, M.; Saeidinia, A.; Mohajeri, S.A.; Madaeni, S. Therapeutic effects of medicinal plants on isoproterenol-induced heart failure in rats. Biomed. Pharmacother. 2021, 134, 111101. [Google Scholar] [CrossRef] [PubMed]
- Canty, J.M., Jr. Myocardial injury, troponin release, and cardiomyocyte death in brief ischemia, failure, and ventricular remodeling. Am. J. Physiol.-Heart Circ. Physiol. 2022, 323, H1–H15. [Google Scholar] [CrossRef]
- Ilmiawati, A.; Mujahidah, U.; Batubara, I.; Nurcholis, W. Exploring the Phytochemical Composition and Pharmacological Activities of Cerbera manghas and Cerbera odollam: A Comprehensive Review. Trop. J. Nat. Prod. Res. 2024, 8, 5715. [Google Scholar] [CrossRef]
- Chisty, T.T.E.; Sarif, S.; Jahan, I.; Ismail, I.N.; Chowdhury, F.I.; Siddiqua, S.; Yasmin, T.; Islam, M.N.; Khan, F.; Alam, M.A.; et al. Protective effects of l-carnitine on isoprenaline-induced heart and kidney dysfunctions: Modulation of inflammation and oxidative stress-related gene expression in rats. Heliyon 2024, 10, e25057. [Google Scholar] [CrossRef]
- Miro, O.; Mojarro, E.M.; Hure, G.; Llorens, P.; Gil, V.; Alquezar-Arbe, A.; Bibiano, C.; González, N.C.; Massó, M.; Mueller, C.; et al. Digoxin initiation after an acute heart failure episode and its association with post-discharge outcomes: An international multicenter analysis. Intern. Emerg. Med. 2025, 20, 65–76. [Google Scholar] [CrossRef]
- Maigida, H.H.; Bukar, B.B. Cardioprotective and antioxidant properties of the arils of the fruit of blighia sapidakd koenig (Sapindaceae) in Isoprenaline-induced myocardial infarction in Wistar rats. Afr. J. Pharm. Res. Dev. 2024, 16, 1–13. [Google Scholar] [CrossRef]
- Salim, S.M.; Yunos, N.M.; Jauri, M.H.; Kamisah, Y. Cardiotonic effects of cardiac glycosides from plants of Apocynaceae family. Chulalongkorn Med. J. 2020, 64, 459–466. [Google Scholar] [CrossRef]
- Zhou, R.; Ma, P.; Xiong, A.; Xu, Y.; Wang, Y.; Xu, Q. Protective effects of low-dose rosuvastatin on isoproterenol-induced chronic heart failure in rats by regulation of DDAH-ADMA-NO pathway. Cardiovasc. Ther. 2017, 35, e12241. [Google Scholar] [CrossRef]
- da Silva Ferreira, R.; Fernandes, P.B.U.; da Cruz, J.P.O.; Silva, F.L.A.; Lempek, M.R.; Canta, G.N.; Veado, J.C.C.; Mantovani, M.M.; Botelho, A.F.M.; Melo, M.M. Comparative therapeutic potential of cardioactive glycosides in doxorubicin model of heart failure. Cardiovasc. Toxicol. 2022, 22, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liang, S.; Wang, Q.; Zheng, Q.; Wang, M.; Qian, J.; Yu, T.; Lou, S.; Luo, W.; Liang, G.; et al. JOSD2 mediates isoprenaline-induced heart failure by deubiquitinating CaMKIIδ in cardiomyocytes. Cell. Mol. Life Sci. 2024, 81, 18. [Google Scholar] [CrossRef] [PubMed]
- Balderas-Villalobos, J.; Medina-Contreras, J.M.L.; Lynch, C.; Kabadi, R.; Ramirez, R.J.; Tan, A.Y.; Eltit, J.M. Alterations of sarcoplasmic reticulum-mediated Ca2+ uptake in a model of premature ventricular contraction (PVC)-induced cardiomyopathy. Mol. Cell. Biochem. 2023, 478, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, A.; Zainalabidin, S.; Kamisah, Y.; Jauri, M.H.; Yunos, N.M. Cardiac Glycoside 17βH-Neriifolin Isolated from Cerbera odollam Enhances Left Ventricular Contractility in Normal and Failing Hearts ex vivo. J. Evol. Biochem. Physiol. 2020, 56, 277–281. [Google Scholar] [CrossRef]
- Baloglu, E. Hypoxic stress-dependent regulation of Na, K-ATPase in ischemic heart disease. Int. J. Mol. Sci. 2023, 24, 7855. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Wu, Q.; Wang, L.; Hu, B. Alleviation of isoprenaline hydrochloride induced myocardial ischemia injury by brucine through the inhibition of Na+/K+-ATPase. Exp. Gerontol. 2021, 149, 111332. [Google Scholar] [CrossRef]
- Matzer, I.; Voglhuber, J.; Kiessling, M.; Djalinac, N.; Trummer-Herbst, V.; Mabotuwana, N.; Rech, L.; Holzer, M.; Sossalla, S.; Ljubojevic-Holzer, S.; et al. β-Adrenergic receptor stimulation maintains NCX-CaMKII axis and prevents overactivation of IL6R-signaling in cardiomyocytes upon increased workload. Biomedicines 2022, 10, 1648. [Google Scholar] [CrossRef]
- Botelho, A.F.M.; Pierezan, F.; Soto-Blanco, B.; Melo, M.M. A review of cardiac glycosides: Structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon 2019, 158, 63–68. [Google Scholar] [CrossRef]
- David, M.N.V.; Shetty, M. Digoxin. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2024. [Google Scholar]
| Group | Heart Weight (HW/g) | HW/TL (g/cm) | LV/TL (g/cm) | Troponin T (Pg/mL) | NT-ProBNP (Pg/mL) | TBARS (nmoL/mg Protein) | GSH (nmoL/mg Protein) |
|---|---|---|---|---|---|---|---|
| CONTROL | 0.85 ± 0.04 | 0.24 ± 0.01 | 0.16 ± 0.01 | 40.49 ± 2.74 | 157.00 ± 29.08 | 3.653 ± 0.61 | 0.655 ± 0.05 |
| SNA 5.0 | 0.93 ± 0.04 | 0.26 ± 0.01 | 0.15 ± 0.01 | 28.54 ± 5.11 | 183.60 ± 9.90 | 2.456 ± 0.38 | 0.527 ± 0.07 |
| ISO | 1.08 ± 0.10 * | 0.30 ± 0.01 * | 0.21 ± 0.01 * | 58.51 ± 2.61 * | 395.90 ± 26.11 * | 17.66 ± 7.13 * | 0.381 ± 0.03 * |
| ISO + SNA 5.0 | 1.03 ± 0.04 | 0.29 ± 0.01 | 0.21 ± 0.01 | 30.56 ± 4.39 ** | 165.80 ± 42.60 ** | 2.377 ± 0.64 ** | 0.428 ± 0.04 * |
| ISO + DIG | 1.05 ± 0.04 | 0.29 ± 0.01 | 0.20 ± 0.01 | 57.03 ± 4.84 | 227.40 ± 16.26 ** | 2.765 ± 0.22 ** | 0.449 ± 0.02 * |
| Group | ST Elevation (mV) | RR (s) | QRS-Complex (ms) | QT-Interval (ms) |
|---|---|---|---|---|
| CONTROL | 0.14 ± 0.01 | 0.33 ± 0.01 | 0.025 ± 0.001 | 0.026 ± 0.001 |
| SNA 5.0 | 0.24 ± 0.03 | 0.33 ± 0.01 | 0.026 ± 0.001 | 0.024 ± 0.002 |
| ISO | 0.38 ± 0.02 * | 0.54 ± 0.01 * | 0.039 ± 0.001 * | 0.035 ± 0.001 * |
| ISO + SNA 5.0 | 0.17 ± 0.01 ** | 0.38 ± 0.01 ** | 0.029 ± 0.001 ** | 0.028 ± 0.001 ** |
| ISO + DIG | 0.22 ± 0.02 ** | 0.37 ± 0.01 ** | 0.028 ± 0.001 ** | 0.028 ± 0.001 ** |
| Group | Systolic Blood Pressure (SBP) (mmHg) | Diastolic Blood Pressure (DBP) (mmHg) | Mean Arterial Pressure (MAP) (mmHg) | Heart Rate (bpm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0th | 14th | 28th | 0th | 14th | 28th | 0th | 14th | 28th | 0th | 14th | 28th | |
| Control | 129.63 ± 6.87 | 126.42 ± 1.68 | 128.09 ± 5.05 | 88.67 ± 6.23 | 86.07 ± 2.93 | 85.52 ± 2.83 | 102.32 ± 6.44 | 99.52 ± 2.28 | 99.71 ± 3.57 | 390.83 ± 24.42 | 370.79 ± 27.24 | 359.17 ± 48.45 |
| SNA 5.0 | 129.88 ± 2.83 | 130.51 ± 9.93 | 129.22 ± 13.13 a | 88.12 ± 3.88 | 87.50 ± 5.60 | 89.23 ± 10.57 a | 102.02 ± 3.23 | 101.68 ± 7.20 | 102.63 ± 10.79 a | 374.02 ± 29.45 | 349.67 ± 53.13 | 329.54 ± 60.31 a |
| ISO | 127.29 ± 5.81 | 139.07 ± 14.33 a | 140.28 ± 7.01 a | 86.00 ± 5.10 | 96.14 ± 4.06 a | 103.11 ± 10.09 a | 99.96 ± 5.21 | 111.39 ± 6.35 a | 114.47 ± 10.01 a | 380.61 ± 23.72 | 283.78 ± 65.01 a | 267.29 ± 42.02 a |
| ISO + SNA 5.0 | 127.40 ± 3.45 | 140.63 ± 3.17 | 127.52 ± 4.15 b | 85.46 ± 2.21 b | 100.30 ± 8.15 | 85.61 ± 4.39 b | 99.44 ± 2.83 | 113.74 ± 8.50 | 99.58 ± 2.42 b | 409.37 ± 24.82 | 262.81 ± 37.33 b | 333.15 ± 33.10 b |
| ISO + DIG | 128.50 ± 4.22 | 138.31 ± 7.94 | 131.3 ±1 0.77 b | 86.64 ± 4.17 | 97.39 ± 10.44 | 88.19 ± 6.08 b | 100.60 ± 3.15 | 111.03 ± 10.80 | 102.56 ± 6.01 b | 391.36 ± 19.70 | 279.60 ± 41.55 b | 333.08 ± 38.84 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anamalley, R.; Kamisah, Y.; Yunos, N.M.; Zainalabidin, S. 17βH-Neriifolin Improves Cardiac Remodeling Through Modulation of Calcium Handling Proteins in the Heart Failure Rat Model. Biomedicines 2025, 13, 2115. https://doi.org/10.3390/biomedicines13092115
Anamalley R, Kamisah Y, Yunos NM, Zainalabidin S. 17βH-Neriifolin Improves Cardiac Remodeling Through Modulation of Calcium Handling Proteins in the Heart Failure Rat Model. Biomedicines. 2025; 13(9):2115. https://doi.org/10.3390/biomedicines13092115
Chicago/Turabian StyleAnamalley, Rajasegar, Yusof Kamisah, Nurhanan Murni Yunos, and Satirah Zainalabidin. 2025. "17βH-Neriifolin Improves Cardiac Remodeling Through Modulation of Calcium Handling Proteins in the Heart Failure Rat Model" Biomedicines 13, no. 9: 2115. https://doi.org/10.3390/biomedicines13092115
APA StyleAnamalley, R., Kamisah, Y., Yunos, N. M., & Zainalabidin, S. (2025). 17βH-Neriifolin Improves Cardiac Remodeling Through Modulation of Calcium Handling Proteins in the Heart Failure Rat Model. Biomedicines, 13(9), 2115. https://doi.org/10.3390/biomedicines13092115






