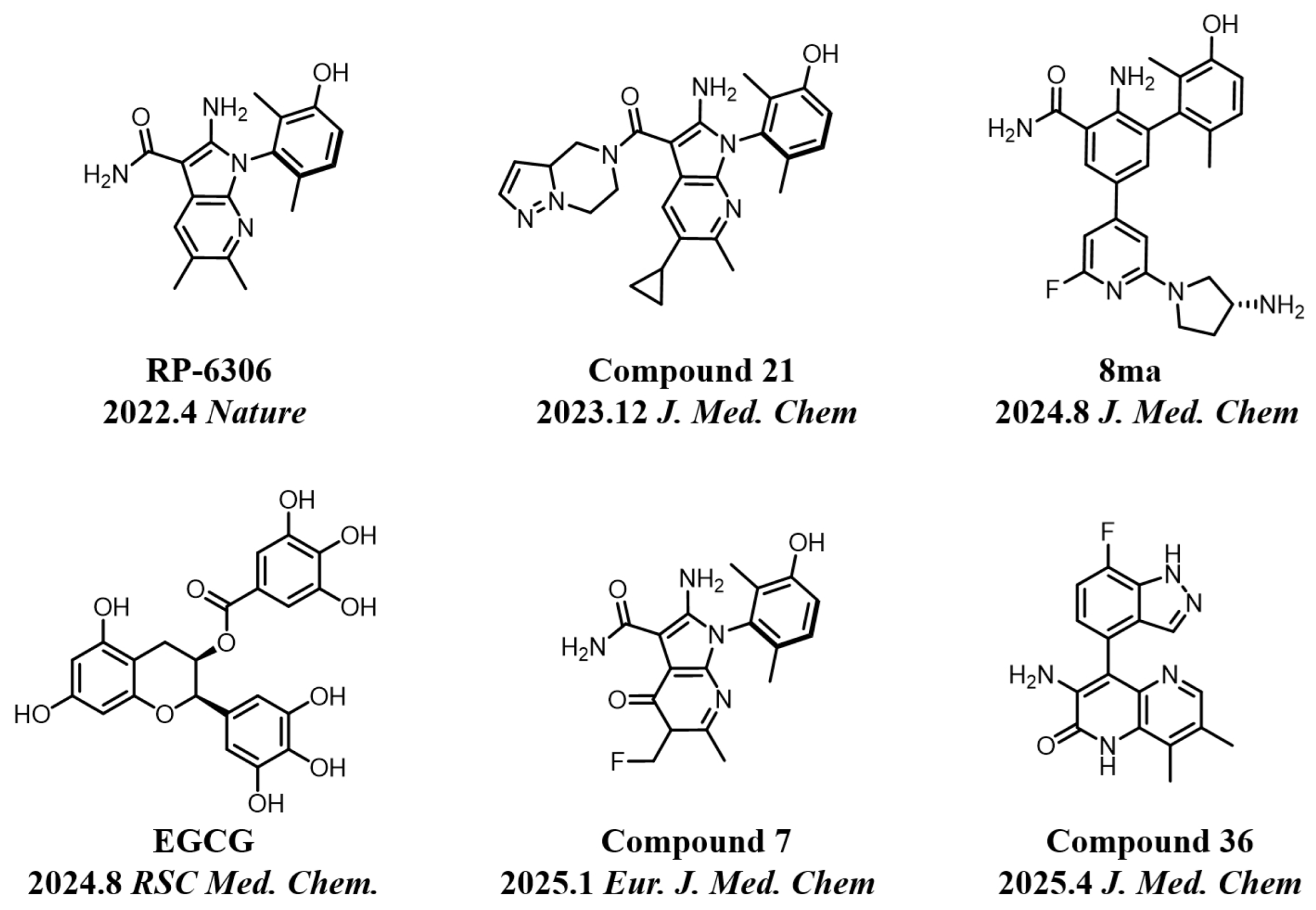
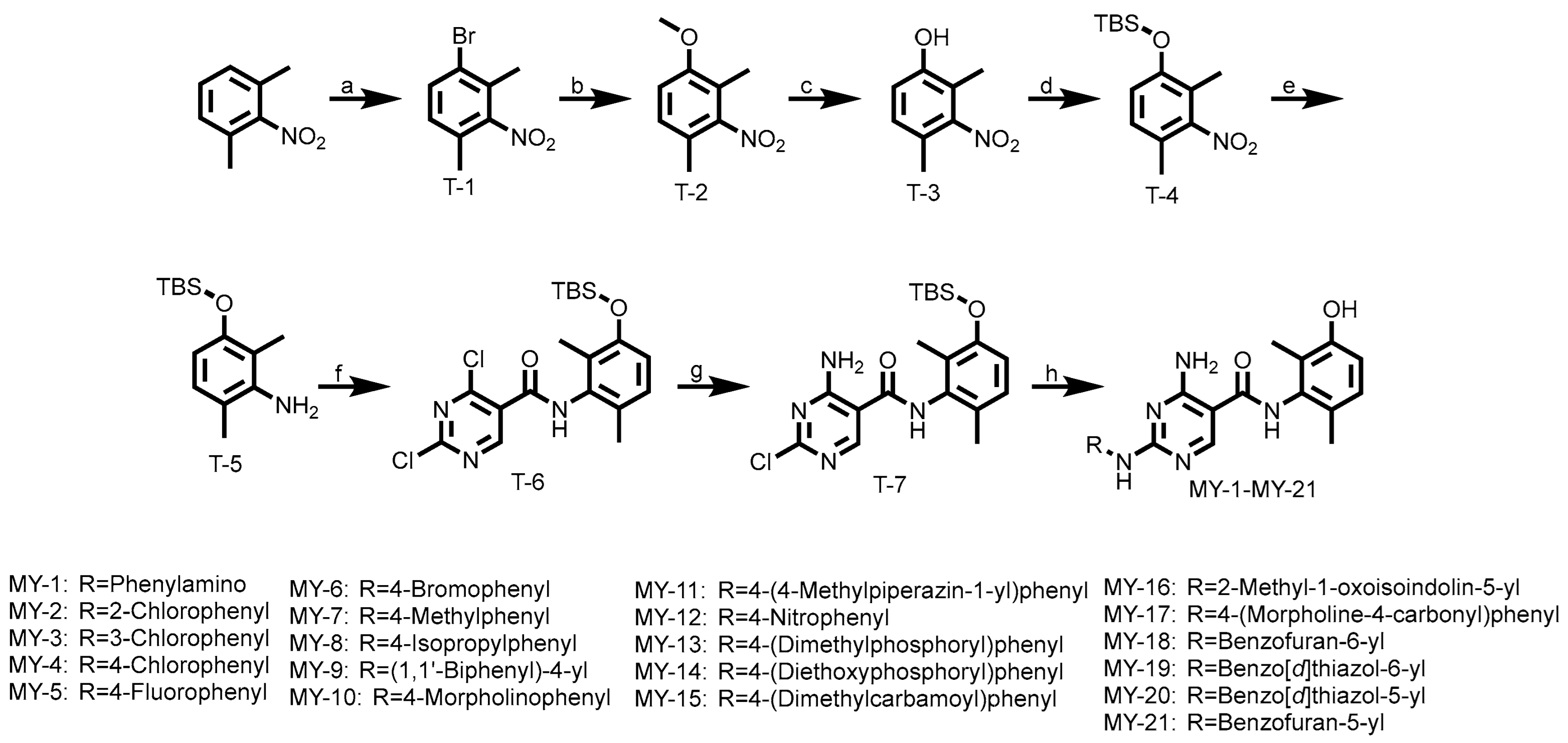
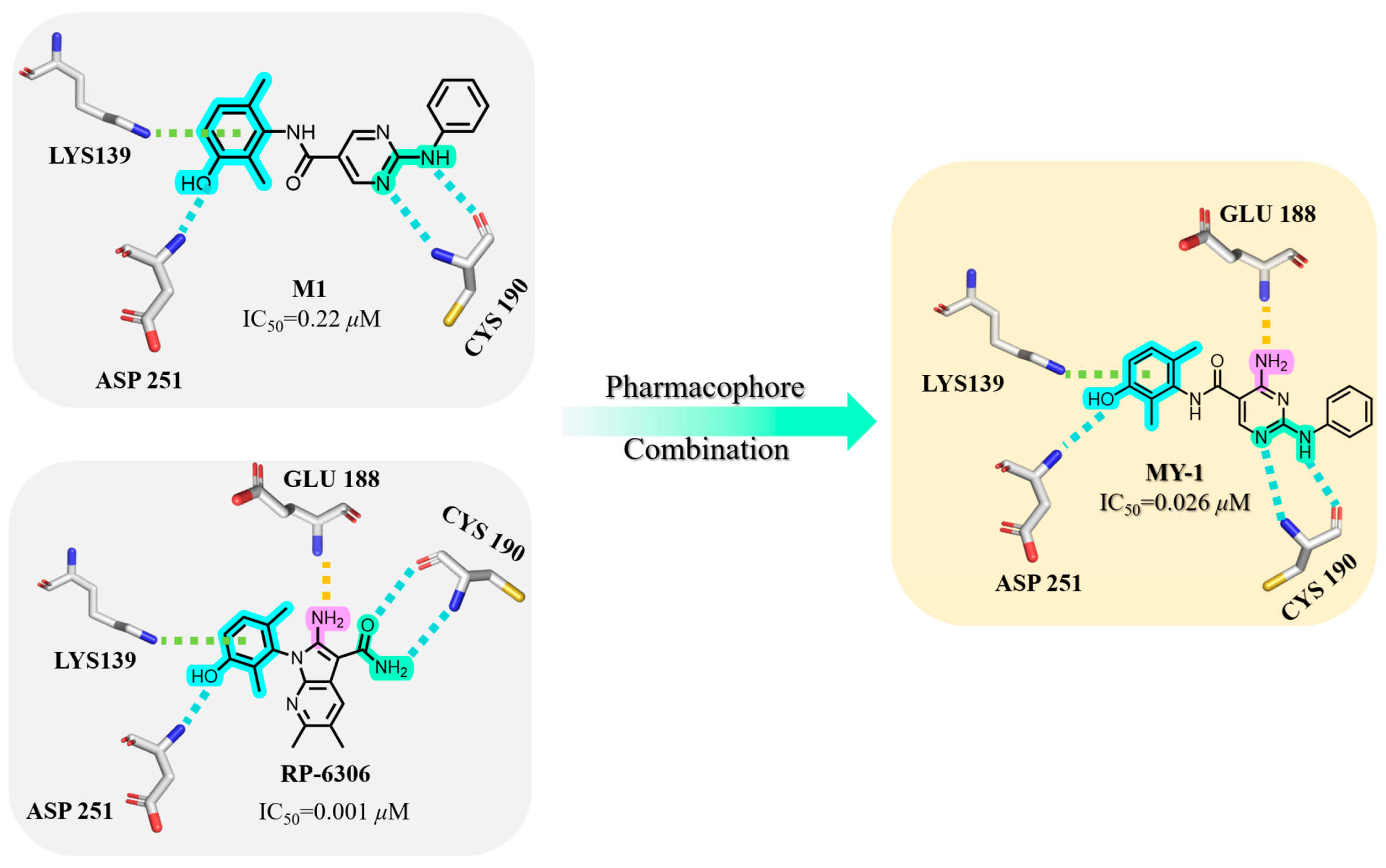
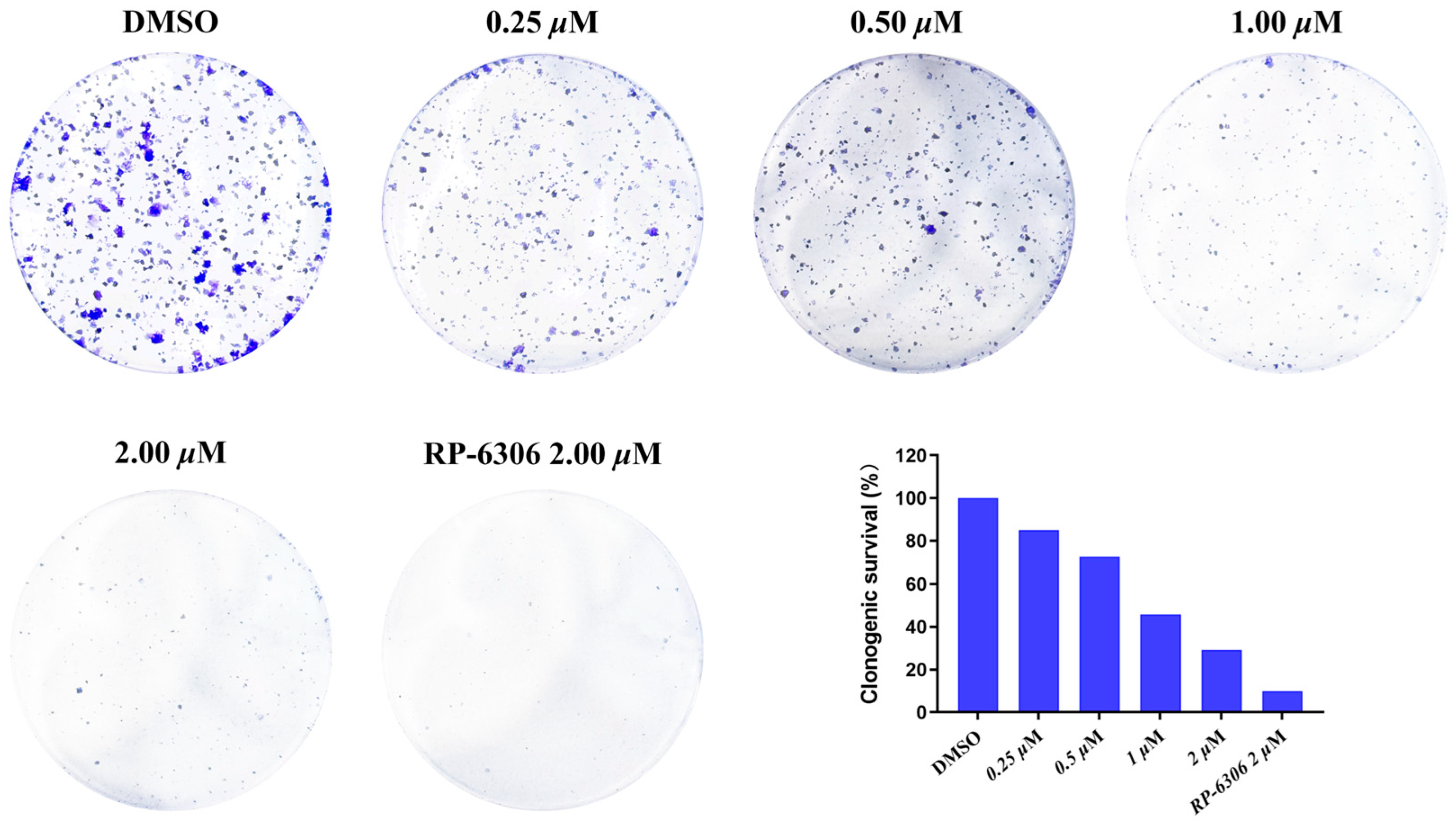
2.1. Chemistry
All reagents and solvents utilized in the experiments were acquired from commercial suppliers and did not undergo any additional purification. Thin-layer chromatography (TLC) plates were employed to monitor the reactions, which were examined under ultraviolet light at 254 or 365 nm. NMR spectra were recorded on Bruker Avance spectrometers operating at either 600 or 400 MHz, utilizing TMS as the internal standard, with CDCl
3 (7.26 ppm) and DMSO-
d6 (2.50 ppm) serving as reference standards; these spectra were obtained in solutions of CDCl
3 or DMSO-
d6. The Agilent 1100 LC-MS spectrometer was used to obtain low-resolution ESI-MS spectra, whereas high-resolution mass spectrometry (HRMS) data were gathered with the Agilent Q-TOF 6530 or 6550 mass spectrometer. The melting point was determined using the BÜCHI B-540 apparatus in an open capillary setup. All compounds were confirmed to possess a purity exceeding 95%. The purity was determined by HPLC using a Hua Spectrum S6000PLUS ultra-high-performance liquid chromatography system, equipped with an Agilent ZORBAX Eclipse XDB-C18 column. The sample injection conditions were specified as a methanol-to-water ratio of 8:2, with a column temperature of 25 °C. Spectral data for all compounds were provided in the
Supplementary Materials.
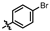
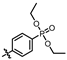
1-bromo-2,4-dimethyl-3-nitrobenzene (T-1): A solution of 2,6-dimethylnitrobenzene (4.00 g, 26.48 mmol), bromine (6.35 g, 39.72 mmol), FeBr3 (0.16 g, 0.53 mmol), and iron powder (0.44 g, 7.94 mmol) were dissolved in chloroform (50 mL). After stirring at 0 °C for 12 h, the mixture was poured into a saturated sodium thiosulfate solution and extracted with methylene chloride. The organic phases were combined, washed with saturated brine, dried with anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by petroleum ether pulping to obtain the white solid compound T-1 (3.25 g, 53.6% yield). 1H NMR (600 MHz, Chloroform-d) δ 7.55 (d, J = 8.2 Hz, 1H), 7.01 (d, J = 8.2 Hz, 1H), 2.34 (s, 3H), 2.25 (s, 3H).
1-methoxy-2,4-dimethyl-3-nitrobenzene (T-2): A solution of T-1 (3.25 g, 14.19 mmol), sodium methoxide (7.89 mL, 42.57 mmol), and copper(I) bromide (0.81 g, 5.68 mmol) in anhydrous N,N-dimethylformamide (DMF, 10 mL) was stirred at 90 °C under an argon atmosphere for 16 h. After cooling to room temperature, the reaction mixture was carefully poured into ice water (100 mL), resulting in the formation of a black-brown precipitate. The solid was collected by vacuum filtration and washed with cold water (3 × 20 mL). The crude product was purified by column chromatography on silica gel to afford T-2 as a light greenish-yellow crystalline solid (2.40 g, 93.4% yield). 1H NMR (600 MHz, Chloroform-d) δ 7.06 (d, J = 8.4, 1.2 Hz, 1H), 6.84 (d, J = 8.4 Hz, 1H), 3.84 (s, 3H), 2.22 (d, J = 0.8 Hz, 3H), 2.13 (s, 3H).
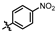
2,4-dimethyl-3-nitrophenol (T-3): A solution of T-2 (2.40 g, 10.48 mmol) in anhydrous dichloromethane (DCM, 8 mL) was cooled to −40 °C. To this stirred solution, a 2 M solution of boron trichloride (13.25 mL, 26.50 mmol) in DCM was added dropwise over 10 min. After complete addition, the reaction mixture was maintained at −40 °C for 15 min, then gradually warmed to room temperature and stirred for an additional 1.5 h. The reaction was carefully quenched by the addition of ice-cold methanol, and the solvent was evaporated under reduced pressure. The resulting residue was extracted with ethyl acetate (3 × 15 mL), and the combined organic layers were dried over anhydrous sodium sulfate (Na2SO4), filtered, and concentrated in vacuo to afford T-3 as a red oil (1.95 g, 88.1% yield). 1H NMR (600 MHz, Chloroform-d) δ 6.98 (d, J = 8.2 Hz, 1H), 6.79 (d, J = 8.3 Hz, 1H), 5.01 (s, 1H), 2.22 (s, 3H), 2.16 (s, 3H).
tert-butyl(2,4-dimethyl-3-nitrophenoxy)dimethylsilane (T-4): A solution of T-3 (1.95 g, 11.67 mmol), anhydrous potassium carbonate (K2CO3, 3.23 g, 23.34 mmol), and tert-butyldimethylsilyl chloride (TBSCl, 3.52 g, 23.34 mmol) in anhydrous dichloromethane (DCM, 8 mL) was stirred at room temperature under an inert atmosphere for 6 h. After completion, the reaction mixture was diluted with DCM (20 mL) and washed with water (20 mL) followed by brine (20 mL). The organic layer was separated, dried over anhydrous sodium sulfate (Na2SO4), filtered, and concentrated under reduced pressure to afford T-4 as a yellow oil (2.45 g, 74.7% yield). 1H NMR (600 MHz, Chloroform-d) δ 6.73 (d, 1H), 6.56 (d, J = 8.3 Hz, 1H), 1.99 (s, 3H), 1.91 (s, 3H), 0.79 (s, 9H), 0.00 (s, 6H).
3-((tert-butyldimethylsilyl)oxy)-2,6-dimethylaniline (T-5): A solution of T-4 (2.45 g, 8.71 mmol) in ethanol (8 mL) and saturated aqueous ammonium chloride (2 mL) was treated with iron powder (2.44 g, 43.55 mmol). The reaction mixture was stirred vigorously at 80 °C for 4 h. After cooling to room temperature, the mixture was filtered, and the filter cake was washed thoroughly with ethanol (3 × 15 mL). The combined filtrates were concentrated under reduced pressure, and the resulting residue was extracted with ethyl acetate (3 × 50 mL). The combined organic layers were washed with brine (50 mL), dried over anhydrous sodium sulfate (Na2SO4), filtered, and concentrated in vacuo. Purification by flash column chromatography afforded T-5 as a pale yellow oil (1.95 g, 89.1% yield). 1H NMR (600 MHz, Chloroform-d) δ 6.57 (d, 1H), 6.04 (d, J = 8.1 Hz, 1H), 1.92 (d, J = 0.7 Hz, 3H), 1.86 (s, 3H), 0.82 (s, 9H), 0.00 (s, 6H).
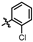
2,4-dichloro-N-(3-((tert-butyldimethylsilyl)oxy)-2,6-dimethylphenyl)pyrimidine-5-carboxamide (T-6): A mixture of T-5 (1.61 g, 6.40 mmol) and 2,4-dichloro-5-pyrimidinecarbonyl chloride (1 g, 6.40 mmol) in anhydrous THF (10 mL) was stirred at room temperature for 4 h. Upon completion, the resulting precipitate was collected by vacuum filtration and further purified by column chromatography to afford T-6 as a white solid (1.50 g, 55.1% yield). 1H NMR (600 MHz, Chloroform-d) δ 8.80 (s, 1H), 7.51 (s, 1H), 6.76 (d, J = 8.3 Hz, 1H), 6.52 (d, J = 8.2 Hz, 1H), 2.00 (s, 3H), 1.93 (s, 3H), 0.79 (s, 9H), 0.00 (s, 6H).
4-amino-2-chloro-N-(3-((tert-butyldimethylsilyl)oxy)-2,6-dimethylphenyl)pyrimidine-5-carboxamide (T-7): A solution of T-6 (1.5 g, 3.53 mmol) in anhydrous 1,4-dioxane (10 mL) was treated with ammonia solution in dioxane (5 mL). The reaction mixture was stirred for 2 h at ambient temperature. Upon completion, the mixture was concentrated under reduced pressure to remove excess ammonia, then diluted with water (20 mL) and extracted with ethyl acetate (3 × 30 mL). The combined organic layers were washed with brine (30 mL), dried over anhydrous sodium sulfate (Na2SO4), filtered, and concentrated in vacuo. The crude product was purified by trituration with petroleum ether/ethyl acetate (3:1, v/v) to afford T-7 as a white crystalline solid (1.05 g, 73.2% yield). 1H NMR (600 MHz, Chloroform-d) δ 8.38 (s, 1H), 7.26 (s, 1H), 6.76 (d, J = 8.3 Hz, 1H), 6.52 (d, J = 8.2 Hz, 1H), 1.97 (s, 3H), 1.90 (s, 3H), 0.80 (s, 9H), −0.00 (s, 6H).
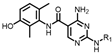
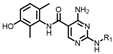
4-amino-2-(phenylamino)-N-(3-hydroxy-2,6-dimethylphenyl)pyrimidine-5-carboxamide (MY-1): White solid; yield: 46.7%; mp: 273.5–274.7 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.45 (d, J = 15.0 Hz, 2H), 9.16 (s, 1H), 8.78 (s, 1H), 7.83 (d, J = 8.0 Hz, 2H), 7.29 –7.24 (m, 2H), 6.95 (t, J = 7.3 Hz, 1H), 6.88 (d, J = 8.2 Hz, 1H), 6.68 (d, J = 8.3 Hz, 1H), 2.06 (s, 3H), 1.99 (s, 3H). 13C NMR (151 MHz, DMSO) δ 165.8, 163.6, 160.6, 157.7, 154.1, 140.9, 136.2, 128.9, 127.3, 126.1, 122.9, 122.0, 119.7, 113.5, 100.5, 18.1, 11.6. HRMS (ESI) for C19H19N5O6 [M+Na]+: calcd, 372.1491, found, 372.1422. HPLC: 99.421%.
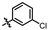
4-amino-2-((2-chlorophenyl)amino)-N-(3-hydroxy-2,6-dimethylphenyl)pyrimidine-5-carboxamide (MY-2): White solid; yield: 39.1% mp: 256.9–257.9 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.46 (s, 1H), 9.16 (s, 1H), 8.73 (s, 1H), 8.51 (s, 1H), 7.94 (d, J = 8.3 Hz, 1H), 7.49 (d, J = 8.0 Hz, 1H), 7.33 (t, J = 7.8 Hz, 1H), 7.15 (t, J = 7.7 Hz, 1H), 6.88 (d, J = 8.2 Hz, 1H), 6.68 (d, J = 8.2 Hz, 1H), 2.05 (s, 3H), 1.98 (s, 3H). 13C NMR (151 MHz, DMSO) δ 165.7, 163.7, 161.0, 157.8, 154.1, 136.8, 136.1, 129.8, 127.9, 127.4, 127.3, 126.4, 126.1, 125.7, 122.9, 113.6, 101.1, 18.0, 11.6. HRMS (ESI) for C19H18ClN5O2 [M+Na]+: calcd, 406.1047, found, 406.1022. HPLC: 98.066%.
4-amino-2-((3-chlorophenyl)amino)-N-(3-hydroxy-2,6-dimethylphenyl)pyrimidine-5-carboxamide (MY-3): White solid; yield: 42.3%; mp: 261.3–262.9 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.67 (s, 1H), 9.48 (s, 1H), 9.17 (s, 1H), 8.80 (s, 1H), 8.02 (t, J = 2.1 Hz, 1H), 7.77 (d, J = 8.3, 2.1 Hz, 1H), 7.28 (t, J = 8.1 Hz, 1H), 6.98 (d, J = 8.0, 2.1 Hz, 1H), 6.88 (d, J = 8.2 Hz, 1H), 6.69 (d, J = 8.2 Hz, 1H), 2.07 (s, 3H), 1.99 (s, 3H). 13C NMR (151 MHz, DMSO) δ 165.6, 163.5, 160.4, 157.7, 154.1, 142.5, 136.1, 133.4, 130.5, 127.3, 126.1, 122.8, 121.4, 118.7, 117.9, 113.6, 101.0, 18.0, 11.6. HRMS (ESI) for C19H18ClN5O2 [M+Na]+: calcd, 406.1047, found, 406.1031. HPLC: 96.054%.
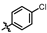
4-amino-2-((4-chlorophenyl)amino)-N-(3-hydroxy-2,6-dimethylphenyl) (MY-4): White solid; yield: 37.6%; mp: 238.5–240.9 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.63 (s, 1H), 9.46 (s, 1H), 8.78 (s, 1H), 7.88 (d, J = 9.0 Hz, 2H), 7.30 (d, J = 8.8 Hz, 2H), 6.88 (d, J = 8.2 Hz, 1H), 6.68 (d, J = 8.3 Hz, 1H), 2.06 (s, 3H), 1.98 (s, 3H). 13C NMR (151 MHz, DMSO) δ 164.6, 162.4, 158.8, 156.6, 153.1, 138.9, 135.1, 127.1, 126.2, 125.0, 124.3, 121.8, 120.6, 111.9, 99.7, 54.9, 17.9, 15.6, 9.7. HRMS (ESI) for C19H18ClN5O2 [M+Na]+: calcd, 406.1047, found, 406.1041. HPLC: 97.481%.
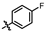
4-amino-2-((4-fluorophenyl)amino)-N-(3-hydroxy-2,6-dimethylphenyl)pyrimidine-5-carboxamide (MY-5): White solid; yield: 48.9%; mp: 282.5–320.2 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.51 (s, 1H), 9.43 (s, 1H), 9.15 (s, 1H), 7.84 (d, J = 9.0, 5.1 Hz, 2H), 7.10 (d, J = 8.9 Hz, 2H), 6.88 (d, J = 8.2 Hz, 1H), 6.68 (d, J = 8.2 Hz, 1H), 2.06 (s, 3H), 1.98 (s, 3H). 13C NMR (151 MHz, DMSO) δ 166.7, 163.5, 160.0, 159.2, 157.7, 156.2, 154.1, 137.3, 135.5, 127.3, 125.5, 123.4, 121.3, 115.4, 115.2, 113.5, 100.5, 17.6, 11.6. HRMS (ESI) for C19H18FN5O2 [M+Na]+: calcd, 390.1343, found, 390.1339. HPLC: 99.218%.
4-amino-2-((4-bromophenyl)amino)-N-(3-hydroxy-2,6-dimethylphenyl)pyrimidine-5-carboxamide (MY-6): Yellow solid; yield: 56.3%; mp: 242.9–245.5 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.63 (s, 1H), 9.46 (s, 1H), 8.78 (s, 1H), 7.83 (d, J = 9.0 Hz, 2H), 7.42 (d, J = 9.0 Hz, 2H), 6.87 (d, J = 8.2 Hz, 1H), 6.68 (d, J = 8.1 Hz, 1H), 2.06 (s, 3H), 1.98 (s, 3H). 13C NMR (151 MHz, DMSO) δ 165.6, 163.5, 160.4, 157.7, 154.2, 140.4, 136.2, 131.6, 127.3, 126.0, 122.9, 121.5, 113.6, 113.3, 100.1, 56.5, 19.0, 18.0, 11.6. HRMS (ESI) for C19H18BrN5O2 [M+Na]+: calcd, 450.0542, found, 450.0574. HPLC: 98.250%.
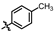
4-amino-2-(p-tolylamino)-N-(3-hydroxy-2,6-dimethylphenyl)pyrimidine-5-carboxamide (MY-7): Light white solid; yield: 52.1% mp: 264.0–264.5 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.41 (s, 1H), 9.36 (s, 1H), 9.15 (s, 1H), 8.76 (s, 1H), 7.70 (d, J = 8.5 Hz, 2H), 7.07 (d, J = 8.2 Hz, 2H), 6.88 (d, J = 8.2 Hz, 1H), 6.68 (d, J = 8.1 Hz, 1H), 2.25 (s, 3H), 2.06 (s, 3H), 1.98 (s, 3H). 13C NMR (151 MHz, DMSO) δ 165.8, 163.6, 160.7, 158.4, 154.7, 139.2, 136.8, 131.8, 129.3, 127.3, 126.1, 122.9, 119.9, 114.4, 100.2, 20.9, 17.4, 10.3. HRMS (ESI) for C20H21N5O2 [M+Na]+: calcd, 386.1593, found, 386.1586. HPLC: 95.956%.
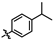
4-amino-2-((4-isopropylphenyl)amino)-N-(3-hydroxy-2,6-dimethylphenyl)pyrimidine-5-carboxamide (MY-8): White solid; yield: 46.8% mp: 248.2–250.2 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.41 (s, 1H), 9.36 (s, 1H), 9.15 (s, 1H), 8.76 (s, 1H), 7.70 (d, J = 8.2 Hz, 2H), 7.13 (d, J = 8.6 Hz, 2H), 6.88 (d, J = 8.2 Hz, 1H), 6.68 (d, J = 8.1 Hz, 1H), 2.83 (hept, J = 6.9 Hz, 1H), 2.06 (s, 3H), 1.98 (s, 3H), 1.19 (d, J = 6.9 Hz, 6H). 13C NMR (151 MHz, DMSO) δ 165.8, 163.6, 161.8, 158.7, 154.1, 143.1, 138.6, 136.3, 127.3, 126.6, 126.1, 122.9, 120.1, 113.5, 100.3, 33.3, 24.5, 18.1, 11.6, 0.6. HRMS (ESI) for C22H25N5O2 [M+Na]+: calcd, 414.1906, found, 414.1925. HPLC: 97.348%.
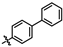
4-amino-2-([1,1′-biphenyl]-4-ylamino)-N-(3-hydroxy-2,6-dimethylphenyl)pyrimidine-5-carboxamide (MY-9): White solid; yield: 39.7% mp: 249.0–249.6 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.61 (s, 1H), 9.46 (s, 1H), 9.18 (s, 1H), 8.80 (s, 1H), 7.94 (d, J = 8.2 Hz, 2H), 7.65 (d, J = 7.7 Hz, 2H), 7.59 (d, J = 8.3 Hz, 2H), 7.44 (t, J = 7.6 Hz, 2H), 7.31 (t, J = 7.4 Hz, 1H), 6.88 (d, J = 8.2 Hz, 1H), 6.69 (d, J = 8.2 Hz, 1H), 2.07 (s, 3H), 1.99 (s, 3H). 13C NMR (151 MHz, DMSO) δ 165.7, 164.0, 160.6, 157.7, 154.1, 141.0, 136.2, 133.6, 130.1, 129.4, 127.3, 127.2, 127.1, 126.6, 126.1, 122.9, 120.0, 113.6, 100.6, 82.7, 18.1, 11.6. HRMS (ESI) for C25H23N5O2 [M+Na]+: calcd, 448.1750, found, 448.1758. HPLC: 96.096%.
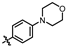
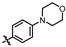
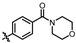
4-amino-2-((4-morpholinophenyl)amino)-N-(3-hydroxy-2,6-dimethylphenyl)pyrimidine-5-carboxamide (MY-10): Yellow solid; yield: 63.6% mp: 270.0–280.0 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.38 (s, 1H), 9.23 (s, 1H), 9.15 (s, 1H), 8.73 (s, 1H), 7.64 (d, J = 8.5 Hz, 2H), 6.88 (d, 1H), 6.86 (s, 2H), 6.68 (d, J = 8.2 Hz, 1H), 3.76–3.71 (m, 4H), 3.05–3.01 (m, 4H), 2.06 (s, 3H), 1.98 (s, 3H). 13C NMR (151 MHz, DMSO) δ 166.4, 164.2, 160.7, 158.7, 154.6, 146.8, 136.3, 132.7, 128.4, 126.7, 123.5, 121.6, 117.3, 114.6, 99.9, 66.2, 49.7, 18.7, 11.2. HRMS (ESI) for C23H26N6O3 [M+Na]+: calcd, 457.1964, found, 457.1971. HPLC: 97.409%.
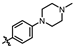
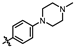
4-amino-2-((4-(4-methylpiperazin-1-yl)phenyl)amino)-N-(3-hydroxy-2,6-dimethylphenyl)pyrimidine-5-carboxamide (MY-11): White solid; yield: 48.8% mp: 276.9–278.7 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.38 (s, 1H), 9.21 (s, 1H), 9.15 (s, 1H), 8.73 (s, 1H), 7.61 (d, J = 8.5 Hz, 2H), 6.87 (d, J = 8.2 Hz, 2H), 6.85 (s, 1H), 6.68 (d, J = 8.2 Hz, 1H), 3.08–3.03 (m, 4H), 2.47–2.43 (m, 4H), 2.22 (s, 3H), 2.06 (s, 3H), 1.98 (s, 3H). 13C NMR (151 MHz, DMSO) δ 207.4, 165.9, 163.6, 160.7, 157.8, 154.1, 146.8, 136.3, 132.9, 127.3, 125.6, 122.9, 121.2, 116.2, 113.5, 100.5, 54.1, 48.7, 46.7, 18.1, 11.6. HRMS (ESI) for C24H29N7O2 [M+Na]+: calcd, 470.2281, found, 470.2271. HPLC: 97.778%.
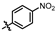
4-amino-2-((4-nitrophenyl)amino)-N-(3-hydroxy-2,6-dimethylphenyl)pyrimidine-5-carboxamide (MY-12): Light yellow solid; yield: 32.2% mp: 278.5–279.3 °C; 1H NMR (600 MHz, DMSO-d6)δ 10.28 (s, 1H), 9.59 (s, 1H), 9.26 (s, 1H), 8.86 (s, 1H), 8.17 (d, J = 8.2 Hz, 2H), 8.13 (d, J = 9.9 Hz, 2), 6.88 (d, J = 8.2 Hz, 1H), 6.70 (d, J = 8.2 Hz, 1H), 2.07 (s, 3H), 1.99 (s, 3H). 13C NMR (151 MHz, DMSO) δ 165.4, 163.5, 160.0, 157.1, 154.2, 147.7, 141.5, 136.0, 127.3, 126.0, 125.3, 122.8, 118.6, 113.7, 102.0, 18.0, 11.6. HRMS (ESI) for C19H18N6O4 [M+Na]+: calcd, 417.1288, found, 417.1275. HPLC: 96.217%.
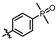
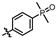
4-amino-2-((4-(dimethylphosphoryl)phenyl)amino)-N-(3-hydroxy-2,6-dimethylphenyl)pyrimidine-5-carboxamide (MY-13): White solid; yield: 54.3% mp: 238.5–239.7 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.75 (s, 1H), 9.50 (s, 1H), 9.20 (s, 1H), 8.81 (s, 1H), 7.98 (dd, 2H), 7.63 (dd, J = 11.0, 8.5 Hz, 2H), 6.88 (d, J = 8.2 Hz, 1H), 6.69 (d, J = 8.2 Hz, 1H), 2.07 (s, 3H), 1.99 (s, 3H), 1.63 (s, 3H), 1.60 (s, 3H). 13C NMR (151 MHz, DMSO) δ 165.2, 162.0, 160.1, 157.9, 153.1, 142.6, 135.7, 129.6, 129.1, 127.3, 126.7, 126.2, 124.5, 121.8, 117.9, 112.5, 100.0, 17.6, 17.1, 17.0, 10.5. HRMS (ESI) for C21H24N5O3P [M+Na]+: calcd, 448.1515, found, 448.1546. HPLC: 98.481%.
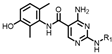
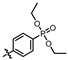
(4-((4-amino-5-((3-hydroxy-2,6-dimethylphenyl)carbamoyl)pyrimidin-2-yl)amino)phenyl)phosphonate (MY-14): White solid; yield: 52.1% mp: 232.1–232.7 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.85 (s, 1H), 9.51 (s, 1H), 9.16 (s, 1H), 8.81 (s, 1H), 8.02 (dd, J = 8.7, 3.6 Hz, 2H), 7.59 (dd, J = 12.6, 8.4 Hz, 2H), 6.88 (d, J = 8.1 Hz, 1H), 6.69 (d, J = 8.1 Hz, 1H), 3.98 (dtq, J = 10.2, 6.5, 3.2 Hz, 4H), 2.07 (s, 3H), 1.99 (s, 3H), 1.23 (t, J = 7.0 Hz, 6H). 13C NMR (101 MHz, DMSO) δ 165.5, 163.5, 160.4, 156.9, 154.1, 144.8, 136.1, 133.5, 132.5, 132.4, 127.3, 126.1, 123.4, 121.0, 119.1, 118.9, 118.8, 113.6, 101.3, 62.7, 19.5, 16.2, 10.4. HRMS (ESI) for C23H28N5O5P [M+Na]+: calcd, 508.1726, found, 508.1732. HPLC: 99.295%.
4-amino-2-((4-(dimethylcarbamoyl)phenyl)amino)-N-(3-hydroxy-2,6-dimethylphenyl)pyrimidine-5-carboxamide (MY-15): White solid; yield: 41.5% mp: 236.3–237.5 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.70 (s, 1H), 9.48 (s, 1H), 9.20 (s, 1H), 8.80 (s, 1H), 7.90 (d, J = 8.2 Hz, 2H), 7.34 (d, J = 8.3 Hz, 2H), 6.88 (d, J = 8.2 Hz, 1H), 6.69 (d, J = 8.2 Hz, 1H), 2.97 (s, 6H), 2.07 (s, 3H), 1.99 (s, 3H). 13C NMR (151 MHz, DMSO) δ 172.4, 165.7, 163.5, 161.4, 158.6, 154.2, 142.7, 135.5, 129.3, 128.3, 127.3, 126.1, 122.9, 118.1, 113.6, 101.4, 29.0, 27.0, 18.0, 10.6. HRMS (ESI) for C22H24N6O3 [M+Na]+: calcd, 443.1808, found, 443.1813. HPLC: 98.055%.
4-amino-2-((4-(morpholine-4-carbonyl)phenyl)amino)-N-(3-hydroxy-2,6-dimethylphenyl)pyrimidine-5-carboxamide (MY-16): White solid; yield: 44.5% mp: 235.4–237.4 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.72 (s, 1H), 9.48 (s, 1H), 9.15 (s, 1H), 8.80 (s, 1H), 7.92 (d, J = 8.3 Hz, 2H), 7.34 (d, J = 8.6 Hz, 2H), 6.88 (d, J = 8.2 Hz, 1H), 6.69 (d, J = 8.2 Hz, 1H), 3.60 (s, 4H), 3.51 (s, 4H), 2.07 (s, 3H), 1.99 (s, 3H). 13C NMR (151 MHz, DMSO) δ 170.6, 165.6, 163.0, 161.2, 157.3, 153.0, 143.6, 135.3, 130.1, 128.5, 128.3, 127.3, 126.1, 122.9, 118.8, 113.6, 100.9, 65.6, 54.7, 18.0, 11.1. HRMS (ESI) for C24H26N6O4 [M+Na]+: calcd, 485.1914, found, 485.1909. HPLC: 98.595%.
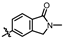
4-amino-2-((2-methyl-1-oxoisoindolin-5-yl)amino)-N-(3-hydroxy-2,6-dimethylphenyl)pyrimidine-5-carboxamide (MY-17): White solid; yield: 48.9% mp: 317.7–318.5 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.85 (s, 1H), 9.49 (s, 1H), 9.17 (s, 1H), 8.82 (s, 1H), 8.23 (s, 1H), 7.83 (dd, J = 8.4, 1.9 Hz, 1H), 7.54 (d, J = 8.4 Hz, 1H), 6.89 (d, J = 8.2 Hz, 1H), 6.69 (d, J = 8.2 Hz, 1H), 4.42 (s, 2H), 3.05 (s, 3H), 2.07 (s, 3H), 1.99 (s, 3H). 13C NMR (151 MHz, DMSO) δ 166.8, 164.5, 163.2, 159.4, 156.6, 153.1, 144.9, 142.2, 135.1, 126.7, 125.0, 124.8, 122.2, 121.8, 117.9, 112.5, 112.0, 100.0, 50.8, 28.5, 17.0, 9.8. HRMS (ESI) for C22H22N6O3 [M+Na]+: calcd, 441.1651, found, 441.1648. HPLC: 98.880%.
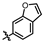
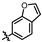
4-amino-2-(benzofuran-6-ylamino)-N-(3-hydroxy-2,6-dimethylphenyl)pyrimidine-5-carboxamide (MY-18): White solid; yield: 53.2% mp: 223.2–224.3 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.68 (s, 1H), 9.48 (s, 1H), 9.16 (s, 1H), 8.80 (s, 1H), 8.44 (s, 1H), 7.88 (d, J = 2.2 Hz, 1H), 7.53–7.46 (m, 2H), 6.88 (d, J = 8.3 Hz, 1H), 6.87 (d, J = 2.5 Hz, 1H), 6.69 (d, J = 8.2 Hz, 1H), 2.07 (s, 3H), 1.99 (s, 3H). 13C NMR (151 MHz, DMSO) δ 164.6, 162.5, 159.2, 154.2, 153.0, 143.0, 137.6, 134.3, 125.7, 124.1, 121.2, 120.6, 119.1, 115.1, 111.9, 105.9, 101.4, 100.0, 54.3, 17.0, 10.5. HRMS (ESI) for C21H19N5O3 [M+Na]+: calcd, 412.1386, found, 412.1393. HPLC: 95.509%.
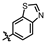
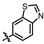
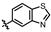
4-amino-2-(benzo[d]thiazol-6-ylamino)-N-(3-hydroxy-2,6-dimethylphenyl)pyrimidine-5-carboxamide (MY-19): White solid; yield: 47.7% mp: 281.9–283.1 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.82 (s, 1H), 9.48 (s, 1H), 9.20 (s, 1H), 9.16 (s, 1H), 8.99 (s, 1H), 8.82 (s, 1H), 7.96 (d, J = 8.8 Hz, 1H), 7.75 (dd, J = 8.8, 2.2 Hz, 1H), 6.89 (d, J = 8.2 Hz, 1H), 6.69 (d, J = 8.2 Hz, 1H), 2.08 (s, 3H), 2.00 (s, 3H). 13C NMR (151 MHz, DMSO) δ 166.7, 163.6, 160.0, 157.2, 154.1, 153.9, 148.4, 139.0, 136.2, 134.8, 127.3, 126.1, 123.0, 122.9, 119.5, 113.6, 111.2, 100.7, 18.1, 11.6. HRMS (ESI) for C20H18N6O2S [M+Na]+: calcd, 429.1110, found, 429.1111. HPLC: 98.832%.
4-amino-2-(benzo[d]thiazol-5-ylamino)-N-(3-hydroxy-2,6-dimethylphenyl)pyrimidine-5-carboxamide (MY-20): White solid; yield: 43.6% mp: 263.5–264.5 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.73 (s, 1H), 9.48 (s, 1H), 9.34 (s, 1H), 9.16 (s, 1H), 8.83 (s, 1H), 8.77 (d, J = 2.1 Hz, 1H), 8.01 (d, J = 8.7 Hz, 1H), 7.86 (dd, J = 8.8, 2.2 Hz, 1H), 6.89 (d, J = 8.2 Hz, 1H), 6.69 (d, J = 8.3 Hz, 1H), 2.08 (s, 3H), 2.00 (s, 3H). 13C NMR (151 MHz, DMSO) δ 165.7, 163.6, 160.7, 157.7, 156.8, 154.8, 154.1, 139.7, 136.2, 127.3, 126.6, 126.1, 123.3, 122.2, 119.6, 114.2, 113.0, 100.7, 18.1, 11.6. HRMS (ESI) for C20H18N6O2S [M+Na]+: calcd, 429.1110, found, 429.1090. HPLC: 97.164%.
4-amino-2-(benzofuran-5-ylamino)-N-(3-hydroxy-2,6-dimethylphenyl)pyrimidine-5-carboxamide (MY-21): White solid; yield: 47.8% mp: 251.9–252.9 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.48 (s, 1H), 9.42 (s, 1H), 9.16 (s, 1H), 8.78 (s, 1H), 8.21 (d, J = 2.3 Hz, 1H), 7.93 (d, J = 2.2 Hz, 1H), 7.61 (dd, J = 8.9, 2.2 Hz, 1H), 7.48 (d, J = 8.9 Hz, 1H), 6.89 (t, 1H), 6.88 (s, 1H), 6.69 (d, J = 8.2 Hz, 1H), 2.07 (s, 3H), 1.99 (s, 3H). 13C NMR (151 MHz, DMSO) δ 166.6, 163.6, 160.8, 158.2, 154.1, 150.6, 147.3, 136.8, 127.6, 127.3, 126.2, 122.9, 118.1, 113.5, 112.0, 111.2, 107.4, 98.6, 55.4, 18.1, 11.6. HRMS (ESI) for C21H19N5O3 [M+Na]+: calcd, 412.1386, found, 412.1384. HPLC: 96.522%.