Mechanistic Insights into a Self-Management Intervention in Young Adults with Irritable Bowel Syndrome: A Pilot Multi-Omics Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants’ Eligibility and Exclusion Criteria
2.3. Measures
2.4. Stool Sample Collection and Gut Microbiome Sequencing
2.5. Blood Sample Collection and RNA Sequencing
2.6. Data Analysis
3. Results
3.1. Demographics, Clinical Symptoms, and Microbial Diversity Profiles
3.2. Multi-Omics Module Function Characteristics at Baseline and Post-Intervention
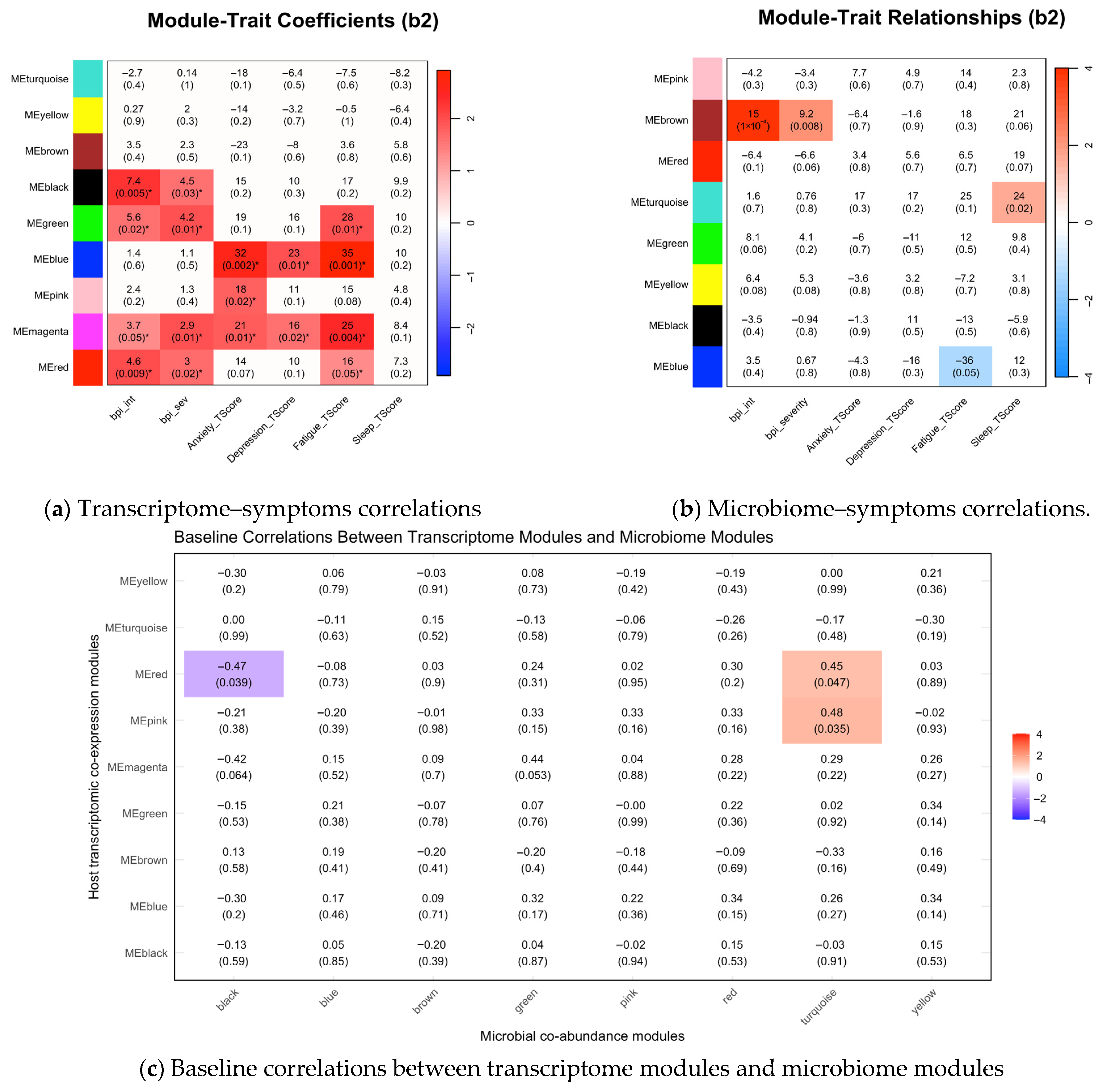
3.3. Associations Between Symptom Phenotypes and Multi-Omics Modules at Baseline
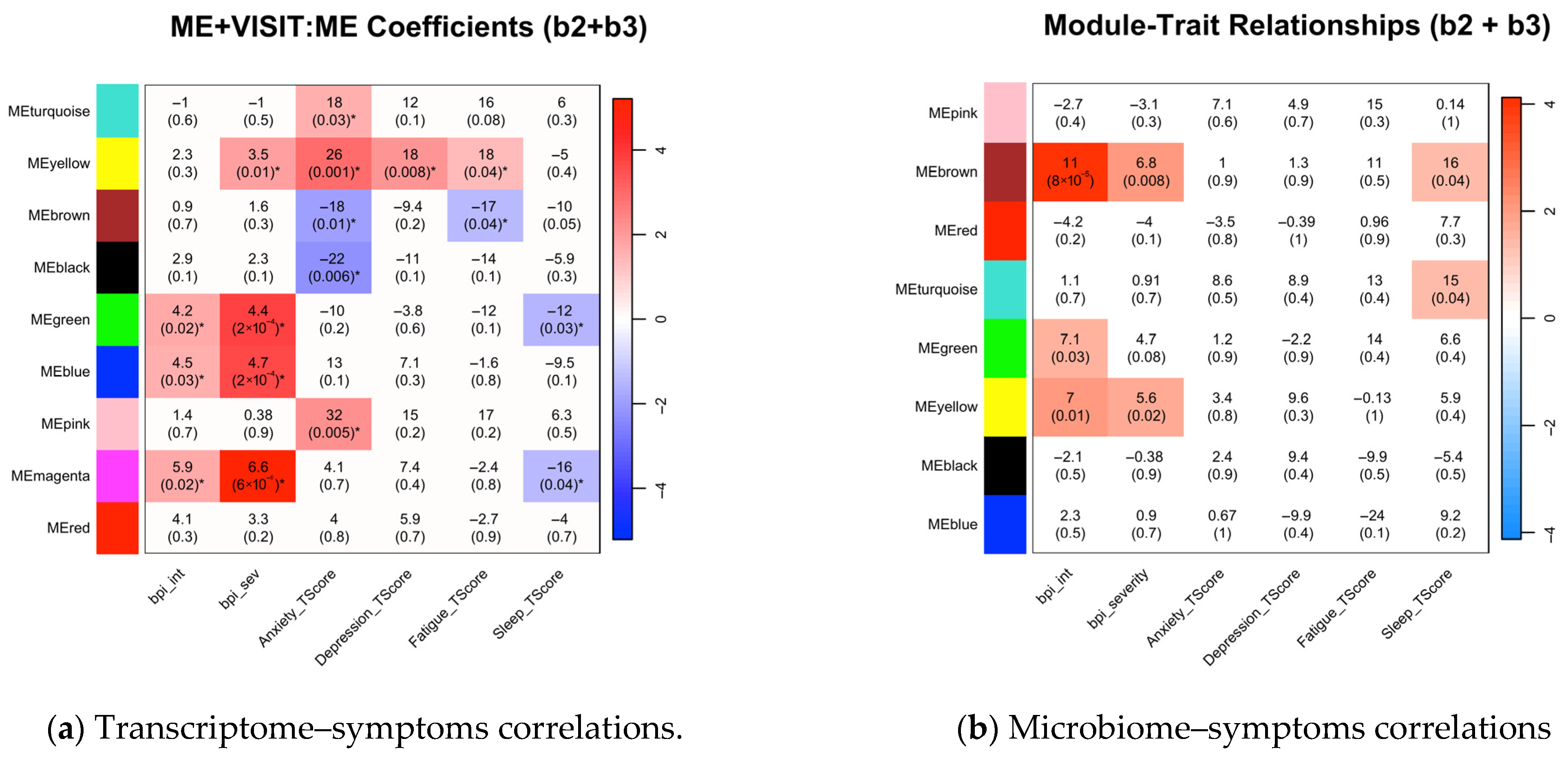
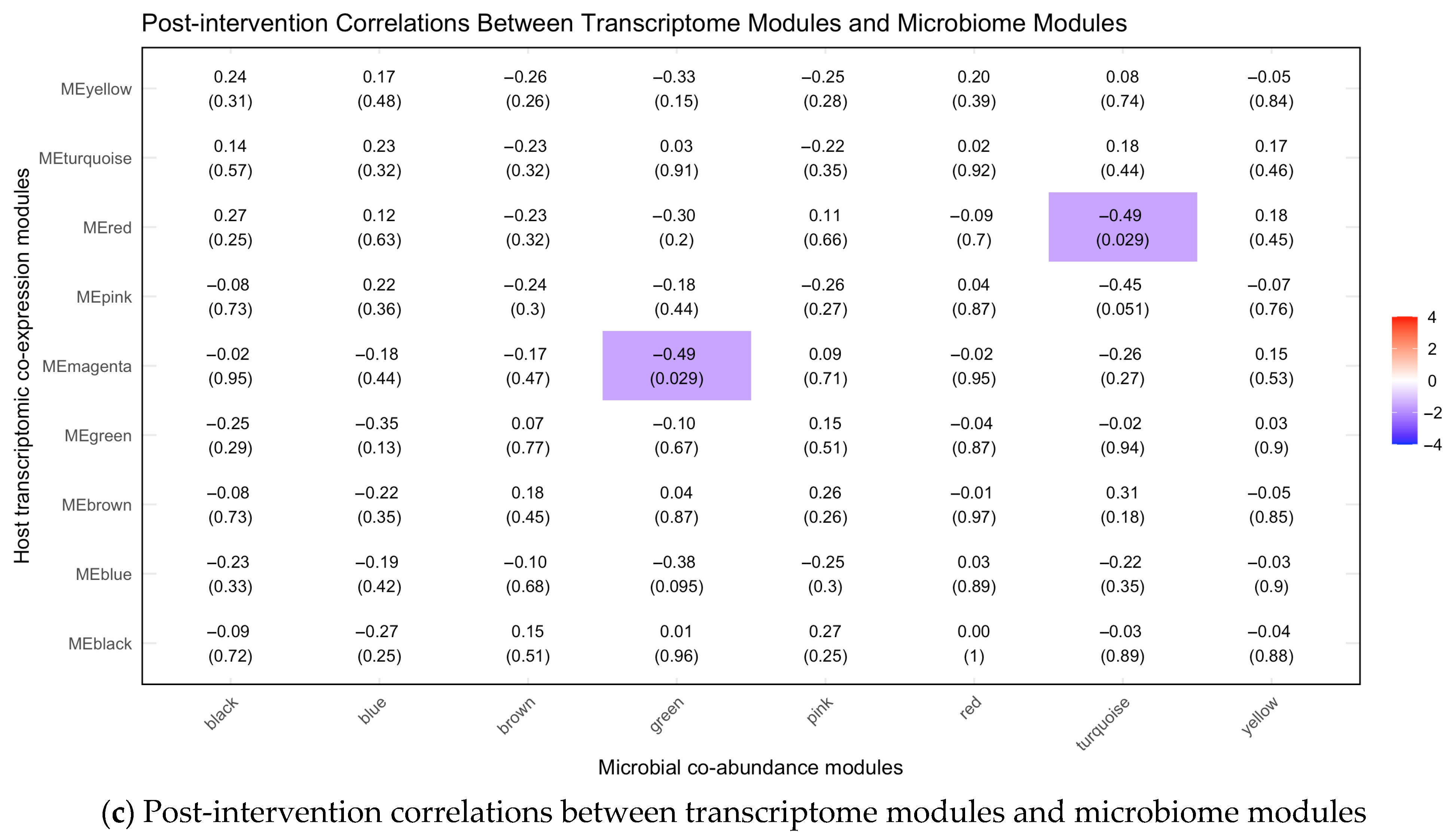
3.4. Associations Between Symptom Phenotypes and Multi-Omics Modules Post-Intervention
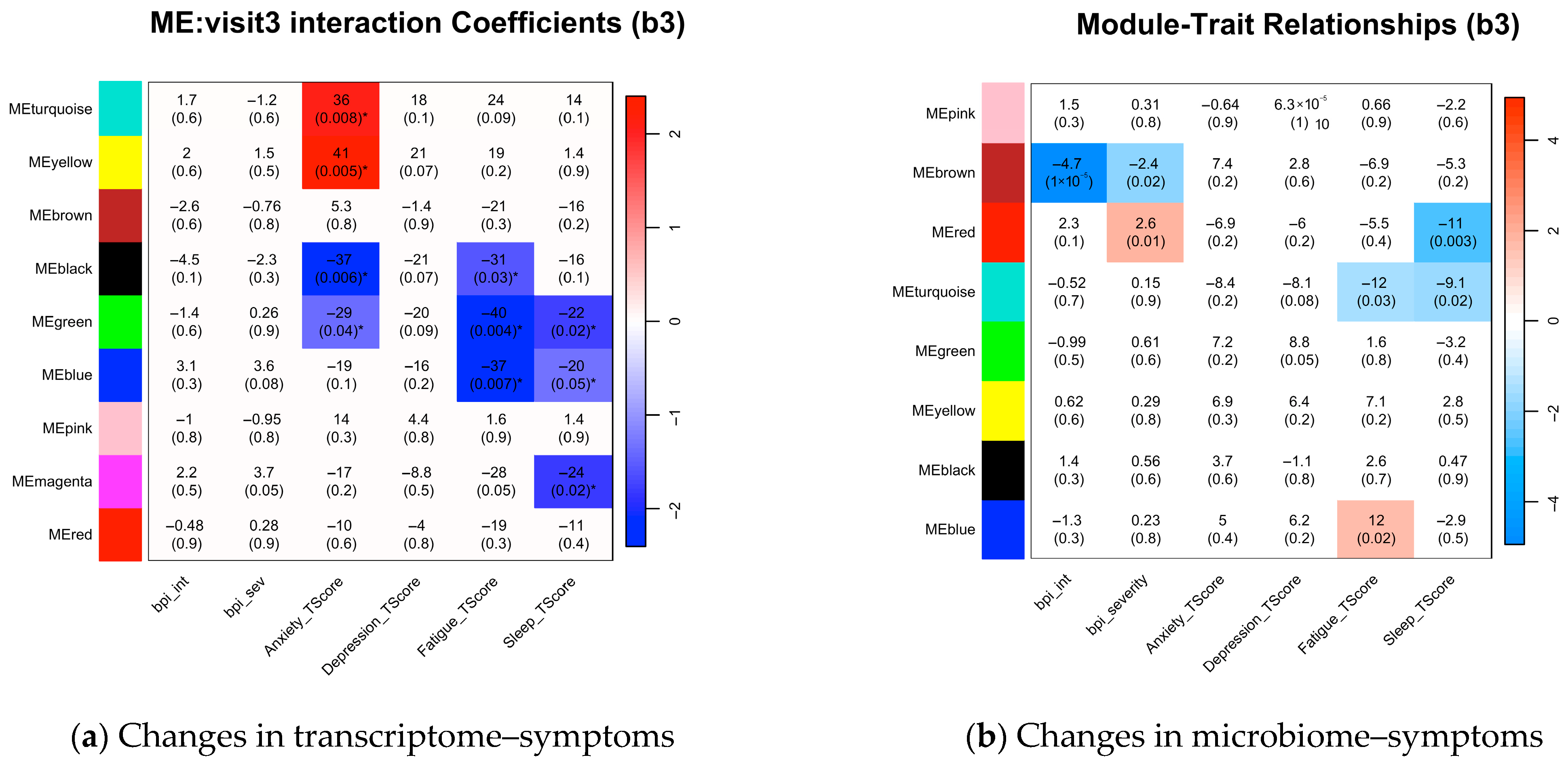
3.5. Intervention-Induced Modulation of the Associations Between Symptoms and Multi-Omics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IBS | Irritable bowel syndrome |
| WGCNA | Weighted gene co-expression network analysis |
| RCT | Randomized controlled trial |
| PROMIS | Patient-Reported Outcomes Measurement Information System |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| ROS | Reactive oxygen species |
| FODMAP | Low-fermentable oligo-, di-, monosaccharides, and polyols |
References
- Huang, K.Y.; Wang, F.Y.; Lv, M.; Ma, X.X.; Tang, X.D.; Lv, L. Irritable bowel syndrome: Epidemiology overlap disorders pathophysiology and treatment. World J. Gastroenterol. 2023, 29, 4120–4135. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Ryu, H.J.; Bhatt, R.R. The neurobiology of irritable bowel syndrome. Mol. Psychiatry 2023, 28, 1451–1465. [Google Scholar] [CrossRef]
- Bosman, M.; Weerts, Z.; Snijkers, J.T.W.; Vork, L.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.; Keszthelyi, D. The socioeconomic impact of irritable bowel syndrome: An analysis of direct and indirect health care costs. Clin. Gastroenterol. Hepatol. 2023, 21, 2660–2669. [Google Scholar] [CrossRef]
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Jensen, E.T.; Kim, H.P.; Egberg, M.D.; Lund, J.L.; Moon, A.M.; Pate, V.; Barnes, E.L.; et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2021. Gastroenterology 2022, 162, 621–644. [Google Scholar] [CrossRef]
- Camilleri, M. Diagnosis and treatment of irritable bowel syndrome: A review. JAMA 2021, 325, 865–877. [Google Scholar] [CrossRef]
- Papale, A.J.; Flattau, R.; Vithlani, N.; Mahajan, D.; Nadella, S. A review of pharmacologic and non-pharmacologic therapies in the management of irritable bowel syndrome: Current recommendations and evidence. J. Clin. Med. 2024, 13, 6948. [Google Scholar] [CrossRef]
- Lembo, A.; Sultan, S.; Chang, L.; Heidelbaugh, J.J.; Smalley, W.; Verne, G.N. AGA clinical practice guideline on the pharmacological management of irritable bowel syndrome with diarrhea. Gastroenterology 2022, 163, 137–151. [Google Scholar] [CrossRef]
- Cong, X.; Perry, M.; Bernier, K.M.; Young, E.E.; Starkweather, A. Effects of self-management interventions in patients with irritable bowel syndrome: Systematic review. West. J. Nurs. Res. 2018, 40, 1698–1720. [Google Scholar] [CrossRef]
- Black, C.J.; Ford, A.C. Best management of irritable bowel syndrome. Frontline Gastroenterol. 2021, 12, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Orock, A.; Yuan, T.; Greenwood-Van Meerveld, B. Importance of non-pharmacological approaches for treating irritable bowel syndrome: Mechanisms and clinical relevance. Front. Pain Res. 2020, 1, 609292. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Burcharth, J.; Pommergaard, H.C.; Rosenberg, J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes 2016, 7, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Barandouzi, Z.A.; Lee, J.; Del Carmen Rosas, M.; Chen, J.; Henderson, W.A.; Starkweather, A.R.; Cong, X.S. Associations of neurotransmitters and the gut microbiome with emotional distress in mixed type of irritable bowel syndrome. Sci. Rep. 2022, 12, 1648. [Google Scholar] [CrossRef] [PubMed]
- Goodoory, V.C.; Mikocka-Walus, A.; Yiannakou, Y.; Houghton, L.A.; Black, C.J.; Ford, A.C. Impact of psychological comorbidity on the prognosis of irritable bowel syndrome. Am. J. Gastroenterol. 2021, 116, 1485–1494. [Google Scholar] [CrossRef]
- Chen, J.; Barandouzi, Z.A.; Lee, J.; Xu, W.; Feng, B.; Starkweather, A.; Cong, X. Psychosocial and sensory factors contribute to self-reported pain and quality of life in young adults with irritable bowel syndrome. Pain Manag. Nurs. 2022, 23, 646–654. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Clarke, G.; Quigley, E.M.; Groeger, J.A.; Dinan, T.G.; Cryan, J.F. Gut memories: Towards a cognitive neurobiology of irritable bowel syndrome. Neurosci. Biobehav. Rev. 2012, 36, 310–340. [Google Scholar] [CrossRef]
- Zhou, Q.; Verne, G.N. New insights into visceral hypersensitivity—Clinical implications in IBS. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Kashyap, P.C. Multi-omics for biomarker approaches in the diagnostic evaluation and management of abdominal pain and irritable bowel syndrome: What lies ahead. Gut Microbes 2023, 15, 2195792. [Google Scholar] [CrossRef]
- Qian, A.; Song, D.; Li, Y.; Liu, X.; Tang, D.; Yao, W.; Yuan, Y. Role of voltage-gated Ca2+ channels in rat visceral hypersensitivity change induced by 2,4,6-trinitrobenzene sulfonic acid. Mol. Pain 2013, 9, 15. [Google Scholar] [CrossRef]
- Wemheuer, F.; Taylor, J.A.; Daniel, R.; Johnston, E.; Meinicke, P.; Thomas, T.; Wemheuer, B. Tax4Fun2: Prediction of habitat-specific functional profiles and functional redundancy based on 16S rRNA gene sequences. Environ. Microbiome 2020, 15, 11. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Barandouzi, Z.A.; Xu, W.; Feng, B.; Chon, K.; Santos, M.; Starkweather, A.; Cong, X. Somatosensory profiles differentiate pain and psychophysiological symptoms among young adults with irritable bowel syndrome: A cluster analysis. Clin. J. Pain 2022, 38, 492–501. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Barandouzi, Z.A.; Lee, J.; Zhao, T.; Xu, W.; Chen, M.H.; Feng, B.; Starkweather, A.; Cong, X. The effect of self-management online modules plus nurse-led support on pain and quality of life among young adults with irritable bowel syndrome: A randomized controlled trial. Int. J. Nurs. Stud. 2022, 132, 104278. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Ramesh, D.; Perry, M.; Xu, W.; Bernier, K.M.; Young, E.E.; Walsh, S.; Starkweather, A. Pain self-management plus nurse-led support in young adults with irritable bowel syndrome: Study protocol for a pilot randomized control trial. Res. Nurs. Health 2018, 41, 121–130. [Google Scholar] [CrossRef]
- Li, J.; Zhou, D.; Qiu, W.; Shi, Y.; Yang, J.J.; Chen, S.; Wang, Q.; Pan, H. Application of weighted gene co-expression network analysis for data from paired design. Sci. Rep. 2018, 8, 622. [Google Scholar] [CrossRef]
- Ryan, P.; Sawin, K.J. The individual and family self-management theory: Background and perspectives on context, process, and outcomes. Nurs. Outlook 2009, 57, 217–225.e6. [Google Scholar] [CrossRef]
- Keller, S.; Bann, C.M.; Dodd, S.L.; Schein, J.; Mendoza, T.R.; Cleeland, C.S. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin. J. Pain 2004, 20, 309–318. [Google Scholar] [CrossRef]
- Cella, D.; Choi, S.W.; Condon, D.M.; Schalet, B.; Hays, R.D.; Rothrock, N.E.; Yount, S.; Cook, K.F.; Gershon, R.C.; Amtmann, D.; et al. PROMIS adult health profiles: Efficient short-form measures of seven health domains. Value Health 2019, 22, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L. Assessing and improving methods used in operational taxonomic unit-based approaches for 16S rRNA gene sequence analysis. Appl. Environ. Microbiol. 2011, 77, 3219–3226. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, T.; Li, H.; Xu, W.; Maas, K.; Singh, V.; Chen, M.H.; Dorsey, S.G.; Starkweather, A.R.; Cong, X.S. Multi-omics analysis of gut microbiota and host transcriptomics reveals dysregulated immune response and metabolism in young adults with irritable bowel syndrome. Int. J. Mol. Sci. 2024, 25, 3514. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Passacatini, L.C.; Ilari, S.; Nucera, S.; Scarano, F.; Macri, R.; Caminiti, R.; Serra, M.; Oppedisano, F.; Maiuolo, J.; Palma, E.; et al. Multiple aspects of irritable bowel syndrome and the role of the immune system: An overview of systematic reviews with a focus on polyphenols. Int. J. Mol. Sci. 2024, 25, 11993. [Google Scholar] [CrossRef]
- Atzler, J.J.; Sahin, A.W.; Gallagher, E.; Zannini, E.; Arendt, E.K. Characteristics and properties of fibres suitable for a low FODMAP diet- an overview. Trends Food Sci. Technol. 2021, 112, 823–836. [Google Scholar] [CrossRef]
- Chu, P.; He, Y.; Hu, F.; Wang, X. The effects of low FODMAP diet on gut microbiota regulation: A systematic review and meta-analysis. J. Food Sci. 2025, 90, e70072. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Thakur, E.; Shapiro, J. Non-pharmaceutical treatments for irritable bowel syndrome. BMJ 2024, 387, e075777. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Cremon, C.; Barbaro, M.R.; Bianco, F.; Stanghellini, V.; Barbara, G. Microbiota modulation in disorders of gut-brain interaction. Dig. Liver Dis. 2024, 56, 1971–1979. [Google Scholar] [CrossRef]
- Lagomarsino, V.N.; Kostic, A.D.; Chiu, I.M. Mechanisms of microbial-neuronal interactions in pain and nociception. Neurobiol. Pain 2021, 9, 100056. [Google Scholar] [CrossRef]
- Lu, J.; Liang, W.; Cui, L.; Mou, S.; Pei, X.; Shen, X.; Shen, Z.; Shen, P. Identifying neuro-inflammatory biomarkers of generalized anxiety disorder from lymphocyte subsets using machine learning approaches. Neuropsychobiology 2025, 84, 74–85. [Google Scholar] [CrossRef]
- Lopizzo, N.; Tosato, S.; Begni, V.; Tomassi, S.; Cattane, N.; Barcella, M.; Turco, G.; Ruggeri, M.; Riva, M.A.; Pariante, C.M.; et al. Transcriptomic analyses and leukocyte telomere length measurement in subjects exposed to severe recent stressful life events. Transl. Psychiatry 2017, 7, e1042. [Google Scholar] [CrossRef]
- Filiou, M.D.; Sandi, C. Anxiety and brain mitochondria: A bidirectional crosstalk. Trends Neurosci. 2019, 42, 573–588. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Scialo, F.; Fernandez-Ayala, D.J.; Sanz, A. Role of mitochondrial reverse electron transport in ROS signaling: Potential roles in health and disease. Front. Physiol. 2017, 8, 428. [Google Scholar] [CrossRef]
- Gerritsen, R.J.S.; Band, G.P.H. Breath of life: The respiratory vagal stimulation model of contemplative activity. Front. Hum. Neurosci. 2018, 12, 397. [Google Scholar] [CrossRef]
- Moloney, R.D.; Johnson, A.C.; O’Mahony, S.M.; Dinan, T.G.; Greenwood-Van Meerveld, B.; Cryan, J.F. Stress and the microbiota-gut-brain axis in visceral pain: Relevance to irritable bowel syndrome. CNS Neurosci. Ther. 2016, 22, 102–117. [Google Scholar] [CrossRef]
- Dhama, K.; Latheef, S.K.; Dadar, M.; Samad, H.A.; Munjal, A.; Khandia, R.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; et al. Biomarkers in stress related diseases/disorders: Diagnostic, prognostic, and therapeutic values. Front. Mol. Biosci. 2019, 6, 91. [Google Scholar] [CrossRef]
- Muro, P.; Zhang, L.; Li, S.; Zhao, Z.; Jin, T.; Mao, F.; Mao, Z. The emerging role of oxidative stress in inflammatory bowel disease. Front. Endocrinol. 2024, 15, 1390351. [Google Scholar] [CrossRef]
- Coyte, K.Z.; Rakoff-Nahoum, S. Understanding competition and cooperation within the mammalian gut microbiome. Curr. Biol. 2019, 29, R538–R544. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M. Intestinal bacteria associated with irritable bowel syndrome and chronic fatigue. Neurogastroenterol. Motil. 2023, 35, e14621. [Google Scholar] [CrossRef]
- Li, Y.; Hao, Y.; Fan, F.; Zhang, B. The role of microbiome in insomnia, circadian disturbance and depression. Front. Psychiatry 2018, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Varesi, A.; Deumer, U.S.; Ananth, S.; Ricevuti, G. The emerging role of gut microbiota in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): Current evidence and potential therapeutic applications. J. Clin. Med. 2021, 10, 5077. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef]
| Mean | SD | |
|---|---|---|
| Age | 22.05 | 2.74 |
| Year of IBS diagnosis | 2.25 | 1.89 |
| N | Percentage | |
| Sex | ||
| Female | 13 | 65.0 |
| Male | 7 | 35.0 |
| Race | ||
| White | 15 | 75.0 |
| Asian | 2 | 10.0 |
| Black or African American | 3 | 15.0 |
| Ethnicity | ||
| Not Hispanic or Latino | 15 | 75.0 |
| Hispanic or Latino | 3 | 15.0 |
| Not reported | 2 | 10.0 |
| Education | ||
| High school or below | 0 | 0 |
| College or associate degree | 11 | 55.0 |
| Bachelor’s degree | 5 | 25.0 |
| Graduate or higher | 4 | 20.0 |
| Employment Status | ||
| Student | 13 | 65.0 |
| Working now | 7 | 35.0 |
| Unemployed or other | 0 | 0 |
| Marital Status | ||
| Never married | 18 | 90.0 |
| Married | 2 | 10.0 |
| Self-Reported Symptom | Baseline (M ± SD) | Post-Intervention (M ± SD) | 95% CI | p |
|---|---|---|---|---|
| Pain interference | 2.65 ± 2.77 | 1.52 ± 1.88 | [−1.99, −0.27] | 0.013 * |
| Pain severity | 3.05 ± 2.14 | 2.29 ± 1.78 | [−1.39, −0.14] | 0.019 * |
| Anxiety | 58.76 ± 8.30 | 56.43 ± 9.02 | [−6.28, 1.61] | 0.231 |
| Depression | 52.53 ± 8.07 | 50.52 ± 8.48 | [−5.03, 1.02] | 0.181 |
| Fatigue | 53.53 ± 9.63 | 52.91 ± 10.98 | [−4.44, 3.19] | 0.735 |
| Sleep disturbance | 49.90 ± 5.84 | 48.40 ± 5.15 | [−4.1, 1.1] | 0.243 |
| Metric | Baseline (M ± SD) | Post-Intervention (M ± SD) | Wilcox p |
|---|---|---|---|
| Alpha Diversity | |||
| invsimpson | 9.51 ± 4.64 | 9.72 ± 5.2 | 0.756 |
| shannon | 2.95 ± 0.68 | 3.01 ± 0.63 | 0.596 |
| sobs | 228.69 ± 57.63 | 238.31 ± 52.2 | 0.202 |
| Beta Diversity | |||
| Within-group Bray–Curtis distance | 0.67 ± 0.18 | 0.65 ± 0.16 | 0.482 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Chen, J.; Li, A.; Chen, M.-H.; Starkweather, A.; Cong, X. Mechanistic Insights into a Self-Management Intervention in Young Adults with Irritable Bowel Syndrome: A Pilot Multi-Omics Study. Biomedicines 2025, 13, 2102. https://doi.org/10.3390/biomedicines13092102
Wu W, Chen J, Li A, Chen M-H, Starkweather A, Cong X. Mechanistic Insights into a Self-Management Intervention in Young Adults with Irritable Bowel Syndrome: A Pilot Multi-Omics Study. Biomedicines. 2025; 13(9):2102. https://doi.org/10.3390/biomedicines13092102
Chicago/Turabian StyleWu, Weizi, Jie Chen, Aolan Li, Ming-Hui Chen, Angela Starkweather, and Xiaomei Cong. 2025. "Mechanistic Insights into a Self-Management Intervention in Young Adults with Irritable Bowel Syndrome: A Pilot Multi-Omics Study" Biomedicines 13, no. 9: 2102. https://doi.org/10.3390/biomedicines13092102
APA StyleWu, W., Chen, J., Li, A., Chen, M.-H., Starkweather, A., & Cong, X. (2025). Mechanistic Insights into a Self-Management Intervention in Young Adults with Irritable Bowel Syndrome: A Pilot Multi-Omics Study. Biomedicines, 13(9), 2102. https://doi.org/10.3390/biomedicines13092102







