Abstract
Background/Objectives: Nicotine-free electronic cigarettes (NFECs) are becoming increasingly popular, especially among youth and non-smokers, yet their effects on the gastrointestinal tract (GIT) remain poorly understood. This systematic review synthesizes available in vitro, in vivo, and limited human evidence on NFEC-associated changes in gastrointestinal health and function. Methods: Literature searches were conducted in Medline, Web of Science, Cochrane, and Scopus in July 2025, following PRISMA guidelines. Eligible studies examined NFEC effects on any GIT segment, including the oral cavity, liver, intestines, and microbiome. Data on study design, exposure characteristics, and main outcomes were extracted and narratively synthesized. Results: Of 111 identified records, 94 full-text articles were retrieved, and 21 studies met the inclusion criteria. Most were preclinical, with only one human pilot study. Evidence from oral cell and microbial models suggests that NFEC aerosols can induce pro-inflammatory cytokine production, impair cell viability, and disrupt microbial metabolism through their base constituents (propylene glycol, vegetable glycerine, and flavourings). Animal studies indicate possible hepatic oxidative stress, altered lipid metabolism, and gut barrier dysfunction, with some data suggesting more pronounced steatosis in nicotine-free exposures compared to nicotine-containing counterparts. Microbiome studies report reduced tight junction expression and altered neutrophil function. Conclusions: Current evidence is limited and predominantly preclinical but indicates that NFEC exposure can affect multiple aspects of gastrointestinal health. Robust longitudinal and interventional human studies are urgently needed to determine the clinical relevance of these findings and to inform regulation and public health policy.
1. Introduction
Cigarette smoking remains a leading global cause of preventable morbidity and mortality and is responsible for over 8 million deaths annually, primarily due to its established links with cardiovascular disease, chronic respiratory conditions, and multiple cancers [1,2,3,4]. In response to tobacco control efforts and increasing public health awareness, the past two decades have seen a sharp rise in the popularity of electronic cigarettes (e-cigarettes, ECs), marketed as a safer alternative to combustible tobacco and a smoking cessation aid [5,6,7]. The concept of e-cigarettes dates back to the early 1960s, following growing recognition of tobacco’s toxic effects. One example is the British American Tobacco-initiated “Project Ariel,” an early attempt to develop a smokeless electronic device aimed at delivering nicotine while filtering out harmful combustion products [8]. Gilbert patented a “Smokeless Non-Tobacco Cigarette,” proposing vaporization of flavoured liquids without combustion in 1962 [9]. However, these early projects were abandoned due to immature technology, unfavourable market conditions, and industry reluctance.
Modern ECs were developed in 2003 by Chinese pharmacist Hon Lik and introduced commercially in 2004 [10], based on the vaporization of an e-liquid base constituent—most commonly composed of propylene glycol (PG) and vegetable glycerine (VG) as the primary base solvents for nicotine and flavourings—using a battery-powered heating coil [11,12].
Global EC use has since expanded dramatically: by 2021, the global vaping market was valued at over USD 18 billion, with more than 400 different brands of e-cigarettes available, and usage among adolescents and young adults outpaced that of older populations in many regions [13,14,15,16].
While much attention has focused on nicotine-containing e-cigarettes, a growing number of users, particularly youth and never-smokers, now consume nicotine-free e-cigarettes (NFECs) [17,18]. Marketing these products through various platforms that are easily accessible to youth further drives the perception of e-cigarette smoking as a harmless trend [19,20,21,22]. Furthermore, dual use of both traditional and electronic cigarettes is increasing, and electronic cigarettes are increasingly used more as a complementary rather than a substitutional habit to traditional tobacco smoking [23,24].
Regulatory oversight of e-cigarettes varies widely. The U.S. Food and Drug Administration (FDA) regulates electronic nicotine delivery systems (ENDSs) under the Tobacco Control Act, but enforcement for nicotine-free devices remains inconsistent [25,26,27,28]. In the European Union, the Tobacco Products Directive excludes non-nicotine liquids, leaving gaps in standardization. In 2015, the European Commission implemented two acts and a commission report related to e-cigarettes, establishing a common format for e-cigarette labelling, technical standards for e-cigarette refilling mechanisms, and their current established risks to public health [29]. Many countries have prohibited the sale of nicotine e-cigarettes to minors but permit or inadequately regulate nicotine-free variants, most commonly through flavour restrictions [30,31,32]. This lack of a regulatory patchwork complicates safety evaluation, consumer awareness, and research harmonization [33,34].
Despite their inhalational route, e-cigarette vapours interact with the gastrointestinal tract (GIT) in several ways: via direct contact with the oral mucosa, swallowing aerosol condensates, systemic absorption of volatile compounds, and hepatic metabolism. The GIT, with its immunological, epithelial, and microbial complexity, may be particularly vulnerable to such exposures. Several studies have shown e-cigarette vapours to exert various biological effects, even in the absence of nicotine [35,36,37,38].
Given the rapid growth in NFEC usage, a comprehensive review of the effects of NFECs on the gastrointestinal system is both timely and necessary. This systematic review aims to evaluate available evidence on the impact of nicotine-free e-cigarette exposure on the gastrointestinal tract, defined broadly from the oral cavity to the rectum, including the liver, pancreas, and gut–brain axis.
2. Materials and Methods
2.1. Information Source and Search Strategies
This systematic review was conducted in accordance with the Cochrane Handbook and adhered to Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines to ensure appropriate quality of the assessment. This review was registered on the Open Science Framework (OFS) (https://doi.org/10.17605/OSF.IO/MXNY8). The protocol outlines the objectives, search strategy, and inclusion criteria for evaluating the effects of nicotine-free e-cigarettes on the gastrointestinal system.
A comprehensive literature search was conducted in July 2025 via the Medline, Web of Science, Cochrane, and Scopus electronic databases to identify relevant studies exploring the gastrointestinal effects of nicotine-free electronic cigarette vapour exposure.
In vitro and in vivo studies of the effects of nicotine-free e-cigarette exposure were included.
Both free-text terms and MeSH-indexed terms were used in combination, with Boolean operators (AND, OR) applied to maximize the retrieval of desired reports. Example search strings included “nicotine-zero e-cigarette” OR “nicotine-free e-cigarette” AND “gastrointestinal health”.
The search aimed to capture all studies, without language barriers, addressing in vitro, in vivo (animal, human, microbiological), and clinical aspects of nicotine-free e-cigarette use and the effects of PG, VG, and flavouring agents in the context of gastrointestinal function. Reference lists of selected articles were manually screened to identify additional relevant publications. Duplicate records were removed. Duplicate records were identified by matching the title, author list, year of publication, journal name, and DOI. Records meeting all these criteria were considered duplicates, and only one copy was retained for screening. The screening and selection process was independently conducted by three authors (I.J., J.V., I.M.). In cases where initial inclusion or exclusion decisions were not unanimous, disagreements were discussed collectively until consensus was reached. If consensus could not be achieved, the decision was made by majority vote. First, titles and abstracts were evaluated for relevance, and full texts of potentially eligible studies were obtained and reviewed in detail to determine final inclusion based on predefined criteria. From each eligible study, we extracted information on the study design, applied model (in vitro, in vivo, microbiological, clinical), sample characteristics, exposure details (including PG/VG ratio and flavourings when reported), measured outcomes, and key findings. Data extraction was performed independently by all three reviewers (I.J., I.M., J.V.), and any discrepancies were resolved through discussion until consensus was reached. A narrative synthesis was performed to summarize findings across different studies due to the heterogeneity of available data. Data were organized by gastrointestinal tract segments and presented as both descriptive text and structured tables with a focus on key findings and gastrointestinal health implications.
The complete search strategies for each database, including all search terms, Boolean operators, and applied filters, are provided in Supplementary File S1.
This review did not include searches of grey literature sources, preprint servers, or conference abstracts. While the database search strategy was designed to capture the widest possible range of published evidence, we acknowledge that relevant emerging studies on NFECs—particularly given the rapid pace of e-cigarette research—may exist outside the peer-reviewed literature.
2.2. Eligibility Criteria
This systematic review reviewed all in vitro and in vivo available studies that investigated the effects of nicotine-free e-cigarette liquids on the gastrointestinal system.
Inclusion criteria for this review were as follows: (1) peer-reviewed original research articles without language barriers; (2) studies evaluating the effects of nicotine-free e-cigarettes or isolated PG/VG on the gastrointestinal tract; (3) in vitro studies using gastrointestinal cell models; (4) in vivo studies in animals or humans assessing gastrointestinal outcomes; (5) research on e-liquid pharmacokinetics, metabolism, and excretion; (6) studies involving paediatric, adolescent, or adult populations.
Exclusion criteria were as follows: (1) studies focusing solely on nicotine-containing e-cigarettes; (2) articles not related to gastrointestinal endpoints; (3) narrative reviews, editorials, or commentaries; (5) articles without full-text availability; and (6) duplicated publications or non-original data (Table 1).

Table 1.
Eligibility criteria.
3. Results
A flow diagram of the database search and selection process is presented in Figure 1. We identified a total of 111 studies potentially eligible for this review, out of which 94 were successfully retrieved. Following further inspection, 36 studies were excluded because they did not distinguish between nicotine-free (NF) and nicotine-containing (NC) e-cigarette effects. An additional 37 studies were excluded as they did not explore the GIT effects of NFECs. Finally, a total of 21 studies met the full inclusion criteria for this systematic review [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59].
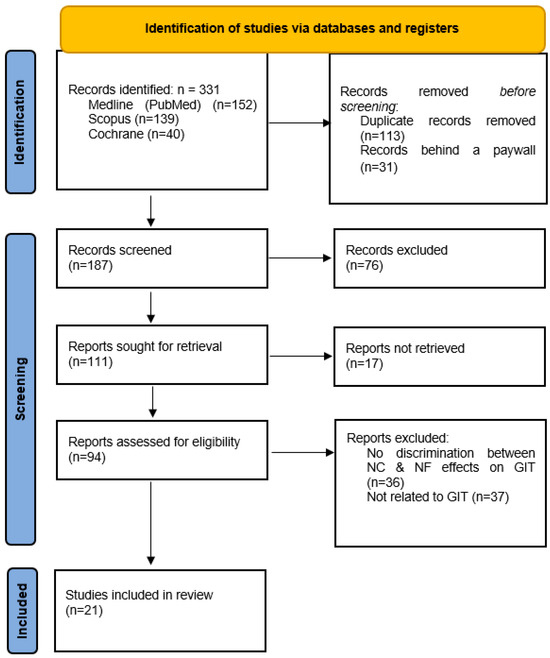
Figure 1.
PRISMA flow diagram of database search and selection. Legend: GIT—gastrointestinal tract; NC—nicotine-containing; NF—nicotine-free.
3.1. Nicotine-Free E-Cigarettes and Oral Health
Table 2 summarizes the characteristics of current available studies on oral health in the context of NFEC exposure [39,40,41,42,43,44,45,46,47,48,49,50,51].

Table 2.
NFEC and oral health.
Nine in vitro studies of human oral cell lines observed the effects of EC exposure with regard to nicotine content [39,40,41,42,43,44,45,46,49]. A study carried out by Yu et al. in 2016 [39] investigated the cytotoxic potential of EC condensate exposure in human oral keratinocytes and head and neck squamous cell carcinoma (HNSCC) lines via several different assays and control exposure to determine nicotine-independent effects of EC exposure. They reported similar levels of DNA strand breaks, apoptosis, and decreased viability in both cell lines, as well as increased HNSCC resistance to chemotherapeutic treatment [39]. Manyanga et al. studied the chemotherapeutic resistance of HNSCC to platinum-based treatment and reported similar results [46]. Sancilio et al. contributed two studies in consideration of the effects of NFEC exposure on human gingival fibroblasts and reported similar rates of geno- and cytotoxicity in both NC and NF groups in both studies [40,41].
NFECs also impaired wound healing and cell migration. Alanzi et al. (2018) observed reduced fibroblast proliferation and delayed wound closure [42], while Rouabhia et al. (2019) reported reduced adhesion and mineralization of osteoblasts on titanium implants, suggesting a potential negative impact on dental implant integration [43]. A study performed by Tsai et al. in 2020 [44] sought to investigate how different flavours may interact with human oral cell lines and found a spectrum of effects. Some flavours were correlated with decreased neoplastic invasion, whereas others enhanced it. Beklen et al. (2021) [45] examined the effects of unflavoured nicotine-free e-liquid base constituents, PG and VG, on gingival epithelial cells. Increased cytotoxicity was found in all groups, but it was most pronounced with ECs containing more PG than VG. In vivo data were limited. Tolba et al. (2023) observed epithelial changes and taste bud damage in rats injected with NFEC liquids, although the non-physiological exposure route limits generalizability [48]. Ma et al. (2024) confirmed NFEC-induced cytotoxicity and DNA damage in oral keratinocytes, with effects comparable to acrolein exposure [49]. A rare pilot human study by Vamos et al. (2024) found no significant changes in palatal blood flow following acute exposure to NFECs, although long-term effects were not assessed [50].
Two microbiological studies were included. A study carried out by Haghighi et al. (2022) on C. albicans found that while nicotine enhanced C. albicans virulence and biofilm formation, NFECs had a minimal effect, suggesting that nicotine plays a more prominent role in fungal pathogenicity [47]. Lee-Scot Beverly et al. (2025) showed that NFEC aerosols are metabolized by the oral microbiome, with alterations in microbial metabolism, gene expression, and biofilm structure in human saliva samples [51].
3.2. NFEC and Liver Health and Function
Table 3 summarizes the current evidence regarding the impact of NFECs and liver health and function [52,53,54,55,56,57]. Five in vivo studies performed on rodent models demonstrated that NFECs may alter hepatic metabolism. Golli et al. compared the effects of both NC and NFEC exposure, as well as nicotine alone, on hepatic parameters, lipid peroxidation, and antioxidant activity [52]. They found altered hepatic metabolism, as evidenced by increases in liver function test (LFT) probes, enzyme activity, and lipid peroxidation in all groups, which were more pronounced in EC groups than those with nicotine alone. Nicotine presence in ECs worsened the histopathological outcomes. Chen et al. and Li et al. performed studies on dams and their offspring to identify how EC exposure may affect metabolism during and after pregnancy [53,54]. Both studies found signs of altered lipid metabolism, increased body fat percentage, and liver steatosis. Li et al. compared the effects with and without nicotine and found more pronounced liver steatosis in the NF group compared to the NC group, suggesting a potential protective effect of nicotine in counteracting liver steatosis induced by EC exposure. Rickard et al. performed a study on hepatocellular carcinoma G2 cells (HepG2) to identify the effects of different common flavourings found in EC liquids, as well as their base constituents [55]. They found chronic exposure to EC liquids decreased HepG2 cell viability. Chen et al. performed additional studies to compare the effects of a high-fat diet (HFD) to EC exposure and concluded EC exposure may induce more potent changes, promoting systemic inflammation, particularly in the liver [56].

Table 3.
NFEC and liver health and function.
3.3. NFEC and Gastrointestinal Microbiome
Table 4 presents the current available data on the effects of NCEFs on the gastrointestinal microbiome [58,59]. Two studies were identified as relevant for this review. Sharma et al. performed an in vitro study using murine and human enteroid-derived cells to examine the effects of EC use on the gut barrier. They found that chronic NFEC exposure may promote inflammation and reduce tight junction expression. Co-culture of these cell lines with bacteria showed signs of barrier disruption. A study carried out on human neutrophils by Jasper et al. showed EC exposure may significantly alter neutrophil function, reducing their phagocytic potential as evidenced by their reduced phagocytosis of E. coli and promoting excessive filamentous actin polymerization [59].

Table 4.
NFEC and gastrointestinal microbiome.
3.4. Nicotine-Free E-Cigarettes and Implications for Gastrointestinal Health
Figure 2 visually represents the effects implied in the studies included in this review. While most findings are based on in vitro or animal models, the consistency of harmful cellular responses across organ systems raises concerns about the long-term safety of NFECs. These results underscore the need for more human studies and mechanistic investigations to clarify dose–response relationships, exposure thresholds, and the role of specific e-liquid constituents in gastrointestinal toxicity.
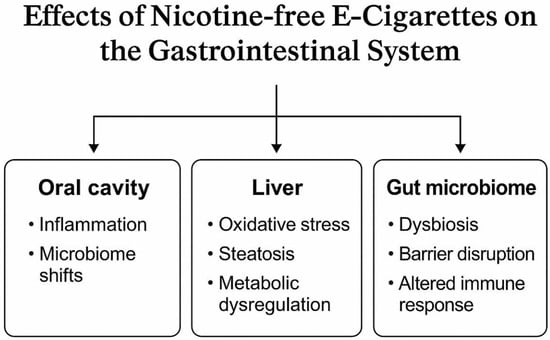
Figure 2.
Effects of NFECs on the gastrointestinal system.
4. Discussion
This systematic review provides a comprehensive synthesis of the existing evidence on the effects of nicotine-free electronic cigarette (NFEC) exposure on the gastrointestinal tract (GIT), encompassing oral health, microbial homeostasis, epithelial function, and metabolic processes. While the current body of literature remains limited, the findings suggest that NFECs are not biologically inert and may exert adverse effects on multiple levels of gastrointestinal function, even in the absence of nicotine.
The most consistent evidence emerged in relation to the oral cavity, which is the GIT region most directly exposed to EC aerosols. Several in vitro studies demonstrated that exposure to NFEC vapour leads to increased production of inflammatory mediators (IL-6, IL-8), reduced cell viability, and impaired intercellular signalling in gingival and epithelial cells. These effects were comparable to those observed with nicotine-containing e-cigarettes, supporting the hypothesis that e-liquid base constituents such as propylene glycol, vegetable glycerine, and flavouring agents may independently contribute to mucosal irritation and cytotoxicity. Notably, these effects were observed even with unflavoured formulations, suggesting that base solvents alone may have pro-inflammatory potential.
Alterations in oral microbiota were also observed, with in vitro and ex vivo studies reporting increased biofilm formation, shifts in bacterial metabolic activity, and changes in gene expression linked to xenobiotic degradation and quorum sensing. These microbial changes may have downstream implications for oral and systemic health, including enhanced risk of caries, gingivitis, and disruption of host–microbiome homeostasis. Such dysbiosis could extend beyond the oral cavity, potentially affecting the entire gastrointestinal ecosystem through microbial migration or inflammatory signalling.
The evidence regarding the effects of NFECs on the liver and intestinal segments of the GIT remains sparse and heterogeneous. Preliminary in vivo animal studies have indicated potential hepatic enzyme induction, oxidative stress, and metabolic dysregulation following chronic NFEC exposure. However, inconsistencies in study design, exposure protocols, and outcome measures preclude firm conclusions. Similarly, while some animal studies have demonstrated histological alterations in intestinal tissues and changes in gut microbiota composition, these findings require replication and validation in human models.
Importantly, only one human study met the inclusion criteria, highlighting a significant gap in clinical research on NFECs and gastrointestinal health. This paucity of clinical data limits the translational relevance of preclinical findings and underscores the need for carefully designed human studies, particularly longitudinal and interventional research, to assess both acute and chronic impacts. An important limitation of the present review is that only one eligible human pilot study was identified, with the majority of included research being preclinical in nature. While in vitro and animal studies provide valuable mechanistic insights, their findings cannot be directly extrapolated to human health outcomes due to species differences, controlled exposure conditions, and the use of supraphysiological concentrations in some experiments. This translational gap highlights the urgent need for well-designed, adequately powered longitudinal and interventional studies in diverse human populations to assess both the short- and long-term effects of nicotine-free e-cigarettes on gastrointestinal health.
The results of this review also raise broader concerns regarding regulatory oversight and public perception. NFECs are often marketed as harmless alternatives due to the absence of nicotine, despite accumulating evidence that non-nicotine constituents may exert biological effects of their own. The current regulatory landscape, particularly in regions where NFECs fall outside established tobacco control frameworks, may contribute to underestimation of risk among users—especially adolescents and never-smokers. Given the rapid evolution of vaping technologies and formulations, there is an urgent need for regulatory harmonization, safety testing standards, and transparent labelling practices.
There are several limits to this systematic review. There is a lack of cohort studies on human health, preclinical trials, and observational and interventional studies regarding the impact of NFECs on the GIT, as well as a clear differentiation of NC- and NFEC effects. Commonly confounding factors, such as alcohol and traditional tobacco use, diminish adequate quantification and quality of data. In our review, although several studies examined the effects of NFEC liquid exposure, only three specified the PG/VG ratio used in these e-liquid base constituents. The exact constitution of EC liquids is commonly omitted from both consumers and researchers. Currently, only nicotine content is most commonly displayed, and even then, some ECs labelled as NF, particularly non-refillable ECs, may contain nicotine [60]. The lack of regulation and standardization of EC liquid contents, as well as the ability of customization of both heating coils and EC liquids, poses an additional and unprecedented risk to human health [61,62,63]. Additionally, some consumers resort to constructing their own EC liquids, most commonly combining PG and VG in various ratios with the addition of flavours and nicotine on their own terms.
To our knowledge, this is the first systematic review to specifically evaluate the effects of nicotine-free e-cigarette exposure on the gastrointestinal system. The inclusion of both in vitro and in vivo studies allows for a broad yet focused overview of the current evidence. However, several limitations must be acknowledged. The overall number of eligible studies was low, and heterogeneity in experimental designs limited the possibility of meta-analysis. Furthermore, many in vitro studies used supraphysiological concentrations of e-liquids or isolated components, potentially overestimating biological effects. The lack of standardized exposure protocols and variability in cell models and outcome measures also complicates direct comparison between studies.
5. Conclusions
This systematic review demonstrates that NFECs are capable of inducing biological effects within the gastrointestinal system, despite the absence of nicotine. The evidence, though limited and primarily preclinical, suggests that components such as propylene glycol, vegetable glycerine, and flavouring agents may contribute to pro-inflammatory, cytotoxic, and microbiota-altering effects, particularly in the oral cavity. Findings from in vitro and in vivo models indicate that NFEC exposure can impair epithelial integrity, alter microbial metabolism, and potentially influence host–microbiome interactions. While data on deeper gastrointestinal segments, including the liver and intestines, remain scarce and heterogeneous, early signals of hepatic metabolic changes and intestinal dysbiosis warrant further investigation.
The prevailing assumption that nicotine-free products are inherently safe is not substantiated by current evidence. Given the increasing popularity of these products, especially among young and previously non-smoking individuals, a more cautious and evidence-based approach to their safety evaluation is essential. Current regulatory frameworks often fail to address the potential risks of NFECs, highlighting a pressing need for policy reassessment and targeted research.
Future Directions
A key limitation of the current evidence base is the near-complete absence of clinical data, with only one small human pilot study meeting our inclusion criteria. This represents a substantial translational gap, as findings from preclinical models—while critical for understanding underlying mechanisms—cannot be directly extrapolated to real-world human health outcomes due to differences in physiology, exposure patterns, and environmental factors. To address this gap, future research should prioritize well-designed, adequately powered longitudinal and interventional studies in diverse human populations. These studies should examine both acute and chronic gastrointestinal effects of NFEC exposure, use standardized exposure protocols, and assess clinically relevant endpoints such as gastrointestinal symptoms, biomarkers of inflammation, microbial diversity, and epithelial integrity.
In parallel, preclinical models require greater standardization in exposure protocols, substance concentrations, and outcome measures to improve comparability. Mechanistic studies are needed to clarify how e-liquid base constituents (propylene glycol, vegetable glycerine, flavourings) and their thermal degradation products interact with epithelial cells, immune pathways, and the gut microbiome. Given the interconnection of the gastrointestinal system, liver, and central nervous system, research should also address potential effects on the gut–liver–brain axis, with particular attention to microbial dysbiosis and systemic inflammation.
In addition, future studies should adopt a multidisciplinary approach, integrating expertise from toxicology, microbiology, gastroenterology, and public health to ensure a comprehensive evaluation of NFEC-related risks. Continuous monitoring of novel e-cigarette formulations, device technologies, and patterns of use will also be essential to capture emerging hazards and adapt research priorities accordingly.
Finally, research progress should be matched by policy action, including regulatory harmonization, clearer labelling, and transparent communication of potential risks—especially to youth and non-smokers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines13081998/s1, File S1: Complete search strategies for each database.
Author Contributions
Conceptualization, I.J.; methodology, I.J., I.M., and J.V.; resources, I.J., I.M., and J.V.; writing—original draft preparation, I.M.; I.J., and J.V.; writing—review and editing, I.J. and J.V.; supervision, I.J. and J.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CCL20 | C-C motif chemokine ligand 20 |
| CCL12 | C-C motif chemokine ligand 12 |
| CCL5 | C-C motif chemokine ligand 5 |
| CSC | Cigarette smoke condensate |
| CX3CL1 | C-X3-C motif chemokine ligand I |
| ECC | E-cigarette smoke condensate |
| EC | Electronic cigarette, e-cigarette |
| ENDS | Electronic nicotine delivery system |
| EU | European Union |
| EVALI | E-vaping-associated lung injury |
| FDA | Food and Drug Administration |
| GIT | Gastrointestinal tract |
| HFD | High-fat diet |
| HNSCC | Head and neck squamous cell carcinoma |
| HGF | Human gingival fibroblast |
| LFT | Liver function test |
| IFN-ɣ | Interferon gamma |
| IL-10 | Interleukin-10 |
| MMP | Matrix metalloproteinase |
| NC | Nicotine-containing |
| NF | Nicotine-free |
| NFEC | Nicotine-free e-cigarette |
| SCC | Squamous cell carcinoma |
| OSCC | Oral squamous cell carcinoma |
| PG | Propylene glycol |
| VG | Vegetable glycerine |
References
- Reitsma, M.B.; Fullman, N.; Ng, M.; Salama, J.S.; Abajobir, A.; Abate, K.H.; Abbafati, C.; Abera, S.F.; Abraham, B.; Abyu, G.Y.; et al. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: A systematic analysis from the Global Burden of Disease Study 2015. Lancet 2017, 389, 1885–1906. [Google Scholar] [CrossRef]
- Le Foll, B.; Piper, M.E.; Fowler, C.D.; Tonstad, S.; Bierut, L.; Lu, L.; Jha, P.; Hall, W.D. Tobacco and nicotine use. Nat. Rev. Dis. Primers 2022, 8, 19. [Google Scholar] [CrossRef]
- Sharma, R.; Rakshit, B. Global burden of cancers attributable to tobacco smoking, 1990–2019: An ecological study. EPMA J. 2022, 14, 167–182. [Google Scholar] [CrossRef]
- West, R. Tobacco smoking: Health impact, prevalence, correlates and interventions. Psychol. Health 2017, 32, 1018–1036. [Google Scholar] [CrossRef] [PubMed]
- Kotz, D.; Batra, A.; Kastaun, S. Smoking cessation attempts and common strategies employed. Dtsch. Arztebl. Int. 2020, 117, 7–13. [Google Scholar] [CrossRef]
- McAlinden, K.D.; Eapen, M.S.; Lu, W.; Sharma, P.; Sohal, S.S. The rise of electronic nicotine delivery systems and the emergence of electronic-cigarette-driven disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L585–L595. [Google Scholar] [CrossRef]
- Khanal, G.; Karna, A.; Kandel, S.; Sharma, H.K. Prevalence, correlates, and perception of e-cigarettes among undergraduate students of Kathmandu Metropolitan City, Nepal: A cross-sectional study. J. Smok. Cessat. 2023, 2023, 1330946. [Google Scholar] [CrossRef]
- Battelle Memorial Institute. Research Proposal Regarding Project Ariel; Battelle Memorial Institute: Columbus, OH, USA, 1962; Bates No. 301120592–301120600; Available online: https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=hlbx0199 (accessed on 10 July 2025).
- Gilbert, H.A. Smokeless Non-Tobacco Cigarette. U.S. Patent 3,200,819, 17 August 1965. [Google Scholar]
- Li, H. Non-Combustible Electronic Atomizing Cigarette. CN Patent 2643681, 29 September 2004. [Google Scholar]
- DeVito, E.E.; Krishnan-Sarin, S. E-cigarettes: Impact of e-liquid components and device characteristics on nicotine exposure. Curr. Neuropharmacol. 2018, 16, 438–459. [Google Scholar] [CrossRef]
- Gordon, T.; Karey, E.; Rebuli, M.E.; Escobar, Y.H.; Jaspers, I.; Chen, L.C. E-cigarette toxicology. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Fadus, M.C.; Smith, T.T.; Squeglia, L.M. The rise of e-cigarettes, pod mod devices, and JUUL among youth: Factors influencing use, health implications, and downstream effects. Drug Alcohol Depend. 2019, 201, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Mao, T.; Zhen, S.; Xu, Y.; Qu, C. Comparative analysis of e-cigarette prevalence and influencing factors among adolescents in Jiangsu Province, China. Front. Public Health 2023, 11, 1221334. [Google Scholar] [CrossRef]
- Gades, M.S.; Alcheva, A.; Riegelman, A.L.; Hatsukami, D.K. The role of nicotine and flavor in the abuse potential and appeal of electronic cigarettes for adult current and former cigarette and electronic cigarette users: A systematic review. Nicotine Tob. Res. 2022, 24, 1332–1343. [Google Scholar] [CrossRef]
- Davis, D.R.; Bold, K.W.; Camenga, D.; Kong, G.; Jackson, A.; Lee, J.; Rajesh-Kumar, L.; Krishnan-Sarin, S.; Morean, M.E. Use and product characteristics of “tobacco free nicotine” e-cigarettes among young adults. Nicotine Tob. Res. 2023, 25, 379–385. [Google Scholar] [CrossRef]
- Kinnunen, J.M.; Rimpelä, A.H.; Lindfors, P.L.; Clancy, L.; Alves, J.; Hoffmann, L.; Richter, M.; Kunst, A.E.; Lorant, V. Electronic cigarette use among 14- to 17-year-olds in Europe. Eur. J. Public Health 2021, 31, 402–408. [Google Scholar] [CrossRef]
- Davis, D.R.; Rajesh-Kumar, L.; Morean, M.E.; Kong, G.; Bold, K.W.; Krishnan-Sarin, S.; Camenga, D.E. Why young adults use tobacco-free nicotine e-cigarettes: An analysis of qualitative data. Addict. Behav. 2024, 150, 107925. [Google Scholar] [CrossRef] [PubMed]
- Vassey, J.; Valente, T.; Barker, J.; Stanton, C.; Li, D.; Laestadius, L.; Cruz, T.B.; Unger, J.B. E-cigarette brands and social media influencers on Instagram: A social network analysis. Tob. Control 2023, 32, e184–e191. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Schott, A.S.; Lee, J.; Dashtian, H.; Murthy, D. Understanding e-cigarette content and promotion on YouTube through machine learning. Tob. Control 2023, 32, 739–746. [Google Scholar] [CrossRef]
- Lyu, J.C.; Huang, P.; Jiang, N.; Ling, P.M. A systematic review of e-cigarette marketing communication: Messages, communication channels, and strategies. Int. J. Environ. Res. Public Health 2022, 19, 9263. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Kuguru, K.E.; Bhatti, H.; Sen, I.; Morean, M.E. Marketing content on e-cigarette brand-sponsored Facebook profile pages. Subst. Use Misuse 2021, 56, 442–448. [Google Scholar] [CrossRef]
- Kalkhoran, S.; Glantz, S.A. E-cigarettes and smoking cessation in real-world and clinical settings: A systematic review and meta-analysis. Lancet Respir. Med. 2016, 4, 116–128. [Google Scholar] [CrossRef]
- Park, J.Y.; Seo, D.C.; Lin, H.C. E-cigarette use and intention to initiate or quit smoking among US youths. Am. J. Public Health 2016, 106, 672–678. [Google Scholar] [CrossRef]
- Zhu, S.H.; Sun, J.Y.; Bonnevie, E.; Cummins, S.E.; Gamst, A.; Yin, L.; Lee, M. Four hundred and sixty brands of e-cigarettes and counting: Implications for product regulation. Tob. Control 2014, 23 (Suppl. S3), iii3–iii9. [Google Scholar] [CrossRef]
- Mayo-Wilson, E. Should e-cigarette regulation be based on randomized trials or observational studies? Am. J. Public Health 2021, 111, 219–220. [Google Scholar] [CrossRef]
- Center for Tobacco Products. Premarket Tobacco Product Applications; U.S. Food Drug Administration: Silver Spring, MD, USA, 7 September 2023. Available online: https://www.fda.gov/tobacco-products/market-and-distribute-tobacco-product/premarket-tobacco-product-applications (accessed on 14 July 2025).
- Center for Tobacco Products. Regulation and Enforcement of Non-Tobacco Nicotine (NTN) Products; U.S. Food and Drug Administration: Silver Spring, MD, USA, 6 February 2024. Available online: https://www.fda.gov/tobacco-products/products-ingredients-components/regulation-and-enforcement-non-tobacco-nicotine-ntn-products (accessed on 14 July 2025).
- European Commission. Electronic Cigarettes; European Commission: Brussels, Belgium, 2024; Available online: https://health.ec.europa.eu/tobacco/product-regulation/electronic-cigarettes_en (accessed on 14 July 2025).
- New York State Department of Health. New York State Department of Health Announces Statewide Ban of Flavored Nicotine Vapor Products Takes Effect Today; New York State Department of Health: Albany, NY, USA, 2020.
- Kowitt, S.; Meernik, C.; Baker, H.; Osman, A.; Huang, L.-L.; Goldstein, A.O. Perceptions and experiences with flavored non-menthol tobacco products: A systematic review of qualitative studies. Int. J. Environ. Res. Public Health 2017, 14, 338. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D., on New Enforcement Actions and a Youth Tobacco Prevention Plan to Stop Youth Use of, and Access to, JUUL and Other E-Cigarettes; US Food and Drug Administration: Silver Spring, MD, USA, 2018.
- Appleton, S.; Cyrus-Miller, H.; Seltzer, R.; Gilligan, K.; McKinney, W. Market survey of disposable e-cigarette nicotine content and e-liquid volume. BMC Public Health 2022, 22, 1760. [Google Scholar] [CrossRef] [PubMed]
- Etter, J.F.; Bugey, A. E-cigarette liquids: Constancy of content across batches and accuracy of labeling. Addict. Behav. 2017, 73, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Seiler-Ramadas, R.; Sandner, I.; Haider, S.; Grabovac, I.; Dorner, T.E. Health effects of electronic cigarette (e-cigarette) use on organ systems and its implications for public health. Wien. Klin. Wochenschr. 2021, 133, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Qazi, S.U.; Ansari, M.H.; Ghazanfar, S.; Ghazanfar, S.S.; Farooq, M. Comparison of acute effects of e-cigarettes with and without nicotine and tobacco cigarettes on hemodynamic and endothelial parameters: A systematic review and meta-analysis. High Blood Press. Cardiovasc. Prev. 2024, 31, 225–237. [Google Scholar] [CrossRef]
- Dai, Z.; Xie, B.; Jiang, C.; Peng, Y.; Lin, J.; Chen, Q.; Sun, J. Aerosolized nicotine-free e-liquid base constituents exacerbate mitochondrial dysfunction and endothelial glycocalyx shedding via the AKT/GSK3β-mPTP pathway in lung injury models. Respir. Res. 2025, 26, 82. [Google Scholar] [CrossRef]
- Gellatly, S.; Pavelka, N.; Crue, T.; Schweitzer, K.S.; Day, B.J.; Min, E.; Numata, M.; Voelker, D.R.; Scruggs, A.; Petrache, I.; et al. Nicotine-free e-cigarette vapor exposure stimulates IL6 and mucin production in human primary small airway epithelial cells. J. Inflamm. Res. 2020, 13, 175–185. [Google Scholar] [CrossRef]
- Yu, V.; Rahimy, M.; Korrapati, A.; Xuan, Y.; Zou, A.E.; Krishnan, A.R.; Tsui, T.; Aguilera, J.A.; Advani, S.; Crotty Alexander, L.E.; et al. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol. 2016, 52, 58–65. [Google Scholar] [CrossRef]
- Sancilio, S.; Gallorini, M.; Cataldi, A.; Di Giacomo, V. Cytotoxicity and apoptosis induction by e-cigarette fluids in human gingival fibroblasts. Clin. Oral Investig. 2016, 20, 477–483. [Google Scholar] [CrossRef]
- Sancilio, S.; Gallorini, M.; Cataldi, A.; Sancillo, L.; Rana, R.A.; Di Giacomo, V. Modifications in human oral fibroblast ultrastructure, collagen production and lysosomal compartment in response to e-cigarette fluids. J. Periodontol. 2017, 88, 673–680. [Google Scholar] [CrossRef]
- Alanazi, H.; Park, H.J.; Chakir, J.; Semlali, A.; Rouabhia, M. Comparative study of the effects of cigarette smoke and electronic cigarettes on human gingival fibroblast proliferation, migration and apoptosis. Food Chem. Toxicol. 2018, 118, 390–398. [Google Scholar] [CrossRef]
- Rouabhia, M.; Alanazi, H.; Park, H.J.; Gonçalves, R.B. Cigarette smoke and e-cigarette vapor dysregulate osteoblast interaction with titanium dental implant surface. J. Oral Implantol. 2019, 45, 421–426. [Google Scholar] [CrossRef]
- Tsai, K.Y.F.; Hirschi Budge, K.M.; Lepre, A.P.; Rhees, M.S.; Ajdaharian, J.; Geiler, J.; Epperson, D.G.; Astle, K.J.; Winden, D.R.; Arroyo, J.A.; et al. Cell invasion, RAGE expression, and inflammation in oral squamous cell carcinoma (OSCC) cells exposed to e-cigarette flavoring. Clin. Exp. Dent. Res. 2020, 6, 618–625. [Google Scholar] [CrossRef]
- Beklen, A.; Uckan, D. Electronic cigarette liquid substances propylene glycol and vegetable glycerin induce an inflammatory response in gingival epithelial cells. Hum. Exp. Toxicol. 2021, 40, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Manyanga, J.; Ganapathy, V.; Bouharati, C.; Mehta, T.; Sadhasivam, B.; Acharya, P.; Zhao, D.; Queimado, L. Electronic cigarette aerosols alter the expression of cisplatin transporters and increase drug resistance in oral cancer cells. Sci. Rep. 2021, 11, 1821. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, F.; Andriasian, L.; Tran, N.C.; Lux, R. Effect of Cigarette and E-Cigarette Smoke Condensates on Candida albicans Biofilm Formation and Gene Expression. Int. J. Environ. Res. Public Health 2022, 19, 4626. [Google Scholar] [CrossRef] [PubMed]
- Tolba, Y.M.; Omar, S.S.; El Hak, A.R.; Nagui, D.A. Electronic cigarettes can damage lingual papillae and taste buds. Can vitamins C and E supplementation reverse this damage? Life Sci. 2023, 329, 121955. [Google Scholar] [CrossRef]
- Ma, T.; Lee, A.; Eng, B.; Patel, V.; Michel, S.L.J.; Kane, M.A.; Dalby, R.; Schneider, A. Aerosolized e-liquid base constituents induce cytotoxicity and genotoxicity in oral keratinocytes. Oral Dis. 2025, 31, 482–491. [Google Scholar] [CrossRef]
- Vámos, O.; Kulcsár, N.; MikEC, B.; Kelemen, K.; Kaán, R.; Abafalvi, L.; Dinya, E.; Vág, J.; Hermann, P.; Kispélyi, B. Acute effects of traditional and electronic cigarettes on palatal blood flow in smokers: A cross-over pilot study. J. Oral Biol. Craniofac. Res. 2024, 14, 100580. [Google Scholar] [CrossRef] [PubMed]
- Beverly, M.L.S.; Chaudhary, P.P.; Dabdoub, S.M.; Kim, S.; Chatzakis, E.; Williamson, K.; Ganesan, S.M.; Yadav, M.; Ratley, G.; D’Souza, B.N.; et al. Toxic cultures: E-cigarettes and the oral microbial exposome. npj Biofilms Microbiomes 2025, 11, 66. [Google Scholar] [CrossRef]
- El Golli, N.; Jrad-Lamine, A.; Neffati, H.; Rahali, D.; Dallagi, Y.; Dkhili, H.; Ba, N.; El May, M.V.; El Fazaa, S. Impact of e-cigarette refill liquid with or without nicotine on liver function in adult rats. Toxicol. Mech. Methods 2016, 26, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, G.; Chan, Y.L.; Nguyen, T.; van Reyk, D.; Saad, S.; Oliver, B.G. Modulation of Neural Regulators of Energy Homeostasis, and of Inflammation, in the Pups of Mice Exposed to E-Cigarettes. Neurosci. Lett. 2018, 684, 61–66. [Google Scholar] [CrossRef]
- Li, G.; Chan, Y.L.; Wang, B.; Saad, S.; George, J.; Oliver, B.G.; Chen, H. E-Cigarettes Damage the Liver and Alter Nutrient Metabolism in Pregnant Mice and Their Offspring. Ann. N. Y. Acad. Sci. 2020, 1475, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Rickard, B.P.; Ho, H.; Tiley, J.B.; Jaspers, I.; Brouwer, K.L.R. E-Cigarette Flavoring Chemicals Induce Cytotoxicity in HepG2 Cells. ACS Omega 2021, 6, 6462–6470. [Google Scholar] [CrossRef]
- Chen, H.; Li, G.; Chan, Y.L.; Zhang, H.E.; Gorrell, M.D.; Pollock, C.A.; Saad, S.; Oliver, B.G. Differential Effects of ‘Vaping’ on Lipid and Glucose Profiles and Liver Metabolic Markers in Obese Versus Non-obese Mice. Front. Physiol. 2021, 12, 755124. [Google Scholar] [CrossRef]
- Chen, H.; Chan, Y.L.; Thorpe, A.E.; Pollock, C.A.; Saad, S.; Oliver, B.G. Inhaled or Ingested, Which Is Worse, E-Vaping or High-Fat Diet? Front. Immunol. 2022, 13, 913044. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, J.; Fonseca, A.G.; Moshensky, A.; Kothari, T.; Sayed, I.M.; Ibeawuchi, S.-R.; Pranadinata, R.F.; Ear, J.; Sahoo, D.; et al. E-Cigarettes Compromise the Gut Barrier and Trigger Inflammation. iScience 2021, 24, 102035. [Google Scholar] [CrossRef]
- Jasper, A.E.; Faniyi, A.A.; Davis, L.C.; Grudzinska, F.S.; Halston, R.; Hazeldine, J.; Parekh, D.; Sapey, E.; Thickett, D.R.; Scott, A. E-Cigarette Vapor Renders Neutrophils Dysfunctional Due to Filamentous Actin Accumulation. J. Allergy Clin. Immunol. 2024, 153, 320–329.e8. [Google Scholar] [CrossRef]
- Goniewicz, M.L.; Hajek, P.; McRobbie, H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: Regulatory implications. Addiction 2014, 109, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Haworth-Duff, A.; Parkes, G.M.B.; Reed, N.J. A simple approach to analysing trace metals in aerosols produced by e-cigarettes. Drug Test Anal. 2023, 15, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Neu, H.M.; Lee, A.; Brandis, J.E.P.; Patel, V.; Schneider, A.; Kane, M.A.; Dalby, R.N.; Michel, S.L.J. Cigalike electronic nicotine delivery systems e-liquids contain variable levels of metals. Sci. Rep. 2020, 10, 11907. [Google Scholar] [CrossRef] [PubMed]
- Nottage, M.K.; Taylor, E.V.; East, K.A.; McNeill, A.; Thrasher, J.F.; Reid, J.L.; Hammond, D.; Simonavičius, E. Packaging of disposable vaping products and e-liquids in England, Canada and the United States: A content analysis. Addiction 2025, 120, 483–495. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).