Atypical Mitochondrial Phenotype of Colonic CD4+ and CD8+ T Cells During Experimental Chronic Colitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice, Colitis Induction and Disease Scoring
2.2. Histopathology of Colon
2.3. Isolation of Lamina Propria Leukocytes from Colon
2.4. Flow Cytometry
2.5. Uptake of Glucose and Amino Acids
2.6. Measurement of mROS Production and Mitochondrial Volume
2.7. Expression of GLUT1, GLUT3 and CD36
2.8. Re-Analysis of Previously Published RNA-Seq Data
2.9. T Cell Culture and Oligomycin Treatment
2.10. Statistical Analysis
3. Results
3.1. Ulcerative Colitis Is Characterized by a Suppression of Mitochondrial Gene Expression
3.2. Dextran Sulfate Sodium-Induced Colitis as a Preclinical Mouse Model to Study Metabolic Characteristics in Colon Immune Cells
3.3. Abnormal Mitochondrial Phenotype of Colon Lamina Propria CD4+ and CD8+ T Cells During DSS-Induced Chronic Colitis Is Resolved During the Remission Phase
3.4. CD4+ and CD8+ T Cells Show Increased Glucose Uptake During Acute but Defective Glucose Consumption During Chronic DSS Colitis That Persists During Remission
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, N.; Bernstein, C.N. Environmental risk factors for inflammatory bowel disease. United Eur. Gastroenterol. J. 2022, 10, 1047–1053. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nóbrega, V.G.; Silva, I.N.N.; Brito, B.S.; Silva, J.; Silva, M.C.M.D.; Santana, G.O. The onset of clinical manifestations in inflammatory bowel disease patients. Arq. Gastroenterol. 2018, 55, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, T.H.; Allin, K.H.; Keefer, L.; Ananthakrishnan, A.N.; Jess, T. Depression and anxiety in inflammatory bowel disease: Epidemiology, mechanisms and treatment. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Schaubeck, M.; Clavel, T.; Calasan, J.; Lagkouvardos, I.; Haange, S.B.; Jehmlich, N.; Basic, M.; Dupont, A.; Hornef, M.; von Bergen, M.; et al. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut 2016, 65, 225–237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mentella, M.C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. Nutrition, IBD and Gut Microbiota: A Review. Nutrients 2020, 12, 944. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lakatos, P.L.; Fischer, S.; Lakatos, L.; Gal, I.; Papp, J. Current concept on the pathogenesis of inflammatory bowel disease-crosstalk between genetic and microbial factors: Pathogenic bacteria and altered bacterial sensing or changes in mucosal integrity take “toll”? World J. Gastroenterol. 2006, 12, 1829–1841. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gravina, A.G.; Panarese, I.; Trotta, M.C.; D’Amico, M.; Pellegrino, R.; Ferraraccio, F.; Galdiero, M.; Alfano, R.; Grieco, P.; Federico, A. Melanocortin 3,5 receptors immunohistochemical expression in colonic mucosa of inflammatory bowel disease patients: A matter of disease activity? World J. Gastroenterol. 2024, 30, 1132–1142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 2019, 20, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Petagna, L.; Antonelli, A.; Ganini, C.; Bellato, V.; Campanelli, M.; Divizia, A.; Efrati, C.; Franceschilli, M.; Guida, A.M.; Ingallinella, S.; et al. Pathophysiology of Crohn’s disease inflammation and recurrence. Biol. Direct 2020, 15, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zaiatz Bittencourt, V.; Jones, F.; Doherty, G.; Ryan, E.J. Targeting Immune Cell Metabolism in the Treatment of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2021, 27, 1684–1693. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.; Jeon, J.H.; Kim, E.S. Mitochondrial dysfunctions in T cells: Eocus on inflammatory bowel disease. Front. Immunol. 2023, 14, 1219422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, H.; Wang, L.; Liu, F. Immunological Impact of Intestinal T Cells on Metabolic Diseases. Front Immunol. 2021, 12, 639902, Erratum in Front Immunol. 2021, 12, 682376. https://doi.org/10.3389/fimmu.2021.682376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, C.H.; Park, J.; Kim, M. Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune Netw. 2014, 14, 277–288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, J.; Taitz, J.; Sun, S.M.; Langford, L.; Ni, D.; Macia, L. Your Regulatory T Cells Are What You Eat: How Diet and Gut Microbiota Affect Regulatory T Cell Development. Front. Nutr. 2022, 9, 878382. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal. Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cong, J.; Liu, P.; Han, Z.; Ying, W.; Li, C.; Yang, Y.; Wang, S.; Yang, J.; Cao, F.; Shen, J.; et al. Bile acids modified by the intestinal microbiota promote colorectal cancer growth by suppressing CD8+ T cell effector functions. Immunity 2024, 57, 876–889. [Google Scholar] [CrossRef] [PubMed]
- Hang, S.; Paik, D.; Yao, L.; Kim, E.; Trinath, J.; Lu, J.; Ha, S.; Nelson, B.N.; Kelly, S.P.; Wu, L.; et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature 2019, 576, 143–148, Erratum in Nature 2020, 579, E7. https://doi.org/10.1038/s41586-020-2030-5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Haberman, Y.; Karns, R.; Dexheimer, P.J.; Schirmer, M.; Somekh, J.; Jurickova, I.; Braun, T.; Novak, E.; Bauman, L.; Collins, M.H.; et al. Ulcerative colitis mucosal transcriptomes reveal mitochondriopathy and personalized mechanisms underlying disease severity and treatment response. Nat. Commun. 2019, 10, 38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schulz-Kuhnt, A.; Rühle, K.; Javidmehr, A.; Döbrönti, M.; Biwank, J.; Knittel, S.; Neidlinger, P.; Leupold, J.; Liu, L.J.; Dedden, M.; et al. ATP citrate lyase (ACLY)-dependent immunometabolism in mucosal T cells drives experimental colitis In Vivo. Gut 2024, 73, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gelmez, E.; Lehr, K.; Kershaw, O.; Frentzel, S.; Vilchez-Vargas, R.; Bank, U.; Link, A.; Schüler, T.; Jeron, A.; Bruder, D. Characterization of Maladaptive Processes in Acute, Chronic and Remission Phases of Experimental Colitis in C57BL/6 Mice. Biomedicines 2022, 10, 1903. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agrawal, M.; Jess, T. Implications of the changing epidemiology of inflammatory bowel disease in a changing world. United Eur. Gastroenterol. J. 2022, 10, 1113–1120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Windsor, J.W.; Kaplan, G.G. Evolving Epidemiology of IBD. Curr. Gastroenterol. Rep. 2019, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Higashiyama, M.; Hokari, R. New and Emerging Treatments for Inflammatory Bowel Disease. Digestion 2023, 104, 74–81, Erratum in Digestion 2023, 104, 164. [Google Scholar] [CrossRef] [PubMed]
- Novak, E.A.; Mollen, K.P. Mitochondrial dysfunction in inflammatory bowel disease. Front. Cell Dev. Biol. 2015, 3, 62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ho, G.T.; Theiss, A.L. Mitochondria and Inflammatory Bowel Diseases: Toward a Stratified Therapeutic Intervention. Annu. Rev. Physiol. 2022, 84, 435–459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peña-Cearra, A.; Song, D.; Castelo, J.; Palacios, A.; Lavín, J.L.; Azkargorta, M.; Elortza, F.; Fuertes, M.; Pascual-Itoiz, M.A.; Barriales, D.; et al. Mitochondrial dysfunction promotes microbial composition that negatively impacts on ulcerative colitis development and progression. npj Biofilms Microbiomes 2023, 9, 74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alarfaj, S.J.; Bahaa, M.M.; Elmasry, T.A.; Elberri, E.I.; El-Khateeb, E.; Hamouda, A.O.; Salahuddin, M.M.; Kamal, M.; Gadallah, A.A.; Eltantawy, N.; et al. Fenofibrate as an Adjunct Therapy for Ulcerative Colitis: Targeting Inflammation via SIRT1, NLRP3, and AMPK Pathways: A Randomized Controlled Pilot Study. Drug Des. Dev. Ther. 2024, 18, 5239–5253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Erol Doğan, Ö.; Karaca Çelik, K.E.; Baş, M.; Alan, E.H.; Çağın, Y.F. Effects of Mediterranean Diet, Curcumin, and Resveratrol on Mild-to-Moderate Active Ulcerative Colitis: A Multicenter Randomized Clinical Trial. Nutrients 2024, 16, 1504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bordt, E.A.; Clerc, P.; Roelofs, B.A.; Saladino, A.J.; Tretter, L.; Adam-Vizi, V.; Cherok, E.; Khalil, A.; Yadava, N.; Ge, S.X.; et al. The Putative Drp1 Inhibitor mdivi-1 Is a Reversible Mitochondrial Complex I Inhibitor that Modulates Reactive Oxygen Species. Dev. Cell 2017, 40, 583–594.e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al Amir Dache, Z.; Thierry, A.R. Mitochondria-derived cell-to-cell communication. Cell Rep. 2023, 42, 112728. [Google Scholar] [CrossRef] [PubMed]
- Spooner, R.; Yilmaz, O. The role of reactive-oxygen-species in microbial persistence and inflammation. Int. J. Mol. Sci. 2011, 12, 334–352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ballard, J.W.O.; Towarnicki, S.G. Mitochondria, the gut microbiome and ROS. Cell Signal. 2020, 75, 109737. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome-Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirose, M.; Sekar, P.; Eladham, M.W.A.; Albataineh, M.T.; Rahmani, M.; Ibrahim, S.M. Interaction between mitochondria and microbiota modulating cellular metabolism in inflammatory bowel disease. J. Mol. Med. 2023, 101, 1513–1526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Histological Changes | Score: 0 | Score: 1 | Score: 2 | Score: 3 | Score: 4 |
|---|---|---|---|---|---|
| Infiltration of immune cells | No inflammation | Around crypt base | Into mucosa | Extensive mucosal infiltration and edema | Into submucosa |
| Epithelial damage and loss of goblet cells | Intact | Slight loss of goblet cells | Considerable loss of goblet cells and slight loss of intestinal crypts | Extensive loss of intestinal crypts | |
| Extent | None | Mucosa | Mucosa and submucosa | Transmural | |
| Percent involvement | 1–25% | 26–50% | 51–75% | 76–100% |
| Conditions | Day | Histopathological Score | Adj. p-Value (Control Group) | % Tissue with Colon Inflammation | Adj. p-Value (Control Group) |
|---|---|---|---|---|---|
| Control young | 11 | 0 | 0 | ||
| Acute | 6 | 11.8 | 0.0018 (**) | 1.8 | 0.0596 (ns) |
| Remission acute | 25 | 5.9 | >0.9999 (ns) | 1.0 | >0.9999 (ns) |
| Control old | 56 | 0 | 0 | ||
| Chronic | 46 | 13.3 | <0.0001 (****) | 3.3 | <0.0001 (****) |
| Remission chronic | 67 | 7.8 | 0.1845 (ns) | 3 | 0.0003 (***) |
| Acute Colitis | Remission Acute | Chronic Colitis | Remission Chronic | |||||
|---|---|---|---|---|---|---|---|---|
| Mitochondrial ROS production | CD4+ T |  | CD4+ T |  | CD4+ T |  | CD4+ T |  |
| CD8+ T |  | CD8+ T |  | CD8+ T |  | CD8+ T |  | |
| B |  | B |  | B |  | B |  | |
| Neut. |  | Neut. |  | Neut. |  | Neut. |  | |
| Eos. |  | Eos. |  | Eos. |  | Eos. |  | |
| Mitochondrial volume | CD4+ T |  | CD4+ T |  | CD4+ T |  | CD4+ T |  |
| CD8+ T |  | CD8+ T |  | CD8+ T |  | CD8+ T |  | |
| B |  | B |  | B |  | B |  | |
| Neut. |  | Neut. |  | Neut. |  | Neut. |  | |
| Eos. |  | Eos. |  | Eos. |  | Eos. |  | |
| Glucose consumption | CD4+ T | 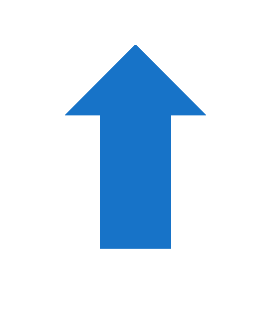 | CD4+ T |  | CD4+ T |  | CD4+ T |  |
| CD8+ T |  | CD8+ T |  | CD8+ T |  | CD8+ T |  | |
| B |  | B |  | B |  | B | 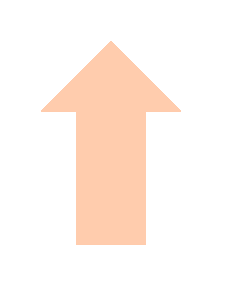 | |
| Neut. |  | Neut. |  | Neut. |  | Neut. | 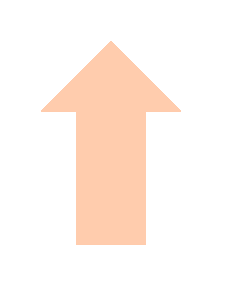 | |
| Eos. |  | Eos. |  | Eos. |  | Eos. | 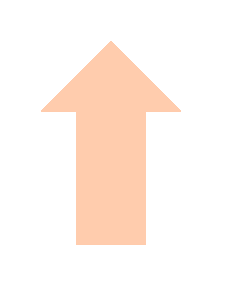 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negele, J.; Franz, T.; Krone, A.; Roder, M.; Gelmez, E.; Jantz-Naeem, N.; Kershaw, O.; Keitel-Anselmino, V.; Jeron, A.; Bruder, D.; et al. Atypical Mitochondrial Phenotype of Colonic CD4+ and CD8+ T Cells During Experimental Chronic Colitis. Biomedicines 2025, 13, 2094. https://doi.org/10.3390/biomedicines13092094
Negele J, Franz T, Krone A, Roder M, Gelmez E, Jantz-Naeem N, Kershaw O, Keitel-Anselmino V, Jeron A, Bruder D, et al. Atypical Mitochondrial Phenotype of Colonic CD4+ and CD8+ T Cells During Experimental Chronic Colitis. Biomedicines. 2025; 13(9):2094. https://doi.org/10.3390/biomedicines13092094
Chicago/Turabian StyleNegele, Jonas, Tobias Franz, Anna Krone, Marc Roder, Elif Gelmez, Nouria Jantz-Naeem, Olivia Kershaw, Verena Keitel-Anselmino, Andreas Jeron, Dunja Bruder, and et al. 2025. "Atypical Mitochondrial Phenotype of Colonic CD4+ and CD8+ T Cells During Experimental Chronic Colitis" Biomedicines 13, no. 9: 2094. https://doi.org/10.3390/biomedicines13092094
APA StyleNegele, J., Franz, T., Krone, A., Roder, M., Gelmez, E., Jantz-Naeem, N., Kershaw, O., Keitel-Anselmino, V., Jeron, A., Bruder, D., & Kahlfuß, S. (2025). Atypical Mitochondrial Phenotype of Colonic CD4+ and CD8+ T Cells During Experimental Chronic Colitis. Biomedicines, 13(9), 2094. https://doi.org/10.3390/biomedicines13092094






