Effectiveness of Exosomes from Different Mesenchymal Stem Cells in the Treatment of Psoriasis: A Murine Study and Meta-Analysis of Experimental Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
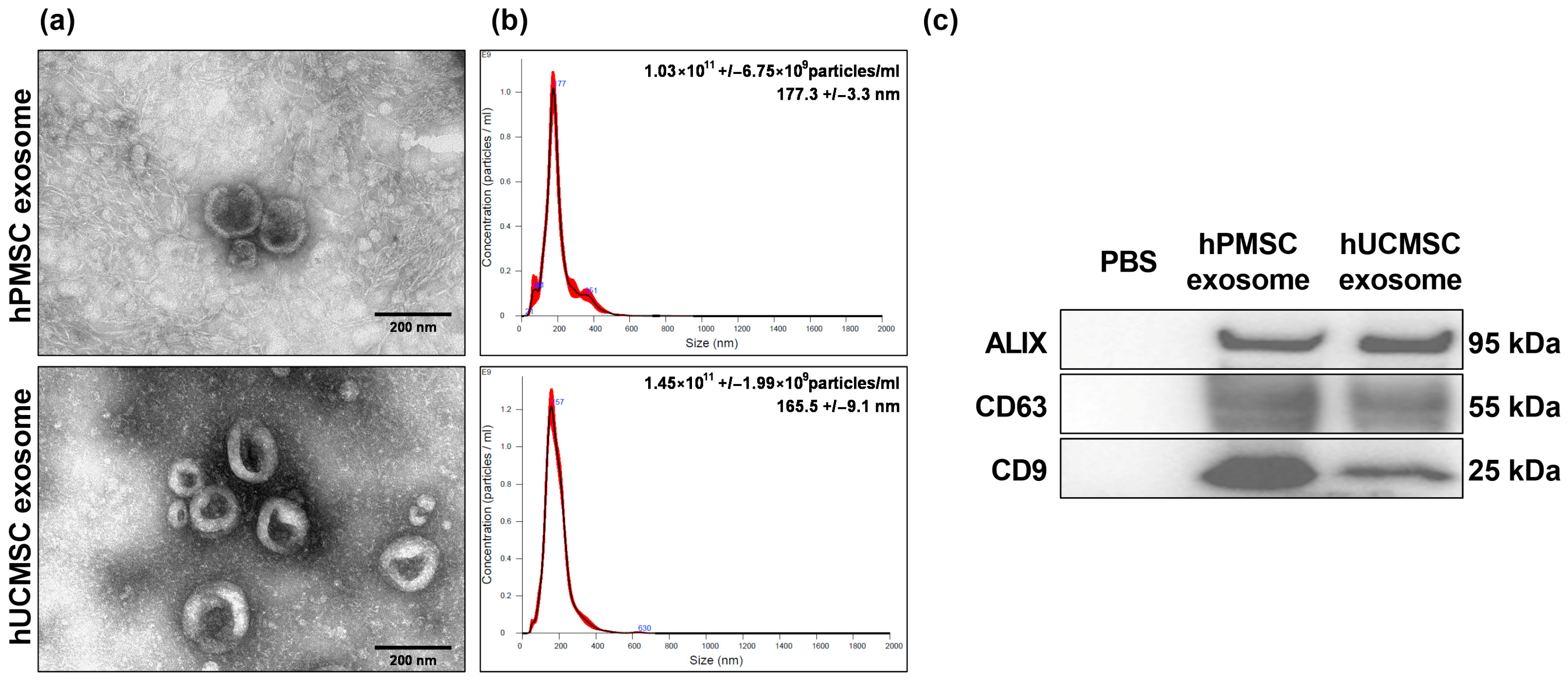
2.1.1. Isolation and Purification of MSC Exosomes
2.1.2. Sizing and Morphology Analyses of MSC Exosomes
2.1.3. Marker Analysis of MSC Exosomes Using Immunoblotting Assays
2.1.4. Animals and Protocol
2.1.5. Psoriasis Area and Severity Index Scores of Animals
2.1.6. Histological Analysis
2.1.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.1.8. Statistical Analysis
2.2. Systematic Review and Meta-Analysis
2.2.1. Data Sources and Search Strategy
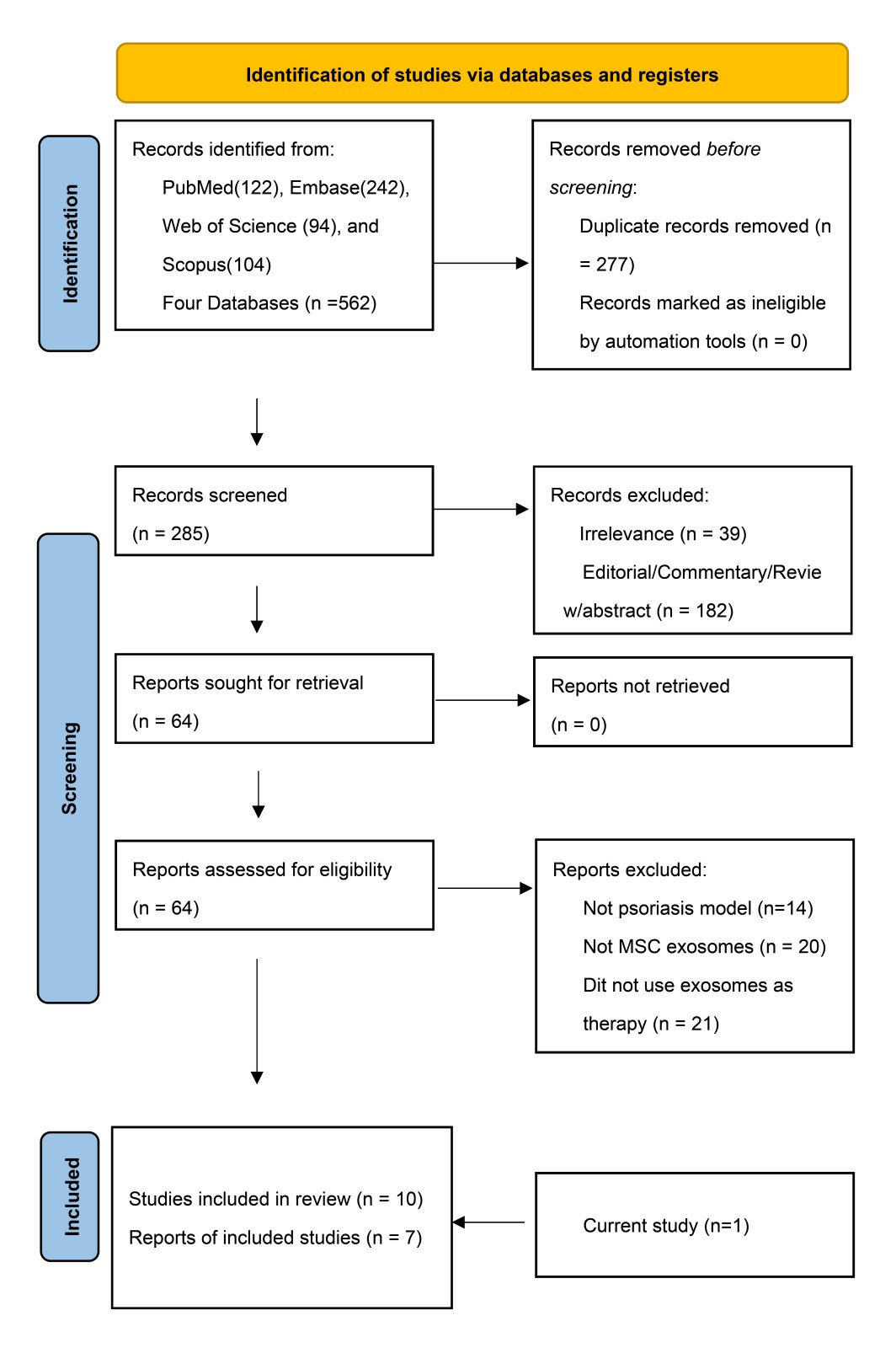
2.2.2. Eligibility Criteria and Study Selection
2.2.3. Quality Assessment
2.2.4. Data Extraction and Definition
| Author | Study Type and Inclusion Criteria | Age (Years) | Origin of MSC-Exo | Grouping and Sample Size | Route of Exo | Protocol | Dosage | Outcome Assessment a | Major Findings |
|---|---|---|---|---|---|---|---|---|---|
| Meybodi et al. [28], 2024 | Phase I/II trial Age > 18 years PASI score 3–10 Duration > 6 M | 36.6 ± 8.07 | Human adipose MSC-Exo | Exo 50: 4 Exo 100: 4 Exo 200: 4 | Intradermal | Single dosage | 50, 100, 200 μg/mL per cm2 | 3 M A, B, C | A single dose of 200 μg significantly improved clinical symptoms and regulated inflammatory and anti-inflammatory markers. |
| Author | Psoriasis Model | Animals | Origin of MSC-Exo | Grouping and Sample Size | Route of Exo | Protocol | Daily Dosage/Mouse | Outcome Assessment a | Major Findings |
|---|---|---|---|---|---|---|---|---|---|
| Rodrigues et al. [23], 2021 | IMQ 6 D | 8–12 weeks, C57BL/6 mice | Human UCB-MNC-sEVs | IMQ: 6 IMQ+vehicle: 6 IMQ+UCB-MNC-sEVs: 6 | Topical (hydrogel) | IMQ D1–6 sEVs one hour after IMQ for D1–6 | 3 × 109 particles/cm2 | D 7 A, B, C, E | UCB-MNC-sEV significantly prevented or reversed acanthosis in IMQ-induced psoriasis and tendentially increased the number of Tregs in the skin. |
| Zhang et al. [22], 2021 | Expt1: IMQ 6 D Expt2: IMQ 3 D | 6–9 weeks, Balb/c male mice | Immortalized E1-MYC 16.3 human ESC-MSC-Exo | Expt1 IMQ+vehicle: 10 IMQ+MSC-Exo: 10 Expt2 IMQ+vehicle: 10 IMQ+MSC-Exo: 10 | Topical (cream) | Expt1: IMQ D1–6+ Exo D4–6 Expt2: IMQ D1–3+ Exo D4–10 | 100 µg/mL, 200 µL | Expt1 D7 Expt2 D11 A, D | MSC-Exo resulted in reduced C5b-9 and IL-17 in a mild model of psoriasis. |
| Xu et al. [27], 2022 | IMQ 4 D | 6–8 weeks, C57BL/6 female mice | Mouse bone marrow MSC-sEVs | IMQ: 6 IMQ+MSC-sEVs: 6 IMQ+MSC-sEVs@PD-L1: 6 IMQ+MSC-sEVs@PD-L1+anti-PD-L1: 6 | Intravenous | IMQ D1–4+ sEVs D1–4 | 50 µg | D5 A, B, C, E | MSC-sEVs-PD-L1 inhibited acanthosis, parakeratosis, and thickening of the stratum corneum and suppressed the inflammatory response by reducing immune cell infiltration, altering their phenotype, activating immunoregulatory cells, and regulating inflammatory cytokines in the skin and peripheral circulation. |
| Zhang et al. [24], 2022 (a) | IMQ 6 D | 8 weeks, C57BL/6 female mice | Human umbilical cord MSC-Exo | IMQ: 6 IMQ+PBS: 6 IMQ+MSC-Exo: 6 | Subcutaneous | IMQ D1–6+ Exo D0, D2, D4 | 50 µg | D7 A, B, D | MSC-Exo ameliorated psoriasis-like skin inflammation in mice by regulating the expression of IL-23 and IL-17 and inhibiting the maturation and activation of DCs. |
| Zhang et al. [25], 2022 (b) | IMQ 6 D | 6–9 weeks, Balb/c male mice | Human umbilical cord MSC-IFNγ-sEVs | IMQ+PBS: 5 IMQ+ MSC-IFNγ-sEVs: 5 IMQ+ASO210: 5 IMQ+ MSC-IFNγ-sEVs+ASO210: 5 IMQ+ MSC-IFNγ-sEVs@ASO210: 5 IMQ+Hal: 5 | Intradermal | IMQ D1–6+ sEVs D3–6 | 25 mg/kg | D7 A, B, C, E | IFNγ-MSC-sEVs reduced thickness, erythema, and scales of skin lesions, exhausted Th17 cells, increased Th2 cells, and reduced inflammatory cytokines. IFNγ-MSC-sEVs significantly improved the delivery efficiency and stability of ASO-210. |
| Zhou et al. [26], 2025 | IMQ 6 D | 8 weeks, C57BL/6 female mice | Human umbilical cord MSC-EVs | IMQ+PBS: 4 IMQ+nor-NOHA: 4 IMQ+MSC-EVs: 4 IMQ+nor@MSC-EVs: 4 IMQ+anti-IL17A: 4 | Intravenous | IMQ D1–6+ EVs D1, 3, 5 | 100 μg | D7 A, B, C, E | MSC-EVs and nor@MSC-EVs displayed notable improvements in skin lesions and a substantial decrease in Ki 67+ cells. nor@MSC-EVs were the most efficacious therapeutic option for mitigating psoriasis. |
| Huang et al. | IMQ 6D | 6–9 weeks, Balb/c mice | Human umbilical cord MSC-Exo Human placenta MSC-Exo | IMQ+vehicle: 6 IMQ+MSC-Exo (P): 6 IMQ+MSC-Exo (U): 6 | Topical (PBS) | IMQ D1–6+ Exo D4–10 | 1 × 108 particles in 25 µL PBS | D11 A, B, C, D | The hPMSC and hUCMSC exosomes both showed better effectiveness in reducing epidermal thickness and skin tissue cytokines than the controls, and no significant difference was observed between the two MSC exosomes. |
2.2.5. Data Synthesis and Statistical Analysis
3. Results
3.1. Experimental Results
3.1.1. Confirmation of MSC Exosomes
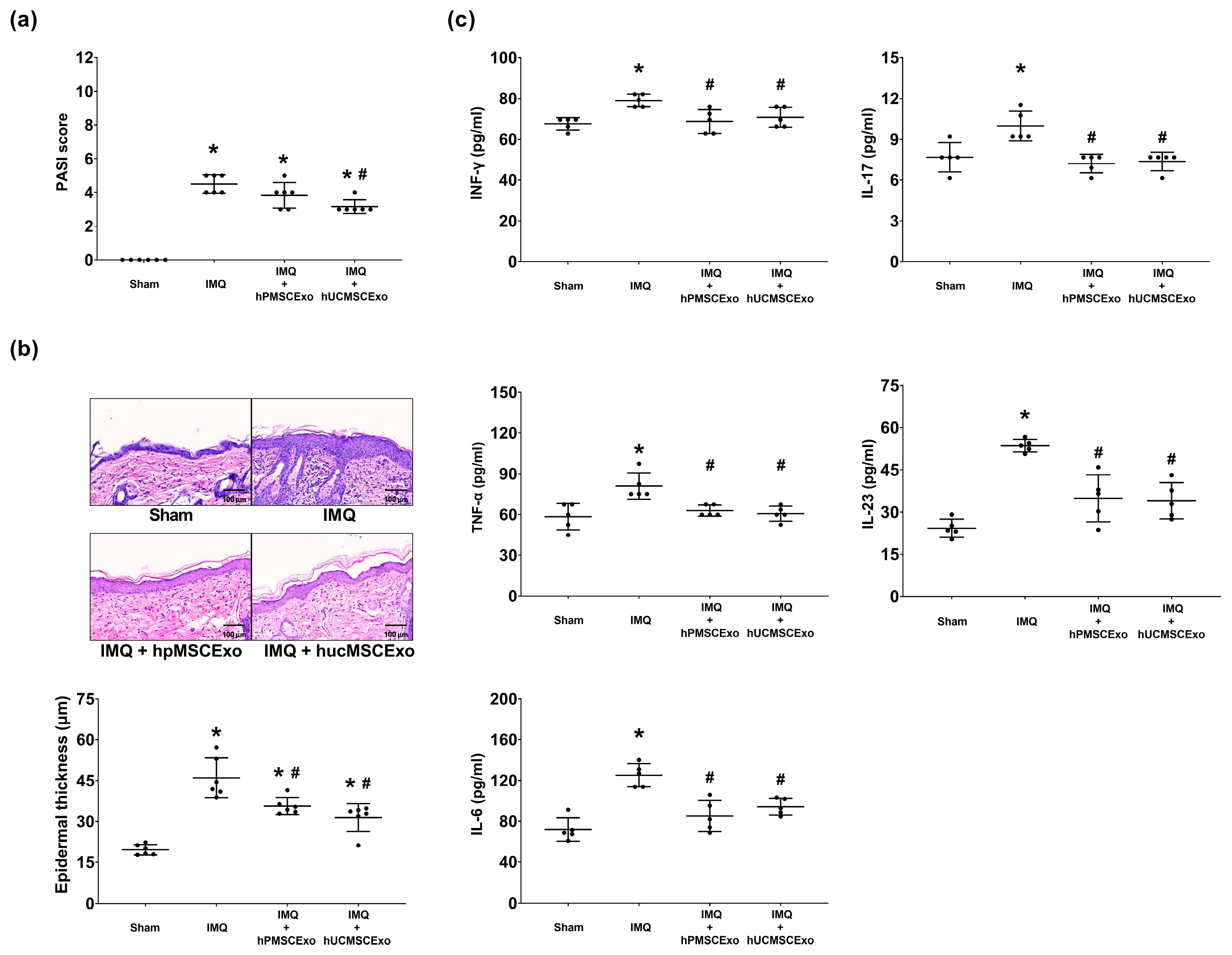
3.1.2. hUCMSC Exosomes Improved Clinical Severity Scores, and Both MSC Exosomes Reduced Epidermal Thickness in the IMQ-Induced Psoriasis Murine Model
3.1.3. Both the hUCMSC and hPMSC Exosomes Mitigated IMQ-Induced Cytokine Upregulation in Mouse Skin Tissues
3.2. Systematic Review and Meta-Analyses
3.2.1. Search Results and Trial Characteristics
| Author | Cell Model | Origin of MSC-Exo | Dosage | Effect of Exo |
|---|---|---|---|---|
| Rodrigues et al. [23], 2021 | THP-1 cells Human PBMCs | Human UCB-MNC sEVs | 1010 particles/mL | UCB-MNC-sEV were shown to shift macrophages toward an anti-inflammatory phenotype, which, in turn, exerted paracrine effects on fibroblasts. The incubation of PBMCs with UCB-MNC-sEV resulted in reduced total CD4+ and CD8+ T cell proliferation and cytokine release while specifically supporting the development of Treg by influencing FOXP3 expression. |
| Xu et al. [27], 2022 | LPS-treated BMDMs and BMDCs of mice AntiCD3/CD28-treated T cells from mouse lymph nodes | Mouse bone marrow MSC-sEVs and MSC-sEVs@PD-L1 | MSC-sEVs@PD-L1 altered the phenotype of various activated immune cells to an immunosuppressed state and inhibited inflammatory cytokine production. MSC-sEVs@PD-L1 resulted in T cell anergy, Treg induction, and effector T cell elimination. | |
| Zhang et al. [24], 2022 (a) | BMDCs of mice IL-17A-treated HaCaT cells | Human umbilical cord MSC-Exo | 2.5 μg/mL | Co-cultured with Exo, the maturation and activation of DCs were suppressed, and the expression level of IL-23 was decreased. Exo suppressed IL-23 and CCL20 secretion of HaCaT cells by inhibiting STAT3 activity. |
| Zhang et al. [25], 2022 (b) | Human PBMC CD3+ T cells from PBMCs | Human umbilical cord MSC-IFNγ-sEVs and MSC-IFNγ-sEVs@ASO210 | IFNγ-sEVs inhibited the proliferation and activation of PBMCs and T cells. | |
| Kim et al. [20], 2023 | Psoriasis serum-derived Exo-treated HaCaT cells | Human ADSC-Exo | 3.7 × 109/mL | ADSC-Exo suppressed pro-inflammatory cytokine and oxidative stress production and restored autophagy in HaCaT cells treated with psoriasis serum-derived exosomes. |
| Abed et al. [21], 2024 | HUVECs | MSC-Exo | 100 μM/mL | The concentration of the TGF-β2 gene in the target cells significantly increased following treatment with Exo. |
| Zhou et al. [26], 2025 | TNF-α- and IL-17A-treated HaCaT cells BMDCs of mice Splenic cells isolated from IMQ-induced mice | Human umbilical cord MSC-EVs and nor@MSC-EVs | 50 μg/mL | nor@MSC-EVs mitigated the psoriatic phenotype by inhibiting the expression of associated antimicrobial peptides, chemokines, cytokines, and inflammatory proteins, as well as those related to polyamine production and cell proliferation. MSC-EVs and nor@MSC-EVs directly inhibited the maturation of BMDCs and the differentiation of Th1 and Th17 in vitro, exerting direct immunomodulatory effects. |
3.2.2. Results of Quality Assessment
3.2.3. Systematic Review
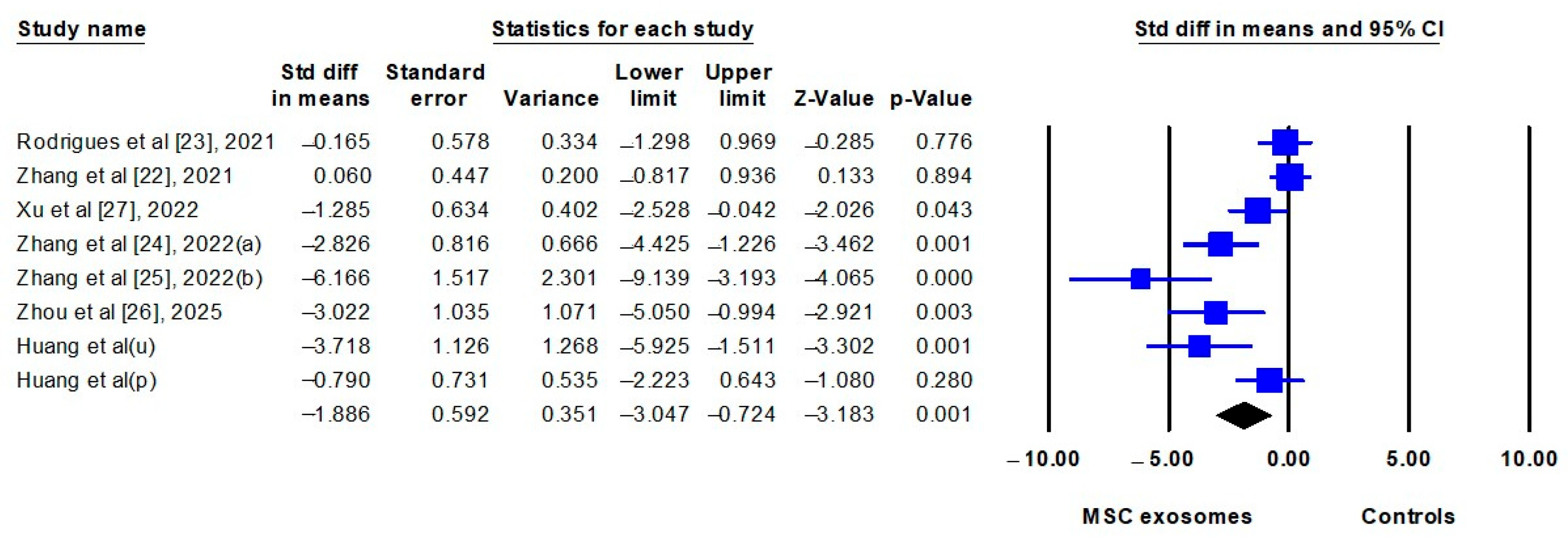
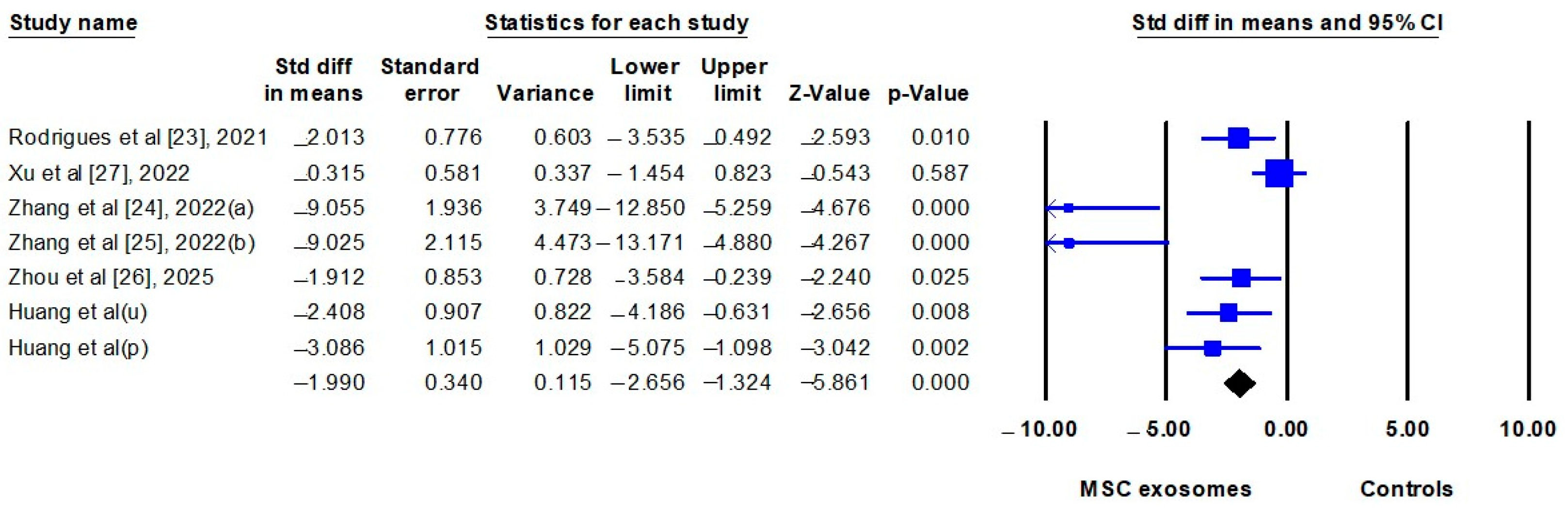
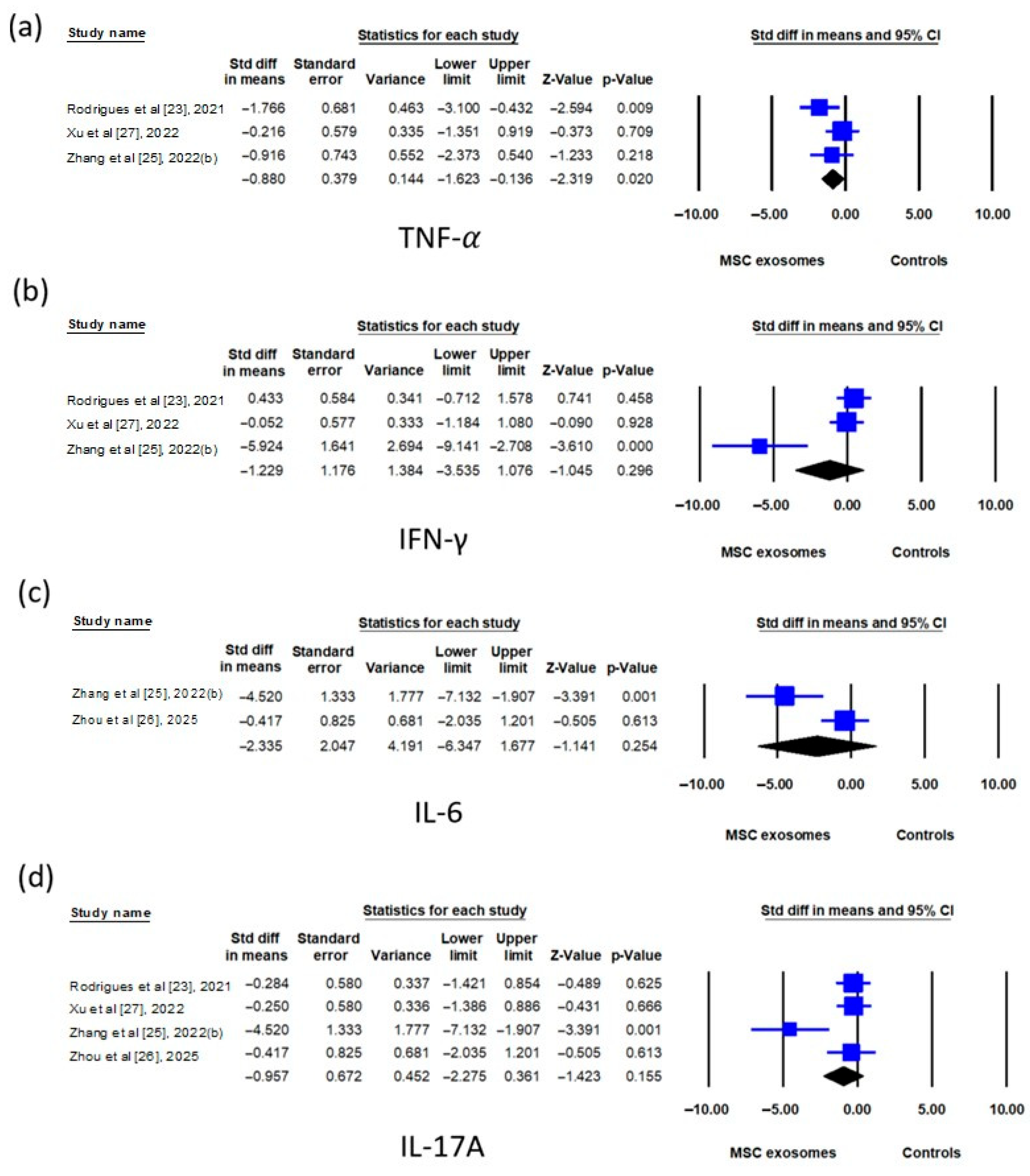
3.2.4. Meta-Analyses
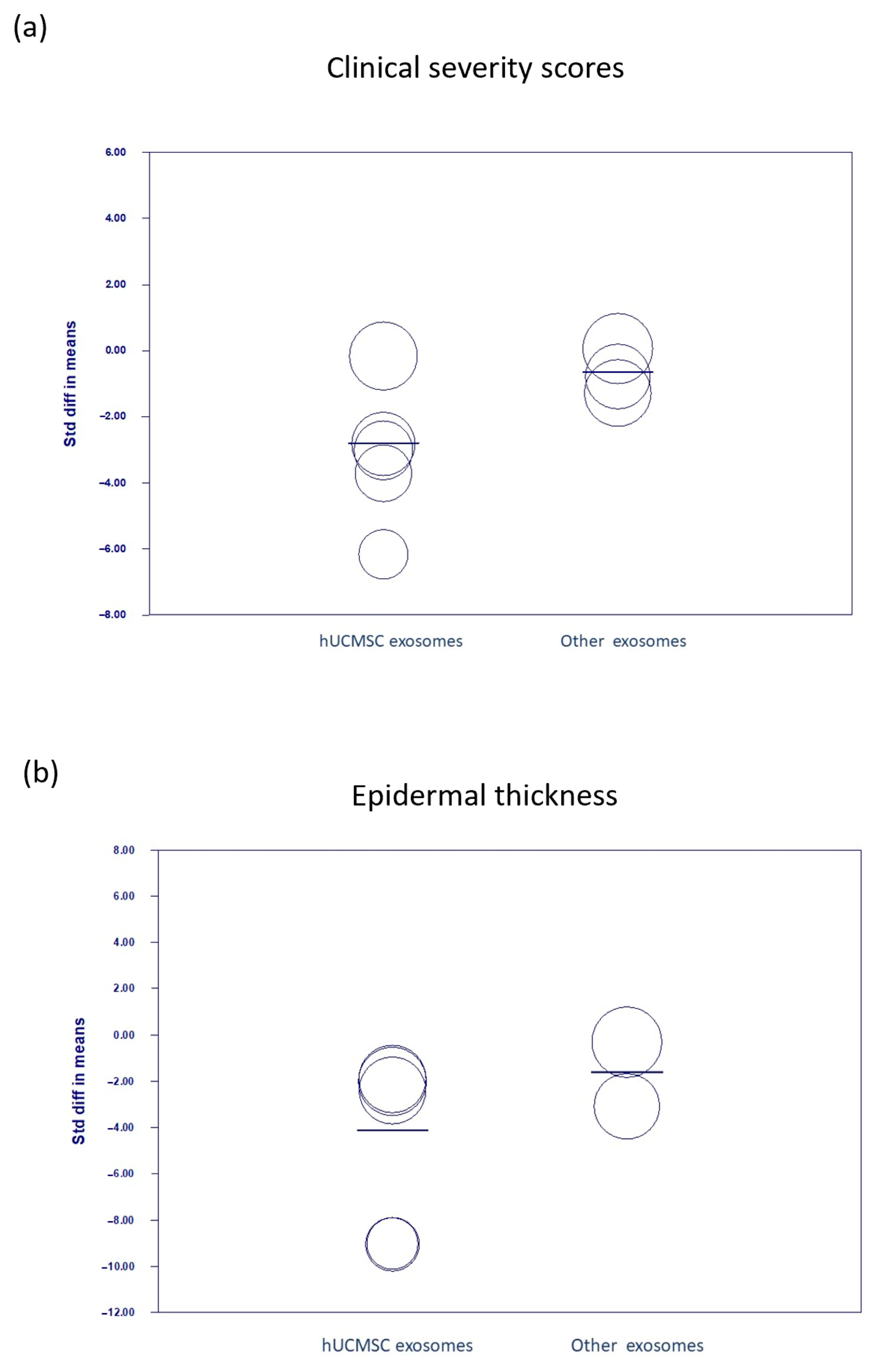
3.3. Comparing the Current Experimental Study with Meta-Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASO210 | antisense oligonucleotides targeting miR-210 |
| CI | confidence interval |
| DC | dendritic cell |
| ELISA | enzyme-linked immunosorbent assay |
| EVs | extracellular vesicles |
| hPMSC | human placenta-derived MSC |
| hUCMSC | human umbilical cord-derived MSC |
| IL | interleukin |
| IMQ | imiquimod |
| INF-γ | interferon-γ |
| miRNAs | microRNAs |
| MSC | mesenchymal stem cell |
| NTA | nanoparticle tracking analysis |
| nor-NOHA | arginase-1 inhibitor |
| PASI | psoriasis area and severity index |
| PD-L1 | programmed cell death-ligand 1 |
| PBMCs | peripheral blood mononuclear cells |
| sEVs | small extracellular vesicles |
| SMD | standardized mean difference |
| SYRCLE | Systematic Review Centre for Laboratory Animal Experimentation |
| TEM | transmission electron microscopy |
| TNF-α | tissue necrosis factor-α |
| ToxRTool | Toxicological Data Reliability Assessment Tool |
| Treg | regulatory T cell |
References
- Branisteanu, D.E.; Cojocaru, C.; Diaconu, R.; Porumb, E.A.; Alexa, A.I.; Nicolescu, A.C.; Brihan, I.; Bogdanici, C.M.; Branisteanu, G.; Dimitriu, A.; et al. Update on the etiopathogenesis of psoriasis. Exp. Ther. Med. 2022, 23, 201. [Google Scholar] [CrossRef]
- Raharja, A.; Mahil, S.K.; Barker, J.N. Psoriasis: A brief overview. Clin. Med. 2021, 21, 170–173. [Google Scholar] [CrossRef]
- Wozniak, E.; Owczarczyk-Saczonek, A.; Placek, W. Psychological stress, mast cells, and psoriasis-is there any relationship? Int. J. Mol. Sci. 2021, 22, 13252. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transpl. 2011, 20, 5–14. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Kou, Z.; Li, B.; Aierken, A.; Tan, N.; Li, C.; Han, M.; Jing, Y.; Li, N.; Zhang, S.; Peng, S.; et al. Mesenchymal stem cells pretreated with collagen promote skin wound-healing. Int. J. Mol. Sci. 2023, 24, 8688. [Google Scholar] [CrossRef]
- Daltro, S.R.T.; Meira, C.S.; Santos, I.P.; Ribeiro dos Santos, R.; Soares, M.B.P. Mesenchymal stem cells and atopic dermatitis: A review. Front. Cell Dev. Biol. 2020, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Niu, J.W.; Ning, H.M.; Pan, X.; Li, X.B.; Li, Y.; Wang, D.H.; Hu, L.D.; Sheng, H.X.; Xu, M.; et al. Treatment of psoriasis with mesenchymal stem cells. Am. J. Med. 2016, 129, e13–e14. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, S.; Peng, C.; Zou, X.; Yang, C.; Mei, H.; Li, C.; Su, X.; Xiao, N.; Ouyang, Q.; et al. Human umbilical cord mesenchymal stem cells for psoriasis: A phase 1/2a, single-arm study. Sig. Trunsduct. Target. Ther. 2022, 7, 263. [Google Scholar] [CrossRef]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transpl. 2019, 28, 801–812. [Google Scholar] [CrossRef]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 24. [Google Scholar] [CrossRef]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and safety issues of stem cell-based therapy. Int. J. Mol. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef]
- Mastrolia, I.; Foppiani, E.M.; Murgia, A.; Candini, O.; Samarelli, A.V.; Grisendi, G.; Veronesi, E.; Horwitz, E.M.; Dominici, M. Challenges in clinical development of mesenchymal stromal/stem cells: Concise review. Stem Cells Transl. Med. 2019, 8, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Riazifar, M.; Pone, E.J.; Lötvall, J.; Zhao, W. Stem cell extracellular vesicles: Extended messages of regeneration. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 125–154. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Shen, H.; Xie, F.; Hu, D.; Jin, Q.; Hu, Y.; Zhong, T. Role of mesenchymal stem cell-derived exosomes in the regeneration of different tissues. J. Biol. Eng. 2024, 18, 36. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem. Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef]
- Kim, H.R.; Lee, S.Y.; You, G.E.; Kim, H.O.; Park, C.W.; Chung, B.Y. Adipose-derived stem cell exosomes alleviate psoriasis serum exosomes-induced inflame mation by regulating autophagy and redox status in keratinocytes. Clin. Cosmet. Investig. Dermatol. 2023, 16, 3699–3711. [Google Scholar] [CrossRef]
- Abed, Z.I.; Arianejad, M.; Azizi, Z. Mesenchymal stem cell-derived exosomes decrease hyperplasia in psoriasis by inducing transforming growth factor β2 (TGF-β2). Mol. Biol. Rep. 2024, 51, 635. [Google Scholar] [CrossRef]
- Zhang, B.; Lai, R.C.; Sim, W.K.; Choo, A.B.H.; Lane, E.B.; Lim, S.K. Topical application of mesenchymal stem cell exosomes alleviates the imiquimod induced psoriasis-like inflammation. Int. J. Mol. Sci. 2021, 22, 720. [Google Scholar] [CrossRef]
- Rodrigues, S.C.; Cardoso, R.M.; Freire, P.C.; Gomes, C.F.; Duarte, F.V.; Neves, R.P.D.; Simões-Correia, J. Immunomodulatory properties of umbilical cord blood-derived small extracellular vesicles and their therapeutic potential for inflammatory skin disorders. Int. J. Mol. Sci. 2021, 22, 9797. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Li, Z.; Zheng, J.; Sun, Q. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate psoriasis-like skin inflammation. J. Interferon Cytokine Res. 2022, 42, 8–18. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, J.; Shi, P.; Su, D.; Cheng, X.; Yi, W.; Yan, J.; Chen, H.; Cheng, F. Small extracellular vesicles derived from MSCs have immunomodulatory effects to enhance delivery of ASO-210 for psoriasis treatment. Front. Cell Dev. Biol. 2022, 10, 842813. [Google Scholar] [CrossRef]
- Zhou, X.; Tang, B.; Huang, Q.; Yang, S.; Jiang, Y.; Xu, L.; Chen, W.; Shan, G.; Liao, X.; Hou, C.; et al. Engineered mesenchymal stem cell-derived extracellular vesicles scavenge self-antigens for psoriasis therapy via modulating metabolic and immunological disorders. Adv. Sci. 2025, 12, e2410067. [Google Scholar] [CrossRef]
- Xu, F.; Fei, Z.; Dai, H.; Xu, J.; Fan, Q.; Shen, S.; Zhang, Y.; Ma, Q.; Chu, J.; Peng, F.; et al. Mesenchymal stem cell-derived extracellular vesicles with high PD-L1 expression for autoimmune diseases treatment. Adv. Mater. 2022, 34, e2106265. [Google Scholar] [CrossRef] [PubMed]
- Meybodi, M.A.M.; Nilforoushzadeh, M.A.; KhandanDezfully, N.; Mansouri, P. The safety and efficacy of adipose tissue-derived exo somes in treating mild to moderate plaque psoriasis: A clinical study. Life Sci. 2024, 353, 122915. [Google Scholar] [CrossRef] [PubMed]
- Su, L.J.; Wu, M.S.; Hui, Y.Y.; Chang, B.M.; Pan, L.; Hsu, P.C.; Chen, Y.T.; Ho, H.N.; Huang, Y.H.; Ling, T.Y.; et al. Fluorescent nanodiamonds enable quantitative tracking of human mesenchymal stem cells in miniature pigs. Sci. Rep. 2017, 7, 45607. [Google Scholar] [CrossRef] [PubMed]
- Lobb, R.J.; Becker, M.; Wen Wen, S.; Wong, C.S.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Cheng, Y.; Fu, Y.; Zhao, H.; Tang, M.; Zhao, H.; Lin, N.; Shi, X.; Lei, Y.; et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res. Ther. 2020, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Hsu, H.J.; Foo, J.; Shih, H.J.; Huang, C.J. Peptide-Based TNF-α-binding decoy therapy mitigates lipopolysaccharide induced liver injury in mice. Pharmaceuticals 2020, 13, 280. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Schneider, K.; Schwarz, M.; Burkholder, I.; Kopp-Schneider, A.; Edler, L.; Kinsner-Ovaskainen, A.; Hartung, T.; Hoffmann, S. “ToxRTool”, a new tool to assess the reliability of toxicological data. Toxicol. Lett. 2009, 189, 138–144. [Google Scholar] [CrossRef]
- Rohatgi, A. WebPlotDigitizer; Pacifica: Rancho Cucamonga, CA, USA, 2021. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H. miRNAs flowing up and down: The concerto of psoriasis. Front. Med. 2021, 8, 646796. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zeng, J.; Yuan, J.; Deng, X.; Huang, Y.; Chen, L.; Zhang, P.; Feng, H.; Liu, Z.; Wang, Z.; et al. MicroRNA-210 overexpression promotes psoriasis-like inflammation by inducing Th1 and Th17 cell differentiation. J. Clin. Investig. 2018, 128, 2551–2568. [Google Scholar] [CrossRef]
- Andreas, K.; Sittinger, M.; Ringe, J. Toward in situ tissue engineering: Chemokine-guided stem cell recruitment. Trends Biotechnol. 2014, 32, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, D.; Fan, Q.; Ma, Q.; Dong, Z.; Tao, W.; Tao, H.; Liu, Z.; Wang, C. The enhanced permeability and retention effect based nanomedicine at the site of injury. Nano Res. 2020, 13, 564–569. [Google Scholar] [CrossRef]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef]
- Paicius, R.; White, Z.S.; Smith, C.; Lightner, A.L.; Ransom, J.T.; Lee, D.W.; Speare, S. Safety and efficacy of intravenous ExoFlo in the treatment of complex regional pain syndrome. Pain Physician 2023, 26, E851–E857. [Google Scholar] [CrossRef] [PubMed]

| Outcomes | Study Included | Effect Size | Effect Estimate (95% CI) | p-Value | I2 |
|---|---|---|---|---|---|
| Before incorporation | |||||
| Clinical severity scores | 6 | SMD | −1.852 (−3.240 to −0.464) | 0.009 | 82.3% |
| Epidermal thickness | 5 | SMD | −3.740 (−6.232 to −1.249) | 0.003 | 84.6% |
| Cytokines in skin tissues | |||||
| mRNA level | |||||
| TNF-α | 3 | SMD | −0.880 (−1.623 to −0.136) | 0.020 | 33.6% |
| IFN-γ | 3 | SMD | −1.229 (−3.535 to 1.076) | 0.296 | 85.1% |
| IL-6 | 2 | SMD | −2.335 (−6.347 to 1.677) | 0.064 | 85.4% |
| IL-17A | 4 | SMD | −0.957 (−2.275 to 0.361) | 0.155 | 67.9% |
| Protein level | |||||
| IL-17A | 3 | SMD | −1.175 (−3.593 to 1.244) | 0.341 | 84.5% |
| IL-23 | 2 | SMD | −7.471 (−24.659 to 9.718) | 0.394 | 92.0% |
| After incorporation | |||||
| Clinical severity scores | 7 | SMD | −1.886 (−3.047 to −0.724) | <0.001 | 79.5% |
| Epidermal thickness | 6 | SMD | −3.258 (−4.987 to −1.529) | <0.001 | 82.4% |
| Cytokines in skin tissues | |||||
| mRNA level | |||||
| TNF-α | 3 | SMD | −0.880 (−1.623 to −0.136) | 0.020 | 33.6% |
| IFN-γ | 3 | SMD | −1.229 (−3.535 to 1.076) | 0.296 | 85.1% |
| IL-6 | 2 | SMD | −2.335 (−6.347 to 1.677) | 0.064 | 85.4% |
| IL-17A | 4 | SMD | −0.957 (−2.275 to 0.361) | 0.155 | 67.9% |
| Protein level | |||||
| TNF-α | 2 | SMD | −1.789 (−3.668 to 0.090) | 0.062 | 71.0% |
| IFN-γ | 2 | SMD | −1.347 (−2.733 to 0.039) | 0.057 | 55.2% |
| IL-17A | 4 | SMD | −2.390 (−4.522 to −0.258) | 0.028 | 85.0% |
| IL-23 | 3 | SMD | −3.517 (−7.242 to 0.207) | 0.064 | 89.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-C.; Chang, C.-Y.; Huang, C.-J. Effectiveness of Exosomes from Different Mesenchymal Stem Cells in the Treatment of Psoriasis: A Murine Study and Meta-Analysis of Experimental Studies. Biomedicines 2025, 13, 2093. https://doi.org/10.3390/biomedicines13092093
Huang Y-C, Chang C-Y, Huang C-J. Effectiveness of Exosomes from Different Mesenchymal Stem Cells in the Treatment of Psoriasis: A Murine Study and Meta-Analysis of Experimental Studies. Biomedicines. 2025; 13(9):2093. https://doi.org/10.3390/biomedicines13092093
Chicago/Turabian StyleHuang, Yu-Chen, Chao-Yuan Chang, and Chun-Jen Huang. 2025. "Effectiveness of Exosomes from Different Mesenchymal Stem Cells in the Treatment of Psoriasis: A Murine Study and Meta-Analysis of Experimental Studies" Biomedicines 13, no. 9: 2093. https://doi.org/10.3390/biomedicines13092093
APA StyleHuang, Y.-C., Chang, C.-Y., & Huang, C.-J. (2025). Effectiveness of Exosomes from Different Mesenchymal Stem Cells in the Treatment of Psoriasis: A Murine Study and Meta-Analysis of Experimental Studies. Biomedicines, 13(9), 2093. https://doi.org/10.3390/biomedicines13092093








