Comparison of Anti-Obesity Effects of Ginger Extract Alone and Mixed with Long Pepper Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Standardization of Ginger and Long Pepper Extracts
2.2. Animals and Treatments
2.3. Fasting Blood Glucose Measurement
2.4. Oral Glucose Tolerance Test and Insulin Tolerance Test
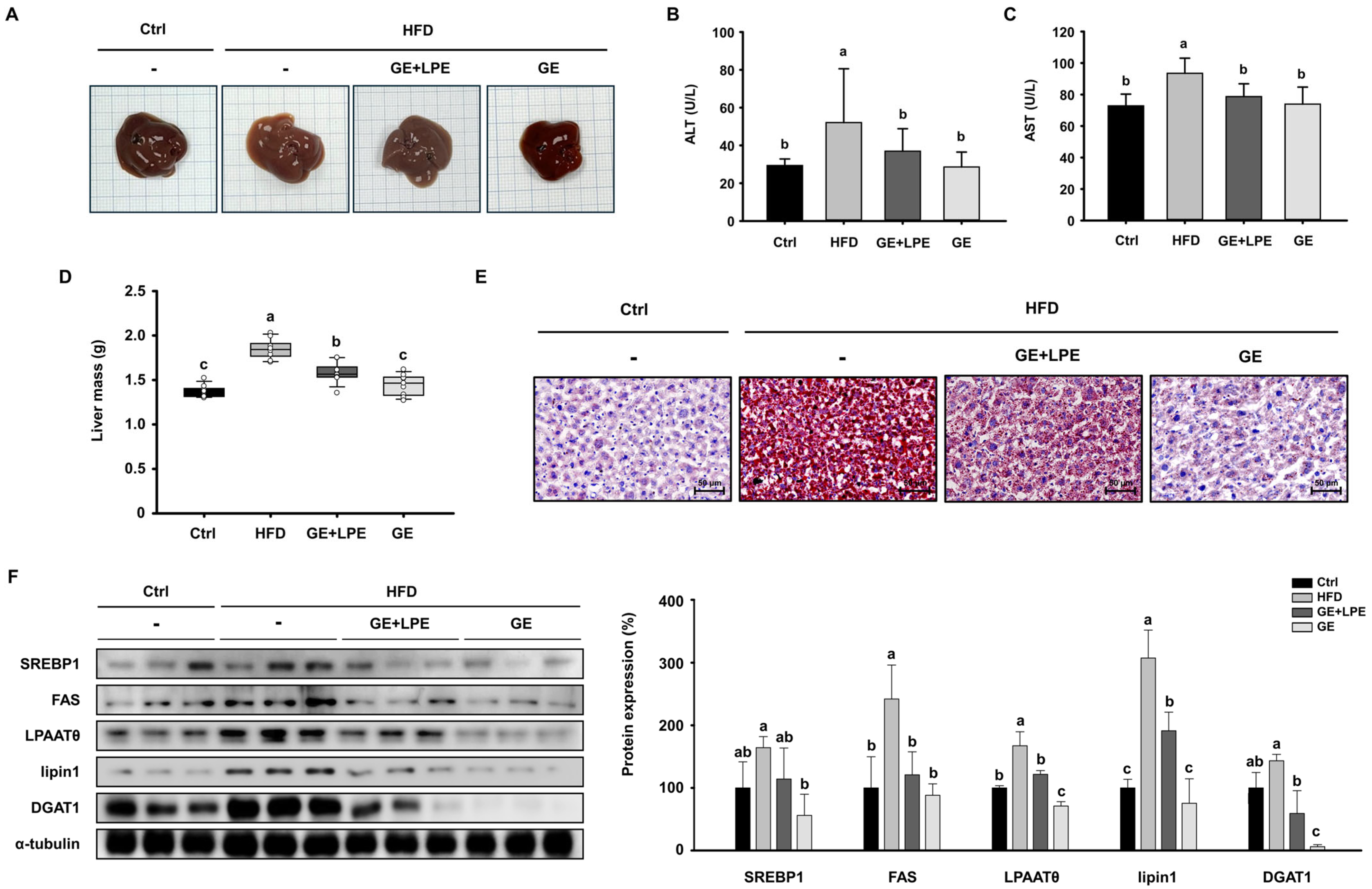
2.5. Histological Analysis
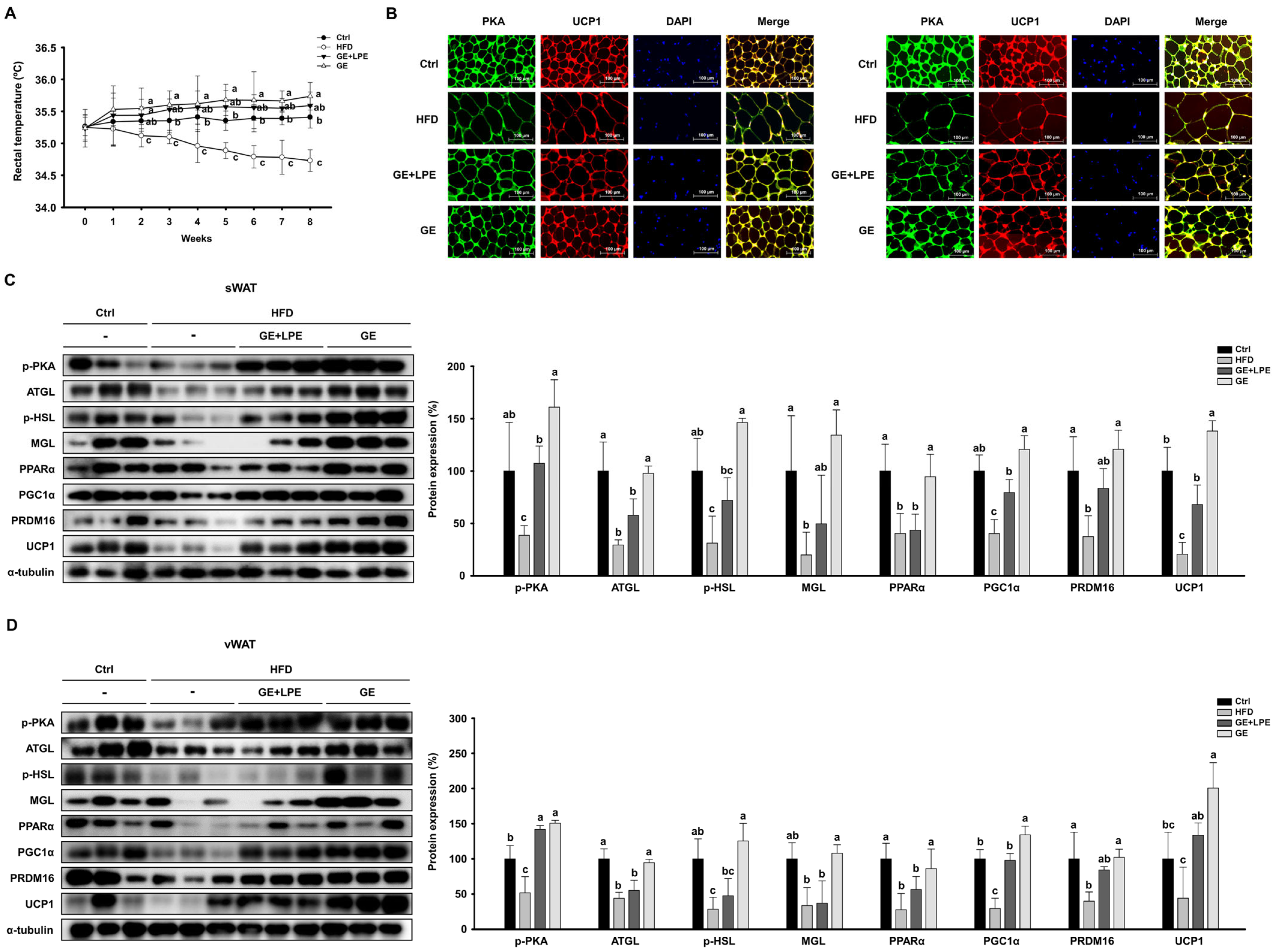
2.6. Rectal Temperature Measurement
2.7. Immunofluorescence Staining
2.8. Biochemical Analysis
2.9. Oil Red O Staining
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
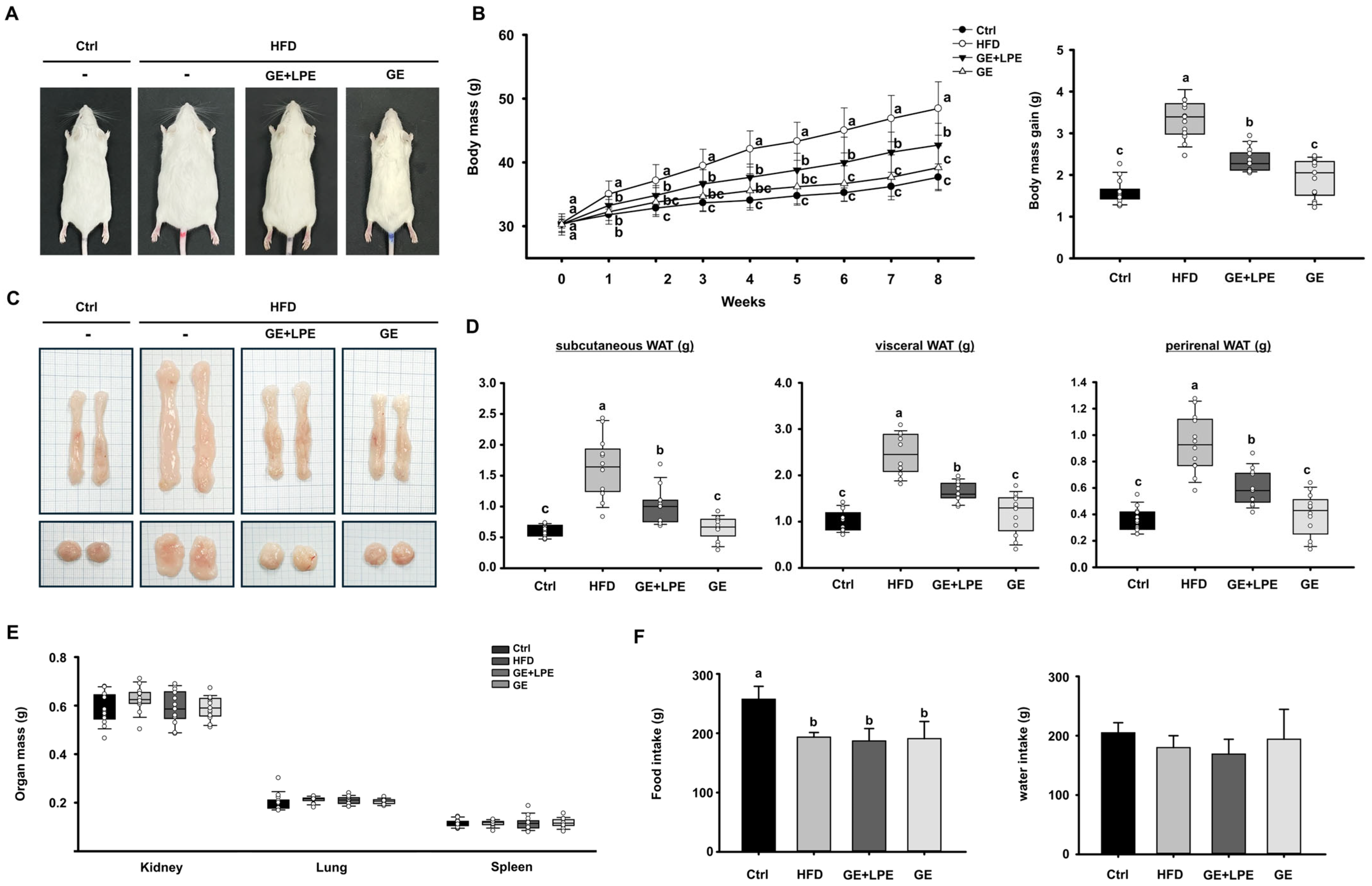
3.1. GE More Effectively Suppresses HFD-Induced Obesity than GE+LPE
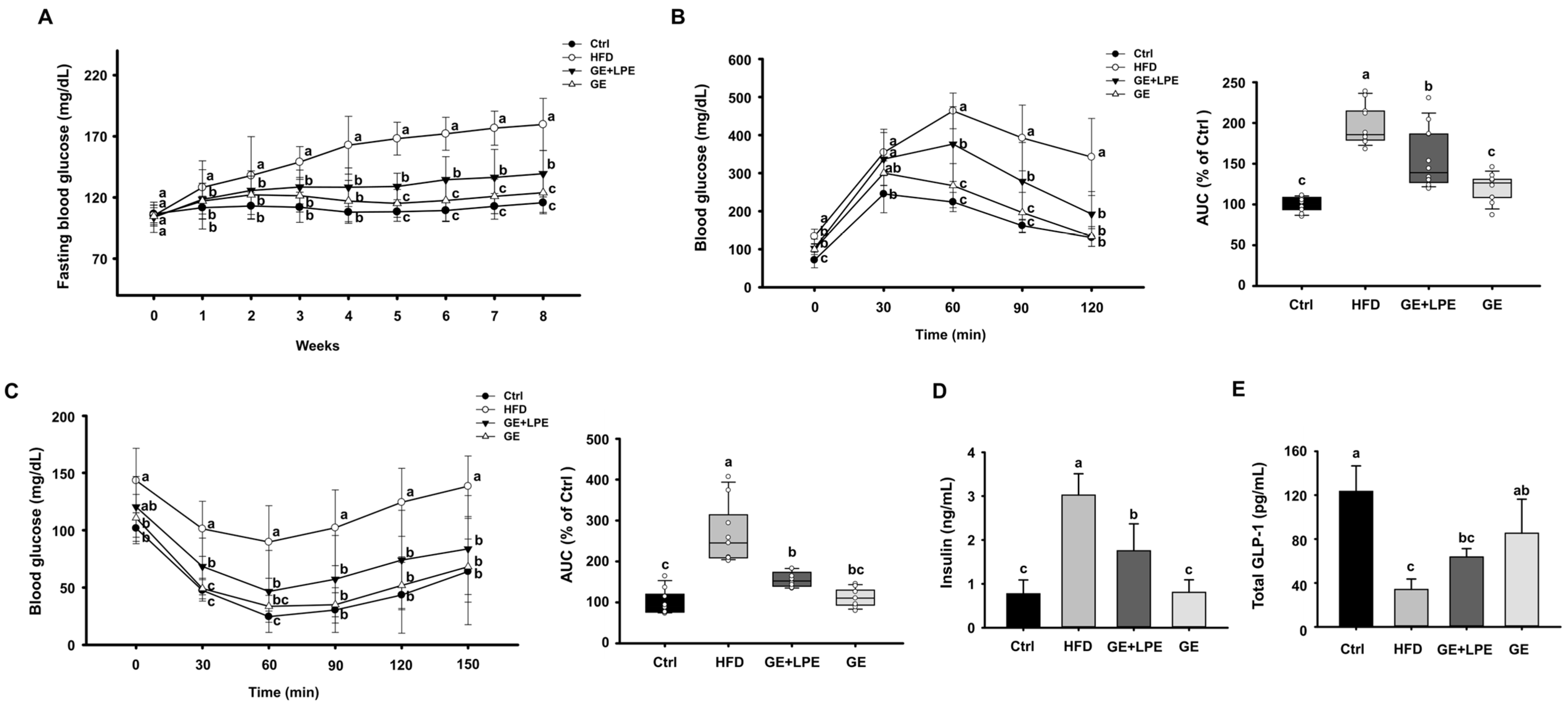
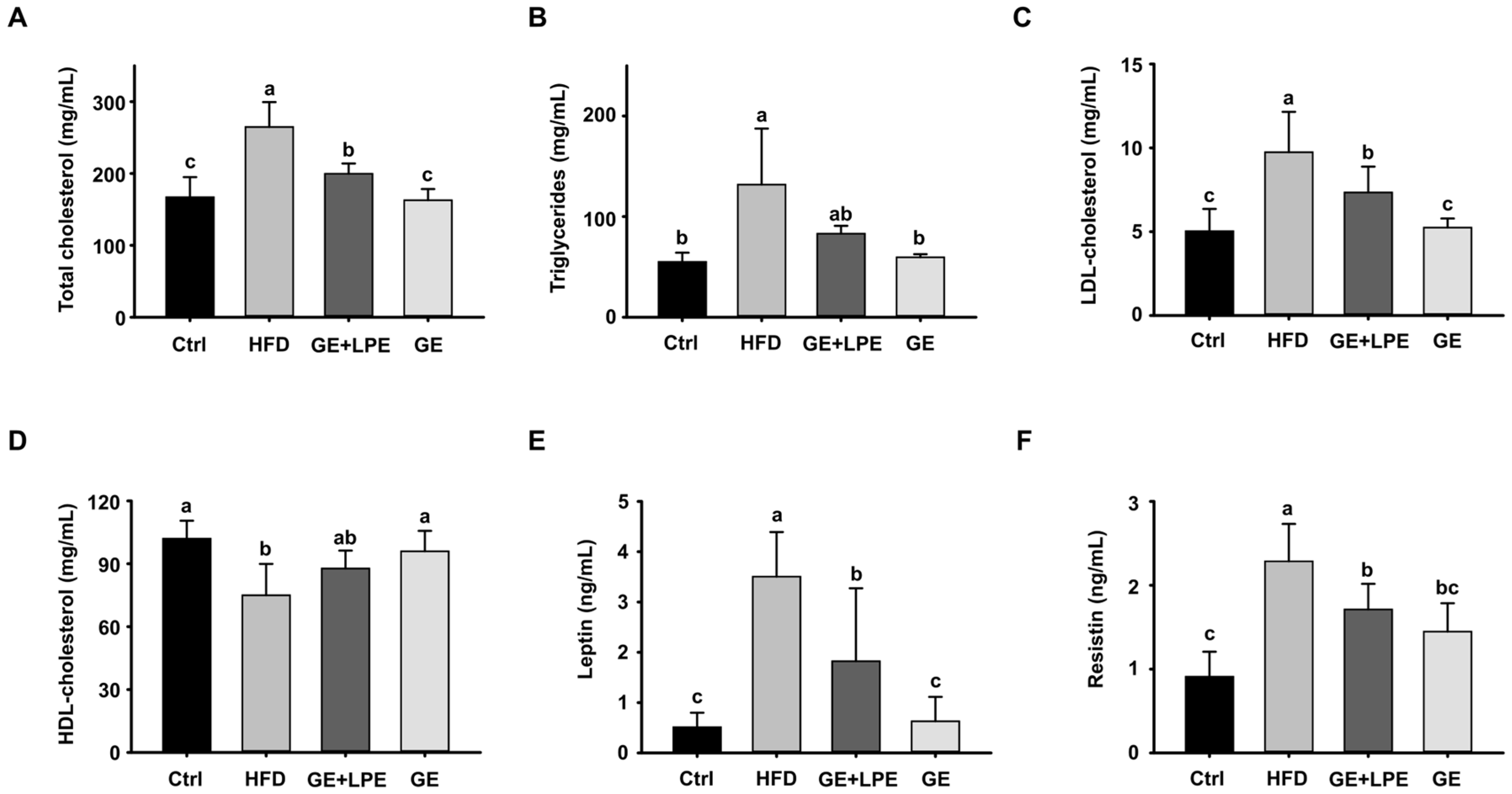
3.2. GE More Effectively Improves Glucose Homeostasis and Metabolic Hormone Regulation than GE+LPE
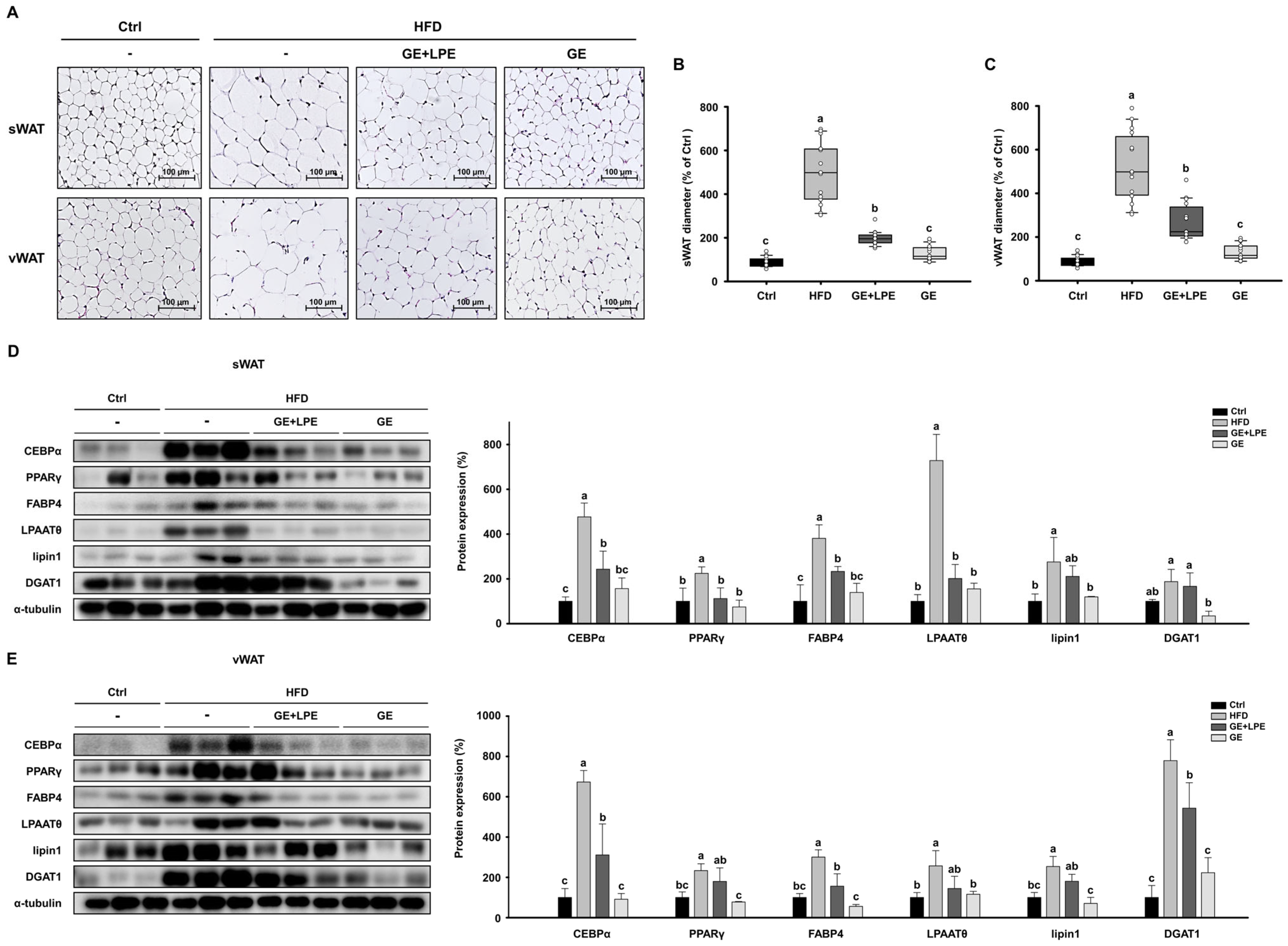
3.3. GE More Effectively Inhibits Adipogenesis and Lipogenesis in WAT than GE+LPE
3.4. GE More Effectively Promotes Lipolysis and Browning in WAT than GE+LPE
3.5. GE More Effectively Ameliorates Hepatic Steatosis than GE+LPE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Chandrasekaran, P.; Weiskirchen, R. The role of obesity in type 2 diabetes mellitus—An overview. Int. J. Mol. Sci. 2024, 25, 1882. [Google Scholar] [CrossRef]
- Kokkorakis, M.; Chakhtoura, M.; Rhayem, C.; Al Rifai, J.; Ghezzawi, M.; Valenzuela-Vallejo, L.; Mantzoros, C.S. Emerging pharmacotherapies for obesity: A systematic review. Pharmacol. Rev. 2024, 77, 100002. [Google Scholar] [CrossRef]
- Jin, X.; Qiu, T.; Li, L.; Yu, R.; Chen, X.; Li, C.; Proud, C.G.; Jiang, T. Pathophysiology of obesity and its associated diseases. Acta Pharm. Sin. B 2023, 13, 2403–2424. [Google Scholar] [CrossRef]
- An, S.-M.; Cho, S.-H.; Yoon, J.C. Adipose tissue and metabolic health. Diabetes Metab. J. 2023, 47, 595–611. [Google Scholar] [CrossRef]
- Janochova, K.; Haluzik, M.; Buzga, M. Visceral fat and insulin resistance—What we know? Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2019, 163, 19–27. [Google Scholar] [CrossRef]
- Radu, F.; Potcovaru, C.-G.; Salmen, T.; Filip, P.V.; Pop, C.; Fierbințeanu-Braticievici, C. The link between NAFLD and metabolic syndrome. Diagnostics 2023, 13, 614. [Google Scholar] [CrossRef]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef]
- Scheja, L.; Heeren, J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol. 2019, 15, 507–524. [Google Scholar] [CrossRef]
- Benomar, Y.; Taouis, M. Molecular mechanisms underlying obesity-induced hypothalamic inflammation and insulin resistance: Pivotal role of resistin/TLR4 pathways. Front. Endocrinol. 2019, 10, 140. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Liu, F.; He, J.; Wang, H.; Zhu, D.; Bi, Y. Adipose morphology: A critical factor in regulation of human metabolic diseases and adipose tissue dysfunction. Obes. Surg. 2020, 30, 5086–5100. [Google Scholar] [CrossRef]
- Al-Sayegh, M.; Mahmood, S.; Khair, S.A.; Xie, X.; El Gindi, M.; Kim, T.; Almansoori, A.; Percipalle, P. β-actin contributes to open chromatin for activation of the adipogenic pioneer factor CEBPA during transcriptional reprograming. Mol. Biol. Cell 2020, 31, 2511–2521. [Google Scholar] [CrossRef]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Prentice, K.J.; Saksi, J.; Hotamisligil, G.S. Adipokine FABP4 integrates energy stores and counterregulatory metabolic responses. J. Lipid Res. 2019, 60, 734–740. [Google Scholar] [CrossRef]
- Lin, S.; Wang, L.; Jia, Y.; Sun, Y.; Qiao, P.; Quan, Y.; Liu, J.; Hu, H.; Yang, B.; Zhou, H. Lipin-1 deficiency deteriorates defect of fatty acid β-oxidation and lipid-related kidney damage in diabetic kidney disease. Transl. Res. 2024, 266, 1–15. [Google Scholar] [CrossRef]
- Yang, W.; Wang, S.; Loor, J.J.; Lopes, M.G.; Zhao, Y.; Ma, X.; Li, M.; Zhang, B.; Xu, C. Role of diacylglycerol O-acyltransferase (DGAT) isoforms in bovine hepatic fatty acid metabolism. J. Dairy Sci. 2022, 105, 3588–3600. [Google Scholar] [CrossRef]
- Farese, R.V.; Walther, T.C. Glycerolipid synthesis and lipid droplet formation in the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2023, 15, a041246. [Google Scholar] [CrossRef]
- Grabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef]
- Kloska, A.; Węsierska, M.; Malinowska, M.; Gabig-Cimińska, M.; Jakóbkiewicz-Banecka, J. Lipophagy and lipolysis status in lipid storage and lipid metabolism diseases. Int. J. Mol. Sci. 2020, 21, 6113. [Google Scholar] [CrossRef]
- Cho, C.H.; Patel, S.; Rajbhandari, P. Adipose tissue lipid metabolism: Lipolysis. Curr. Opin. Genet. Dev. 2023, 83, 102114. [Google Scholar] [CrossRef]
- Vagena, E.; Ryu, J.K.; Baeza-Raja, B.; Walsh, N.M.; Syme, C.; Day, J.P.; Houslay, M.D.; Baillie, G.S. A high-fat diet promotes depression-like behavior in mice by suppressing hypothalamic PKA signaling. Transl. Psychiatry 2019, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Leiria, L.O.; Tseng, Y.-H. Lipidomics of brown and white adipose tissue: Implications for energy metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158788. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef]
- Herz, C.T.; Kiefer, F.W. Adipose tissue browning in mice and humans. J. Endocrinol. 2019, 241, R97–R109. [Google Scholar] [CrossRef]
- Ježek, P.; Jabůrek, M.; Porter, R.K. Uncoupling mechanism and redox regulation of mitochondrial uncoupling protein 1 (UCP1). Biochim. Biophys. Acta Bioenerg. 2019, 1860, 259–269. [Google Scholar] [CrossRef]
- Miller, K.N.; Clark, J.P.; Anderson, R.M. Mitochondrial regulator PGC-1a—Modulating the modulator. Curr. Opin. Endocr. Metab. Res. 2019, 5, 37–44. [Google Scholar] [CrossRef]
- Ebrahimzadeh Attari, V.; Malek Mahdavi, A.; Javadivala, Z.; Mahluji, S.; Zununi Vahed, S.; Ostadrahimi, A. A systematic review of the anti-obesity and weight lowering effect of ginger (Zingiber officinale Roscoe) and its mechanisms of action. Phytother. Res. 2018, 32, 577–585. [Google Scholar] [CrossRef]
- Kim, B.; Kim, H.-J.; Cha, Y.-S. The protective effects of steamed ginger on adipogenesis in 3T3-L1 cells and adiposity in diet-induced obese mice. Nutr. Res. Pract. 2021, 15, 279–293. [Google Scholar] [CrossRef]
- Wang, K.; Kong, L.; Wen, X.; Li, M.; Su, S.; Ni, Y.; Gu, J. The positive effect of 6-Gingerol on High-Fat Diet and Streptozotocin-Induced Prediabetic mice: Potential pathways and underlying mechanisms. Nutrients 2023, 15, 824. [Google Scholar] [CrossRef]
- Preciado-Ortiz, M.E.; Gembe-Olivarez, G.; Martínez-López, E.; Rivera-Valdés, J.J. Immunometabolic Effects of Ginger (Zingiber officinale Roscoe) Supplementation in Obesity: A Comprehensive Review. Molecules 2025, 30, 2933. [Google Scholar] [CrossRef]
- Du, Y.; Chen, Y.; Fu, X.; Gu, J.; Sun, Y.; Zhang, Z.; Xu, J.; Qin, L. Effects of piperine on lipid metabolism in high-fat diet induced obese mice. J. Funct. Foods 2020, 71, 104011. [Google Scholar] [CrossRef]
- Stojanović-Radić, Z.; Pejčić, M.; Dimitrijević, M.; Aleksić, A.; V. Anil Kumar, N.; Salehi, B.; C. Cho, W.; Sharifi-Rad, J. Piperine-a major principle of black pepper: A review of its bioactivity and studies. Appl. Sci. 2019, 9, 4270. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific opinion on flavouring group evaluation 86, revision 2 (FGE.86Rev2): Consideration of aliphatic and arylalkyl amines and amides evaluated by JECFA (65th meeting). EFSA J. 2015, 13, 3998. [Google Scholar] [CrossRef]
- Choi, M.J.; Yu, H.; Kim, J.I.; Seo, H.; Kim, J.G.; Kim, S.-K.; Lee, H.S.; Cheon, H.G. Anti-obesity effects of Lactiplantibacillus plantarum SKO-001 in high-fat diet-induced obese mice. Eur. J. Nutr. 2023, 62, 1611–1622. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Cheng, S.; Zhang, Y.; Yang, M.; Fang, R.; Li, H.; Man, C.; Jiang, Y. A potential synbiotic strategy for the prevention of type 2 diabetes: Lactobacillus paracasei JY062 and exopolysaccharide isolated from Lactobacillus plantarum JY039. Nutrients 2022, 14, 377. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.H.; Lee, S.; Kim, B.; Kim, H.Y. The protective effects of Aster yomena (Kitam.) Honda on high-fat diet-induced obese C57BL/6J mice. Nutr. Res. Pract. 2022, 16, 46–59. [Google Scholar] [CrossRef]
- Glavas, M.M.; Hui, Q.; Miao, I.; Yang, F.; Erener, S.; Prentice, K.J.; Wheeler, M.B.; Kieffer, T.J. Early overnutrition in male mice negates metabolic benefits of a diet high in monounsaturated and omega-3 fats. Sci. Rep. 2021, 11, 14032. [Google Scholar] [CrossRef]
- Jin, H.; Oh, H.J.; Cho, S.; Lee, O.H.; Lee, B.Y. Okra (Abelmoschus esculentus L. Moench) prevents obesity by reducing lipid accumulation and increasing white adipose browning in high-fat diet-fed mice. Food Funct. 2022, 13, 11840–11852. [Google Scholar] [CrossRef]
- Liu, W.; Mao, Y.; Schoenborn, J.; Wang, Z.; Tang, G.; Tang, X. Whole blueberry protects pancreatic beta-cells in diet-induced obese mouse. Nutr. Metab. 2019, 16, 34. [Google Scholar] [CrossRef]
- Lee, K.; Jin, H.; Chei, S.; Oh, H.-J.; Lee, J.-Y.; Lee, B.-Y. Effect of dietary silk peptide on obesity, hyperglycemia, and skeletal muscle regeneration in high-fat diet-fed mice. Cells 2020, 9, 377. [Google Scholar] [CrossRef]
- Bu, T.; Sun, Z.; Pan, Y.; Deng, X.; Yuan, G. Glucagon-like peptide-1: New regulator in lipid metabolism. Diabetes Metab. J. 2024, 48, 354–372. [Google Scholar] [CrossRef]
- Drucker, D.J. GLP-1 physiology informs the pharmacotherapy of obesity. Mol. Metab. 2022, 57, 101351. [Google Scholar] [CrossRef]
- Wharton, S.; Blevins, T.; Connery, L.; Rosenstock, J.; Raha, S.; Liu, R.; Ma, X.; Mather, K.J.; Haupt, A.; Robins, D. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. N. Engl. J. Med. 2023, 389, 877–888. [Google Scholar] [CrossRef]
- Giammanco, M.; Marini, H.R.; Pallio, S.; Giammanco, M.M.; Tomasello, G.; Carini, F.; Venturella, F.; Leto, G.; La Guardia, M. Adipokines in obesity and metabolic diseases. J. Biol. Res. Boll. Della Soc. Ital. Di Biol. Sper. 2020, 93. [Google Scholar] [CrossRef]
- Mishra, B.; Madhu, S.; Aslam, M.; Agarwal, V.; Banerjee, B. Adipose tissue expression of UCP1 and PRDM16 genes and their association with postprandial triglyceride metabolism and glucose intolerance. Diabetes Res. Clin. Pract. 2021, 182, 109115. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, B.; Mun, E.-G.; Jeong, S.-Y.; Cha, Y.-S. The antioxidant activity of steamed ginger and its protective effects on obesity induced by high-fat diet in C57BL/6J mice. Nutr. Res. Pract. 2018, 12, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, M.-S.; Jung, S.; Son, H.-Y.; Park, S.; Kang, B.; Kim, S.-Y.; Kim, I.-H.; Kim, C.-T.; Kim, Y. Ginger extract ameliorates obesity and inflammation via regulating microRNA-21/132 expression and AMPK activation in white adipose tissue. Nutrients 2018, 10, 1567. [Google Scholar] [CrossRef]
- Seo, S.H.; Fang, F.; Kang, I. Ginger (Zingiber officinale) attenuates obesity and adipose tissue remodeling in high-fat diet-fed C57BL/6 mice. Int. J. Environ. Res. Public Health 2021, 18, 631. [Google Scholar] [CrossRef]
- Oliveira, C.T.; Lacerda, D.R.; Zicker, M.C.; Martins, L.B.; Teixeira, M.M.; de Araujo, R.L.B.; Ferreira, A.V.M. Ginger (Zingiber officinale Rosc.) ameliorated metabolic and inflammatory dysfunction induced by high-refined carbohydrate-containing diet in mice. J. Med. Food 2019, 22, 38–45. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Wang, P.; Hu, X.; Chen, F. Ginger prevents obesity through regulation of energy metabolism and activation of browning in high-fat diet-induced obese mice. J. Nutr. Biochem. 2019, 70, 105–115. [Google Scholar] [CrossRef]
- Lee, G.-H.; Peng, C.; Jeong, S.-Y.; Park, S.-A.; Lee, H.-Y.; Hoang, T.-H.; Kim, J.; Chae, H.-J. Ginger extract controls mTOR-SREBP1-ER stress-mitochondria dysfunction through AMPK activation in obesity model. J. Funct. Foods 2021, 87, 104628. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, C.; Wang, L.; Wang, K. Natural piperine improves lipid metabolic profile of high-fat diet-fed mice by upregulating SR-B1 and ABCG8 transporters. J. Nat. Prod. 2021, 84, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zheng, Q.; Song, M.; Xiao, J.; Cao, Y.; Huang, Q.; Ho, C.-T.; Lu, M. A review on the bioavailability, bio-efficacies and novel delivery systems for piperine. Food Funct. 2021, 12, 8867–8881. [Google Scholar] [CrossRef]
| Component | Ginger Extract (GE) | Long Pepper Extract (LPE) |
|---|---|---|
| Energy (kcal/100 g) | 623.49 | 357.85 |
| Carbohydrate (%) | 39.96 | 86.30 |
| Crude fat (%) | 51.21 | 0.45 |
| Crude protein (%) | 0.69 | 2.15 |
| Ash (%) | 5.10 | 5.38 |
| Moisture (%) | 3.04 | 5.72 |
| Total polyphenols (GAE mg/g) | 139.4 ± 2.7 | 5.6 ± 0.1 |
| Marker compound content (mg/g) | 6-gingerol: 132 | piperine: 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, G.; Han, H.; Jin, H.; Kim, J.; Kim, H.; Seo, Y.-S.; Song, H.; Lee, B.-Y. Comparison of Anti-Obesity Effects of Ginger Extract Alone and Mixed with Long Pepper Extract. Biomedicines 2025, 13, 2077. https://doi.org/10.3390/biomedicines13092077
Song G, Han H, Jin H, Kim J, Kim H, Seo Y-S, Song H, Lee B-Y. Comparison of Anti-Obesity Effects of Ginger Extract Alone and Mixed with Long Pepper Extract. Biomedicines. 2025; 13(9):2077. https://doi.org/10.3390/biomedicines13092077
Chicago/Turabian StyleSong, Gunju, Hyein Han, Heegu Jin, Jongwon Kim, Hyeongmin Kim, Yi-Seul Seo, Heewon Song, and Boo-Yong Lee. 2025. "Comparison of Anti-Obesity Effects of Ginger Extract Alone and Mixed with Long Pepper Extract" Biomedicines 13, no. 9: 2077. https://doi.org/10.3390/biomedicines13092077
APA StyleSong, G., Han, H., Jin, H., Kim, J., Kim, H., Seo, Y.-S., Song, H., & Lee, B.-Y. (2025). Comparison of Anti-Obesity Effects of Ginger Extract Alone and Mixed with Long Pepper Extract. Biomedicines, 13(9), 2077. https://doi.org/10.3390/biomedicines13092077






