Glycomics in Human Diseases and Its Emerging Role in Biomarker Discovery
Abstract
1. Introduction
2. Fundamentals of Glycobiology and Glycan Function in Health
2.1. Biosynthesis and Structural Diversity of Glycans
2.1.1. N-Glycan Biosynthesis and Structural Diversity
2.1.2. O-Glycan Biosynthesis and Structural Diversity
2.1.3. Glycosaminoglycans Biosynthesis and Structural Diversity
2.1.4. Glycolipids Biosynthesis and Structural Diversity
2.2. Glycosylation and Cellular Function
2.2.1. Protein Folding, Stability, and Trafficking
2.2.2. Cell–Cell Communication and Immune Response
2.3. Role of Glycans in Homeostasis and Development
Neural Development and Synaptic Plasticity
3. Glycomic Alterations in Diseases
3.1. Cancer Glycomics
3.1.1. Aberrant Glycosylation as a Hallmark of Cancer
3.1.2. Tumor-Associated Carbohydrate Antigens (TACAs)
3.1.3. Aberrant Glycosylation in Cancer Metastasis, Immune Evasion, and Therapy Resistance
Role of Aberrant Glycosylation in Cancer Metastasis
Altered Glycosylation in Cancer Immune Evasion
Aberrant Glycosylation and Resistance to Cancer Therapies
3.2. Glycan Modifications in Neurodegenerative Disease
3.2.1. Glycan Modifications in Alzheimer’s Disease
3.2.2. Dysregulated Glycosylation in Parkinson’s Disease
3.2.3. The Role of Impaired Glycosylation in Neuroinflammation
3.2.4. Role of Impaired Glycosylation in Synaptic Dysfunction
3.3. Role of Glycosylation in Cardiovascular Diseases
3.4. Autoimmune and Inflammatory Disorders
3.4.1. Aberrant Glycosylation in Rheumatoid Arthritis, Lupus, and IBD
Rheumatoid Arthritis
Systemic Lupus Erythematosus
Inflammatory Bowel Disease (IBD)
3.4.2. Glycan-Mediated Immune Modulation and Inflammation
Glycan Roles in Viral, Bacterial, and Parasitic Infections
3.4.3. Glycobiology of SARS-CoV-2, Influenza, and HIV
Glycobiology of SARS-CoV-2
Glycobiology of Influenza Viruses
Glycobiology of HIV
4. Translational Applications of Glycomics in Medicine
4.1. Glycan Biomarkers for Disease Diagnosis
4.1.1. Cancer Glycan Signatures for Early Detection
4.1.2. Glycoproteins as Non-Invasive Biomarkers in Neurodegeneration
4.2. Therapeutic Targeting of Glycans
4.2.1. Glycoengineered Monoclonal Antibodies

4.2.2. Glycan-Based Vaccines and Immunotherapy Strategies
4.3. Glycoengineering for Precision Medicine
4.3.1. Personalized Glycoprofiling in Drug Response and Efficacy
4.3.2. Glycobiology of Biopharmaceuticals and Gene Therapy
5. Challenges and Future Perspectives in Glycomics and Disease Research
5.1. Challenges in Glycan Analysis and Interpretation: Structural Complexity and Isomeric Diversity
Standardization and Reproducibility Issues
5.2. Emerging Trends in Glycomics
5.2.1. Single-Cell Glycomics and Spatial Glycoprofiling
5.2.2. Integrative Multi-Omics Approaches for Unraveling Disease Mechanisms
5.3. Artificial Intelligence in Glycomics
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chandler, K.B.; Leon, D.R.; Kuang, J.; Meyer, R.D.; Rahimi, N.; Costello, C.E. N-Glycosylation regulates ligand-dependent activation and signaling of vascular endothelial growth factor receptor 2 (VEGFR2). J. Biol. Chem. 2019, 294, 13117–13130. [Google Scholar] [CrossRef]
- Perkey, E.; Maurice De Sousa, D.; Carrington, L.; Chung, J.; Dils, A.; Granadier, D.; Koch, U.; Radtke, F.; Ludewig, B.; Blazar, B.R.; et al. GCNT1-Mediated O-Glycosylation of the Sialomucin CD43 Is a Sensitive Indicator of Notch Signaling in Activated T Cells. J. Immunol. 2020, 204, 1674–1688. [Google Scholar] [CrossRef]
- Zeck, A.; Pohlentz, G.; Schlothauer, T.; Peter-Katalinić, J.; Regula, J.T. Cell type-specific and site directed N-glycosylation pattern of FcγRIIIa. J. Proteome Res. 2011, 10, 3031–3039. [Google Scholar] [CrossRef]
- Mathew, C.; Weiß, R.G.; Giese, C.; Lin, C.-w.; Losfeld, M.-E.; Glockshuber, R.; Riniker, S.; Aebi, M. Glycan–protein interactions determine kinetics of N-glycan remodeling. RSC Chem. Biol. 2021, 2, 917–931. [Google Scholar] [CrossRef]
- Chen, S.; Xie, Y.; Alvarez, M.R.; Sheng, Y.; Bouchibti, Y.; Chang, V.; Lebrilla, C.B. Quantitative Glycan-Protein Cross-Linking Mass Spectrometry Using Enrichable Linkers Reveals Extensive Glycan-Mediated Protein Interaction Networks. Anal. Chem. 2025, 97, 1584–1593. [Google Scholar] [CrossRef]
- Lin, P.; Qin, Q.; Gan, X.-y.; Pang, J.-s.; Wen, R.; He, Y.; Yang, H. Integrating single-cell and bulk RNA sequencing data to characterize the heterogeneity of glycan-lipid metabolism polarization in hepatocellular carcinoma. J. Transl. Med. 2025, 23, 358. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Takahashi, M.; Gu, J.G.; Miyoshi, E.; Matsumoto, A.; Kitazume, S.; Taniguchi, N. Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci. 2008, 99, 1304–1310. [Google Scholar] [CrossRef]
- Takahashi, M.; Tsuda, T.; Ikeda, Y.; Honke, K.; Taniguchi, N. Role of N-glycans in growth factor signaling. Glycoconj. J. 2003, 20, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Madunić, K.; Zhang, T.; Mayboroda, O.A.; Lageveen-Kammeijer, G.S.M.; Wuhrer, M. High diversity of glycosphingolipid glycans of colorectal cancer cell lines reflects the cellular differentiation phenotype. Mol. Cell. Proteom. 2022, 21, 100239. [Google Scholar] [CrossRef] [PubMed]
- Maverakis, E.; Kim, K.; Shimoda, M.; Gershwin, M.E.; Patel, F.; Wilken, R.; Raychaudhuri, S.; Ruhaak, L.R.; Lebrilla, C.B. Glycans in the immune system and the Altered Glycan Theory of Autoimmunity: A critical review. J. Autoimmun. 2015, 57, 1–13. [Google Scholar] [CrossRef] [PubMed]
- van Kooyk, Y.; Rabinovich, G.A. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 2008, 9, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Yang, C.; Liu, Z. How hydrophobicity and the glycosylation site of glycans affect protein folding and stability: A molecular dynamics simulation. J. Phys. Chem. B 2012, 116, 390–400. [Google Scholar] [CrossRef]
- Shental-Bechor, D.; Levy, Y. Effect of glycosylation on protein folding: A close look at thermodynamic stabilization. Proc. Natl. Acad. Sci. USA 2008, 105, 8256–8261. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Luan, X.; Melamed, J.; Brockhausen, I. Role of Glycans on Key Cell Surface Receptors That Regulate Cell Proliferation and Cell Death. Cells 2021, 10, 1252. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Spillings, B.L.; Day, C.J.; Garcia-Minambres, A.; Aggarwal, A.; Condon, N.D.; Haselhorst, T.; Purcell, D.F.J.; Turville, S.G.; Stow, J.L.; Jennings, M.P.; et al. Host glycocalyx captures HIV proximal to the cell surface via oligomannose-GlcNAc glycan-glycan interactions to support viral entry. Cell Rep. 2022, 38, 110296. [Google Scholar] [CrossRef]
- Li, Y.; Liu, D.; Wang, Y.; Su, W.; Liu, G.; Dong, W. The Importance of Glycans of Viral and Host Proteins in Enveloped Virus Infection. Front. Immunol. 2021, 12, 638573. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Alves, I.; Gaifem, J.; Rabinovich, G.A. Immune regulatory networks coordinated by glycans and glycan-binding proteins in autoimmunity and infection. Cell. Mol. Immunol. 2023, 20, 1101–1113. [Google Scholar] [CrossRef]
- Mulloy, B.; Hart, G.W.; Stanley, P. Structural Analysis of Glycans. In Essentials of Glycobiology, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009. [Google Scholar]
- Gagneux, P.; Panin, V.; Hennet, T.; Aebi, M.; Varki, A. Evolution of Glycan Diversity. In Essentials of Glycobiology [Internet], 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022. [Google Scholar]
- Huang, Y.; Zhang, H.L.; Li, Z.L.; Du, T.; Chen, Y.H.; Wang, Y.; Ni, H.H.; Zhang, K.M.; Mai, J.; Hu, B.X.; et al. FUT8-mediated aberrant N-glycosylation of B7H3 suppresses the immune response in triple-negative breast cancer. Nat. Commun. 2021, 12, 2672. [Google Scholar] [CrossRef]
- Rømer, T.B.; Aasted, M.K.M.; Dabelsteen, S.; Groen, A.; Schnabel, J.; Tan, E.; Pedersen, J.W.; Haue, A.D.; Wandall, H.H. Mapping of truncated O-glycans in cancers of epithelial and non-epithelial origin. Br. J. Cancer 2021, 125, 1239–1250. [Google Scholar] [CrossRef]
- Dimitroff, C.J. I-branched carbohydrates as emerging effectors of malignant progression. Proc. Natl. Acad. Sci. USA 2019, 116, 13729–13737. [Google Scholar] [CrossRef]
- Onigbinde, S.; Peng, W.; Reddy, A.; Cho, B.G.; Goli, M.; Solomon, J.; Adeniyi, M.; Nwaiwu, J.; Fowowe, M.; Daramola, O.; et al. O-Glycome Profiling of Breast Cancer Cell Lines to Understand Breast Cancer Brain Metastasis. J. Proteome Res. 2024, 23, 1458–1470. [Google Scholar] [CrossRef]
- Peng, W.; Goli, M.; Mirzaei, P.; Mechref, Y. Revealing the Biological Attributes of N-Glycan Isomers in Breast Cancer Brain Metastasis Using Porous Graphitic Carbon (PGC) Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). J. Proteome Res. 2019, 18, 3731–3740. [Google Scholar] [CrossRef] [PubMed]
- Giron, L.B.; Palmer, C.S.; Liu, Q.; Yin, X.; Papasavvas, E.; Sharaf, R.; Etemad, B.; Damra, M.; Goldman, A.R.; Tang, H.-Y.; et al. Non-invasive plasma glycomic and metabolic biomarkers of post-treatment control of HIV. Nat. Commun. 2021, 12, 3922. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Qin, S.; Dai, L.; Tian, Z. The glycosylation in SARS-CoV-2 and its receptor ACE2. Signal Transduct. Target. Ther. 2021, 6, 396. [Google Scholar] [CrossRef] [PubMed]
- Onigbinde, S.; Reyes, C.D.G.; Fowowe, M.; Daramola, O.; Atashi, M.; Bennett, A.I.; Mechref, Y. Variations in O-Glycosylation Patterns Influence Viral Pathogenicity, Infectivity, and Transmissibility in SARS-CoV-2 Variants. Biomolecules 2023, 13, 1467. [Google Scholar] [CrossRef]
- Reyes, C.D.G.; Onigbinde, S.; Sanni, A.; Bennett, A.I.; Jiang, P.; Daramola, O.; Ahmadi, P.; Fowowe, M.; Atashi, M.; Sandilya, V.; et al. N-Glycome Profile of the Spike Protein S1: Systemic and Comparative Analysis from Eleven Variants of SARS-CoV-2. Biomolecules 2023, 13, 1421. [Google Scholar] [CrossRef]
- Holland, M.; Takada, K.; Okumoto, T.; Takahashi, N.; Kato, K.; Adu, D.; Ben-Smith, A.; Harper, L.; Savage, C.O.; Jefferis, R. Hypogalactosylation of serum IgG in patients with ANCA-associated systemic vasculitis. Clin. Exp. Immunol. 2002, 129, 183–190. [Google Scholar] [CrossRef]
- Zhou, X.; Motta, F.; Selmi, C.; Ridgway, W.M.; Gershwin, M.E.; Zhang, W. Antibody glycosylation in autoimmune diseases. Autoimmun. Rev. 2021, 20, 102804. [Google Scholar] [CrossRef] [PubMed]
- Gudelj, I.; Lauc, G.; Pezer, M. Immunoglobulin G glycosylation in aging and diseases. Cell. Immunol. 2018, 333, 65–79. [Google Scholar] [CrossRef]
- Fastenau, C.; Bunce, M.; Keating, M.; Wickline, J.; Hopp, S.C.; Bieniek, K.F. Distinct patterns of plaque and microglia glycosylation in Alzheimer’s disease. Brain Pathol. 2024, 34, e13267. [Google Scholar] [CrossRef]
- Rebelo, A.L.; Drake, R.R.; Marchetti-Deschmann, M.; Saldova, R.; Pandit, A. Changes in tissue protein N-glycosylation and associated molecular signature occur in the human Parkinsonian brain in a region-specific manner. PNAS Nexus 2024, 3, pgad439. [Google Scholar] [CrossRef]
- Orlean, P. Congenital disorders of glycosylation caused by defects in mannose addition during N-linked oligosaccharide assembly. J. Clin. Investig. 2000, 105, 131–132. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhou, X.; Wang, X. Glycosylation: Mechanisms, biological functions and clinical implications. Signal Transduct. Target. Ther. 2024, 9, 194. [Google Scholar] [CrossRef]
- Xu, X.; Peng, Q.; Jiang, X.; Tan, S.; Yang, W.; Han, Y.; Oyang, L.; Lin, J.; Shen, M.; Wang, J.; et al. Altered glycosylation in cancer: Molecular functions and therapeutic potential. Cancer Commun. 2024, 44, 1316–1336. [Google Scholar] [CrossRef]
- Alter, G.; Ottenhoff, T.H.M.; Joosten, S.A. Antibody Glycosylation in Inflammation, Disease and Vaccination; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Qin, R.; Mahal, L.K. The host glycomic response to pathogens. Curr. Opin. Struct. Biol. 2021, 68, 149–156. [Google Scholar] [CrossRef]
- Klaus, C.; Liao, H.; Allendorf, D.H.; Brown, G.C.; Neumann, H. Sialylation acts as a checkpoint for innate immune responses in the central nervous system. Glia 2021, 69, 1619–1636. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Cobb, B.A. Glycans in Immunologic Health and Disease. Annu. Rev. Immunol. 2021, 39, 511–536. [Google Scholar] [CrossRef]
- Stanley, P.; Moremen, K.W.; Lewis, N.E.; Taniguchi, N.; Aebi, M. N-Glycans. In Essentials of Glycobiology [Internet], 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022. [Google Scholar]
- Viinikangas, T.; Khosrowabadi, E.; Kellokumpu, S. N-glycan biosynthesis: Basic principles and factors affecting its outcome. In Antibody Glycosylation; Springer: Berlin/Heidelberg, Germany, 2021; pp. 237–257. [Google Scholar]
- Trombetta, E.S. The contribution of N-glycans and their processing in the endoplasmic reticulum to glycoprotein biosynthesis. Glycobiology 2003, 13, 77R–91R. [Google Scholar] [CrossRef] [PubMed]
- Krasnova, L.; Wong, C.-H. Understanding the Chemistry and Biology of Glycosylation with Glycan Synthesis. Annu. Rev. Biochem. 2016, 85, 599–630. [Google Scholar] [CrossRef] [PubMed]
- Lombard, J. The multiple evolutionary origins of the eukaryotic N-glycosylation pathway. Biol. Direct 2016, 11, 36. [Google Scholar] [CrossRef]
- Toustou, C.; Walet-Balieu, M.-L.; Kiefer-Meyer, M.-C.; Houdou, M.; Lerouge, P.; Foulquier, F.; Bardor, M. Towards understanding the extensive diversity of protein -glycan structures in eukaryotes. Biol. Rev. 2022, 97, 732–748. [Google Scholar] [CrossRef] [PubMed]
- Hossler, P.; Mulukutla, B.C.; Hu, W.-S. Systems Analysis of N-Glycan Processing in Mammalian Cells. PLoS ONE 2007, 2, e713. [Google Scholar] [CrossRef] [PubMed]
- Kizuka, Y.; Taniguchi, N. Enzymes for N-Glycan Branching and Their Genetic and Nongenetic Regulation in Cancer. Biomolecules 2016, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Ohkawa, Y.; Maeda, K.; Taniguchi, N. Glycan quality control in and out of the endoplasmic reticulum of mammalian cells. FEBS J. 2022, 289, 7147–7162. [Google Scholar] [CrossRef]
- Wopereis, S.; Lefeber, D.J.; Morava, E.; Wevers, R.A. Mechanisms in protein O-glycan biosynthesis and clinical and molecular aspects of protein O-glycan biosynthesis defects: A review. Clin. Chem. 2006, 52, 574–600. [Google Scholar] [CrossRef] [PubMed]
- Brockhausen, I.; Schachter, H.; Stanley, P. O-GalNAc Glycans. In Essentials of Glycobiology, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009. [Google Scholar]
- Brockhausen, I. Pathways of O-glycan biosynthesis in cancer cells. Biochim. Biophys. Acta-Gen. Subj. 1999, 1473, 67–95. [Google Scholar] [CrossRef]
- Thompson, N.; Wakarchuk, W. O-glycosylation and its role in therapeutic proteins. Biosci. Rep. 2022, 42, BSR20220094. [Google Scholar] [CrossRef]
- Schachter, H.; Brockhausen, I. The biosynthesis of branched O-glycans. Symp. Soc. Exp. Biol. 1989, 43, 1–26. [Google Scholar]
- Magalhães, A.; Duarte, H.O.; Reis, C.A. The role of O-glycosylation in human disease. Mol. Asp. Med. 2021, 79, 100964. [Google Scholar] [CrossRef]
- DeAngelis, P.L. Glycosaminoglycan polysaccharide biosynthesis and production: Today and tomorrow. Appl. Microbiol. Biotechnol. 2012, 94, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Merry, C.L.; Lindahl, U.; Couchman, J.; Esko, J.D. Proteoglycans and sulfated glycosaminoglycans. In Essentials of Glycobiology [Internet], 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022. [Google Scholar]
- Yao, Z.-Y.; Gong, J.-S.; Jiang, J.-Y.; Su, C.; Zhao, W.-H.; Xu, Z.-H.; Shi, J.-S. Unraveling the intricacies of glycosaminoglycan biosynthesis: Decoding the molecular symphony in understanding complex polysaccharide assembly. Biotechnol. Adv. 2024, 75, 108416. [Google Scholar] [CrossRef] [PubMed]
- Mandawe, J.; Infanzon, B.; Eisele, A.; Zaun, H.; Kuballa, J.; Davari, M.D.; Jakob, F.; Elling, L.; Schwaneberg, U. Directed evolution of hyaluronic acid synthase from Pasteurella multocida towards high-molecular-weight hyaluronic acid. ChemBioChem 2018, 19, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Li, M.; Xu, A.; Zhuo, F. Recent applications and molecular mechanisms of hyaluronic acid in skin aging and wound healing. Med. Nov. Technol. Devices 2024, 23, 100320. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan in tissue injury and repair. Annu. Rev. Cell Dev. Biol. 2007, 23, 435–461. [Google Scholar] [CrossRef]
- Vigetti, D.; Karousou, E.; Viola, M.; Passi, A. Analysis of hyaluronan synthase activity. In Glycosaminoglycans: Chemistry and Biology; Springer: New York, NY, USA, 2014; pp. 201–208. [Google Scholar]
- Viola, M.; Brüggemann, K.; Karousou, E.; Caon, I.; Caravà, E.; Vigetti, D.; Greve, B.; Stock, C.; De Luca, G.; Passi, A.; et al. MDA-MB-231 breast cancer cell viability, motility and matrix adhesion are regulated by a complex interplay of heparan sulfate, chondroitin-/dermatan sulfate and hyaluronan biosynthesis. Glycoconj. J. 2017, 34, 411–420. [Google Scholar] [CrossRef]
- Weigel, P.H.; DeAngelis, P.L. Hyaluronan Synthases: A Decade-plus of Novel Glycosyltransferases. J. Biol. Chem. 2007, 282, 36777–36781. [Google Scholar] [CrossRef]
- Sugahara, K.; Kitagawa, H. Recent advances in the study of the biosynthesis and functions of sulfated glycosaminoglycans. Curr. Opin. Struct. Biol. 2000, 10, 518–527. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, S.; Qiao, M.; Jiao, R.; Li, J.; Song, P.; Zhang, X.; Huang, H. Synthesis of structurally defined chondroitin sulfate: Paving the way to the structure-activity relationship studies. Carbohydr. Polym. 2020, 248, 116796. [Google Scholar] [CrossRef] [PubMed]
- Silbert, J.E.; Sugumaran, G. Biosynthesis of chondroitin/dermatan sulfate. IUBMB Life 2002, 54, 177–186. [Google Scholar] [CrossRef]
- Götting, C.; Kuhn, J.; Zahn, R.; Brinkmann, T.; Kleesiek, K. Molecular cloning and expression of human UDP-D-xylose: Proteoglycan core protein β-D-xylosyltransferase and its first isoform XT-II. J. Mol. Biol. 2000, 304, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Okajima, T.; Yoshida, K.; Kondo, T.; Furukawa, K. Human homolog of Caenorhabditis elegans sqv-3 gene is galactosyltransferase I involved in the biosynthesis of the glycosaminoglycan-protein linkage region of proteoglycans. J. Biol. Chem. 1999, 274, 22915–22918. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhou, D.; Brown, J.R.; Crawford, B.E.; Hennet, T.; Esko, J.D. Biosynthesis of the linkage region of glycosaminoglycans: Cloning and activity of galactosyltransferase II, the sixth member of the β1, 3-galactosyltransferase family (β3GalT6). J. Biol. Chem. 2001, 276, 48189–48195. [Google Scholar] [CrossRef]
- Kitagawa, H.; Tone, Y.; Tamura, J.-i.; Neumann, K.W.; Ogawa, T.; Oka, S.; Kawasaki, T.; Sugahara, K. Molecular cloning and expression of glucuronyltransferase I involved in the biosynthesis of the glycosaminoglycan-protein linkage region of proteoglycans. J. Biol. Chem. 1998, 273, 6615–6618. [Google Scholar] [CrossRef]
- Uyama, T.; Kitagawa, H.; Sugahara, K. Biosynthesis of glycosaminoglycans and proteoglycans. In Comprehensive Glycoscience; Elsevier: Amsterdam, The Netherlands, 2007; pp. 79–104. [Google Scholar]
- Sugahara, K.; Mikami, T.; Uyama, T.; Mizuguchi, S.; Nomura, K.; Kitagawa, H. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr. Opin. Struct. Biol. 2003, 13, 612–620. [Google Scholar] [CrossRef]
- Mikami, T.; Kitagawa, H. Biosynthesis and function of chondroitin sulfate. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 4719–4733. [Google Scholar] [CrossRef]
- Fu, L.; Li, K.; Mori, D.; Hirakane, M.; Lin, L.; Grover, N.; Datta, P.; Yu, Y.; Zhao, J.; Zhang, F.; et al. Enzymatic generation of highly anticoagulant bovine intestinal heparin. J. Med. Chem. 2017, 60, 8673–8679. [Google Scholar] [CrossRef]
- Sugahara, K.; Kitagawa, H. Heparin and Heparan Sulfate Biosynthesis. IUBMB Life 2002, 54, 163–175. [Google Scholar] [CrossRef]
- Sasisekharan, R.; Venkataraman, G. Heparin and heparan sulfate: Biosynthesis, structure and function. Curr. Opin. Chem. Biol. 2000, 4, 626–631. [Google Scholar] [CrossRef]
- Fu, L.; Suflita, M.; Linhardt, R.J. Bioengineered heparins and heparan sulfates. Adv. Drug Deliv. Rev. 2016, 97, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Petitou, M.; Hérault, J.-P.; Bernat, A.; Driguez, P.-A.; Duchaussoy, P.; Lormeau, J.-C.; Herbert, J.-M. Synthesis of thrombin-inhibiting heparin mimetics without side effects. Nature 1999, 398, 417–422. [Google Scholar] [CrossRef]
- Sasisekharan, R.; Ernst, S.; Venkataraman, G. On the regulation of fibroblast growth factor activity by heparin-like glycosaminoglycans. Angiogenesis 1997, 1, 45–54. [Google Scholar] [CrossRef]
- Shukla, D.; Liu, J.; Blaiklock, P.; Shworak, N.W.; Bai, X.; Esko, J.D.; Cohen, G.H.; Eisenberg, R.J.; Rosenberg, R.D.; Spear, P.G. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 1999, 99, 13–22. [Google Scholar] [CrossRef]
- Perrimon, N.; Bernfield, M. Specificities of heparan sulphate proteoglycans in developmental processes. Nature 2000, 404, 725–728. [Google Scholar] [CrossRef]
- Lutsyk, V.; Plazinski, W. Conformational properties of glycosaminoglycan disaccharides: A molecular dynamics study. J. Phys. Chem. B 2021, 125, 10900–10916. [Google Scholar] [CrossRef]
- Funderburgh, J.L. MINI REVIEW Keratan sulfate: Structure, biosynthesis, and function. Glycobiology 2000, 10, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Keratan sulfate: An up-to-date review. Int. J. Biol. Macromol. 2015, 72, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Funderburgh, J.L. Keratan sulfate biosynthesis. IUBMB Life 2002, 54, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Caterson, B.; Melrose, J. Keratan sulfate, a complex glycosaminoglycan with unique functional capability. Glycobiology 2018, 28, 182–206. [Google Scholar] [CrossRef]
- Carlson, E.C.; Sun, Y.; Auletta, J.; Kao, W.W.; Liu, C.Y.; Perez, V.L.; Pearlman, E. Regulation of corneal inflammation by neutrophil-dependent cleavage of keratan sulfate proteoglycans as a model for breakdown of the chemokine gradient. J. Leukoc. Biol. 2010, 88, 517–522. [Google Scholar]
- Miyamoto, T.; Ishii, K.; Asaka, R.; Suzuki, A.; Takatsu, A.; Kashima, H.; Shiozawa, T. Immunohistochemical expression of keratan sulfate: A possible diagnostic marker for carcinomas of the female genital tract. J. Clin. Pathol. 2011, 64, 1058. [Google Scholar] [CrossRef]
- Imagama, S.; Sakamoto, K.; Tauchi, R.; Shinjo, R.; Ohgomori, T.; Ito, Z.; Zhang, H.; Nishida, Y.; Asami, N.; Takeshita, S.; et al. Keratan sulfate restricts neural plasticity after spinal cord injury. J. Neurosci. 2011, 31, 17091–17102. [Google Scholar] [CrossRef]
- Jala, R.C.R.; Vudhgiri, S.; Kumar, C.G. A comprehensive review on natural occurrence, synthesis and biological activities of glycolipids. Carbohydr. Res. 2022, 516, 108556. [Google Scholar] [CrossRef] [PubMed]
- Wennekes, T.; van den Berg, R.J.B.H.N.; Boot, R.G.; van der Marel, G.A.; Overkleeft, H.S.; Aerts, J.M.F.G. Glycosphingolipids—Nature, Function, and Pharmacological Modulation. Angew. Chem. Int. Ed. 2009, 48, 8848–8869. [Google Scholar] [CrossRef]
- Pewzner-Jung, Y.; Ben-Dor, S.; Futerman, A.H. When Do Lasses (Longevity Assurance Genes) Become CerS (Ceramide Synthases)? Insights into the regulation of ceramide synthesis. J. Biol. Chem. 2006, 281, 25001–25005. [Google Scholar] [CrossRef] [PubMed]
- Schnaar, R.L.; Sandhoff, R.; Tiemeyer, M.; Kinoshita, T. Glycosphingolipids. In Essentials of Glycobiology [Internet], 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022. [Google Scholar]
- Platt, F.M. Sphingolipid lysosomal storage disorders. Nature 2014, 510, 68–75. [Google Scholar] [CrossRef]
- Yu, J.; Hung, J.-T.; Wang, S.-H.; Cheng, J.-Y.; Yu, A.L. Targeting glycosphingolipids for cancer immunotherapy. FEBS Lett. 2020, 594, 3602–3618. [Google Scholar] [CrossRef]
- Lou, Y.-W.; Wang, P.-Y.; Yeh, S.-C.; Chuang, P.-K.; Li, S.-T.; Wu, C.-Y.; Khoo, K.-H.; Hsiao, M.; Hsu, T.-L.; Wong, C.-H. Stage-specific embryonic antigen-4 as a potential therapeutic target in glioblastoma multiforme and other cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 2482–2487. [Google Scholar] [CrossRef]
- Belarbi, K.; Cuvelier, E.; Bonte, M.-A.; Desplanque, M.; Gressier, B.; Devos, D.; Chartier-Harlin, M.-C. Glycosphingolipids and neuroinflammation in Parkinson’s disease. Mol. Neurodegener. 2020, 15, 59. [Google Scholar] [CrossRef]
- Hao, C.; Zou, Q.; Bai, X.; Shi, W. Effect of Glycosylation on Protein Folding: From Biological Roles to Chemical Protein Synthesis. iScience 2025, 28, 112605. [Google Scholar] [CrossRef]
- Jayaprakash, N.G.; Surolia, A. Role of glycosylation in nucleating protein folding and stability. Biochem. J. 2017, 474, 2333–2347. [Google Scholar] [CrossRef]
- Parodi, A.J. Protein Glucosylation and Its Role in Protein Folding. Annu. Rev. Biochem. 2000, 69, 69–93. [Google Scholar] [CrossRef]
- Parodi, A.J. Role of N-oligosaccharide endoplasmic reticulum processing reactions in glycoprotein folding and degradation. Biochem. J. 2000, 348 Pt 1, 1–13. [Google Scholar] [CrossRef]
- Braakman, I.; Hebert, D.N. Protein folding in the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2013, 5, a013201. [Google Scholar] [CrossRef]
- Helenius, A.; Aebi, M. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 2004, 73, 1019–1049. [Google Scholar] [CrossRef]
- Braakman, I.; Bulleid, N.J. Protein Folding and Modification in the Mammalian Endoplasmic Reticulum. Annu. Rev. Biochem. 2011, 80, 71–99. [Google Scholar] [CrossRef] [PubMed]
- Wujek, P.; Kida, E.; Walus, M.; Wisniewski, K.E.; Golabek, A.A. N-Glycosylation Is Crucial for Folding, Trafficking, and Stability of Human Tripeptidyl-peptidase I. J. Biol. Chem. 2004, 279, 12827–12839. [Google Scholar] [CrossRef] [PubMed]
- Moharir, A.; Peck, S.H.; Budden, T.; Lee, S.Y. The role of N-glycosylation in folding, trafficking, and functionality of lysosomal protein CLN5. PLoS ONE 2013, 8, e74299. [Google Scholar] [CrossRef] [PubMed]
- Boscher, C.; Dennis, J.W.; Nabi, I.R. Glycosylation, galectins and cellular signaling. Curr. Opin. Cell Biol. 2011, 23, 383–392. [Google Scholar] [CrossRef]
- Gu, J.; Isaji, T.; Xu, Q.; Kariya, Y.; Gu, W.; Fukuda, T.; Du, Y. Potential roles of N-glycosylation in cell adhesion. Glycoconj. J. 2012, 29, 599–607. [Google Scholar] [CrossRef]
- Link-Lenczowski, P.; Bubka, M.; Balog, C.I.; Koeleman, C.A.; Butters, T.D.; Wuhrer, M.; Lityńska, A. The glycomic effect of N-acetylglucosaminyltransferase III overexpression in metastatic melanoma cells. GnT-III modifies highly branched N-glycans. Glycoconj. J. 2018, 35, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Goetze, A.M.; Liu, Y.D.; Zhang, Z.; Shah, B.; Lee, E.; Bondarenko, P.V.; Flynn, G.C. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology 2011, 21, 949–959. [Google Scholar] [CrossRef]
- Kanda, Y.; Yamada, T.; Mori, K.; Okazaki, A.; Inoue, M.; Kitajima-Miyama, K.; Kuni-Kamochi, R.; Nakano, R.; Yano, K.; Kakita, S.; et al. Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: The high-mannose, hybrid, and complex types. Glycobiology 2007, 17, 104–118. [Google Scholar] [CrossRef]
- Huang, L.; Biolsi, S.; Bales, K.R.; Kuchibhotla, U. Impact of variable domain glycosylation on antibody clearance: An LC/MS characterization. Anal. Biochem. 2006, 349, 197–207. [Google Scholar] [CrossRef]
- Newkirk, M.; Novick, J.; Stevenson, M.; Fournier, M.J.; Apostolakos, P. Differential clearance of glycoforms of IgG in normal and autoimmune-prone mice. Clin. Exp. Immunol. 1996, 106, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, X.; Attri, K.S.; Liu, C.; Li, L.; Herring, L.E.; Asara, J.M.; Lei, Y.L.; Singh, P.K.; Gao, C.; et al. O-GlcNAc transferase links glucose metabolism to MAVS-mediated antiviral innate immunity. Cell Host Microbe 2018, 24, 791–803.e6. [Google Scholar] [CrossRef]
- Song, N.; Qi, Q.; Cao, R.; Qin, B.; Wang, B.; Wang, Y.; Zhao, L.; Li, W.; Du, X.; Liu, F.; et al. MAVS O-GlcNAcylation is essential for host antiviral immunity against lethal RNA viruses. Cell Rep. 2019, 28, 2386–2396.e5. [Google Scholar] [CrossRef]
- Ramakrishnan, P.; Clark, P.M.; Mason, D.E.; Peters, E.C.; Hsieh-Wilson, L.C.; Baltimore, D. Activation of the transcriptional function of the NF-κB protein c-Rel by O-GlcNAc glycosylation. Sci. Signal. 2013, 6, ra75. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Weng, C.-L.; Lin, K.-I. O-GlcNAcylation and its role in the immune system. J. Biomed. Sci. 2020, 27, 57. [Google Scholar] [CrossRef]
- Hayes, A.J.; Melrose, J. Glycans and glycosaminoglycans in neurobiology: Key regulators of neuronal cell function and fate. Biochem. J. 2018, 475, 2511–2545. [Google Scholar] [CrossRef] [PubMed]
- Mountford, C.; Quadrelli, S.; Lin, A.; Ramadan, S. Six fucose-α (1–2) sugars and α-fucose assigned in the human brain using in vivo two-dimensional MRS. NMR Biomed. 2015, 28, 291–296. [Google Scholar] [CrossRef]
- Rutishauser, U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat. Rev. Neurosci. 2008, 9, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Ghanimi Fard, M.; Everest-Dass, A.; Packer, N.H.; Parker, L.M. Understanding cellular glycan surfaces in the central nervous system. Biochem. Soc. Trans. 2019, 47, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Haukedal, H.; Freude, K.K. Implications of Glycosylation in Alzheimer’s Disease. Front. Neurosci. 2020, 14, 625348. [Google Scholar] [CrossRef]
- Tu, C.-F.; Wu, M.-Y.; Lin, Y.-C.; Kannagi, R.; Yang, R.-B. FUT8 promotes breast cancer cell invasiveness by remodeling TGF-β receptor core fucosylation. Breast Cancer Res. 2017, 19, 111. [Google Scholar] [CrossRef]
- Al-Alem, L.; Prendergast, J.M.; Clark, J.; Zarrella, B.; Zarrella, D.T.; Hill, S.J.; Growdon, W.B.; Pooladanda, V.; Spriggs, D.R.; Cramer, D.; et al. Sialyl-Tn serves as a potential therapeutic target for ovarian cancer. J. Ovarian Res. 2024, 17, 71. [Google Scholar] [CrossRef]
- Nazha, B.; Inal, C.; Owonikoko, T.K. Disialoganglioside GD2 Expression in Solid Tumors and Role as a Target for Cancer Therapy. Front. Oncol. 2020, 10, 1000. [Google Scholar] [CrossRef]
- Feng, H.; Liang, L.; Deng, W.; Gao, J.; Li, X.; Guan, F. Sialyl Lewis X decorated integrin α3 on small extracellular vesicles promotes metastasis of bladder cancer via enhancing vascular permeability. Angiogenesis 2024, 27, 883–901. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, A.; Relvas-Santos, M.; Azevedo, R.; Santos, L.L.; Ferreira, J.A. Protein Glycosylation and Tumor Microenvironment Alterations Driving Cancer Hallmarks. Front. Oncol. 2019, 9, 380. [Google Scholar] [CrossRef]
- Schultz, M.J.; Swindall, A.F.; Bellis, S.L. Regulation of the metastatic cell phenotype by sialylated glycans. Cancer Metastasis Rev. 2012, 31, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Kurita, T.; Thi, T.N.; Koi, C.; Murakami, M.; Kagami, S.; Izumi, H.; Hachisuga, T. Expression of N-Acetylgalactosaminyltransferase-6 Is Related to Expression of Cell Adhesion Molecules in Endometrial Cancer. Anticancer Res. 2017, 37, 3905–3910. [Google Scholar]
- Sun, L.; Li, Z.; Shu, P.; Lu, M. N-acetylgalactosaminyltransferase GALNT6 is a potential therapeutic target of clear cell renal cell carcinoma progression. Cancer Sci. 2024, 115, 3320–3332. [Google Scholar] [CrossRef]
- Thomas, D.; Rathinavel, A.K.; Radhakrishnan, P. Altered glycosylation in cancer: A promising target for biomarkers and therapeutics. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188464. [Google Scholar] [CrossRef]
- Venkitachalam, S.; and Guda, K. Altered glycosyltransferases in colorectal cancer. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Venkitachalam, S.; Revoredo, L.; Varadan, V.; Fecteau, R.E.; Ravi, L.; Lutterbaugh, J.; Markowitz, S.D.; Willis, J.E.; Gerken, T.A.; Guda, K. Biochemical and functional characterization of glycosylation-associated mutational landscapes in colon cancer. Sci. Rep. 2016, 6, 23642. [Google Scholar] [CrossRef]
- Li, R.; Dong, X.; Chen, S.; Tan, J.; Chen, X.; Liu, J.; Wen, T.; Ru, X. Tn antigen promotes breast cancer metastasis via impairment of CASC4. Cell Biol. Int. 2023, 47, 1854–1867. [Google Scholar] [CrossRef]
- Rajesh, C.; Radhakrishnan, P. The (Sialyl) Tn antigen: Contributions to immunosuppression in gastrointestinal cancers. Front. Oncol. 2023, 12, 1093496. [Google Scholar] [CrossRef]
- Ma, M.; Guo, D.; Tan, Z.; Du, J.; Guan, F.; Li, X. Fucosyltransferase 8 regulation and breast cancer suppression by transcription factor activator protein 2γ. Cancer Sci. 2021, 112, 3190–3204. [Google Scholar] [CrossRef] [PubMed]
- Hait, N.C.; Maiti, A.; Wu, R.; Andersen, V.L.; Hsu, C.-C.; Wu, Y.; Chapla, D.G.; Takabe, K.; Rusiniak, M.E.; Bshara, W.; et al. Extracellular sialyltransferase st6gal1 in breast tumor cell growth and invasiveness. Cancer Gene Ther. 2022, 29, 1662–1675. [Google Scholar] [CrossRef]
- Gc, S.; Tuy, K.; Rickenbacker, L.; Jones, R.; Chakraborty, A.; Miller, C.R.; Beierle, E.A.; Hanumanthu, V.S.; Tran, A.N.; Mobley, J.A.; et al. α2, 6 Sialylation mediated by ST6GAL1 promotes glioblastoma growth. JCI Insight 2022, 7, e158799. [Google Scholar] [CrossRef] [PubMed]
- Kurz, E.; Chen, S.; Vucic, E.; Baptiste, G.; Loomis, C.; Agrawal, P.; Hajdu, C.; Bar-Sagi, D.; Mahal, L.K. Integrated systems analysis of the murine and human pancreatic cancer glycomes reveals a tumor-promoting role for ST6GAL1. Mol. Cell. Proteom. 2021, 20, 100160. [Google Scholar] [CrossRef]
- Takada, A.; Ohmori, K.; Yoneda, T.; Tsuyuoka, K.; Hasegawa, A.; Kiso, M.; Kannagi, R. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res. 1993, 53, 354–361. [Google Scholar]
- Liu, L.; Liu, L.; Wang, Y.; Fang, Z.; Bian, Y.; Zhang, W.; Wang, Z.; Gao, X.; Zhao, C.; Tian, M.; et al. Robust Glycoproteomics Platform Reveals a Tetra-Antennary Site-Specific Glycan Capping with Sialyl-Lewis Antigen for Early Detection of Gastric Cancer. Adv. Sci. 2024, 11, 2306955. [Google Scholar] [CrossRef] [PubMed]
- He, X.-F.; Hu, X.; Wen, G.-J.; Wang, Z.; Lin, W.-J. O-GlcNAcylation in cancer development and immunotherapy. Cancer Lett. 2023, 566, 216258. [Google Scholar] [CrossRef]
- Sun, L.; Lv, S.; Song, T. O-GlcNAcylation links oncogenic signals and cancer epigenetics. Discov. Oncol. 2021, 12, 54. [Google Scholar] [CrossRef]
- Galle, P.R.; Foerster, F.; Kudo, M.; Chan, S.L.; Llovet, J.M.; Qin, S.; Schelman, W.R.; Chintharlapalli, S.; Abada, P.B.; Sherman, M.; et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019, 39, 2214–2229. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Y.; Lu, H.; Ying, J.; Zhao, H.; Cai, J. Serum alpha-fetoprotein as a predictive biomarker for tissue alpha-fetoprotein status and prognosis in patients with hepatocellular carcinoma. Transl. Cancer Res. 2022, 11, 669. [Google Scholar] [CrossRef]
- Scholler, N.; Urban, N. CA125 in ovarian cancer. Biomark. Med. 2007, 1, 513–523. [Google Scholar] [CrossRef]
- Zhang, R.; Siu, M.K.Y.; Ngan, H.Y.S.; Chan, K.K.L. Molecular biomarkers for the early detection of ovarian cancer. Int. J. Mol. Sci. 2022, 23, 12041. [Google Scholar] [CrossRef]
- Lee, T.-H.; Kim, J.-S.; Baek, S.-J.; Kwak, J.-M.; Kim, J. Diagnostic accuracy of carcinoembryonic antigen (CEA) in detecting colorectal cancer recurrence depending on its preoperative level. J. Gastrointest. Surg. 2023, 27, 1694–1701. [Google Scholar] [CrossRef]
- Moertel, C.G.; Fleming, T.R.; Macdonald, J.S.; Haller, D.G.; Laurie, J.A.; Tangen, C. An evaluation of the carcinoembryonic antigen (CEA) test for monitoring patients with resected colon cancer. Jama 1993, 270, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Moradi, A.; Srinivasan, S.; Clements, J.; Batra, J. Beyond the biomarker role: Prostate-specific antigen (PSA) in the prostate cancer microenvironment. Cancer Metastasis Rev. 2019, 38, 333–346. [Google Scholar] [CrossRef]
- Luo, G.; Jin, K.; Deng, S.; Cheng, H.; Fan, Z.; Gong, Y.; Qian, Y.; Huang, Q.; Ni, Q.; Liu, C.; et al. Roles of CA19-9 in pancreatic cancer: Biomarker, predictor and promoter. Biochim. Biophys. Acta-Rev. Cancer 2021, 1875, 188409. [Google Scholar] [CrossRef] [PubMed]
- Angerstein, A.O.; Young, L.E.A.; Thanasupawat, T.; Vriend, J.; Grimsley, G.; Lun, X.; Senger, D.L.; Sinha, N.; Beiko, J.; Pitz, M.; et al. Distinct spatial N-glycan profiles reveal glioblastoma-specific signatures. J. Pathol. 2025, 265, 486–501. [Google Scholar] [CrossRef]
- Silsirivanit, A.; Alvarez, M.R.S.; Grijaldo-Alvarez, S.J.; Gogte, R.; Kitkhuandee, A.; Piyawattanametha, N.; Seubwai, W.; Luang, S.; Panawan, O.; Mahalapbutr, P.; et al. Serum N-Glycomics with Nano-LC-QToF LC-MS/MS Reveals N-Glycan Biomarkers for Glioblastoma, Meningioma, and High-Grade Meningioma. J. Proteome Res. 2025, 24, 1402–1413. [Google Scholar] [CrossRef]
- Cuello, H.A.; Ferreira, G.M.; Gulino, C.A.; Toledo, A.G.; Segatori, V.I.; Gabri, M.R. Terminally sialylated and fucosylated complex N-glycans are involved in the malignant behavior of high-grade glioma. Oncotarget 2020, 11, 4822. [Google Scholar] [CrossRef]
- Mittal, P.; Briggs, M.; Klingler-Hoffmann, M.; Kaur, G.; Packer, N.H.; Oehler, M.K.; Hoffmann, P. Altered N-linked glycosylation in endometrial cancer. Anal. Bioanal. Chem. 2021, 413, 2721–2733. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, Y.; Wang, X.; Yan, B.; Lou, W.; Di, W. Serum immunoglobulin G N-glycome: A potential biomarker in endometrial cancer. Ann. Transl. Med. 2020, 8, 748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cao, Z.; Wang, J.; Li, Z.; Wang, T.; Xiang, Y. Serum protein N-glycome patterns reveal alterations associated with endometrial cancer and its phenotypes of differentiation. Front. Endocrinol. 2023, 14, 1157487. [Google Scholar] [CrossRef]
- Kyselova, Z.; Mechref, Y.; Kang, P.; Goetz, J.A.; Dobrolecki, L.E.; Sledge, G.W.; Schnaper, L.; Hickey, R.J.; Malkas, L.H.; Novotny, M.V. Breast Cancer Diagnosis and Prognosis through Quantitative Measurements of Serum Glycan Profiles. Clin. Chem. 2008, 54, 1166–1175. [Google Scholar] [CrossRef]
- Vreeker, G.C.M.; Vangangelt, K.M.H.; Bladergroen, M.R.; Nicolardi, S.; Mesker, W.E.; Wuhrer, M.; van der Burgt, Y.E.M.; Tollenaar, R.A.E.M. Serum N-glycan profiles differ for various breast cancer subtypes. Glycoconj. J. 2021, 38, 387–395. [Google Scholar] [CrossRef]
- Goldman, R.; Ressom, H.W.; Varghese, R.S.; Goldman, L.; Bascug, G.; Loffredo, C.A.; Abdel-Hamid, M.; Gouda, I.; Ezzat, S.; Kyselova, Z. Detection of hepatocellular carcinoma using glycomic analysis. Clin. Cancer Res. 2009, 15, 1808–1813. [Google Scholar] [CrossRef]
- Cheng, L.; Gao, S.; Song, X.; Dong, W.; Zhou, H.; Zhao, L.; Jia, L. Comprehensive N-glycan profiles of hepatocellular carcinoma reveal association of fucosylation with tumor progression and regulation of FUT8 by microRNAs. Oncotarget 2016, 7, 61199. [Google Scholar] [CrossRef]
- Feng, D.; Shaikh, A.S.; Wang, F. Recent Advance in Tumor-associated Carbohydrate Antigens (TACAs)-based Antitumor Vaccines. ACS Chem. Biol. 2016, 11, 850–863. [Google Scholar] [CrossRef]
- Monzavi-Karbassi, B.; Pashov, A.; Kieber-Emmons, T. Tumor-Associated Glycans and Immune Surveillance. Vaccines 2013, 1, 174–203. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Mantuano, N.; Natoli, M.; Zippelius, A.; Läubli, H. Tumor-associated carbohydrates and immunomodulatory lectins as targets for cancer immunotherapy. J. Immunother. Cancer 2020, 8, e001222. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.; Gnanapragassam, V.S.; Jain, M.; Rachagani, S.; Ponnusamy, M.P.; Batra, S.K. Pathobiological implications of mucin glycans in cancer: Sweet poison and novel targets. Biochim. Biophys. Acta 2015, 1856, 211–225. [Google Scholar] [CrossRef]
- Yin, Z.; Huang, X. Recent Development in Carbohydrate Based Anti-cancer Vaccines. J. Carbohydr. Chem. 2012, 31, 143–186. [Google Scholar] [CrossRef]
- Wang, Y.; Ju, T.; Ding, X.; Xia, B.; Wang, W.; Xia, L.; He, M.; Cummings, R.D. Cosmc is an essential chaperone for correct protein O-glycosylation. Proc. Natl. Acad. Sci. USA 2010, 107, 9228–9233. [Google Scholar] [CrossRef]
- Munkley, J. The Role of Sialyl-Tn in Cancer. Int. J. Mol. Sci. 2016, 17, 275. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yang, H.; Peng, L.; Kuhn, C.; Chelariu-Raicu, A.; Mahner, S.; Jeschke, U.; von Schönfeldt, V. Expression of the Carbohydrate Lewis Antigen, Sialyl Lewis A, Sialyl Lewis X, Lewis X, and Lewis Y in the Placental Villi of Patients with Unexplained Miscarriages. Front. Immunol. 2021, 12, 679424. [Google Scholar] [CrossRef]
- Blanas, A.; Sahasrabudhe, N.M.; Rodríguez, E.; van Kooyk, Y.; van Vliet, S.J. Fucosylated Antigens in Cancer: An Alliance toward Tumor Progression, Metastasis, and Resistance to Chemotherapy. Front. Oncol. 2018, 8, 39. [Google Scholar] [CrossRef]
- Eller, C.H.; Chao, T.Y.; Singarapu, K.K.; Ouerfelli, O.; Yang, G.; Markley, J.L.; Danishefsky, S.J.; Raines, R.T. Human Cancer Antigen Globo H Is a Cell-Surface Ligand for Human Ribonuclease 1. ACS Cent. Sci. 2015, 1, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Sigal, D.S.; Hermel, D.J.; Hsu, P.; Pearce, T. The role of Globo H and SSEA-4 in the Development and Progression of Cancer, and their Potential as Therapeutic Targets. Future Oncol. 2022, 18, 117–134. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Lan, R.; Liu, P.; Xiong, K.; Teng, H.; Tao, L.; Yu, S.; Han, G. Transmembrane mucins in lung adenocarcinoma: Understanding of current molecular mechanisms and clinical applications. Cell Death Discov. 2025, 11, 163. [Google Scholar] [CrossRef]
- Mercanoglu, B.; Karstens, K.F.; Giannou, A.D.; Meiners, J.; Lücke, J.; Seeger, P.; Brackrock, V.; Güngör, C.; Izbicki, J.R.; Bockhorn, M.; et al. A Comprehensive Analysis of Tn and STn Antigen Expression in Esophageal Adenocarcinoma. Cancers 2024, 16, 240. [Google Scholar] [CrossRef] [PubMed]
- Indellicato, R.; Zulueta, A.; Caretti, A.; Trinchera, M. Complementary Use of Carbohydrate Antigens Lewis a, Lewis b, and Sialyl-Lewis a (CA19.9 Epitope) in Gastrointestinal Cancers: Biological Rationale Towards A Personalized Clinical Application. Cancers 2020, 12, 1509. [Google Scholar] [CrossRef]
- Kim, Y.S.; Yuan, M.; Itzkowitz, S.H.; Sun, Q.B.; Kaizu, T.; Palekar, A.; Trump, B.F.; Hakomori, S. Expression of LeY and extended LeY blood group-related antigens in human malignant, premalignant, and nonmalignant colonic tissues. Cancer Res. 1986, 46, 5985–5992. [Google Scholar]
- Hsu, H.T.; Kuo, T.M.; Wei, C.Y.; Huang, J.Y.; Liu, T.W.; Hsing, M.T.; Lai, M.T.; Chen, C.T. Investigation of the impact of Globo-H expression on the progression of gastric cancer. Am. J. Cancer Res. 2023, 13, 2969–2983. [Google Scholar] [PubMed]
- Chang, W.W.; Lee, C.H.; Lee, P.; Lin, J.; Hsu, C.W.; Hung, J.T.; Lin, J.J.; Yu, J.C.; Shao, L.E.; Yu, J.; et al. Expression of Globo H and SSEA3 in breast cancer stem cells and the involvement of fucosyl transferases 1 and 2 in Globo H synthesis. Proc. Natl. Acad. Sci. USA 2008, 105, 11667–11672. [Google Scholar] [CrossRef]
- Cheng, J.J.; Matsumoto, Y.; Dombek, G.E.; Stackhouse, K.A.; Ore, A.S.; Glickman, J.N.; Heimburg-Molinaro, J.; Cummings, R.D. Differential expression of CD175 and CA19-9 in pancreatic adenocarcinoma. Sci. Rep. 2025, 15, 4177. [Google Scholar] [CrossRef]
- Madunić, K.; Mayboroda, O.A.; Zhang, T.; Weber, J.; Boons, G.J.; Morreau, H.; van Vlierberghe, R.; van Wezel, T.; Lageveen-Kammeijer, G.S.M.; Wuhrer, M. Specific (sialyl-)Lewis core 2 O-glycans differentiate colorectal cancer from healthy colon epithelium. Theranostics 2022, 12, 4498–4512. [Google Scholar] [CrossRef]
- Guo, H.B.; Johnson, H.; Randolph, M.; Pierce, M. Regulation of homotypic cell-cell adhesion by branched N-glycosylation of N-cadherin extracellular EC2 and EC3 domains. J. Biol. Chem. 2009, 284, 34986–34997. [Google Scholar] [CrossRef]
- Harosh-Davidovich, S.B.; Khalaila, I. O-GlcNAcylation affects β-catenin and E-cadherin expression, cell motility and tumorigenicity of colorectal cancer. Exp. Cell Res. 2018, 364, 42–49. [Google Scholar] [CrossRef]
- Hamester, F.; Legler, K.; Wichert, B.; Kelle, N.; Eylmann, K.; Rossberg, M.; Ding, Y.; Kürti, S.; Schmalfeldt, B.; Milde-Langosch, K.; et al. Prognostic relevance of the Golgi mannosidase MAN1A1 in ovarian cancer: Impact of N-glycosylation on tumour cell aggregation. Br. J. Cancer 2019, 121, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.; Catarino, T.A.; Dias, A.M.; Kato, M.; Almeida, A.; Hessling, B.; Figueiredo, J.; Gärtner, F.; Sanches, J.M.; Ruppert, T.; et al. Preventing E-cadherin aberrant N-glycosylation at Asn-554 improves its critical function in gastric cancer. Oncogene 2016, 35, 1619–1631. [Google Scholar] [CrossRef]
- Geng, F.; Shi, B.Z.; Yuan, Y.F.; Wu, X.Z. The expression of core fucosylated E-cadherin in cancer cells and lung cancer patients: Prognostic implications. Cell Res. 2004, 14, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gao, Z.; Yue, L. Fucosyltransferase 8 deficiency suppresses breast cancer cell migration by interference of the FAK/integrin pathway. Cancer Biomark. 2019, 25, 303–311. [Google Scholar] [CrossRef]
- Reticker-Flynn, N.E.; Bhatia, S.N. Aberrant glycosylation promotes lung cancer metastasis through adhesion to galectins in the metastatic niche. Cancer Discov. 2015, 5, 168–181. [Google Scholar] [CrossRef]
- Chen, X.; Sandrine, I.K.; Yang, M.; Tu, J.; Yuan, X. MUC1 and MUC16: Critical for immune modulation in cancer therapeutics. Front. Immunol. 2024, 15, 1356913. [Google Scholar] [CrossRef]
- Julien, S.; Ivetic, A.; Grigoriadis, A.; QiZe, D.; Burford, B.; Sproviero, D.; Picco, G.; Gillett, C.; Papp, S.L.; Schaffer, L.; et al. Selectin ligand sialyl-Lewis x antigen drives metastasis of hormone-dependent breast cancers. Cancer Res. 2011, 71, 7683–7693. [Google Scholar] [CrossRef]
- Gianchecchi, E.; Arena, A.; Fierabracci, A. Sialic acid-siglec axis in human immune regulation, involvement in autoimmunity and cancer and potential therapeutic treatments. Int. J. Mol. Sci. 2021, 22, 5774. [Google Scholar] [CrossRef]
- Yu, X.; Qian, J.; Ding, L.; Yin, S.; Zhou, L.; Zheng, S. Galectin-1: A Traditionally Immunosuppressive Protein Displays Context-Dependent Capacities. Int. J. Mol. Sci. 2023, 24, 6501. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, H.C.; Zhao, J.; Wu, M.H.; Shih, T.C. Immunosuppressive Roles of Galectin-1 in the Tumor Microenvironment. Biomolecules 2021, 11, 1398. [Google Scholar] [CrossRef] [PubMed]
- Very, N.; Lefebvre, T.; El Yazidi-Belkoura, I. Drug resistance related to aberrant glycosylation in colorectal cancer. Oncotarget 2018, 9, 1380–1402. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.A.H.; Bertozzi, C.R. The clinical impact of glycobiology: Targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discov. 2021, 20, 217–243. [Google Scholar] [CrossRef]
- Goode, E.A.; Orozco-Moreno, M.; Hodgson, K.; Nabilah, A.; Murali, M.; Peng, Z.; Merx, J.; Rossing, E.; Pijnenborg, J.F.A.; Boltje, T.J.; et al. Sialylation Inhibition Can Partially Revert Acquired Resistance to Enzalutamide in Prostate Cancer Cells. Cancers 2024, 16, 2953. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gopal, S.; Pocock, R.; Xiao, Z. Glycan mimetics from natural products: New therapeutic opportunities for neurodegenerative disease. Molecules 2019, 24, 4604. [Google Scholar] [CrossRef] [PubMed]
- Akasaka-Manya, K.; Manya, H.; Sakurai, Y.; Wojczyk, B.S.; Kozutsumi, Y.; Saito, Y.; Taniguchi, N.; Murayama, S.; Spitalnik, S.L.; Endo, T. Protective effect of N-glycan bisecting GlcNAc residues on β-amyloid production in Alzheimer’s disease. Glycobiology 2010, 20, 99–106. [Google Scholar] [CrossRef]
- Tena, J.; Maezawa, I.; Barboza, M.; Wong, M.; Zhu, C.; Alvarez, M.R.; Jin, L.-W.; Zivkovic, A.M.; Lebrilla, C.B. Regio-specific N-glycome and N-glycoproteome map of the elderly human brain with and without Alzheimer’s disease. Mol. Cell. Proteom. 2022, 21, 100427. [Google Scholar] [CrossRef]
- Frenkel-Pinter, M.; Shmueli, M.D.; Raz, C.; Yanku, M.; Zilberzwige, S.; Gazit, E.; Segal, D. Interplay between protein glycosylation pathways in Alzheimer’s disease. Sci. Adv. 2017, 3, e1601576. [Google Scholar] [CrossRef]
- Gaunitz, S.; Tjernberg, L.O.; Schedin-Weiss, S. The N-glycan profile in cortex and hippocampus is altered in Alzheimer disease. J. Neurochem. 2021, 159, 292–304. [Google Scholar] [CrossRef]
- Quaranta, A.; Karlsson, I.; Ndreu, L.; Marini, F.; Ingelsson, M.; Thorsén, G. Glycosylation profiling of selected proteins in cerebrospinal fluid from Alzheimer’s disease and healthy subjects. Anal. Methods 2019, 11, 3331–3340. [Google Scholar] [CrossRef]
- Liang, C.; Yuan, Z.; Yang, S.; Zhu, Y.; Chen, Z.; Can, D.; Lei, A.; Li, H.; Leng, L.; Zhang, J. Mannose Promotes β-Amyloid Pathology by Regulating BACE1 Glycosylation in Alzheimer’s Disease. Adv. Sci. 2025, 12, 2409105. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, C.; Chin, L.-S.; Pan, S.; Li, L. Human brain glycoform coregulation network and glycan modification alterations in Alzheimer’s disease. Sci. Adv. 2024, 10, eadk6911. [Google Scholar] [CrossRef]
- Losev, Y.; Paul, A.; Frenkel-Pinter, M.; Abu-Hussein, M.; Khalaila, I.; Gazit, E.; Segal, D. Novel model of secreted human tau protein reveals the impact of the abnormal N-glycosylation of tau on its aggregation propensity. Sci. Rep. 2019, 9, 2254. [Google Scholar] [CrossRef] [PubMed]
- Rawal, P.; Zhao, L. Sialometabolism in Brain Health and Alzheimer’s Disease. Front. Neurosci. 2021, 15, 648617. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.G.; Veillon, L.; Mechref, Y. N-Glycan Profile of Cerebrospinal Fluids from Alzheimer’s Disease Patients Using Liquid Chromatography with Mass Spectrometry. J. Proteome Res. 2019, 18, 3770–3779. [Google Scholar] [CrossRef]
- Levine, P.M.; Galesic, A.; Balana, A.T.; Mahul-Mellier, A.-L.; Navarro, M.X.; De Leon, C.A.; Lashuel, H.A.; Pratt, M.R. α-Synuclein O-GlcNAcylation alters aggregation and toxicity, revealing certain residues as potential inhibitors of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2019, 116, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Wani, W.Y.; Ouyang, X.; Benavides, G.A.; Redmann, M.; Cofield, S.S.; Shacka, J.J.; Chatham, J.C.; Darley-Usmar, V.; Zhang, J. O-GlcNAc regulation of autophagy and α-synuclein homeostasis; implications for Parkinson’s disease. Mol. Brain 2017, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Videira, P.A.Q.; Castro-Caldas, M. Linking glycation and glycosylation with inflammation and mitochondrial dysfunction in Parkinson’s disease. Front. Neurosci. 2018, 12, 381. [Google Scholar] [CrossRef]
- Schneider, J.S.; Singh, G. Altered expression of glycobiology-related genes in Parkinson’s disease brain. Front. Mol. Neurosci. 2022, 15, 1078854. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Jin, H.; Wu, Z.; Han, Y.; Chen, J.; Mao, C.; Hao, P.; Zhang, X.; Liu, C.-F.; Yang, S. Mass spectrometry-based analysis of serum N-glycosylation changes in patients with Parkinson’s disease. ACS Chem. Neurosci. 2022, 13, 1719–1726. [Google Scholar] [CrossRef]
- Xu, M.; Jin, H.; Ge, W.; Zhao, L.; Liu, Z.; Guo, Z.; Wu, Z.; Chen, J.; Mao, C.; Zhang, X.; et al. Mass spectrometric analysis of urinary N-glycosylation changes in patients with Parkinson’s disease. ACS Chem. Neurosci. 2023, 14, 3507–3517. [Google Scholar] [CrossRef]
- Rebelo, A.L.; Chevalier, M.T.; Russo, L.; Pandit, A. Role and therapeutic implications of protein glycosylation in neuroinflammation. Trends Mol. Med. 2022, 28, 270–289. [Google Scholar] [CrossRef]
- Puigdellívol, M.; Allendorf, D.H.; Brown, G.C. Sialylation and galectin-3 in microglia-mediated neuroinflammation and neurodegeneration. Front. Cell. Neurosci. 2020, 14, 162. [Google Scholar] [CrossRef]
- Kizuka, Y.; Nakano, M.; Kitazume, S.; Saito, T.; Saido, T.C.; Taniguchi, N. Bisecting GlcNAc modification stabilizes BACE1 protein under oxidative stress conditions. Biochem. J. 2016, 473, 21–30. [Google Scholar] [CrossRef]
- Balana, A.T.; Pratt, M.R. Mechanistic roles for altered O-GlcNAcylation in neurodegenerative disorders. Biochem. J. 2021, 478, 2733–2758. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, M.; Yin, X.; Chen, K.; Hu, Z.; Zhou, Q.; Cao, X.; Chen, Z.; Liu, D. The role of pathological tau in synaptic dysfunction in Alzheimer’s diseases. Transl. Neurodegener. 2021, 10, 45. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef]
- Wielgat, P.; Braszko, J.J. Significance of the cell adhesion molecules and sialic acid in neurodegeneration. Adv. Med. Sci. 2012, 57, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Varbanov, H.; Dityatev, A. Regulation of extrasynaptic signaling by polysialylated NCAM: Impact for synaptic plasticity and cognitive functions. Mol. Cell. Neurosci. 2017, 81, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Fan, Q.; Zhou, J.; Vassar, R. Inhibiting BACE1 to reverse synaptic dysfunctions in Alzheimer’s disease. Neurosci. Biobehav. Rev. 2016, 65, 326–340. [Google Scholar] [CrossRef]
- Chahinian, H.; Yahi, N.; Fantini, J. Glutamate, Gangliosides, and the Synapse: Electrostatics at Work in the Brain. Int. J. Mol. Sci. 2024, 25, 8583. [Google Scholar] [CrossRef] [PubMed]
- Nachman, E.; Verstreken, P. Synaptic proteostasis in Parkinson’s disease. Curr. Opin. Neurobiol. 2022, 72, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, L. α-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef]
- Palmano, K.; Rowan, A.; Guillermo, R.; Guan, J.; Mc Jarrow, P. The role of gangliosides in neurodevelopment. Nutrients 2015, 7, 3891–3913. [Google Scholar] [CrossRef]
- Chatham, J.C.; Patel, R.P. Protein glycosylation in cardiovascular health and disease. Nat. Rev. Cardiol. 2024, 21, 525–544. [Google Scholar] [CrossRef]
- Ferro, F.; Spelat, R.; Pandit, A.; Martin-Ventura, J.L.; Rabinovich, G.A.; Contessotto, P. Glycosylation of blood cells during the onset and progression of atherosclerosis and myocardial infarction. Trends Mol. Med. 2024, 30, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Gudelj, I.; Lauc, G. Protein N-glycosylation in cardiovascular diseases and related risk factors. Curr. Cardiovasc. Risk Rep. 2018, 12, 16. [Google Scholar] [CrossRef]
- Menni, C.; Gudelj, I.; Macdonald-Dunlop, E.; Mangino, M.; Zierer, J.; Bešić, E.; Joshi, P.K.; Trbojević-Akmačić, I.; Chowienczyk, P.J.; Spector, T.D.; et al. Glycosylation Profile of Immunoglobulin G Is Cross-Sectionally Associated with Cardiovascular Disease Risk Score and Subclinical Atherosclerosis in Two Independent Cohorts. Circ. Res. 2018, 122, 1555–1564. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, Z.; Wang, A.; Ge, S.; Wang, H.; Zhang, X.; Sun, Q.; Cao, W.; Sun, M.; Wu, L.; et al. Ischemic stroke is associated with the pro-inflammatory potential of N-glycosylated immunoglobulin G. J. Neuroinflamm. 2018, 15, 123. [Google Scholar] [CrossRef]
- Wattchow, N.E.; Pullen, B.J.; Indraratna, A.D.; Nankivell, V.; Everest-Dass, A.; Psaltis, P.J.; Kolarich, D.; Nicholls, S.J.; Packer, N.H.; Bursill, C.A. The emerging role of glycans and the importance of sialylation in cardiovascular disease. Atherosclerosis 2025, 403, 119172. [Google Scholar] [CrossRef]
- Ngoh, G.A.; Facundo, H.T.; Zafir, A.; Jones, S.P. O-GlcNAc signaling in the cardiovascular system. Circ. Res. 2010, 107, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Lunde, I.G.; Aronsen, J.M.; Kvaløy, H.; Qvigstad, E.; Sjaastad, I.; Tønnessen, T.; Christensen, G.; Grønning-Wang, L.M.; Carlson, C.R. Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure. Physiol. Genom. 2012, 44, 162–172. [Google Scholar] [CrossRef]
- Shaheen, W.A.; Quraishi, M.N.; Iqbal, T.H. Gut microbiome and autoimmune disorders. Clin. Exp. Immunol. 2022, 209, 161–174. [Google Scholar] [CrossRef]
- M’Koma, A.E. The Multifactorial Etiopathogeneses Interplay of Inflammatory Bowel Disease: An Overview. Gastrointest. Disord. 2019, 1, 75–105. [Google Scholar] [CrossRef] [PubMed]
- Ghodke-Puranik, Y.; Olferiev, M.; Crow, M.K. Systemic lupus erythematosus genetics: Insights into pathogenesis and implications for therapy. Nat. Rev. Rheumatol. 2024, 20, 635–648. [Google Scholar] [CrossRef]
- Xu, H.; Liu, M.; Cao, J.; Li, X.; Fan, D.; Xia, Y.; Lu, X.; Li, J.; Ju, D.; Zhao, H. The Dynamic Interplay between the Gut Microbiota and Autoimmune Diseases. J. Immunol. Res. 2019, 2019, 7546047. [Google Scholar] [CrossRef]
- Rosser, E.C.; Mauri, C. A clinical update on the significance of the gut microbiota in systemic autoimmunity. J. Autoimmun. 2016, 74, 85–93. [Google Scholar] [CrossRef]
- Baum, L.G.; Cobb, B.A. The direct and indirect effects of glycans on immune function. Glycobiology 2017, 27, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Radovani, B.; Gudelj, I. N-Glycosylation and Inflammation; the Not-So-Sweet Relation. Front. Immunol. 2022, 13, 893365. [Google Scholar] [CrossRef] [PubMed]
- Cummings, R.D. Stuck on sugars-how carbohydrates regulate cell adhesion, recognition, and signaling. Glycoconj. J. 2019, 36, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Gravallese, E.M.; Firestein, G.S. Rheumatoid Arthritis-Common Origins, Divergent Mechanisms. N. Engl. J. Med. 2023, 388, 529–542. [Google Scholar] [CrossRef]
- Di Matteo, A.; Bathon, J.M.; Emery, P. Rheumatoid arthritis. Lancet 2023, 402, 2019–2033. [Google Scholar] [CrossRef]
- Smith, M.H.; Berman, J.R. What Is Rheumatoid Arthritis? Jama 2022, 327, 1194. [Google Scholar] [CrossRef]
- Komatsu, N.; Takayanagi, H. Mechanisms of joint destruction in rheumatoid arthritis-immune cell-fibroblast-bone interactions. Nat. Rev. Rheumatol. 2022, 18, 415–429. [Google Scholar] [CrossRef]
- Parekh, R.; Isenberg, D.; Rook, G.; Roitt, I.; Dwek, R.; Rademacher, T. A comparative analysis of disease-associated changes in the galactosylation of serum IgG. J. Autoimmun. 1989, 2, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Parekh, R.B.; Dwek, R.A.; Sutton, B.J.; Fernandes, D.L.; Leung, A.; Stanworth, D.; Rademacher, T.W.; Mizuochi, T.; Taniguchi, T.; Matsuta, K.; et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 1985, 316, 452–457. [Google Scholar] [CrossRef]
- Noris, M.; Remuzzi, G. Overview of complement activation and regulation. Semin. Nephrol. 2013, 33, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Xie, Q.; Wang, Y.; Li, Y. Abberant Immunoglobulin G Glycosylation in Rheumatoid Arthritis by LTQ-ESI-MS. Int. J. Mol. Sci. 2020, 21, 2045. [Google Scholar] [CrossRef]
- Huang, C.; Liu, Y.; Wu, H.; Sun, D.; Li, Y. Characterization of IgG glycosylation in rheumatoid arthritis patients by MALDI-TOF-MS(n) and capillary electrophoresis. Anal. Bioanal. Chem. 2017, 409, 3731–3739. [Google Scholar] [CrossRef]
- Rook, G.A.; Steele, J.; Brealey, R.; Whyte, A.; Isenberg, D.; Sumar, N.; Nelson, J.L.; Bodman, K.B.; Young, A.; Roitt, I.M.; et al. Changes in IgG glycoform levels are associated with remission of arthritis during pregnancy. J. Autoimmun. 1991, 4, 779–794. [Google Scholar] [CrossRef]
- Kissel, T.; Toes, R.E.M.; Huizinga, T.W.J.; Wuhrer, M. Glycobiology of rheumatic diseases. Nat. Rev. Rheumatol. 2023, 19, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Shkunnikova, S.; Mijakovac, A.; Sironic, L.; Hanic, M.; Lauc, G.; Kavur, M.M. IgG glycans in health and disease: Prediction, intervention, prognosis, and therapy. Biotechnol. Adv. 2023, 67, 108169. [Google Scholar] [CrossRef]
- Li, D.; Lou, Y.; Zhang, Y.; Liu, S.; Li, J.; Tao, J. Sialylated immunoglobulin G: A promising diagnostic and therapeutic strategy for autoimmune diseases. Theranostics 2021, 11, 5430–5446. [Google Scholar] [CrossRef] [PubMed]
- Vattepu, R.; Sneed, S.L.; Anthony, R.M. Sialylation as an Important Regulator of Antibody Function. Front. Immunol. 2022, 13, 818736. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Sanda, M.; Wu, J.; Koomen, J.; Goldman, R. Quantitative analysis of immunoglobulin subclasses and subclass specific glycosylation by LC-MS-MRM in liver disease. J. Proteom. 2015, 116, 24–33. [Google Scholar] [CrossRef]
- Sanda, M.; Pompach, P.; Brnakova, Z.; Wu, J.; Makambi, K.; Goldman, R. Quantitative liquid chromatography-mass spectrometry-multiple reaction monitoring (LC-MS-MRM) analysis of site-specific glycoforms of haptoglobin in liver disease. Mol. Cell. Proteom. 2013, 12, 1294–1305. [Google Scholar] [CrossRef]
- Ma, J.; Sanda, M.; Wei, R.; Zhang, L.; Goldman, R. Quantitative analysis of core fucosylation of serum proteins in liver diseases by LC-MS-MRM. J. Proteom. 2018, 189, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Yau, L.F.; Liu, J.; Jiang, M.; Bai, G.; Wang, J.R.; Jiang, Z.H. An integrated approach for comprehensive profiling and quantitation of IgG-Fc glycopeptides with application to rheumatoid arthritis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1122–1123, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Zhu, R.; Hammoud, Z.T.; Mechref, Y. LC-MS/MS quantitation of esophagus disease blood serum glycoproteins by enrichment with hydrazide chemistry and lectin affinity chromatography. J. Proteome Res. 2014, 13, 4808–4820. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Liu, Q.; Xu, X.; Qin, W.; Pan, Y.; Qin, R.; Zhao, R.; Gu, Y.; Gu, J.; Ren, S. Relative Quantitation of Subclass-Specific Murine IgG Fc N-Glycoforms by Multiple Reaction Monitoring. ACS Omega 2020, 5, 8564–8571. [Google Scholar] [CrossRef]
- Pilkington, C.; Yeung, E.; Isenberg, D.; Lefvert, A.K.; Rook, G.A. Agalactosyl IgG and antibody specificity in rheumatoid arthritis, tuberculosis, systemic lupus erythematosus and myasthenia gravis. Autoimmunity 1995, 22, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Tomana, M.; Schrohenloher, R.E.; Reveille, J.D.; Arnett, F.C.; Koopman, W.J. Abnormal galactosylation of serum IgG in patients with systemic lupus erythematosus and members of families with high frequency of autoimmune diseases. Rheumatol. Int. 1992, 12, 191–194. [Google Scholar] [CrossRef]
- Vučković, F.; Krištić, J.; Gudelj, I.; Teruel, M.; Keser, T.; Pezer, M.; Pučić-Baković, M.; Štambuk, J.; Trbojević-Akmačić, I.; Barrios, C.; et al. Association of systemic lupus erythematosus with decreased immunosuppressive potential of the IgG glycome. Arthritis Rheumatol. 2015, 67, 2978–2989. [Google Scholar] [CrossRef]
- Magorivska, I.; Muñoz, L.E.; Janko, C.; Dumych, T.; Rech, J.; Schett, G.; Nimmerjahn, F.; Bilyy, R.; Herrmann, M. Sialylation of anti-histone immunoglobulin G autoantibodies determines their capabilities to participate in the clearance of late apoptotic cells. Clin. Exp. Immunol. 2016, 184, 110–117. [Google Scholar] [CrossRef]
- Bacalao, M.A.; Satterthwaite, A.B. Recent Advances in Lupus B Cell Biology: PI3K, IFNγ, and Chromatin. Front. Immunol. 2020, 11, 615673. [Google Scholar] [CrossRef] [PubMed]
- Rönnblom, L.; Elkon, K.B. Cytokines as therapeutic targets in SLE. Nat. Rev. Rheumatol. 2010, 6, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J.V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006, 313, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Anthony, R.M.; Ravetch, J.V. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc. Natl. Acad. Sci. USA 2007, 104, 8433–8437. [Google Scholar] [CrossRef]
- Karsten, C.M.; Pandey, M.K.; Figge, J.; Kilchenstein, R.; Taylor, P.R.; Rosas, M.; McDonald, J.U.; Orr, S.J.; Berger, M.; Petzold, D.; et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat. Med. 2012, 18, 1401–1406. [Google Scholar] [CrossRef]
- McDowell, C.; Farooq, U.; Haseeb, M. Inflammatory Bowel Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Rubin, D.C.; Shaker, A.; Levin, M.S. Chronic intestinal inflammation: Inflammatory bowel disease and colitis-associated colon cancer. Front. Immunol. 2012, 3, 107. [Google Scholar] [CrossRef]
- Trbojević Akmačić, I.; Ventham, N.T.; Theodoratou, E.; Vučković, F.; Kennedy, N.A.; Krištić, J.; Nimmo, E.R.; Kalla, R.; Drummond, H.; Štambuk, J.; et al. Inflammatory bowel disease associates with proinflammatory potential of the immunoglobulin G glycome. Inflamm. Bowel Dis. 2015, 21, 1237–1247. [Google Scholar] [CrossRef]
- Šimurina, M.; de Haan, N.; Vučković, F.; Kennedy, N.A.; Štambuk, J.; Falck, D.; Trbojević-Akmačić, I.; Clerc, F.; Razdorov, G.; Khon, A.; et al. Glycosylation of Immunoglobulin G Associates with Clinical Features of Inflammatory Bowel Diseases. Gastroenterology 2018, 154, 1320–1333.e10. [Google Scholar] [CrossRef]
- Gaifem, J.; Rodrigues, C.S.; Petralia, F.; Alves, I.; Leite-Gomes, E.; Cavadas, B.; Dias, A.M.; Moreira-Barbosa, C.; Revés, J.; Laird, R.M.; et al. A unique serum IgG glycosylation signature predicts development of Crohn’s disease and is associated with pathogenic antibodies to mannose glycan. Nat. Immunol. 2024, 25, 1692–1703. [Google Scholar] [CrossRef]
- Lewis, A.J.; Richards, A.C.; Mulvey, M.A. Invasion of Host Cells and Tissues by Uropathogenic Bacteria. Microbiol. Spectr. 2016, 4, 23. [Google Scholar] [CrossRef]
- Cooke, C.L.; An, H.J.; Kim, J.; Canfield, D.R.; Torres, J.; Lebrilla, C.B.; Solnick, J.V. Modification of gastric mucin oligosaccharide expression in rhesus macaques after infection with Helicobacter pylori. Gastroenterology 2009, 137, 1061–1071, 1071.e1–e8. [Google Scholar] [CrossRef]
- Naegeli, A.; Bratanis, E.; Karlsson, C.; Shannon, O.; Kalluru, R.; Linder, A.; Malmström, J.; Collin, M. Streptococcus pyogenes evades adaptive immunity through specific IgG glycan hydrolysis. J. Exp. Med. 2019, 216, 1615–1629. [Google Scholar] [CrossRef] [PubMed]
- Freire-de-Lima, L.; Alisson-Silva, F.; Carvalho, S.T.; Takiya, C.M.; Rodrigues, M.M.; DosReis, G.A.; Mendonça-Previato, L.; Previato, J.O.; Todeschini, A.R. Trypanosoma cruzi subverts host cell sialylation and may compromise antigen-specific CD8+ T cell responses. J. Biol. Chem. 2010, 285, 13388–13396. [Google Scholar] [CrossRef]
- Poole, J.; Day, C.J.; von Itzstein, M.; Paton, J.C.; Jennings, M.P. Glycointeractions in bacterial pathogenesis. Nat. Rev. Microbiol. 2018, 16, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Chung, N.P.; Matthews, K.; Kim, H.J.; Ketas, T.J.; Golabek, M.; de Los Reyes, K.; Korzun, J.; Yasmeen, A.; Sanders, R.W.; Klasse, P.J.; et al. Stable 293 T and CHO cell lines expressing cleaved, stable HIV-1 envelope glycoprotein trimers for structural and vaccine studies. Retrovirology 2014, 11, 33. [Google Scholar] [CrossRef]
- Behrens, A.J.; Vasiljevic, S.; Pritchard, L.K.; Harvey, D.J.; Andev, R.S.; Krumm, S.A.; Struwe, W.B.; Cupo, A.; Kumar, A.; Zitzmann, N.; et al. Composition and Antigenic Effects of Individual Glycan Sites of a Trimeric HIV-1 Envelope Glycoprotein. Cell Rep. 2016, 14, 2695–2706. [Google Scholar] [CrossRef]
- Chang, Y.C.; Olson, J.; Beasley, F.C.; Tung, C.; Zhang, J.; Crocker, P.R.; Varki, A.; Nizet, V. Group B Streptococcus engages an inhibitory Siglec through sialic acid mimicry to blunt innate immune and inflammatory responses in vivo. PLoS Pathog. 2014, 10, e1003846. [Google Scholar] [CrossRef]
- Chang, Y.C.; Nizet, V. The interplay between Siglecs and sialylated pathogens. Glycobiology 2014, 24, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Heindel, D.W.; Koppolu, S.; Zhang, Y.; Kasper, B.; Meche, L.; Vaiana, C.A.; Bissel, S.J.; Carter, C.E.; Kelvin, A.A.; Elaish, M.; et al. Glycomic analysis of host response reveals high mannose as a key mediator of influenza severity. Proc. Natl. Acad. Sci. USA 2020, 117, 26926–26935. [Google Scholar] [CrossRef]
- Ash, M.K.; Bhimalli, P.P.; Cho, B.K.; Mattamana, B.B.; Gambut, S.; Tarhoni, I.; Fhied, C.L.; Reyes, A.F.; Welninski, S.J.; Arivalagan, J.; et al. Bulk IgG glycosylation predicts COVID-19 severity and vaccine antibody response. Cell Rep. 2022, 41, 111799. [Google Scholar] [CrossRef]
- Váradi, C. The Glycosylation of Serum IgG Antibodies in Post-COVID-19 and Post-Vaccination Patients. Int. J. Mol. Sci. 2025, 26, 807. [Google Scholar] [CrossRef]
- Agarwal, K.; Choudhury, B.; Robinson, L.S.; Morrill, S.R.; Bouchibiti, Y.; Chilin-Fuentes, D.; Rosenthal, S.B.; Fisch, K.M.; Peipert, J.F.; Lebrilla, C.B.; et al. Resident microbes shape the vaginal epithelial glycan landscape. Sci. Transl. Med. 2023, 15, eabp9599. [Google Scholar] [CrossRef] [PubMed]
- Shio, M.T.; Hassani, K.; Isnard, A.; Ralph, B.; Contreras, I.; Gomez, M.A.; Abu-Dayyeh, I.; Olivier, M. Host cell signalling and leishmania mechanisms of evasion. J. Trop. Med. 2012, 2012, 819512. [Google Scholar] [CrossRef]
- Tedford, E.; McConkey, G. Neurophysiological Changes Induced by Chronic Toxoplasma gondii Infection. Pathogens 2017, 6, 19. [Google Scholar] [CrossRef]
- Engevik, M.A.; Engevik, A.C.; Engevik, K.A.; Auchtung, J.M.; Chang-Graham, A.L.; Ruan, W.; Luna, R.A.; Hyser, J.M.; Spinler, J.K.; Versalovic, J. Mucin-Degrading Microbes Release Monosaccharides That Chemoattract Clostridioides difficile and Facilitate Colonization of the Human Intestinal Mucus Layer. ACS Infect. Dis. 2021, 7, 1126–1142. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.M.; Bibollet-Ruche, F.; Hahn, B.H. Plasmodium falciparum CyRPA Glycan Binding Does Not Explain Adaptation to Humans. Genome Biol. Evol. 2025, 17, evaf016. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.J.; Gupta, P.; Naaz, T.; Rai, R.; Gupta, S.; Gupta, S.; Chaurasiya, S.K.; Purwar, S.; Biswas, D.; Vyas, A.K.; et al. Role of Streptococcus pneumoniae extracellular glycosidases in immune evasion. Front. Cell. Infect. Microbiol. 2023, 13, 1109449. [Google Scholar] [CrossRef]
- O’Riordan, K.; Lee, J.C. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 2004, 17, 218–234. [Google Scholar] [CrossRef] [PubMed]
- López-Ramírez, L.A.; Martínez-Álvarez, J.A.; Martínez-Duncker, I.; Lozoya-Pérez, N.E.; Mora-Montes, H.M. Silencing of Sporothrix schenckii GP70 Reveals Its Contribution to Fungal Adhesion, Virulence, and the Host-Fungus Interaction. J. Fungi. 2024, 10, 302. [Google Scholar] [CrossRef]
- Mattos, E.C.; Tonelli, R.R.; Colli, W.; Alves, M.J. The Gp85 surface glycoproteins from Trypanosoma cruzi. Subcell. Biochem. 2014, 74, 151–180. [Google Scholar]
- Watanabe, Y.; Allen, J.D.; Wrapp, D.; McLellan, J.S.; Crispin, M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science 2020, 369, 330–333. [Google Scholar] [CrossRef]
- Karmakar, J.; Roy, S.; Mandal, C. Modulation of TLR4 Sialylation Mediated by a Sialidase Neu1 and Impairment of Its Signaling in Leishmania donovani Infected Macrophages. Front. Immunol. 2019, 10, 2360. [Google Scholar] [CrossRef]
- Carlin, A.F.; Lewis, A.L.; Varki, A.; Nizet, V. Group B streptococcal capsular sialic acids interact with siglecs (immunoglobulin-like lectins) on human leukocytes. J. Bacteriol. 2007, 189, 1231–1237. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, J.Y.; Yang, J.S.; Kim, J.W.; Kim, V.N.; Chang, H. The Architecture of SARS-CoV-2 Transcriptome. Cell 2020, 181, 914–921.e10. [Google Scholar] [CrossRef] [PubMed]
- Shajahan, A.; Pepi, L.E.; Rouhani, D.S.; Heiss, C.; Azadi, P. Glycosylation of SARS-CoV-2: Structural and functional insights. Anal. Bioanal. Chem. 2021, 413, 7179–7193. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Krammer, F. SARS-CoV-2 Vaccines: Status Report. Immunity 2020, 52, 583–589. [Google Scholar] [CrossRef]
- Cao, L.; Diedrich, J.K.; Kulp, D.W.; Pauthner, M.; He, L.; Park, S.R.; Sok, D.; Su, C.Y.; Delahunty, C.M.; Menis, S.; et al. Global site-specific N-glycosylation analysis of HIV envelope glycoprotein. Nat. Commun. 2017, 8, 14954. [Google Scholar] [CrossRef]
- Behrens, A.J.; Harvey, D.J.; Milne, E.; Cupo, A.; Kumar, A.; Zitzmann, N.; Struwe, W.B.; Moore, J.P.; Crispin, M. Molecular Architecture of the Cleavage-Dependent Mannose Patch on a Soluble HIV-1 Envelope Glycoprotein Trimer. J. Virol. 2017, 91, e01894-16. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Kearns, F.L.; Sandoval, D.R.; Casalino, L.; Clausen, T.M.; Rosenfeld, M.A.; Spliid, C.B.; Amaro, R.E.; Esko, J.D. Spike-heparan sulfate interactions in SARS-CoV-2 infection. Curr. Opin. Struct. Biol. 2022, 76, 102439. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.O.; Jette, C.A.; Abernathy, M.E.; Dam, K.A.; Esswein, S.R.; Gristick, H.B.; Malyutin, A.G.; Sharaf, N.G.; Huey-Tubman, K.E.; Lee, Y.E.; et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 2020, 588, 682–687. [Google Scholar] [CrossRef]
- Huang, H.Y.; Liao, H.Y.; Chen, X.; Wang, S.W.; Cheng, C.W.; Shahed-Al-Mahmud, M.; Liu, Y.M.; Mohapatra, A.; Chen, T.H.; Lo, J.M.; et al. Vaccination with SARS-CoV-2 spike protein lacking glycan shields elicits enhanced protective responses in animal models. Sci. Transl. Med. 2022, 14, eabm0899. [Google Scholar] [CrossRef]
- Bayani, F.; Safaei Hashkavaei, N.; Uversky, V.N.; Mozaffari-Jovin, S.; Sefidbakht, Y. Insights into the structural peculiarities of the N-terminal and receptor binding domains of the spike protein from the SARS-CoV-2 Omicron variant. Comput. Biol. Med. 2022, 147, 105735. [Google Scholar] [CrossRef] [PubMed]
- Kosik, I.; Yewdell, J.W. Influenza Hemagglutinin and Neuraminidase: Yin–Yang Proteins Coevolving to Thwart Immunity. Viruses 2019, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Cao, L.; Ma, Y.; Wang, X.; Diedrich, J.K.; Kikuchi, C.; Willis, S.; Worth, C.; McBride, R.; Yates, J.R.; et al. Human Influenza Virus Hemagglutinins Contain Conserved Oligomannose N-Linked Glycans Allowing Potent Neutralization by Lectins. Cell Host Microbe 2020, 27, 725–735.e5. [Google Scholar] [CrossRef]
- Kumlin, U.; Olofsson, S.; Dimock, K.; Arnberg, N. Sialic acid tissue distribution and influenza virus tropism. Influenza Other Respir. Viruses 2008, 2, 147–154. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, C.Y.; Wan, X.F. Antigenic characterization of influenza and SARS-CoV-2 viruses. Anal. Bioanal. Chem. 2022, 414, 2841–2881. [Google Scholar] [CrossRef]
- Suzuki, Y. Sialobiology of influenza: Molecular mechanism of host range variation of influenza viruses. Biol. Pharm. Bull. 2005, 28, 399–408. [Google Scholar] [CrossRef]
- Doores, K.J. The HIV glycan shield as a target for broadly neutralizing antibodies. FEBS J. 2015, 282, 4679–4691. [Google Scholar] [CrossRef]
- Bandres, J.C.; Wang, Q.F.; O’Leary, J.; Baleaux, F.; Amara, A.; Hoxie, J.A.; Zolla-Pazner, S.; Gorny, M.K. Human immunodeficiency virus (HIV) envelope binds to CXCR4 independently of CD4, and binding can be enhanced by interaction with soluble CD4 or by HIV envelope deglycosylation. J. Virol. 1998, 72, 2500–2504. [Google Scholar] [CrossRef]
- Lapham, C.K.; Ouyang, J.; Chandrasekhar, B.; Nguyen, N.Y.; Dimitrov, D.S.; Golding, H. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science 1996, 274, 602–605. [Google Scholar] [CrossRef]
- Trkola, A.; Dragic, T.; Arthos, J.; Binley, J.M.; Olson, W.C.; Allaway, G.P.; Cheng-Mayer, C.; Robinson, J.; Maddon, P.J.; Moore, J.P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 1996, 384, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.L.; Williamson, C. Approaches to the induction of HIV broadly neutralizing antibodies. Curr. Opin. HIV AIDS 2016, 11, 569–575. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, D.T.; Choi, N.M.; Briney, B.; Garces, F.; Ver, L.S.; Landais, E.; Murrell, B.; Wrin, T.; Kilembe, W.; Liang, C.H.; et al. Early Antibody Lineage Diversification and Independent Limb Maturation Lead to Broad HIV-1 Neutralization Targeting the Env High-Mannose Patch. Immunity 2016, 44, 1215–1226. [Google Scholar] [CrossRef]
- Bonsignori, M.; Kreider, E.F.; Fera, D.; Meyerhoff, R.R.; Bradley, T.; Wiehe, K.; Alam, S.M.; Aussedat, B.; Walkowicz, W.E.; Hwang, K.K.; et al. Staged induction of HIV-1 glycan-dependent broadly neutralizing antibodies. Sci. Transl. Med. 2017, 9, eaai7514. [Google Scholar] [CrossRef]
- Hu, M.; Lan, Y.; Lu, A.; Ma, X.; Zhang, L. Glycan-based biomarkers for diagnosis of cancers and other diseases: Past, present, and future. Prog. Mol. Biol. Transl. Sci. 2019, 162, 1–24. [Google Scholar]
- Ohtsubo, K.; Marth, J.D. Glycosylation in Cellular Mechanisms of Health and Disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Detection of inflammatory biomarkers in saliva and urine: Potential in diagnosis, prevention, and treatment for chronic diseases. Exp. Biol. Med. 2016, 241, 783–799. [Google Scholar] [CrossRef]
- Zhou, R.Z.; Gaunitz, S.; Kirsebom, B.E.; Lundin, B.; Hellström, M.; Jejcic, A.; Sköldunger, A.; Wimo, A.; Winblad, B.; Fladby, T.; et al. Blood N-glycomics reveals individuals at risk for cognitive decline and Alzheimer’s disease. EBioMedicine 2025, 113, 105598. [Google Scholar] [CrossRef]
- Hu, M.; Lan, Y.; Lu, A.; Ma, X.; Zhang, L. Chapter One-Glycan-Based Biomarkers for Diagnosis Of Cancers and Other Diseases: Past, Present, and Future. In Progress in Molecular Biology and Translational Science; Zhang, L., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 162, pp. 1–24. [Google Scholar]
- Jiang, T.; Zhang, Y.; Dai, F.; Liu, C.; Hu, H.; Zhang, Q. Advanced glycation end products and diabetes and other metabolic indicators. Diabetol. Metab. Syndr. 2022, 14, 104. [Google Scholar] [CrossRef]
- Hu, D.S.; Zhu, S.H.; Li, X.; Chen, Q.F.; Lin, C.J.; Fang, D.H.; Wu, J.S. Association between Hemoglobin Glycation Index and NAFLD in Chinese Nondiabetic Individuals. Can. J. Gastroenterol. Hepatol. 2019, 2019, 8748459. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Edmondson, A.; Meyers, G.D.; Izumi, K.; Ackermann, A.M.; Morava, E.; Ficicioglu, C.; Bennett, M.J.; He, M. Increased Clinical Sensitivity and Specificity of Plasma Protein N-Glycan Profiling for Diagnosing Congenital Disorders of Glycosylation by Use of Flow Injection–Electrospray Ionization–Quadrupole Time-of-Flight Mass Spectrometry. Clin. Chem. 2019, 65, 653–663. [Google Scholar] [CrossRef] [PubMed]
- van der Burgt, Y.; Wuhrer, M. The Role of Clinical Glyco(proteo)mics in Precision Medicine. Mol. Cell. Proteom. 2023, 22, 100565. [Google Scholar] [CrossRef]
- Tzeng, S.F.; Tsai, C.H.; Chao, T.K.; Chou, Y.C.; Yang, Y.C.; Tsai, M.H.; Cha, T.L.; Hsiao, P.W. O-Glycosylation-mediated signaling circuit drives metastatic castration-resistant prostate cancer. FASEB J. 2018, 32, fj201800687. [Google Scholar] [CrossRef] [PubMed]
- de Vreede, G.; Morrison, H.A.; Houser, A.M.; Boileau, R.M.; Andersen, D.; Colombani, J.; Bilder, D. A Drosophila Tumor Suppressor Gene Prevents Tonic TNF Signaling through Receptor N-Glycosylation. Dev. Cell 2018, 45, 595–605.e4. [Google Scholar] [CrossRef] [PubMed]
- Oyama, M.; Kariya, Y.; Kariya, Y.; Matsumoto, K.; Kanno, M.; Yamaguchi, Y.; Hashimoto, Y. Biological role of site-specific O-glycosylation in cell adhesion activity and phosphorylation of osteopontin. Biochem. J. 2018, 475, 1583–1595. [Google Scholar] [CrossRef]
- Singh, C.; Shyanti, R.K.; Singh, V.; Kale, R.K.; Mishra, J.P.N.; Singh, R.P. Integrin expression and glycosylation patterns regulate cell-matrix adhesion and alter with breast cancer progression. Biochem. Biophys. Res. Commun. 2018, 499, 374–380. [Google Scholar] [CrossRef]
- Sperandio, M.; Gleissner, C.A.; Ley, K. Glycosylation in immune cell trafficking. Immunol. Rev. 2009, 230, 97–113. [Google Scholar] [CrossRef]
- Veillon, L.; Huang, Y.; Peng, W.; Dong, X.; Cho, B.G.; Mechref, Y. Characterization of isomeric glycan structures by LC-MS/MS. Electrophoresis 2017, 38, 2100–2114. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Cao, Q.; Liao, R.; Chen, X.; Li, X.; Bai, L.; Ma, C.; Deng, X.; Dai, Z.; Li, J.; et al. Elevated transcription and glycosylation of B3GNT5 promotes breast cancer aggressiveness. J. Exp. Clin. Cancer Res. 2022, 41, 169. [Google Scholar] [CrossRef]
- He, L.; Guo, Z.; Wang, W.; Tian, S.; Lin, R. FUT2 inhibits the EMT and metastasis of colorectal cancer by increasing LRP1 fucosylation. Cell Commun. Signal 2023, 21, 63. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.; Archer Goode, E.; Garnham, R.; Hodgson, K.; Orozco-Moreno, M.; Turner, H.; Livermore, K.; Putri Nangkana, K.; Frame, F.M.; Bermudez, A.; et al. ST6GAL1-mediated aberrant sialylation promotes prostate cancer progression. J. Pathol. 2023, 261, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, L.; Lumen, N.; Van Praet, C.; Delanghe, J.; Rottey, S.; Vermassen, T. A comprehensive view of N-glycosylation as clinical biomarker in prostate cancer. Biochim. Biophys. Acta Rev. Cancer 2025, 1880, 189239. [Google Scholar] [CrossRef] [PubMed]
- Vermassen, T.; De Bruyne, S.; Himpe, J.; Lumen, N.; Callewaert, N.; Rottey, S.; Delanghe, J. N-Linked Glycosylation and Near-Infrared Spectroscopy in the Diagnosis of Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 1592. [Google Scholar] [CrossRef]
- Kawahara, R.; Recuero, S.; Srougi, M.; Leite, K.R.M.; Thaysen-Andersen, M.; Palmisano, G. The Complexity and Dynamics of the Tissue Glycoproteome Associated with Prostate Cancer Progression. Mol. Cell. Proteom. 2021, 20, 100026. [Google Scholar] [CrossRef]
- Butler, W.; McDowell, C.; Yang, Q.; He, Y.; Zhao, Y.; Hauck, J.S.; Zhou, Y.; Zhang, H.; Armstrong, A.J.; George, D.J.; et al. Rewiring of the N-Glycome with prostate cancer progression and therapy resistance. NPJ Precis. Oncol. 2023, 7, 22. [Google Scholar] [CrossRef]
- Scott, E.; Munkley, J. Glycans as Biomarkers in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 1389. [Google Scholar] [CrossRef]
- Liao, C.; An, J.; Yi, S.; Tan, Z.; Wang, H.; Li, H.; Guan, X.; Liu, J.; Wang, Q. FUT8 and Protein Core Fucosylation in Tumours: From Diagnosis to Treatment. J. Cancer 2021, 12, 4109–4120. [Google Scholar] [CrossRef]
- Saldova, R.; Fan, Y.; Fitzpatrick, J.M.; Watson, R.W.; Rudd, P.M. Core fucosylation and alpha2–3 sialylation in serum N-glycome is significantly increased in prostate cancer comparing to benign prostate hyperplasia. Glycobiology 2011, 21, 195–205. [Google Scholar] [CrossRef]
- Yoneyama, T.; Tobisawa, Y.; Kaneko, T.; Kaya, T.; Hatakeyama, S.; Mori, K.; Sutoh Yoneyama, M.; Okubo, T.; Mitsuzuka, K.; Duivenvoorden, W.; et al. Clinical significance of the LacdiNAc-glycosylated prostate-specific antigen assay for prostate cancer detection. Cancer Sci. 2019, 110, 2573–2589. [Google Scholar] [CrossRef] [PubMed]
- Ideo, H.; Kondo, J.; Nomura, T.; Nonomura, N.; Inoue, M.; Amano, J. Study of glycosylation of prostate-specific antigen secreted by cancer tissue-originated spheroids reveals new candidates for prostate cancer detection. Sci. Rep. 2020, 10, 2708. [Google Scholar] [CrossRef]
- Park, D.D.; Phoomak, C.; Xu, G.; Olney, L.P.; Tran, K.A.; Park, S.S.; Haigh, N.E.; Luxardi, G.; Lert-Itthiporn, W.; Shimoda, M.; et al. Metastasis of cholangiocarcinoma is promoted by extended high-mannose glycans. Proc. Natl. Acad. Sci. USA 2020, 117, 7633–7644. [Google Scholar] [CrossRef]
- Chu, Y.D.; Fan, T.C.; Lai, M.W.; Yeh, C.T. GALNT14-mediated O-glycosylation on PHB2 serine-161 enhances cell growth, migration and drug resistance by activating IGF1R cascade in hepatoma cells. Cell Death Dis. 2022, 13, 956. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhou, H.; Wu, L.; Lai, Z.; Geng, D.; Yang, W.; Zhang, J.; Fan, Z.; Qin, W.; Wang, Y.; et al. O-GlcNAcylation promotes pancreatic tumor growth by regulating malate dehydrogenase 1. Nat. Chem. Biol. 2022, 18, 1087–1095. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, H.; Chai, S.; Xu, L.; Lin, B.; Yi, W.; Wu, L. O-GlcNAcylation promotes tumor immune evasion by inhibiting PD-L1 lysosomal degradation. Proc. Natl. Acad. Sci. USA 2023, 120, e2216796120. [Google Scholar] [CrossRef]
- Delafield, D.G.; Li, L. Recent Advances in Analytical Approaches for Glycan and Glycopeptide Quantitation. Mol. Cell. Proteom. 2021, 20, 100054. [Google Scholar] [CrossRef]
- Gutierrez Reyes, C.D.; Sanni, A.; Adeniyi, M.; Mogut, D.; Najera Gonzalez, H.R.; Ahmadi, P.; Atashi, M.; Onigbinde, S.; Mechref, Y. Targeted Glycoproteomics Analysis Using MRM/PRM Approaches. Methods Mol. Biol. 2024, 2762, 231–250. [Google Scholar] [PubMed]
- Gutierrez Reyes, C.D.; Sanni, A.; Mogut, D.; Adeniyi, M.; Ahmadi, P.; Atashi, M.; Onigbinde, S.; Mechref, Y. Targeted Analysis of Permethylated N-Glycans Using MRM/PRM Approaches. Methods Mol. Biol. 2024, 2762, 251–266. [Google Scholar] [PubMed]
- Cho, B.G.; Gutierrez Reyes, C.D.; Goli, M.; Gautam, S.; Banazadeh, A.; Mechref, Y. Targeted N-glycan analysis with parallel reaction monitoring using a quadrupole-orbitrap hybrid mass spectrometer. Anal. Chem. 2022, 94, 15215–15222. [Google Scholar] [CrossRef]
- Georgopoulou, N.; McLaughlin, M.; McFarlane, I.; Breen, K.C. The role of post-translational modification in ϐ-amyloid precursor protein processing. Biochem. Soc. Symp. 2001, 67, 23–36. [Google Scholar]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Z.; Wang, R.; Zhang, X.; Zhang, S.; Wu, Y.; Staufenbiel, M.; Cai, F.; Song, W. Amyloid-β protein (Aβ) Glu11 is the major β-secretase site of β-site amyloid-β precursor protein-cleaving enzyme 1(BACE1), and shifting the cleavage site to Aβ Asp1 contributes to Alzheimer pathogenesis. Eur. J. Neurosci. 2013, 37, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Belloy, M.E.; Napolioni, V.; Greicius, M.D. A Quarter Century of APOE and Alzheimer’s Disease: Progress to Date and the Path Forward. Neuron 2019, 101, 820–838. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Liu, F.; Zaidi, T.; Iqbal, K.; Grundke-Iqbal, I.; Merkle, R.K.; Gong, C.-X. Role of glycosylation in hyperphosphorylation of tau in Alzheimer’s disease. FEBS Lett. 2002, 512, 101–106. [Google Scholar] [CrossRef]
- Liu, F.; Iqbal, K.; Grundke-Iqbal, I.; Hart, G.W.; Gong, C.X. O-GlcNAcylation regulates phosphorylation of tau: A mechanism involved in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 10804–10809. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.M.; Zhou, M.T.; Li, S.W.; Zhen, X.C.; Yang, S. Glycoproteins as diagnostic and prognostic biomarkers for neurodegenerative diseases: A glycoproteomic approach. J. Neurosci. Res. 2021, 99, 1308–1324. [Google Scholar] [CrossRef] [PubMed]
- Marotta, N.P.; Lin, Y.H.; Lewis, Y.E.; Ambroso, M.R.; Zaro, B.W.; Roth, M.T.; Arnold, D.B.; Langen, R.; Pratt, M.R. O-GlcNAc modification blocks the aggregation and toxicity of the protein α-synuclein associated with Parkinson’s disease. Nat. Chem. 2015, 7, 913–920. [Google Scholar] [CrossRef]
- Lewis, Y.E.; Galesic, A.; Levine, P.M.; De Leon, C.A.; Lamiri, N.; Brennan, C.K.; Pratt, M.R. O-GlcNAcylation of α-Synuclein at Serine 87 Reduces Aggregation without Affecting Membrane Binding. ACS Chem. Biol. 2017, 12, 1020–1027. [Google Scholar] [CrossRef]
- Váradi, C.; Nehéz, K.; Hornyák, O.; Viskolcz, B.; Bones, J. Serum N-Glycosylation in Parkinson’s Disease: A Novel Approach for Potential Alterations. Molecules 2019, 24, 2220. [Google Scholar] [CrossRef]
- Fang, T.; Dai, Y.; Hu, X.; Xu, Y.; Qiao, J. Evaluation of serum neurofilament light chain and glial fibrillary acidic protein in the diagnosis of Alzheimer’s disease. Front. Neurol. 2024, 15, 1320653. [Google Scholar] [CrossRef]
- Zou, Y.; Li, L.; Guan, L.; Ma, C.; Yu, S.; Ma, X.; Mao, C.; Gao, J.; Qiu, L. Research trends and hotspots of glial fibrillary acidic protein within the area of Alzheimer’s disease: A bibliometric analysis. Front. Aging Neurosci. 2023, 15, 1196272. [Google Scholar] [CrossRef]
- Samadzadeh, S.; Sleator, R.D. The role of Neurofilament light (NfL) and glial fibrillary acidic protein (GFAP) in MS and AQP4-NMOSD: Advancing clinical applications. eNeurologicalSci 2025, 38, 100550. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Z.; Qiu, Y.; Sun, D.; Zhou, H. Peripheral GFAP and NfL as early biomarkers for dementia: Longitudinal insights from the UK Biobank. BMC Med. 2024, 22, 192. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.S.; Chen, I.C.; Chen, H.C.; Lee, Y.C.; Wu, S.C. Glycan Masking of Epitopes in the NTD and RBD of the Spike Protein Elicits Broadly Neutralizing Antibodies Against SARS-CoV-2 Variants. Front. Immunol. 2021, 12, 795741. [Google Scholar] [CrossRef]
- Jefferis, R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 2009, 8, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Reusch, D.; Tejada, M.L. Fc glycans of therapeutic antibodies as critical quality attributes. Glycobiology 2015, 25, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.L.; Lai, J.; Keck, R.; O’Connell, L.Y.; Hong, K.; Meng, Y.G.; Weikert, S.H.A.; Presta, L.G. Lack of Fucose on Human IgG1 N-Linked Oligosaccharide Improves Binding to Human FcγRIII and Antibody-dependent Cellular Toxicity. J. Biol. Chem. 2002, 277, 26733–26740. [Google Scholar] [PubMed]
- Shinkawa, T.; Nakamura, K.; Yamane, N.; Shoji-Hosaka, E.; Kanda, Y.; Sakurada, M.; Uchida, K.; Anazawa, H.; Satoh, M.; Yamasaki, M.; et al. The Absence of Fucose but Not the Presence of Galactose or Bisecting N-Acetylglucosamine of Human IgG1 Complex-type Oligosaccharides Shows the Critical Role of Enhancing Antibody-dependent Cellular Cytotoxicity. J. Biol. Chem. 2003, 278, 3466–3473. [Google Scholar] [CrossRef]
- Anthony, R.M.; Wermeling, F.; Karlsson, M.C.I.; Ravetch, J.V. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc. Natl. Acad. Sci. USA 2008, 105, 19571–19578. [Google Scholar] [CrossRef]
- Yamane-Ohnuki, N.; Satoh, M. Production of therapeutic antibodies with controlled fucosylation. MAbs 2009, 1, 230–236. [Google Scholar] [CrossRef]
- Hossler, P.; Khattak, S.F.; Li, Z.J. Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiology 2009, 19, 936–949. [Google Scholar] [CrossRef]
- Zhang, P.; Woen, S.; Wang, T.; Liau, B.; Zhao, S.; Chen, C.; Yang, Y.; Song, Z.; Wormald, M.R.; Yu, C.; et al. Challenges of glycosylation analysis and control: An integrated approach to producing optimal and consistent therapeutic drugs. Drug Discov. Today 2016, 21, 740–765. [Google Scholar] [CrossRef]
- Zhou, Q.; Shankara, S.; Roy, A.; Qiu, H.; Estes, S.; McVie-Wylie, A.; Culm-Merdek, K.; Park, A.; Pan, C.; Edmunds, T. Development of a simple and rapid method for producing non-fucosylated oligomannose containing antibodies with increased effector function. Biotechnol. Bioeng. 2008, 99, 652–665. [Google Scholar] [CrossRef]
- Sehn, L.H.; Goy, A.; Offner, F.C.; Martinelli, G.; Caballero, M.D.; Gadeberg, O.; Baetz, T.; Zelenetz, A.D.; Gaidano, G.; Fayad, L.E.; et al. Randomized Phase II Trial Comparing Obinutuzumab (GA101) with Rituximab in Patients with Relapsed CD20+ Indolent B-Cell Non-Hodgkin Lymphoma: Final Analysis of the GAUSS Study. J. Clin. Oncol. 2015, 33, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Joh, T.; Uike, N.; Yamamoto, K.; Utsunomiya, A.; Yoshida, S.; Saburi, Y.; Miyamoto, T.; Takemoto, S.; Suzushima, H.; et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: A multicenter phase II study. J. Clin. Oncol. 2012, 30, 837–842. [Google Scholar] [CrossRef]
- Musolino, A.; Naldi, N.; Bortesi, B.; Pezzuolo, D.; Capelletti, M.; Missale, G.; Laccabue, D.; Zerbini, A.; Camisa, R.; Bisagni, G.; et al. Immunoglobulin G Fragment C Receptor Polymorphisms and Clinical Efficacy of Trastuzumab-Based Therapy in Patients With HER-2/Neu-Positivepositive Metastatic Breast Cancer. J. Clin. Oncol. 2008, 26, 1789–1796. [Google Scholar] [CrossRef]
- Rugo, H.S.; Im, S.A.; Cardoso, F.; Cortés, J.; Curigliano, G.; Musolino, A.; Pegram, M.D.; Wright, G.S.; Saura, C.; Escrivá-de-Romaní, S.; et al. Efficacy of Margetuximab vs Trastuzumab in Patients with Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 573–584. [Google Scholar] [CrossRef]
- Zeng, Y.; Tang, F.; Shi, W.; Dong, Q.; Huang, W. Recent advances in synthetic glycoengineering for biological applications. Curr. Opin. Biotechnol. 2022, 74, 247–255. [Google Scholar] [CrossRef]
- García-Alija, M.; van Moer, B.; Sastre, D.E.; Azzam, T.; Du, J.J.; Trastoy, B.; Callewaert, N.; Sundberg, E.J.; Guerin, M.E. Modulating antibody effector functions by Fc glycoengineering. Biotechnol. Adv. 2023, 67, 108201. [Google Scholar] [CrossRef]
- Martina, C.E.; Crowe, J.E.; Meiler, J. Glycan masking in vaccine design: Targets, immunogens and applications. Front. Immunol. 2023, 14, 1126034. [Google Scholar] [CrossRef]
- Rappuoli, R. Reverse vaccinology, a genome-based approach to vaccine development. Vaccine 2001, 19, 2688–2691. [Google Scholar] [CrossRef]
- Pilishvili, T.; Lexau, C.; Farley, M.M.; Hadler, J.; Harrison, L.H.; Bennett, N.M.; Reingold, A.; Thomas, A.; Schaffner, W.; Craig, A.S.; et al. Sustained Reductions in Invasive Pneumococcal Disease in the Era of Conjugate Vaccine. J. Infect. Dis. 2010, 201, 32–41. [Google Scholar] [CrossRef]
- Holmberg, L.A.; Sandmaier, B.M. Vaccination with Theratope (STn-KLH) as treatment for breast cancer. Expert Rev. Vaccines 2004, 3, 655–663. [Google Scholar] [CrossRef]
- Schlom, J. Therapeutic cancer vaccines: Current status and moving forward. J. Natl. Cancer Inst. 2012, 104, 599–613. [Google Scholar] [CrossRef]
- Cui, Z.; Shi, C.; An, R.; Tang, Y.; Li, Y.; Cao, X.; Jiang, X.; Liu, C.C.; Xiao, M.; Xu, L. In Silico-Guided Discovery of Polysaccharide Derivatives as Adjuvants in Nanoparticle Vaccines for Cancer Immunotherapy. ACS Nano 2025, 19, 2099–2116. [Google Scholar] [CrossRef] [PubMed]
- Slovin, S.F.; Ragupathi, G.; Adluri, S.; Ungers, G.; Terry, K.; Kim, S.; Spassova, M.; Bornmann, W.G.; Fazzari, M.; Dantis, L.; et al. Carbohydrate vaccines in cancer: Immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc. Natl. Acad. Sci. USA 1999, 96, 5710–5715. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Furukawa, K.; Fortunato, S.R.; Livingston, P.O.; Lloyd, K.O.; Oettgen, H.F.; Old, L.J. Human monoclonal antibody with dual GM2/GD2 specificity derived from an immunized melanoma patient. Proc. Natl. Acad. Sci. USA 1990, 87, 3333–3337. [Google Scholar] [CrossRef]
- Bärenwaldt, A.; Läubli, H. The sialoglycan-Siglec glyco-immune checkpoint–a target for improving innate and adaptive anti-cancer immunity. Expert Opin. Ther. Targets 2019, 23, 839–853. [Google Scholar] [CrossRef]
- Läubli, H.; Nalle, S.C.; Maslyar, D. Targeting the siglec–sialic acid immune axis in cancer: Current and future approaches. Cancer Immunol. Res. 2022, 10, 1423–1432. [Google Scholar] [CrossRef]
- Ibarlucea-Benitez, I.; Weitzenfeld, P.; Smith, P.; Ravetch, J.V. Siglecs-7/9 function as inhibitory immune checkpoints in vivo and can be targeted to enhance therapeutic antitumor immunity. Proc. Natl. Acad. Sci. USA 2021, 118, e2107424118. [Google Scholar] [CrossRef]
- Sun, J.; Lu, Q.; Sanmamed, M.F.; Wang, J. Siglec-15 as an emerging target for next-generation cancer immunotherapy. Clin. Cancer Res. 2021, 27, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Berois, N.; Pittini, A.; Osinaga, E. Targeting Tumor Glycans for Cancer Therapy: Successes, Limitations, and Perspectives. Cancers 2022, 14, 645. [Google Scholar] [CrossRef]
- Ren, X.; Lin, S.; Guan, F.; Kang, H. Glycosylation Targeting: A Paradigm Shift in Cancer Immunotherapy. Int. J. Biol. Sci. 2024, 20, 2607–2621. [Google Scholar] [CrossRef]
- Wang, R.C.; Wang, Z. Precision Medicine: Disease Subtyping and Tailored Treatment. Cancers 2023, 15, 3837. [Google Scholar] [CrossRef]
- Solá, R.J.; Griebenow, K. Glycosylation of therapeutic proteins: An effective strategy to optimize efficacy. BioDrugs 2010, 24, 9–21. [Google Scholar] [CrossRef]
- Koury, M.J. Sugar coating extends half-lives and improves effectiveness of cytokine hormones. Trends Biotechnol. 2003, 21, 462–464. [Google Scholar] [CrossRef]
- Egrie, J.C.; Dwyer, E.; Browne, J.K.; Hitz, A.; Lykos, M.A. Darbepoetin alfa has a longer circulating half-life and greater in vivo potency than recombinant human erythropoietin. Exp. Hematol. 2003, 31, 290–299. [Google Scholar] [CrossRef]
- Farley, S. Glycoengineered to last longer. Nat. Rev. Drug Discov. 2003, 2, 339. [Google Scholar] [CrossRef]
- Leung, J.C.K.; Tsang, A.W.L.; Chan, D.T.M.; Lai, K.N. Absence of CD89, polymeric immunoglobulin receptor, and asialoglycoprotein receptor on human mesangial cells. J. Am. Soc. Nephrol. 2000, 11, 241–249. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, H.; Su, S.; Wang, T.; Zhang, C.; Fida, G.; Cui, S.; Zhao, J.; Gu, Y. Galactose as Broad Ligand for Multiple Tumor Imaging and Therapy. J. Cancer 2015, 6, 658–670. [Google Scholar] [CrossRef]
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment. J. Med. Chem. 2018, 61, 5108–5121. [Google Scholar] [CrossRef]
- Li, S.; McCraw, A.J.; Gardner, R.A.; Spencer, D.I.R.; Karagiannis, S.N.; Wagner, G.K. Glycoengineering of Therapeutic Antibodies with Small Molecule Inhibitors. Antibodies 2021, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G.; Jefferis, R. Post-translational modifications in the context of therapeutic proteins. Nat. Biotechnol. 2006, 24, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M.; Mero, A. The Impact of PEGylation on Biological Therapies. BioDrugs 2008, 22, 315–329. [Google Scholar] [CrossRef]
- Zhou, Y.; Priya, S.; Ong, J.Y. Characterizing Glycosylation of Adeno-Associated Virus Serotype 9 Capsid Proteins Generated from HEK293 Cells through Glycopeptide Mapping and Released Glycan Analysis. Microorganisms 2024, 12, 946. [Google Scholar] [CrossRef] [PubMed]
- Murtada, R.; Fabijanczuk, K.; Gaspar, K.; Dong, X.; Alzarieni, K.Z.; Calix, K.; Manriquez, E.; Bakestani, R.M.; Kenttämaa, H.I.; Gao, J. Free-radical-mediated glycan isomer differentiation. Anal. Chem. 2020, 92, 13794–13802. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H. Essentials of Glycobiology [Internet]; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015. [Google Scholar]
- Makrydaki, E.; Kotidis, P.; Polizzi, K.M.; Kontoravdi, C. Hitting the sweet spot with capillary electrophoresis: Advances in N-glycomics and glycoproteomics. Curr. Opin. Biotechnol. 2021, 71, 182–190. [Google Scholar] [CrossRef]
- Wei, J.; Tang, Y.; Ridgeway, M.E.; Park, M.A.; Costello, C.E.; Lin, C. Accurate identification of isomeric glycans by trapped ion mobility spectrometry-electronic excitation dissociation tandem mass spectrometry. Anal. Chem. 2020, 92, 13211–13220. [Google Scholar] [CrossRef]
- Morris, C.B.; Poland, J.C.; May, J.C.; McLean, J.A. Fundamentals of ion mobility-mass spectrometry for the analysis of biomolecules. In Ion Mobility-Mass Spectrometry: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–31. [Google Scholar]
- Patidar, A.; Kamble, P. A Comprehensive Review on Liquid Chromatography-Mass Spectrometry (LC-MS): A Hyphenated Technique. Asian J. Pharm. Res. Dev. 2025, 13, 95–103. [Google Scholar]
- Gautam, S.; Banazadeh, A.; Cho, B.G.; Goli, M.; Zhong, J.; Mechref, Y. Mesoporous graphitized carbon column for efficient isomeric separation of permethylated glycans. Anal. Chem. 2021, 93, 5061–5070. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.I.; Daramola, O.; Bhuiyan, M.M.A.A.; Sandilya, V.; Mechref, Y. Analysis of Native and Permethylated N-Glycan Isomers Using MGC-LC-MS Techniques. In Recombinant Glycoproteins: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2024; pp. 219–230. [Google Scholar]
- Reyes, C.D.G.; Hakim, M.A.; Atashi, M.; Goli, M.; Gautam, S.; Wang, J.; Bennett, A.I.; Zhu, J.; Lubman, D.M.; Mechref, Y. LC-MS/MS isomeric profiling of N-Glycans derived from low-abundant serum glycoproteins in mild cognitive impairment patients. Biomolecules 2022, 12, 1657. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dong, X.; Yu, A.; Huang, Y.; Peng, W.; Mechref, Y. Isomeric separation of permethylated glycans by extra-long reversed-phase liquid chromatography (RPLC)-MS/MS. Analyst 2022, 147, 2048–2059. [Google Scholar] [CrossRef] [PubMed]
- Bangarh, R.; Khatana, C.; Kaur, S.; Sharma, A.; Kaushal, A.; Siwal, S.S.; Tuli, H.S.; Dhama, K.; Thakur, V.K.; Saini, R.V.; et al. Aberrant protein glycosylation: Implications on diagnosis and Immunotherapy. Biotechnol. Adv. 2023, 66, 108149. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Li, L. Recent Advances in Labeling-Based Quantitative Glycomics: From High-Throughput Quantification to Structural Elucidation. Proteomics 2025, 25, e202400057. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, S.; Yu, C.-Y.; Tang, H.; Mechref, Y. Automated annotation and quantitation of glycans by liquid chromatography/electrospray ionization mass spectrometric analysis using the MultiGlycan-ESI computational tool. Rapid Commun. Mass Spectrom. 2015, 29, 135–142. [Google Scholar] [CrossRef]
- Apte, A.; Meitei, N.S. Bioinformatics in glycomics: Glycan characterization with mass spectrometric data using SimGlycan. Methods Mol. Biol. 2010, 600, 269–281. [Google Scholar]
- Guan, Y.; Zhao, S.; Fu, C.; Zhang, J.; Yang, F.; Luo, J.; Dai, L.; Li, X.; Schlüter, H.; Wang, J.; et al. nQuant Enables Precise Quantitative N-Glycomics. Anal. Chem. 2024, 96, 15531–15539. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, E.; Tan, Y.; Tan, Y.; Hu, H.; Benson, G.; Aizikov, K.; Conley, S.; Staples, G.O.; Slysz, G.W.; Smith, R.D.; et al. GlycReSoft: A software package for automated recognition of glycans from LC/MS data. PLoS ONE 2012, 7, e45474. [Google Scholar] [CrossRef] [PubMed]
- Kronewitter, S.R.; De Leoz, M.L.; Strum, J.S.; An, H.J.; Dimapasoc, L.M.; Guerrero, A.; Miyamoto, S.; Lebrilla, C.B.; Leiserowitz, G.S. The glycolyzer: Automated glycan annotation software for high performance mass spectrometry and its application to ovarian cancer glycan biomarker discovery. Proteomics 2012, 12, 2523–2538. [Google Scholar] [CrossRef]
- Kelly, M.I.; Ashwood, C. GlyCombo enables rapid, complete glycan composition identification across diverse glycomic sample types. J. Am. Soc. Mass Spectrom. 2024, 35, 2324–2330. [Google Scholar] [CrossRef]
- Campbell, M.P.; Nguyen-Khuong, T.; Hayes, C.A.; Flowers, S.A.; Alagesan, K.; Kolarich, D.; Packer, N.H.; Karlsson, N.G. Validation of the curation pipeline of UniCarb-DB: Building a global glycan reference MS/MS repository. Biochim. Biophys. Acta 2014, 1844, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Lisacek, F.; Mariethoz, J.; Alocci, D.; Rudd, P.M.; Abrahams, J.L.; Campbell, M.P.; Packer, N.H.; Ståhle, J.; Widmalm, G.; Mullen, E.; et al. Databases and Associated Tools for Glycomics and Glycoproteomics. Methods Mol. Biol. 2017, 1503, 235–264. [Google Scholar]
- Donohoo, K.B.; Wang, J.; Goli, M.; Yu, A.; Peng, W.; Hakim, M.A.; Mechref, Y. Advances in mass spectrometry-based glycomics—An update covering the period 2017–2021. Electrophoresis 2022, 43, 119–142. [Google Scholar] [CrossRef]
- Liu, C.; Otsuka, K.; Kawai, T. Recent advances in microscale separation techniques for glycome analysis. J. Sep. Sci. 2024, 47, 2400170. [Google Scholar] [CrossRef]
- Lu, J.; Guo, S.; Liu, Q.; Tursumamat, N.; Liu, S.; Wu, S.; Li, H.; Wei, J. Recent advances in analytical methods and bioinformatic tools for quantitative glycomics. Anal. Bioanal. Chem. 2025, 417, 1947–1959. [Google Scholar] [CrossRef]
- He, K.; Baniasad, M.; Kwon, H.; Caval, T.; Xu, G.; Lebrilla, C.; Hommes, D.W.; Bertozzi, C. Decoding the glycoproteome: A new frontier for biomarker discovery in cancer. J. Hematol. Oncol. 2024, 17, 12. [Google Scholar] [CrossRef]
- Oh, S.; Cao, W.; Song, M. Twin Scholarships of Glycomedicine and Precision Medicine in Times of Single-Cell Multiomics. OMICS A J. Integr. Biol. 2024, 28, 319–323. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Assessing the Importance and Impact of Glycomics and Glycosciences. Transforming Glycoscience: A Roadmap for the Future; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Keisham, S.; Tateno, H. Emerging technologies for single-cell glycomics. BBA Adv. 2024, 6, 100125. [Google Scholar] [CrossRef]
- Ma, T.; McGregor, M.; Giron, L.; Xie, G.; George, A.F.; Abdel-Mohsen, M.; Roan, N.R. Single-cell glycomics analysis by CyTOF-Lec reveals glycan features defining cells differentially susceptible to HIV. eLife 2022, 11, e78870. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, J.; Zhu, R.; Huang, G.; Zeng, J.; Yu, H.; He, Z.; Han, C. Integrating TCGA and Single-Cell Sequencing Data for Hepatocellular Carcinoma: A Novel Glycosylation (GLY)/Tumor Microenvironment (TME) Classifier to Predict Prognosis and Immunotherapy Response. Metabolites 2024, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, Y.; Song, W. Single-cell transcriptome analysis identifies subclusters and signature with N-glycosylation in endometrial cancer. Clin. Transl. Oncol. 2025, 27, 2467–2483. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhao, Z.; Yu, W.; Liu, S.; Zhou, M.; Jiang, N.; Du, X.; Yang, X.; Chen, J.; Guo, H. Single-Cell Multiomics Identifies Glycan Epitope LacNAc as a Potential Cell-Surface Effector Marker of Peripheral T Cells in Bladder Cancer Patients. ACS Chem. Biol. 2024, 19, 2535–2547. [Google Scholar] [CrossRef]
- Grzeski, M.; Taube, E.T.; Braicu, E.I.; Sehouli, J.; Blanchard, V.; Klein, O. In situ N-glycosylation signatures of epithelial ovarian cancer tissue as defined by MALDI mass spectrometry imaging. Cancers 2022, 14, 1021. [Google Scholar] [CrossRef]
- Riera, R.; Hogervorst, T.P.; Doelman, W.; Ni, Y.; Pujals, S.; Bolli, E.; Codée, J.D.C.; van Kasteren, S.I.; Albertazzi, L. Single-molecule imaging of glycan–lectin interactions on cells with Glyco-PAINT. Nat. Chem. Biol. 2021, 17, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Malaker, S.A.; Quanico, J.; Raffo-Romero, A.; Kobeissy, F.; Aboulouard, S.; Tierny, D.; Bertozzi, C.R.; Fournier, I.; Salzet, M. On-tissue spatially resolved glycoproteomics guided by N-glycan imaging reveal global dysregulation of canine glioma glycoproteomic landscape. Cell Chem. Biol. 2022, 29, 30–42. [Google Scholar] [CrossRef]
- Wang, M.; Grauzam, S.; Bayram, M.F.; Dressman, J.; DelaCourt, A.; Blaschke, C.; Liang, H.; Scott, D.; Huffman, G.; Black, A.; et al. Spatial omics-based machine learning algorithms for the early detection of hepatocellular carcinoma. Commun. Med. 2024, 4, 258. [Google Scholar] [CrossRef]
- Hawkinson, T.R.; Clarke, H.A.; Young, L.E.A.; Conroy, L.R.; Markussen, K.H.; Kerch, K.M.; Johnson, L.A.; Nelson, P.T.; Wang, C.; Allison, D.B.; et al. In situ spatial glycomic imaging of mouse and human Alzheimer’s disease brains. Alzheimer’s Dement. 2022, 18, 1721–1735. [Google Scholar] [CrossRef]
- Zhan, C.; Tang, T.; Wu, E.; Zhang, Y.; He, M.; Wu, R.; Bi, C.; Wang, J.; Zhang, Y.; Shen, B. From multi-omics approaches to personalized medicine in myocardial infarction. Front. Cardiovasc. Med. 2023, 10, 1250340. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez Reyes, C.D.; Alejo-Jacuinde, G.; Perez Sanchez, B.; Chavez Reyes, J.; Onigbinde, S.; Mogut, D.; Hernández-Jasso, I.; Calderón-Vallejo, D.; Quintanar, J.L.; Mechref, Y. Multi omics applications in biological systems. Curr. Issues Mol. Biol. 2024, 46, 5777–5793. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, K.; Das, D.; Huang, M. Advancing the Metabolic Disfunction-Associated Steatotic Liver Disease Proteome: A Post-Translational Outlook. Genes 2025, 16, 334. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Zhu, R.; Zhou, S.; Mirzaei, P.; Mechref, Y. Integrated transcriptomics, proteomics, and glycomics reveals the association between up-regulation of sialylated N-glycans/integrin and breast cancer brain metastasis. Sci. Rep. 2019, 9, 17361. [Google Scholar] [CrossRef]
- Rudd, P.M.; Karlsson, N.G.; Khoo, K.-H.; Thaysen-Andersen, M.; Wells, L.; Packer, N.H. Glycomics and Glycoproteomics. In Essentials of Glycobiology [Internet], 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022. [Google Scholar]
- Lim, S.Y.; Lim, F.L.S.; Criado-Navarro, I.; Yeo, X.H.; Dayal, H.; Vemulapalli, S.D.; Seah, S.J.; Laserna, A.K.C.; Yang, X.; Tan, S.H.; et al. Multi-omics investigation into acute myocardial infarction: An integrative method revealing interconnections amongst the metabolome, lipidome, glycome, and metallome. Metabolites 2022, 12, 1080. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Ju, T. Aberrant glycosylation as immune therapeutic targets for solid tumors. Cancers 2023, 15, 3536. [Google Scholar] [CrossRef]
- Urban, J.; Jin, C.; Thomsson, K.A.; Karlsson, N.G.; Ives, C.M.; Fadda, E.; Bojar, D. Predicting glycan structure from tandem mass spectrometry via deep learning. Nat. Methods 2024, 21, 1206–1215. [Google Scholar] [CrossRef]
- Bojar, D.; Lisacek, F. Glycoinformatics in the Artificial Intelligence Era. Chem. Rev. 2022, 122, 15971–15988. [Google Scholar] [CrossRef]
- Myszczynska, M.A.; Ojamies, P.N.; Lacoste, A.M.B.; Neil, D.; Saffari, A.; Mead, R.; Hautbergue, G.M.; Holbrook, J.D.; Ferraiuolo, L. Applications of machine learning to diagnosis and treatment of neurodegenerative diseases. Nat. Rev. Neurol. 2020, 16, 440–456. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Shu, J.; Wang, J.; Guo, Y.; Zhang, Y.; Yue, L.; Yu, H.; Chen, W.; Zhang, C.; Ma, J.; et al. Machine learning reveals salivary glycopatterns as potential biomarkers for the diagnosis and prognosis of papillary thyroid cancer. Int. J. Biol. Macromol. 2022, 215, 280–289. [Google Scholar] [CrossRef]
- Burkholz, R.; Quackenbush, J.; Bojar, D. Using graph convolutional neural networks to learn a representation for glycans. Cell Rep. 2021, 35, 109251. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.; Kim, J.; Jung, S.H.; Woerner, J.; Suh, E.H.; Lee, D.G.; Shivakumar, M.; Lee, M.E.; Kim, D. Harnessing Artificial Intelligence in Multimodal Omics Data Integration: Paving the Path for the Next Frontier in Precision Medicine. Annu. Rev. Biomed. Data Sci. 2024, 7, 225–250. [Google Scholar] [CrossRef] [PubMed]
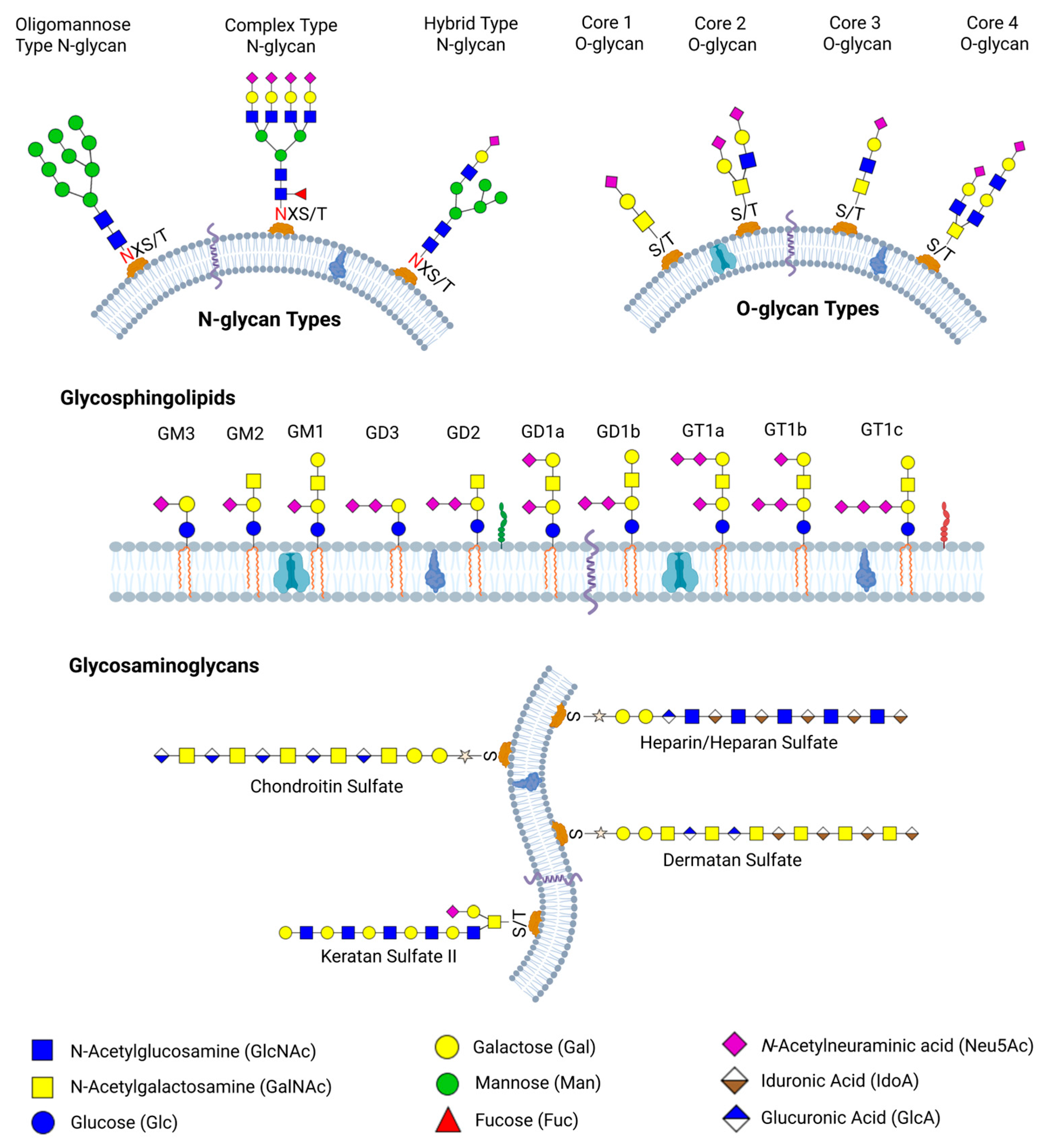
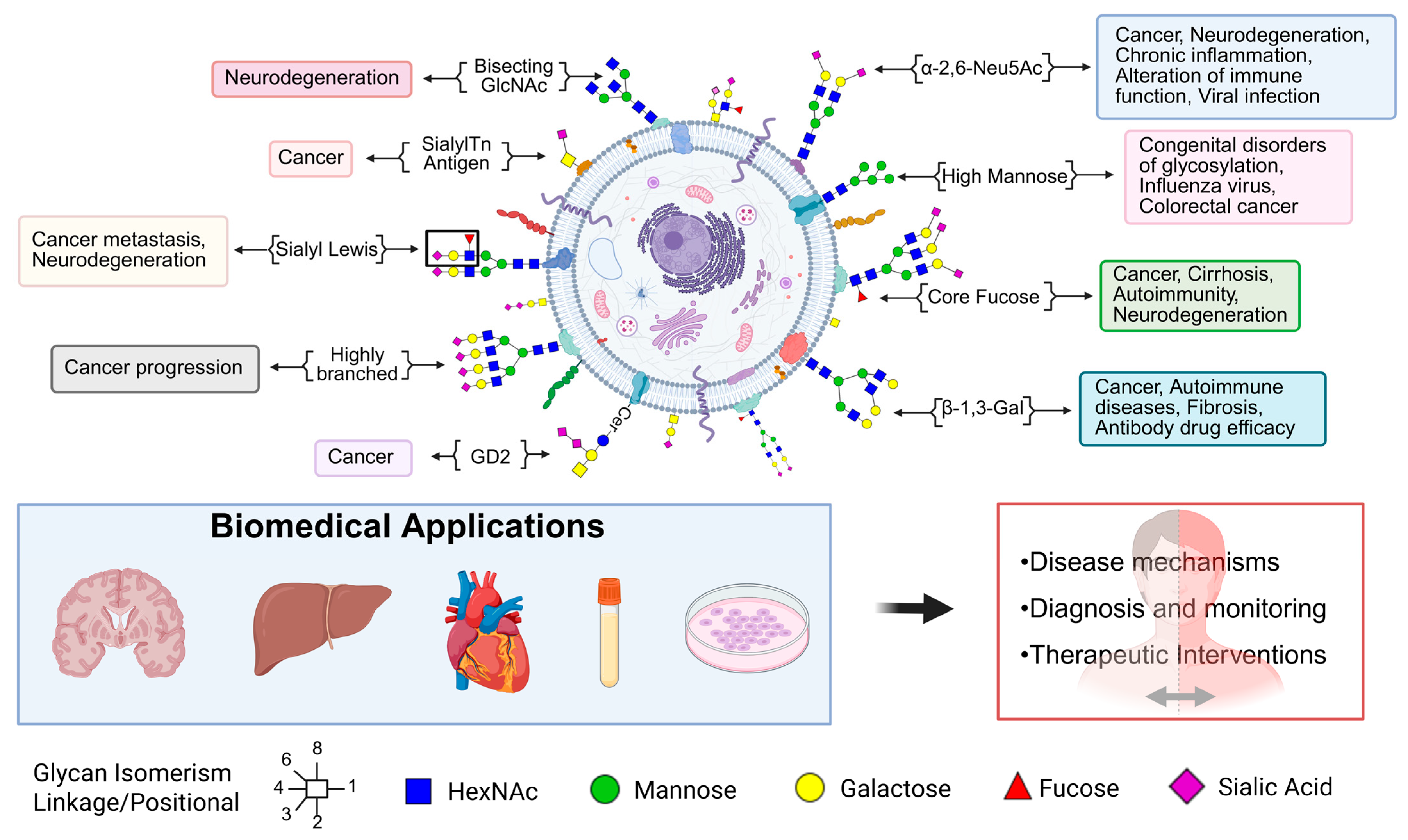
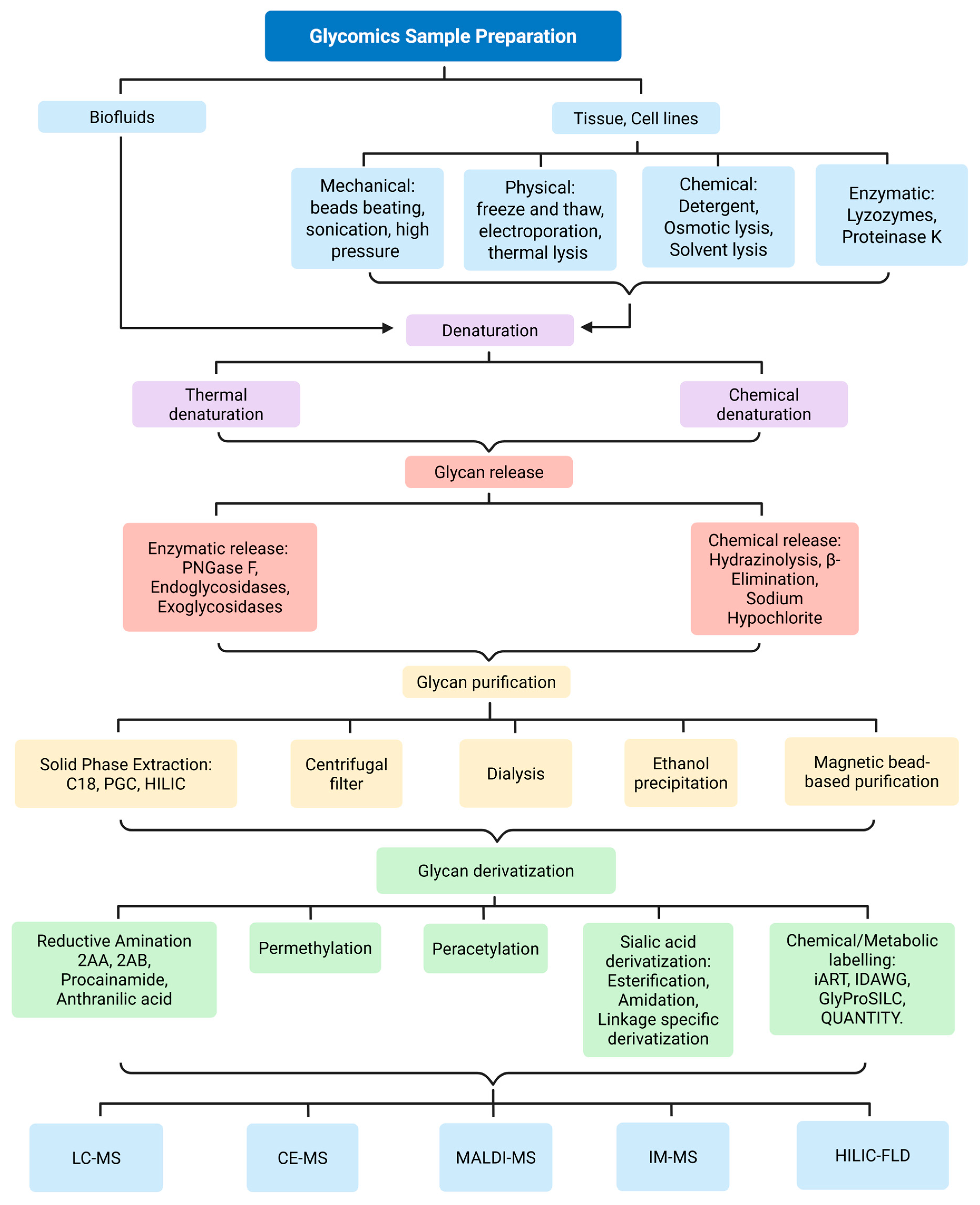

| Glycan and Glycoconjugates Biomarkers | Associated Diseases and Clinical Relevance |
|---|---|
| Tn antigen (GalNAcα1-O-Ser/Thr) | Found in breast, colon, lung, and esophageal cancers; indicates incomplete O-glycosylation and is a marker of immune evasion [22,172]. |
| Sialyl-Tn (STn) | Detected in gastric, colon, and breast cancers; associated with metastasis and poor prognosis [24,129,173]. |
| Carcinoembryonic Antigen (CEA) | Colorectal, lung, breast cancers; serum glycoprotein marker for cancer detection and recurrence monitoring [153,154]. |
| Alpha-fetoprotein (AFP) | Liver cancer; diagnostic and prognostic serum marker [149,150]. |
| Prostate-Specific Antigen (PSA) | Prostate cancer; widely used clinical tumor marker [155]. |
| Sialyl Lewis X (sLeX)/Sialyl Lewis A (sLeA) | Expressed in pancreatic, colorectal cancers; promotes tumor cell adhesion and metastasis via selectin binding [131,145,185]. |
| CA19-9 (Sialyl-Lewis A) | Pancreatic and gastrointestinal cancers; widely used serum tumor biomarker [156,184]. |
| CA125 (MUC16) | Ovarian cancer; used for diagnosis and treatment monitoring [151,152]. |
| Globo H | Highly expressed in breast, prostate, ovarian, and lung cancers; enhances tumor growth and immune evasion; target for cancer vaccines [176,182]. |
| GD2 ganglioside | Present in neuroblastoma, glioma, and melanoma; a therapeutic target of monoclonal antibodies (e.g., dinutuximab) [130]. |
| CA19-9 (sLeA) | Clinically used biomarker for pancreatic and GI cancers; reflects sialylated Lewis A expression [184]. |
| High-mannose N-glycans | Elevated in congenital disorders of glycosylation (CDGs), cancer, and viral infections, and is used in diagnosis and pathogen recognition [35,291]. |
| Bisecting GlcNAc | Increased in neurological disorders such as Alzheimer’s disease and cancer; affects tau glycosylation and immune signaling; detectable in CSF and serum [127,201,220]. |
| Core fucose | Altered in SLE, RA, Alzheimer’s; affects IgG function and cancer immune evasion [21,73,269]. |
| Hypogalactosylated IgG (G0 glycan) | Found in RA and SLE; promotes inflammation via complement activation and Fc receptor binding [30,31,251]. |
| IgG sialylation | Decreased in Rheumatoid arthritis (RA), systematic lupus erythematosus (SLE), and Inflammatory Bowel Disease (IBD); correlates with pro-inflammatory activity and disease flares [254,267,270]. |
| Polysialic acid (PolySia) | Reduced in Parkinson’s and Alzheimer’s disease; regulates synaptic plasticity and neuroinflammation [126,215]. |
| GM1, GD1a (gangliosides) | Depleted in Parkinson’s and Alzheimer’s; linked to synaptic dysfunction and neurodegeneration [215]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onigbinde, S.; Adeniyi, M.; Daramola, O.; Chukwubueze, F.; Bhuiyan, M.M.A.A.; Nwaiwu, J.; Bhattacharjee, T.; Mechref, Y. Glycomics in Human Diseases and Its Emerging Role in Biomarker Discovery. Biomedicines 2025, 13, 2034. https://doi.org/10.3390/biomedicines13082034
Onigbinde S, Adeniyi M, Daramola O, Chukwubueze F, Bhuiyan MMAA, Nwaiwu J, Bhattacharjee T, Mechref Y. Glycomics in Human Diseases and Its Emerging Role in Biomarker Discovery. Biomedicines. 2025; 13(8):2034. https://doi.org/10.3390/biomedicines13082034
Chicago/Turabian StyleOnigbinde, Sherifdeen, Moyinoluwa Adeniyi, Oluwatosin Daramola, Favour Chukwubueze, Md Mostofa Al Amin Bhuiyan, Judith Nwaiwu, Tuli Bhattacharjee, and Yehia Mechref. 2025. "Glycomics in Human Diseases and Its Emerging Role in Biomarker Discovery" Biomedicines 13, no. 8: 2034. https://doi.org/10.3390/biomedicines13082034
APA StyleOnigbinde, S., Adeniyi, M., Daramola, O., Chukwubueze, F., Bhuiyan, M. M. A. A., Nwaiwu, J., Bhattacharjee, T., & Mechref, Y. (2025). Glycomics in Human Diseases and Its Emerging Role in Biomarker Discovery. Biomedicines, 13(8), 2034. https://doi.org/10.3390/biomedicines13082034







