Advancement in Clinical Glycomics and Glycoproteomics for Congenital Disorders of Glycosylation: Progress and Challenges Ahead
Abstract
1. Introduction
2. Technological Advancements
3. Integration with Multi-Omics
4. Biomarker Discovery
5. Standardization of Diagnostic Practices
6. Challenges in Clinical Translation
7. Role of Glycan Databases and Bioinformatics
8. Future Directions and Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDG | congenital disorders of glycosylation |
| PMM2-CDG | phosphomannomutase 2-CDG |
| COG6-CDG | component of oligomeric golgi complex 6-CDG |
| B4GALT1-CDG | beta-1,4-galactosyltransferase 1-CDG |
| PGM1-CDG | phosphoglucomutase 1-CDG |
| ALG1-CDG | beta-1,4-mannosyltransferase/asparagine-linked glycosylation 1-CDG |
| SRD5A3-CDG | steroid 5-alpha-reductase 3-CDG |
| SLC10A7-CDG | solute carrier family 10, member 7-CDG |
| MOGS-CDG | mannosyl-oligosaccharide glucosidase-CDG |
| MS | mass spectrometry |
| ApoCIII | apolipoprotein C-III |
| WES | whole-exome sequencing |
| PGC | porous graphitized carbon |
| LC | liquid chromatography |
| QTOF | quadrupole time-of-flight |
| ESI | electrospray ionization |
| MS/MS | tandem mass spectrometry |
| DBS | dried blood spots |
| ACMG | American College of Medical Genetics and Genomics |
| MALDI TOF | matrix-assisted laser desorption ionization time of flight |
| HILIC | hydrophilic interaction chromatography |
| UPLC | ultra-performance liquid chromatography |
| DIA | data-independent acquisition |
| ERNDIM | European Research Network for Evaluation and Improvement of Screening, Diagnosis and Treatment of Inherited Disorders of Metabolism |
| MetabERN | European Reference Network for Hereditary Metabolic Disorders |
| AI | artificial intelligence |
| ML | machine learning |
| IgG | immunoglobulin G |
References
- Jaeken, J.; Hennet, T.; Matthijs, G.; Freeze, H.H. CDG Nomenclature: Time for a Change! Biochim. Biophys. Acta Mol. Basis Dis. 2009, 1792, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Francisco, R.; Brasil, S.; Poejo, J.; Jaeken, J.; Pascoal, C.; Videira, P.A.; dos Reis Ferreira, V. Congenital Disorders of Glycosylation (CDG): State of the Art in 2022. Orphanet J. Rare Dis. 2023, 18, 329. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, N.; Lefeber, D.J.; van Scherpenzeel, M. Clinical Glycomics for the Diagnosis of Congenital Disorders of Glycosylation. J. Inherit. Metab. Dis. 2018, 41, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Malsagova, K.; Kopylov, A.; Stepanov, A.; Butkova, T.; Izotov, A.; Kaysheva, A. Dried Blood Spot in Laboratory: Directions and Prospects. Diagnostics 2020, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Zamani, M.; Shariati, G.; Sedaghat, A.; Seifi, T.; Negahdari, S.; Sedighzadeh, S.S.; Zeighami, J.; Saberi, A.; Hamid, M.; et al. Whole Exome Sequencing Reveals Several Novel Variants in Congenital Disorders of Glycosylation and Glycogen Storage Diseases in Seven Patients from Iran. Mol. Genet. Genom. Med. 2023, 11, e2099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, T.L.; Ma, J.; He, W.J.; Gu, H. Clinical and Whole-Exome Sequencing Findings in Two Siblings from Hani Ethnic Minority with Congenital Glycosylation Disorders. BMC Med. Genet. 2019, 20, 181. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.G.; Freeze, H.H. Perspectives on Glycosylation and Its Congenital Disorders. Trends Genet. 2018, 34, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, N.; Voermans, N.C.; Marquardt, T.; Thiel, C.; Janssen, M.C.H.; Hansikova, H.; Crushell, E.; Sykut-Cegielska, J.; Bowling, F.; MØrkrid, L.; et al. Intact Transferrin and Total Plasma Glycoprofiling for Diagnosis and Therapy Monitoring in Phosphoglucomutase-I Deficiency. Transl. Res. 2018, 199, 62–76. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Baniasad, M.; Kwon, H.; Caval, T.; Xu, G.; Lebrilla, C.; Hommes, D.W.; Bertozzi, C. Decoding the Glycoproteome: A New Frontier for Biomarker Discovery in Cancer. J. Hematol. Oncol. 2024, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Altassan, R.; Radenkovic, S.; Edmondson, A.C.; Barone, R.; Brasil, S.; Cechova, A.; Coman, D.; Donoghue, S.; Falkenstein, K.; Ferreira, V.; et al. International Consensus Guidelines for Phosphoglucomutase 1 Deficiency (PGM1-CDG): Diagnosis, Follow-up, and Management. J. Inherit. Metab. Dis. 2021, 44, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Condina, M.R.; Briggs, M.T.; Moh, E.S.X.; Kaur, G.; Oehler, M.K.; Hoffmann, P. In-House Packed Porous Graphitic Carbon Columns for Liquid Chromatography-Mass Spectrometry Analysis of N-Glycans. Front. Chem. 2021, 9, 653959. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Lin, Y.P.; Ren, C.T.; Shivatare, S.S.; Lin, N.H.; Wu, C.Y.; Chen, C.H.; Lin, J.L. Enhancement of Fucosylated N-Glycan Isomer Separation with an Ultrahigh Column Temperature in Porous Graphitic Carbon Liquid Chromatography-Mass Spectrometry. J. Chromatogr. A 2020, 1632, 461610. [Google Scholar] [CrossRef] [PubMed]
- Van Scherpenzeel, M.; Steenbergen, G.; Morava, E.; Wevers, R.A.; Lefeber, D.J. High-Resolution Mass Spectrometry Glycoprofiling of Intact Transferrin for Diagnosis and Subtype Identification in the Congenital Disorders of Glycosylation. Transl. Res. 2015, 166, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Kadoya, M.; Okamoto, N. Mass Spectrometry of Transferrin and Apolipoprotein CIII from Dried Blood Spots for Congenital Disorders of Glycosylation. Mass Spectrom. 2022, 11, A0113. [Google Scholar] [CrossRef] [PubMed]
- Casetta, B.; Malvagia, S.; Funghini, S.; Martinelli, D.; Dionisi-Vici, C.; Barone, R.; Fiumara, A.; Donati, M.A.; Guerrini, R.; La Marca, G. A New Strategy Implementing Mass Spectrometry in the Diagnosis of Congenital Disorders of N-Glycosylation (CDG). Clin. Chem. Lab. Med. 2020, 59, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.L.; Lam, C.; Wolfe, L.; Edmondson, A.; ACMG Laboratory Quality Assurance Committee. Biochemical Testing for Congenital Disorders of Glycosylation: A Technical Standard of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2025, 27, 101328. [Google Scholar] [CrossRef] [PubMed]
- Trbojević-Akmačić, I.; Lageveen-Kammeijer, G.S.M.; Heijs, B.; Petrović, T.; Deriš, H.; Wuhrer, M.; Lauc, G. High-Throughput Glycomic Methods. Chem. Rev. 2022, 122, 15865–15913. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Okamoto, N. Apolipoprotein C-III O-Glycoform Profiling of 500 Serum Samples by Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry for Diagnosis of Congenital Disorders of Glycosylation. J. Mass Spectrom. 2021, 56, e4597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, W.; Wuhrer, M.; de Haan, N. Comprehensive O-Glycan Analysis by Porous Graphitized Carbon Nanoliquid Chromatography-Mass Spectrometry. Anal. Chem. 2024, 96, 8942–8948. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Okamoto, N. Electrospray Ionization Mass Spectrometry of Apolipoprotein CIII to Evaluate O-Glycan Site Occupancy and Sialylation in Congenital Disorders of Glycosylation. Mass Spectrom. 2022, 11, A0104. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yang, W.S.; An, D.; Lee, S.G.; Baek, J.H. Fast and Straightforward Simultaneous Quantification of Multiple Apolipoproteins in Human Serum on a High-Throughput LC-MS/MS Platform. Proteom. Clin. Appl. 2023, 17, 2200056. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhou, Y.; Li, X.; Lin, S.; Tan, Z.; Guan, F. Integrating Transcriptomics, Proteomics, Glycomics and Glycoproteomics to Characterize Paclitaxel Resistance in Breast Cancer Cells. J. Proteom. 2021, 243, 104266. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Li, W.; Jiang, D.; Wu, J.; Xie, J.; Wu, Y. Advances in Multi-Omics Applications in HBV-Associated Hepatocellular Carcinoma. Front. Med. 2021, 8, 754709. [Google Scholar] [CrossRef] [PubMed]
- Garapati, K.; Ranatunga, W.; Joshi, N.; Budhraja, R.; Sabu, S.; Kantautas, K.A.; Preston, G.; Perlstein, E.O.; Kozicz, T.; Morava, E.; et al. N-Glycoproteomic and Proteomic Alterations in SRD5A3-Deficient Fibroblasts. Glycobiology 2024, 34, cwae076. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.R.S.; Holmes, X.A.; Oloumi, A.; Grijaldo-Alvarez, S.J.; Schindler, R.; Zhou, Q.; Yadlapati, A.; Silsirivanit, A.; Lebrilla, C.B. Integration of RNAseq Transcriptomics and N -Glycomics Reveal Biosynthetic Pathways and Predict Structure-Specific N -Glycan Expression. Chem. Sci. 2025, 16, 7155–7172. [Google Scholar] [CrossRef] [PubMed]
- Van Der Burgt, Y.; Wuhrer, M. The Role of Clinical Glyco(Proteo)Mics in Precision Medicine. Mol. Cell. Proteom. 2023, 22, 100565. [Google Scholar] [CrossRef] [PubMed]
- Radenkovic, S.; Bird, M.J.; Emmerzaal, T.L.; Wong, S.Y.; Felgueira, C.; Stiers, K.M.; Sabbagh, L.; Himmelreich, N.; Poschet, G.; Windmolders, P.; et al. The Metabolic Map into the Pathomechanism and Treatment of PGM1-CDG. Am. J. Hum. Genet. 2019, 104, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Mangione, R.; Cirnigliaro, L.; Saab, M.W.; Pettinato, F.; Barbato, A.; Distefano, A.; Spina, E.L.; Lazzarino, G.; Volti, G.L.; Longhitano, L.; et al. Targeted Metabolomic Evaluation of Peripheral Blood Mononucleated Cells from Patients with PMM2-CDG. Sci. Rep. 2025, 15, 15929. [Google Scholar] [CrossRef] [PubMed]
- Conte, F.; Noga, M.J.; van Scherpenzeel, M.; Veizaj, R.; Scharn, R.; Sam, J.E.; Palumbo, C.; van den Brandt, F.C.A.; Freund, C.; Soares, E.; et al. Isotopic Tracing of Nucleotide Sugar Metabolism in Human Pluripotent Stem Cells. Cells 2023, 12, 1765. [Google Scholar] [CrossRef] [PubMed]
- Radenkovic, S.; Adant, I.; Bird, M.J.; Swinnen, J.V.; Cassiman, D.; Kozicz, T.; Gruenert, S.C.; Ghesquière, B.; Morava, E. Complex Metabolomic Changes in a Combined Defect of Glycosylation and Oxidative Phosphorylation in a Patient with Pathogenic Variants in PGM1 and NDUFA13. Cells 2025, 14, 638. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.A.; Veenvliet, A.R.J.; Engelke, U.F.H.; Kluijtmans, L.A.J.; Huigen, M.C.D.G.; Hoegen, B.; de Boer, L.; de Vries, M.C.; van Bon, B.W.; Leenders, E.; et al. Diagnosing, Discarding, or de-VUSsing: A Practical Guide to (Un)Targeted Metabolomics as Variant-Transcending Functional Tests. Genet. Med. 2023, 25, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Tian, Q.; Guo, S.; Xie, D.; Cai, Y.; Wang, Z.; Chu, H.; Qiu, S.; Tang, S.; Zhang, A. Metabolomics for Clinical Biomarker Discovery and Therapeutic Target Identification. Molecules 2024, 29, 2198. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez Reyes, C.D.; Alejo-Jacuinde, G.; Perez Sanchez, B.; Chavez Reyes, J.; Onigbinde, S.; Mogut, D.; Hernández-Jasso, I.; Calderón-Vallejo, D.; Quintanar, J.L.; Mechref, Y. Multi Omics Applications in Biological Systems. Curr. Issues Mol. Biol. 2024, 46, 5777–5793. [Google Scholar] [CrossRef] [PubMed]
- Budhraja, R.; Joshi, N.; Radenkovic, S.; Kozicz, T.; Morava, E.; Pandey, A. Dysregulated Proteome and N-Glycoproteome in ALG1-Deficient Fibroblasts. Proteomics 2024, 24, 2400012. [Google Scholar] [CrossRef] [PubMed]
- Baerenfaenger, M.; Post, M.A.; Langerhorst, P.; Huijben, K.; Zijlstra, F.; Jacobs, J.F.M.; Verbeek, M.M.; Wessels, H.J.C.T.; Lefeber, D.J. Glycoproteomics in Cerebrospinal Fluid Reveals Brain-Specific Glycosylation Changes. Int. J. Mol. Sci. 2023, 24, 1937. [Google Scholar] [CrossRef] [PubMed]
- Ashikov, A.; Bakar, N.A.; Wen, X.Y.; Niemeijer, M.; Osorio, G.R.P.; Brand-Arzamendi, K.; Hasadsri, L.; Hansikova, H.; Raymond, K.; Vicogne, D.; et al. Integrating Glycomics and Genomics Uncovers SLC10A7 as Essential Factor for Bone Mineralization by Regulating Post-Golgi Protein Transport and Glycosylation. Hum. Mol. Genet. 2018, 27, 3029–3045. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, N.; Ashikov, A.; Brum, J.M.; Smeets, R.; Kersten, M.; Huijben, K.; Keng, W.T.; Speck-Martins, C.E.; de Carvalho, D.R.; de Rizzo, I.M.P.O.; et al. Synergistic Use of Glycomics and Single-Molecule Molecular Inversion Probes for Identification of Congenital Disorders of Glycosylation Type-1. J. Inherit. Metab. Dis. 2022, 45, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Post, M.A.; de Wit, I.; Zijlstra, F.S.M.; Engelke, U.F.H.; van Rooij, A.; Christodoulou, J.; Tan, T.Y.; Le Fevre, A.; Jin, D.; Yaplito-Lee, J.; et al. MOGS-CDG: Quantitative Analysis of the Diagnostic Glc3Man Tetrasaccharide and Clinical Spectrum of Six New Cases. J. Inherit. Metab. Dis. 2023, 46, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Wessels, H.J.C.T.; Kulkarni, P.; van Dael, M.; Suppers, A.; Willems, E.; Zijlstra, F.; Kragt, E.; Gloerich, J.; Schmit, P.-O.; Pengelley, S.; et al. Plasma Glycoproteomics Delivers High-Specificity Disease Biomarkers by Detecting Site-Specific Glycosylation Abnormalities. J. Adv. Res. 2024, 61, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Garapati, K.; Budhraja, R.; Saraswat, M.; Kim, J.; Joshi, N.; Sachdeva, G.S.; Jain, A.; Ligezka, A.N.; Radenkovic, S.; Ramarajan, M.G.; et al. A Complement C4–Derived Glycopeptide Is a Biomarker for PMM2-CDG. JCI Insight 2024, 9, e172509. [Google Scholar] [CrossRef] [PubMed]
- Seo, N.; Lee, H.; Oh, M.J.; Kim, G.H.; Lee, S.G.; Ahn, J.K.; Cha, H.S.; Kim, K.H.; Kim, J.; An, H.J. Isomer-Specific Monitoring of Sialylated N-Glycans Reveals Association of A2,3-Linked Sialic Acid Epitope With Behcet’s Disease. Front. Mol. Biosci. 2021, 8, 778851. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Palmigiano, A.; Esposito, F.; Fiumara, A.; Bordugo, A.; Barone, R.; Sturiale, L.; Jaeken, J.; Garozzo, D. HILIC-UPLC-MS for High Throughput and Isomeric N-Glycan Separation and Characterization in Congenital Disorders Glycosylation and Human Diseases. Glycoconj. J. 2021, 38, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez Reyes, C.D.; Hakim, M.A.; Atashi, M.; Goli, M.; Gautam, S.; Wang, J.; Bennett, A.I.; Zhu, J.; Lubman, D.M.; Mechref, Y. LC-MS/MS Isomeric Profiling of N-Glycans Derived from Low-Abundant Serum Glycoproteins in Mild Cognitive Impairment Patients. Biomolecules 2022, 12, 1657. [Google Scholar] [CrossRef]
- Perales-Clemente, E.; Liedtke, K.; Studinski, A.; Radenkovic, S.; Gavrilov, D.; Oglesbee, D.; Matern, D.; Rinaldo, P.; Tortorelli, S.; Morava, E.; et al. A New D-Galactose Treatment Monitoring Index for PGM1-CDG. J. Inherit. Metab. Dis. 2021, 44, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Conte, F.; Morava, E.; Bakar, N.A.; Wortmann, S.B.; Poerink, A.J.; Grunewald, S.; Crushell, E.; Al-Gazali, L.; de Vries, M.C.; Mørkrid, L.; et al. Phosphoglucomutase-1 Deficiency: Early Presentation, Metabolic Management and Detection in Neonatal Blood Spots. Mol. Genet. Metab. 2020, 131, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; James, P.M.; Ng, B.G.; Li, X.; Xia, B.; Rong, J.; Asif, G.; Raymond, K.; Jones, M.A.; Hegde, M.; et al. A Novel N-Tetrasaccharide in Patients with Congenital Disorders of Glycosylation, Including Asparagine-Linked Glycosylation Protein 1, Phosphomannomutase 2, and Mannose Phosphate Isomerase Deficiencies. Clin. Chem. 2016, 62, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Bengtson, P.; Ng, B.G.; Jaeken, J.; Matthijs, G.; Freeze, H.H.; Eklund, E.A. Serum Transferrin Carrying the Xeno-Tetrasaccharide NeuAc-Gal-GlcNAc2 Is a Biomarker of ALG1-CDG. J. Inherit. Metab. Dis. 2016, 39, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Kitata, R.B.; Yang, J.C.; Chen, Y.J. Advances in Data-Independent Acquisition Mass Spectrometry towards Comprehensive Digital Proteome Landscape. Mass Spectrom. Rev. 2023, 42, 2324–2348. [Google Scholar] [CrossRef] [PubMed]
- Mathis, D.; Croft, J.; Chrastina, P.; Fowler, B.; Vianey-Saban, C.; Ruijter, G.J.G. The Role of ERNDIM Diagnostic Proficiency Schemes in Improving the Quality of Diagnostic Testing for Inherited Metabolic Diseases. J. Inherit. Metab. Dis. 2022, 45, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Péanne, R.; de Lonlay, P.; Foulquier, F.; Kornak, U.; Lefeber, D.J.; Morava, E.; Pérez, B.; Seta, N.; Thiel, C.; Van Schaftingen, E.; et al. Congenital Disorders of Glycosylation (CDG): Quo Vadis? Eur. J. Med. Genet. 2018, 61, 643–663. [Google Scholar] [CrossRef] [PubMed]
- Hertzog, A.; Selvanathan, A.; Devanapalli, B.; Ho, G.; Bhattacharya, K.; Tolun, A.A. A Narrative Review of Metabolomics in the Era of “-Omics”: Integration into Clinical Practice for Inborn Errors of Metabolism. Transl. Pediatr. 2022, 11, 1704–1716. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.P.; Peterson, R.; Mariethoz, J.; Gasteiger, E.; Akune, Y.; Aoki-Kinoshita, K.F.; Lisacek, F.; Packer, N.H. UniCarbKB: Building a Knowledge Platform for Glycoproteomics. Nucleic Acids Res. 2014, 42, D215–D221. [Google Scholar] [CrossRef] [PubMed]
- Alocci, D.; Mariethoz, J.; Gastaldello, A.; Gasteiger, E.; Karlsson, N.G.; Kolarich, D.; Packer, N.H.; Lisacek, F. GlyConnect: Glycoproteomics Goes Visual, Interactive, and Analytical. J. Proteome Res. 2019, 18, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Aoki-Kinoshita, K.; Agravat, S.; Aoki, N.P.; Arpinar, S.; Cummings, R.D.; Fujita, A.; Fujita, N.; Hart, G.M.; Haslam, S.M.; Kawasaki, T.; et al. GlyTouCan 1.0—The International Glycan Structure Repository. Nucleic Acids Res. 2016, 44, D1237–D1242. [Google Scholar] [CrossRef] [PubMed]
- Ceroni, A.; Maass, K.; Geyer, H.; Geyer, R.; Dell, A.; Haslam, S.M. GlycoWorkbench: A Tool for the Computer-Assisted Annotation of Mass Spectra of Glycans. J. Proteome Res. 2008, 7, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Aoki-Kinoshita, K.F.; Lisacek, F.; Karlsson, N.; Kolarich, D.; Packer, N.H. GlycoBioinformatics. Beilstein J. Org. Chem. 2021, 17, 2726–2728. [Google Scholar] [CrossRef] [PubMed]
- Packer, N.H.; Von Der Lieth, C.W.; Aoki-Kinoshita, K.F.; Lebrilla, C.B.; Paulson, J.C.; Raman, R.; Rudd, P.; Sasisekharan, R.; Taniguchi, N.; York, W.S. Frontiers in Glycomics: Bioinformatics and Biomarkers in Disease: An NIH White Paper Prepared from Discussions by the Focus Groups at a Workshop on the NIH Campus, Bethesda MD (September 11–13, 2006). Proteomics 2008, 8, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Bojar, D.; Lisacek, F. Glycoinformatics in the Artificial Intelligence Era. Chem. Rev. 2022, 122, 15971–15988. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chiang, A.W.T.; Lewis, N.E. Artificial Intelligence in the Analysis of Glycosylation Data. Biotechnol. Adv. 2022, 60, 108008. [Google Scholar] [CrossRef] [PubMed]
- Van Scherpenzeel, M.; Willems, E.; Lefeber, D.J. Clinical Diagnostics and Therapy Monitoring in the Congenital Disorders of Glycosylation. Glycoconj. J. 2016, 33, 345–358. [Google Scholar] [CrossRef] [PubMed]

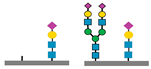
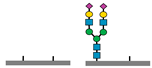
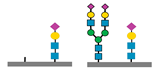
| CDG Subtype | Glycomarkers Identified | Reference(s) |
|---|---|---|
| PGM1-CDG |
| [8,38] |
| ||
| SLC10-CDG |
| [36] |
| PMM2-CDG |
| [37,39] |
| ALG1-CDG |
| [37,39,40] |
 sialic acids,
sialic acids,  galactose,
galactose,  N-acetyl glucosamine,
N-acetyl glucosamine,  mannose.
mannose.| CDG Subtype | Key Glycomarkers | Clinical Features | Diagnostic Tools |
|---|---|---|---|
| PGM1-CDG | ↓ Sialylation, ↑ fucosylation, abnormal transferrin N-glycoforms | Hepatopathy, hypoglycemia, myopathy, endocrine abnormalities | Transferrin glycoprofiling, total N-glycome, WES |
| PMM2-CDG | ↓ N-Tetrasaccharide, ↑ high mannose glycans | Developmental delay, hypotonia, ataxia, multiorgan involvement | Transferrin glycoprofiling, total N-glycome, WES |
| ALG1-CDG | N-Tetrasaccharide, fucosylated glycans | Seizures, developmental delay, muscular hypotonia | Transferrin glycoprofiling, total N-glycome, Sanger sequencing |
| SRD5A3-CDG | Abnormal N-glycan and glycopeptide profiles | Ocular anomalies, intellectual disability, cerebellar atrophy | Glycoproteomics (fibroblasts), WES |
| MOGS-CDG | Diagnostic Glc3Man tetrasaccharide | Dysmorphic features, developmental delay, feeding problems | Glycan tetrasaccharide screening, enzymatic assay |
| SLC10A7-CDG | Abnormal N-glycans and abnormal transferrin N-glycoforms | Skeletal dysplasia, dental anomalies, hypotonia | Transferrin glycoprofiling, total N-glycome, WES |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Bakar, N.; Hamzan, N.I. Advancement in Clinical Glycomics and Glycoproteomics for Congenital Disorders of Glycosylation: Progress and Challenges Ahead. Biomedicines 2025, 13, 1964. https://doi.org/10.3390/biomedicines13081964
Abu Bakar N, Hamzan NI. Advancement in Clinical Glycomics and Glycoproteomics for Congenital Disorders of Glycosylation: Progress and Challenges Ahead. Biomedicines. 2025; 13(8):1964. https://doi.org/10.3390/biomedicines13081964
Chicago/Turabian StyleAbu Bakar, Nurulamin, and Nurul Izzati Hamzan. 2025. "Advancement in Clinical Glycomics and Glycoproteomics for Congenital Disorders of Glycosylation: Progress and Challenges Ahead" Biomedicines 13, no. 8: 1964. https://doi.org/10.3390/biomedicines13081964
APA StyleAbu Bakar, N., & Hamzan, N. I. (2025). Advancement in Clinical Glycomics and Glycoproteomics for Congenital Disorders of Glycosylation: Progress and Challenges Ahead. Biomedicines, 13(8), 1964. https://doi.org/10.3390/biomedicines13081964