The Epigenetics of Sepsis: How Gene Modulation Shapes Outcomes
Abstract
1. Introduction
2. Search Strategy
3. Epigenetics
- Citrullination: Mediated by peptidyl arginine deaminases (PADs), converts arginine to citrulline.
- Ubiquitination: Involves mono/poly-ubiquitin attachment to lysines, critical for genome stability.
- Lactylation: A newly characterized acylation where lactyl groups modify lysines via P300, functionally distinct from acetylation or succinylation.
- O-GlcNAcylation: A post-translational modification involving the attachment of O-linked N-acetylglucosamine moieties to serine or threonine hydroxyl groups.
4. Epigenetic Methodologies in Sepsis Research
5. The Immunological Spectrum of Sepsis
6. DNA Methylation
7. Histone Modifications
8. Non-Coding RNAs
9. Clinical Aspects: Patient Endotypes
10. Epigenetic Modifications as Biomarkers
11. Influence on Therapy Response
12. Epigenetic-Targeted Therapies
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DNMT | DNA methyltransferases |
| CpG | cytosine residues adjacent to guanines |
| 5mC | 5-methylcytosine |
| PAD | peptidyl arginine deaminase |
| HPTM | histone post-translational modifications |
| ncRNA | non-coding RNA |
| miRNA | MicroRNAs |
| lncRNA | long non-coding RNA |
| circRNA | circular RNA |
| TNFα | tumor necrosis factor alfa |
| IL | interleukine |
| CARS | compensatory anti-inflammatory response syndrome |
| PICS | persistent inflammation, immunosuppression, and catabolism syndrome |
| Tregs | regulatory T cells |
| MDSCs | myeloid-derived suppressor cells |
| Foxp3+ | forkhead box P3 |
| TGF-β | transforming grow factor-β |
| TIM-3 | T-cell immunoglobulin and mucin-domain containing-3 |
| PD-1 | programmed cell death protein 1 |
| TIGIT | T cell immunoreceptor with Ig and ITIM domains |
| CTLA-4 | cytotoxic T-lymphocyte antigen 4 |
| MHC | major histocompatibility complex |
| JAK3 | janus kinase 3 |
| STAT5 | signal transducer and activator of transcription 5 |
| LPS | lipopolysaccharide |
| GPR84 | G protein-coupled receptor 84 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| DAMPs | damage-associated molecular patterns |
| PAMPS | pathogen-associated molecular patterns |
| NEAT1 | Nuclear Paraspeckle Assembly Transcript |
| m6A | N6-methyladenosine |
| METTL3 | methyltransferase-like 3 |
| FTO | fat mass and obesity-associated protein |
| YTHDF1 | YTH N6-Methyladenosine RNA Binding Protein 1 |
| NETs | neutrophil extracellular traps |
| AKI | acute kidney injury |
| ALI | acute lung injury |
| SICD | sepsis-induced cardiac dysfunction |
| AQP5 | aquaporin-5 |
| MERS | Middle East respiratory syndrome |
| CoV | Coronavirus |
| TREM1 | triggering receptor expressed on myeloid cells 1 |
| TNFAIP8 | TNFα-Induced Protein 8 |
| SIRS | systemic inflammatory response syndrome |
| SOFA | sequential organ failure assessment |
| TLR4 | toll-like receptor 4 |
| PADs | peptidylarginine deaminases |
| HDAC1 | histone deacetylase 1 |
| JMJD3 | jumonji domain-containing protein 3 |
| ACLY | ATP citrate lyase |
| MLL1 | mixed-lineage leukemia 1 |
| GATA3 | guanine–adenine–thymine–adenine binding protein 3 |
| LLO | listeriolysin O |
| NUE | nuclear ubiquitin E3 ligase |
| OspF | outer surface protein F |
| IpaH9.8 | invasion plasmid antigen H 9.8 |
| Kla | lysine lactylation |
| APACHE | acute physiology and chronic health evaluation |
| ICU | intensive care unit |
| CLP | cecal ligation and puncture |
| EZH2 | enhancer of zeste 2 polycomb repressive complex 2 subunit |
| Sox9 | SRY-box transcription factor 9 |
| EGR1 | early growth response protein 1 |
| HPSE | heparanase |
| ARDS | acute distress respiratory syndrome |
| SIRT | sirtuin |
| NAD | nicotinamide adenine dinucleotide |
| IRAK1 | IL-1 receptor-associated kinase 1 |
| TRAF6 | TNF receptor-associated factor 6 |
| NLRP3 | NOD-, LRR-, and Pyrin domain-containing protein 3 |
| SOCS1 | suppressor of cytokine signaling 1 |
| Bcl6 | B-cell lymphoma 6 |
| JNK | c-Jun N-terminal kinase |
| CRP | C-reactive protein |
| NEAT1 | nuclear-enriched abundant transcript 1 |
| RhoA | Ras homolog gene family A |
| ROCK | Rho-associated coiled-coil-containing protein kinase |
| MALAT1 | metastasis-associated lung adenocarcinoma transcript 1 |
| ICAM-1 | intercellular adhesion molecule 1 |
| TUG1 | taurine upregulated gene 1 |
| MIAT | myocardial infarction-associated transcript |
| HOTAIR | HOX transcript antisense intergenic RNA |
| circ-RSF1 | circular remodeling and spacing factor 1 |
| PPM1F | protein phosphatase, Mg2+/Mn2+ dependent 1F |
| EPC | endothelial progenitor cells |
| ALKBH5 | AlkB homolog 5, RNA demethylase |
| MAPK | mitogen-activated protein kinase |
| MARS | modular acute response system |
| SRS1 | sepsis response signatures |
| eQTL | expression quantitative trait loci |
| EWAS | epigenome-wide association study |
| DMRs | differentially methylated regions |
| SERPINA1 | serine protease inhibitor, clade A, member 1 |
| MPO | myeloperoxidase |
| ATAC-seq | assay for transposase-accessible chromatin using sequencing |
| CEBPB | CCAAT/enhancer-binding protein beta |
| NPS | Neutrophilic-Suppressive |
| INF | Inflammatory |
| IHD | Innate Host Defense |
| IFN | Interferon |
| ADA | Adaptive |
| CALCA | calcitonin-related polypeptide alpha |
| ChIP-seq | chromatin immunoprecipitation sequencing |
| PCT | procalcitonin |
| cfDNA | cell-free DNA |
| G-CSF | granulocyte colony-stimulating factor |
| HDACi | histone deacetylase inhibitors |
| SAHA | suberoylanilide hydroxamic acid |
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Backer, D.; Deutschman, C.; Hellman, J.; Myatra, S.; Ostermann, M.; Prescott, H.; Talmor, D.; Antonelli, M.; Pontes Azevedo, L.; Bauer, S.; et al. Surviving Sepsis Campaign Research Priorities 2023. Crit. Care Med. 2024, 52, 268–296. [Google Scholar] [CrossRef]
- Tammen, S.A.; Friso, S.; Choi, S.W. Epigenetics: The link between nature and nurture. Mol. Asp. Med. 2013, 34, 753–764. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Falcão-Holanda, R.B.; Brunialti, M.K.C.; Jasiulionis, M.G.; Salomão, R. Epigenetic Regulation in Sepsis, Role in Pathophysiology and Therapeutic Perspective. Front. Med. 2021, 8, 685333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, K.; Zhang, K.; Wang, Y.; Gu, Y. Comprehensive review of histone lactylation: Structure, function, and therapeutic targets. Biochem. Pharmacol. 2024, 225, 116331. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Shi, Y.; Zhang, H.; Miao, C. Epigenetic mechanisms of immune remodeling in sepsis: Targeting histone modification. Cell Death Dis. 2023, 14, 112. [Google Scholar] [CrossRef]
- Yao, W.; Hu, X.; Wang, X. Crossing epigenetic frontiers: The intersection of novel histone modifications and diseases. Signal Transduct. Target. Ther. 2024, 9, 232. [Google Scholar] [CrossRef]
- Zhang, T.N.; Li, D.; Xia, J.; Wu, Q.J.; Wen, R.; Yang, N.; Liu, C.F. Non-coding RNA: A potential biomarker and therapeutic target for sepsis. Oncotarget 2017, 8, 91765–91778. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krueger, F.; Kreck, B.; Franke, A.; Andrews, S.R. DNA methylome analysis using short bisulfite sequencing data. Nat. Methods 2012, 9, 145–151. [Google Scholar] [CrossRef]
- Johnson, D.S.; Mortazavi, A.; Myers, R.M.; Wold, B. Genome-wide mapping of in vivo protein-DNA interactions. Science 2007, 316, 1497–1502. [Google Scholar] [CrossRef]
- Kaya-Okur, H.S.; Wu, S.J.; Codomo, C.A.; Pledger, E.S.; Bryson, T.D.; Henikoff, J.G.; Ahmad, K.; Henikoff, S. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat. Commun. 2019, 10, 1930. [Google Scholar] [CrossRef] [PubMed]
- Buenrostro, J.D.; Giresi, P.G.; Zaba, L.C.; Chang, H.Y.; Greenleaf, W.J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 2013, 10, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; Huntley, M.H.; Durand, N.C.; Stamenova, E.K.; Bochkov, I.D.; Robinson, J.T.; Sanborn, A.L.; Machol, I.; Omer, A.D.; Lander, E.S.; et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 2014, 159, 1665–1680. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Qian, X.; Harris, K.D.; Hauling, T.; Nicoloutsopoulos, D.; Muñoz-Manchado, A.B.; Skene, N.; Hjerling-Leffler, J.; Nilsson, M. Probabilistic cell typing enables fine mapping of closely related cell types in situ. Nat. Methods 2020, 17, 101–106. [Google Scholar] [CrossRef]
- Satpathy, A.T.; Granja, J.M.; Yost, K.E.; Qi, Y.; Meschi, F.; McDermott, G.P.; Olsen, B.N.; Mumbach, M.R.; Pierce, S.E.; Corces, M.R.; et al. Massively parallel single-cell chromatin landscapes of human immune cell development and intratumoral T cell exhaustion. Nat. Biotechnol. 2019, 37, 925–936. [Google Scholar] [CrossRef]
- Green, M.D.; Sabatinos, S.A.; Forsburg, S.L. Microscopy techniques to examine DNA replication in fission yeast. Methods Mol. Biol. 2015, 1300, 13–41. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef]
- Silva, E.E.; Skon-Hegg, C.; Badovinac, V.P.; Griffith, T.S. The Calm after the Storm: Implications of Sepsis Immunoparalysis on Host Immunity. J. Immunol. 2023, 211, 711–719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Essandoh, K.; Li, Y.; Huo, J.; Fan, G.C. MiRNA-Mediated Macrophage Polarization and its Potential Role in the Regulation of Inflammatory Response. Shock 2016, 46, 122–131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Córneo, E.D.S.; Michels, M.; Dal-Pizzol, F. Sepsis, immunosuppression and the role of epigenetic mechanisms. Expert Rev. Clin. Immunol. 2021, 17, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, S.Y.; Sun, J.H.; Zhang, H.C.; Cai, Q.L.; Gao, C.; Li, L.; Cao, J.; Xu, F.; Zhou, Y.; et al. Sepsis-induced immunosuppression: Mechanisms, diagnosis and current treatment options. Mil. Med. Res. 2022, 9, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Q.; Wang, Y.; Zheng, Q.; Dong, X.; Xie, Z.; Panayi, A.; Bai, X.; Li, Z. MicroRNA-150 inhibits myeloid-derived suppressor cells proliferation and function through negative regulation of ARG-1 in sepsis. Life Sci. 2021, 278, 119626. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-García, J.; Osca-Verdegal, R.; Romá-Mateo, C.; Carbonell, N.; Ferreres, J.; Rodríguez, M.; Mulet, S.; García-López, E.; Pallardó, F.V.; García-Giménez, J.L. Epigenetic biomarkers for human sepsis and septic shock: Insights from immunosuppression. Epigenomics 2020, 12, 617–646. [Google Scholar] [CrossRef] [PubMed]
- Venet, F.; Monneret, G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 2018, 14, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.; Drury, R.; Hill, J.; Pollard, A.J. Epigenetics in Sepsis: Understanding Its Role in Endothelial Dysfunction, Immunosuppression, and Potential Therapeutics. Front. Immunol. 2019, 10, 1363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, Y.; Zhao, Y.; Zhang, X. Factors affecting neutrophil functions during sepsis: Human microbiome and epigenetics. J. Leukoc. Biol. 2024, 116, 672–688. [Google Scholar] [CrossRef] [PubMed]
- Bierne, H.; Hamon, M.; Cossart, P. Epigenetics and bacterial infections. Cold Spring Harb. Perspect. Med. 2012, 2, a010272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Binnie, A.; Tsang, J.L.Y.; Hu, P.; Carrasqueiro, G.; Castelo-Branco, P.; Dos Santos, C.C. Epigenetics of Sepsis. Crit. Care Med. 2020, 48, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-García, J.; Casabó-Vallés, G.; Osca-Verdegal, R.; Navarrete-López, P.; Rodriguez-Gimillo, M.; Nacher-Sendra, E.; Ferrando-Sánchez, C.; García-López, E.; Pallardó, F.V.; Carbonell, N.; et al. Alterations in leukocyte DNA methylome are associated to immunosuppression in severe clinical phenotypes of septic patients. Front. Immunol. 2024, 14, 1333705. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ismail, N.; Wang, Y.; Dakhlallah, D.; Moldovan, L.; Agarwal, K.; Batte, K.; Shah, P.; Wisler, J.; Eubank, T.D.; Tridandapani, S.; et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood 2013, 121, 984–995. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wisler, J.R.; Singh, K.; Mccarty, A.R.; Abouhashem, A.S.E.; Christman, J.W.; Sen, C.K. Proteomic Pathway Analysis of Monocyte-Derived Exosomes during Surgical Sepsis Identifies Immunoregulatory Functions. Surg. Infect. 2020, 21, 101–111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harkless, R.; Singh, K.; Christman, J.; McCarty, A.; Sen, C.; Jalilvand, A.; Wisler, J. Microvesicle-mediated transfer of DNA methyltransferase proteins results in recipient cell immunosuppression. J. Surg. Res. 2023, 283, 368–376. [Google Scholar] [CrossRef]
- Wisler, J.R.; Singh, K.; McCarty, A.; Harkless, R.; Karpurapu, M.; Hernandez, E.; Mukherjee, D.; Abouhashem, A.S.; Christman, J.W.; Sen, C.K. Exosomal transfer of DNA methyl-transferase mRNA induces an immunosuppressive phenotype in human monocytes. Shock 2022, 57, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Dakhlallah, D.A.; Wisler, J.; Gencheva, M.; Brown, C.M.; Leatherman, E.R.; Singh, K.; Brundage, K.; Karsies, T.; Dakhlallah, A.; Witwer, K.W.; et al. Circulating extracellular vesicle content reveals de novo DNA methyltransferase expression as a molecular method to predict septic shock. J. Extracell. Vesicles 2019, 8, 1669881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, X.; Liu, Y.; Gao, Y.; Shou, S.; Chai, Y. The roles of macrophage polarization in the host immune response to sepsis. Int. Immunopharmacol. 2021, 96, 107791. [Google Scholar] [CrossRef] [PubMed]
- Carson, W.F., 4th; Kunkel, S.L. Regulation of Cellular Immune Responses in Sepsis by Histone Modifications. Adv. Protein Chem. Struct. Biol. 2017, 106, 191–225. [Google Scholar] [CrossRef] [PubMed]
- Urdinguio, R.G.; Lopez, V.; Bayón, G.F.; Diaz de la Guardia, R.; Sierra, M.I.; García-Toraño, E.; Perez, R.F.; García, M.G.; Carella, A.; Pruneda, P.C.; et al. Chromatin regulation by Histone H4 acetylation at Lysine 16 during cell death and differentiation in the myeloid compartment. Nucleic Acids Res. 2019, 47, 5016–5037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cicchinelli, S.; Pignataro, G.; Gemma, S.; Piccioni, A.; Picozzi, D.; Ojetti, V.; Franceschi, F.; Candelli, M. PAMPs and DAMPs in Sepsis: A Review of Their Molecular Features and Potential Clinical Implications. Int. J. Mol. Sci. 2024, 25, 962. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davis, F.M.; Schaller, M.A.; Dendekker, A.; Joshi, A.D.; Kimball, A.S.; Evanoff, H.; Wilke, C.; Obi, A.T.; Melvin, W.J.; Cavassani, K.; et al. Sepsis Induces Prolonged Epigenetic Modifications in Bone Marrow and Peripheral Macrophages Impairing Inflammation and Wound Healing. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2353–2366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jentho, E.; Ruiz-Moreno, C.; Novakovic, B.; Kourtzelis, I.; Megchelenbrink, W.L.; Martins, R.; Chavakis, T.; Soares, M.P.; Kalafati, L.; Guerra, J.; et al. Trained innate immunity, long-lasting epigenetic modulation, and skewed myelopoiesis by heme. Proc. Natl. Acad. Sci. USA 2021, 118, e2102698118. [Google Scholar] [CrossRef]
- Carson, W.F., 4th; Cavassani, K.A.; Ito, T.; Schaller, M.; Ishii, M.; Dou, Y.; Kunkel, S.L. Impaired CD4+ T-cell proliferation and effector function correlates with repressive histone methylation events in a mouse model of severe sepsis. Eur. J. Immunol. 2010, 40, 998–1010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, Z.; Song, Y.; Li, J.; Li, Y.; Yu, Y.; Wang, X. Potential biomarker for diagnosis and therapy of sepsis: Lactylation. Immun. Inflamm. Dis. 2023, 11, e1042. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, S.; Yang, T.; Jiang, Q.; Zhang, L.; Shi, X.; Liu, X.; Li, X. Lactate and Lactylation in Sepsis: A Comprehensive Review. J. Inflamm. Res. 2024, 17, 4405–4417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chu, X.; Di, C.; Chang, P.; Li, L.; Feng, Z.; Xiao, S.; Yan, X.; Xu, X.; Li, H.; Qi, R.; et al. Lactylated Histone H3K18 as a Potential Biomarker for the Diagnosis and Predicting the Severity of Septic Shock. Front. Immunol. 2022, 12, 786666. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qiao, J.; Tan, Y.; Liu, H.; Yang, B.; Zhang, Q.; Liu, Q.; Sun, W.; Li, Z.; Wang, Q.; Feng, W.; et al. Histone H3K18 and Ezrin Lactylation Promote Renal Dysfunction in Sepsis-Associated Acute Kidney Injury. Adv. Sci. 2024, 11, e2307216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Zhang, Y.; Yuan, S.; Zhang, J. The potential immunological mechanisms of sepsis. Front. Immunol. 2024, 15, 1434688. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, B.; Xia, Y.; Mei, S.; Ye, Z.; Song, B.; Yan, X.; Lin, F.; Rao, T.; Yu, W.; Mei, C.; et al. Histone H3K27 methyltransferase EZH2 regulates apoptotic and inflammatory responses in sepsis-induced AKI. Theranostics 2023, 13, 1860–1875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, Z.; Fang, P.; Li, S.; Xia, D.; Zhang, J.; Wu, X.; Pan, J.; Cai, H.; Fu, L.; Sun, G.; et al. Lactylation of Histone H3k18 and Egr1 Promotes Endothelial Glycocalyx Degradation in Sepsis-Induced Acute Lung Injury. Adv. Sci. 2025, 12, e2407064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gandhirajan, A.; Roychowdhury, S.; Vachharajani, V. Sirtuins and Sepsis: Cross Talk between Redox and Epigenetic Pathways. Antioxidants 2021, 11, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vachharajani, V.T.; Liu, T.; Brown, C.M.; Wang, X.; Buechler, N.L.; Wells, J.D.; Yoza, B.K.; McCall, C.E. SIRT1 inhibition during the hypoinflammatory phenotype of sepsis enhances immunity and improves outcome. J. Leukoc. Biol. 2014, 96, 785–796. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vachharajani, V.; McCall, C.E. Epigenetic and metabolic programming of innate immunity in sepsis. Innate Immun. 2019, 25, 267–279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qin, K.; Han, C.; Zhang, H.; Li, T.; Li, N.; Cao, X. NAD+ dependent deacetylase Sirtuin 5 rescues the innate inflammatory response of endotoxin tolerant macrophages by promoting acetylation of p65. J. Autoimmun. 2017, 81, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Buechler, N.L.; Woodruff, A.G.; Long, D.L.; Zabalawi, M.; Yoza, B.K.; McCall, C.E.; Vachharajani, V. Sirtuins and Immuno-Metabolism of Sepsis. Int. J. Mol. Sci. 2018, 19, 2738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hashemian, S.M.; Pourhanifeh, M.H.; Fadaei, S.; Velayati, A.A.; Mirzaei, H.; Hamblin, M.R. Non-coding RNAs and Exosomes: Their Role in the Pathogenesis of Sepsis. Mol. Ther. Nucleic Acids 2020, 21, 51–74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Z.; Wang, Z. The role of macrophages polarization in sepsis-induced acute lung injury. Front. Immunol. 2023, 14, 1209438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manetti, A.C.; Maiese, A.; Paolo, M.D.; De Matteis, A.; La Russa, R.; Turillazzi, E.; Frati, P.; Fineschi, V. MicroRNAs and Sepsis-Induced Cardiac Dysfunction: A Systematic Review. Int. J. Mol. Sci. 2020, 22, 321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beltrán-García, J.; Osca-Verdegal, R.; Nacher-Sendra, E.; Pallardó, F.V.; García-Giménez, J.L. Circular RNAs in Sepsis: Biogenesis, Function, and Clinical Significance. Cells 2020, 9, 1544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maiese, A.; Scatena, A.; Costantino, A.; Chiti, E.; Occhipinti, C.; La Russa, R.; Di Paolo, M.; Turillazzi, E.; Frati, P.; Fineschi, V. Expression of MicroRNAs in Sepsis-Related Organ Dysfunction: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 9354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bi, C.F.; Liu, J.; Hu, X.D.; Yang, L.S.; Zhang, J.F. Novel insights into the regulatory role of N6-methyladenosine methylation modified autophagy in sepsis. Aging 2023, 15, 15676–15700. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, T.; Sun, L.; Zhu, J.; Li, N.; Gu, H.; Zhang, Y.; Li, M.; Xu, J. MicroRNA-23a-3p promotes macrophage M1 polarization and aggravates lipopolysaccharide-induced acute lung injury by regulating PLK1/STAT1/STAT3 signalling. Int. J. Exp. Pathol. 2022, 103, 198–207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, W.; Wang, H.; Sun, T. N6-methyladenosine modification: Regulatory mechanisms and therapeutic potential in sepsis. Biomed. Pharmacother. 2023, 168, 115719. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Zhang, D.; Li, Y. The Role of Long Non-coding RNAs in Sepsis-Induced Cardiac Dysfunction. Front. Cardiovasc. Med. 2021, 8, 684348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, H.; Zhang, X.; Su, H.; Zeng, J.; Chan, H.; Li, Q.; Liu, X.; Zhang, L.; Wu, W.K.K.; Chan, M.T.V.; et al. Immune dysregulation and RNA N6-methyladenosine modification in sepsis. Wiley Interdiscip. Rev. RNA 2023, 14, e1764. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, M.; Yue, P.; Zhang, D.; Tong, J.; Li, Y. Novel Insights into the Potential Mechanisms of N6-Methyladenosine RNA Modification on Sepsis-Induced Cardiovascular Dysfunction: An Update Summary on Direct and Indirect Evidences. Front. Cell Dev. Biol. 2021, 9, 772921. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scicluna, B.P.; van Vught, L.A.; Zwinderman, A.H.; Wiewel, M.A.; Davenport, E.E.; Burnham, K.L.; Nürnberg, P.; Schultz, M.J.; Horn, J.; Cremer, O.L.; et al. Classification of patients with sepsis according to blood genomic endotype: A prospective cohort study. Lancet Respir. Med. 2017, 5, 816–826. [Google Scholar] [CrossRef]
- Davenport, E.E.; Burnham, K.L.; Radhakrishnan, J.; Humburg, P.; Hutton, P.; Mills, T.C.; Rautanen, A.; Gordon, A.C.; Garrard, C.; Hill, A.V.; et al. Genomic landscape of the individual host response and outcomes in sepsis: A prospective cohort study. Lancet Respir. Med. 2016, 4, 259–271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sweeney, T.E.; Azad, T.D.; Donato, M.; Haynes, W.A.; Perumal, T.M.; Henao, R.; Bermejo-Martin, J.F.; Almansa, R.; Tamayo, E.; Howrylak, J.A.; et al. Unsupervised analysis of transcriptomics in bacterial sepsis across multiple datasets reveals three robust clusters. Crit. Care Med. 2018, 46, 915–925. [Google Scholar] [CrossRef]
- Binnie, A.; Walsh, C.J.; Hu, P.; Dwivedi, D.J.; Fox-Robichaud, A.; Liaw, P.C.; Tsang, J.L.Y.; Batt, J.; Carrasqueiro, G.; Gupta, S.; et al. Epigenetic Profiling in Severe Sepsis: A Pilot Study of DNA Methylation Profiles in Critical Illness. Crit. Care Med. 2020, 48, 142–150. [Google Scholar] [CrossRef] [PubMed]
- López-Cruz, I.; García-Giménez, J.L.; Madrazo, M.; García-Guallarte, J.; Piles, L.; Pallardó, F.V.; Artero, A. Epigenome-wide DNA methylation profiling in septic and non-septic patients with similar infections: Potential use as sepsis biomarkers. Front. Cell. Infect. Microbiol. 2025, 14, 1532417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kwok, A.J.; Allcock, A.; Ferreira, R.C.; Cano-Gamez, E.; Smee, M.; Burnham, K.L.; Zurke, Y.X.; Emergency Medicine Research Oxford (EMROx); McKechnie, S.; Mentzer, A.J.; et al. Neutrophils and emergency granulopoiesis drive immune suppression and an extreme response endotype during sepsis. Nat. Immunol. 2023, 24, 767–779. [Google Scholar] [CrossRef]
- Severino, P.; Silva, E.; Baggio-Zappia, G.L.; Brunialti, M.K.; Nucci, L.A.; Rigato, O., Jr.; da Silva, I.D.; Machado, F.R.; Salomao, R. Patterns of gene expression in peripheral blood mononuclear cells and outcomes from patients with sepsis secondary to community acquired pneumonia. PLoS ONE 2014, 9, e91886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burnham, K.L.; Davenport, E.E.; Radhakrishnan, J.; Humburg, P.; Gordon, A.C.; Hutton, P.; Svoren-Jabalera, E.; Garrard, C.; Hill, A.V.S.; Hinds, C.J.; et al. Shared and Distinct Aspects of the Sepsis Transcriptomic Response to Fecal Peritonitis and Pneumonia. Am. J. Respir. Crit. Care Med. 2017, 196, 328–339. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balch, J.A.; Chen, U.I.; Liesenfeld, O.; Starostik, P.; Loftus, T.J.; Efron, P.A.; Brakenridge, S.C.; Sweeney, T.E.; Moldawer, L.L. Defining critical illness using immunological endotypes in patients with and without sepsis: A cohort study. Crit. Care 2023, 27, 292. [Google Scholar] [CrossRef] [PubMed]
- Bodinier, M.; Monneret, G.; Casimir, M.; Fleurie, A.; Conti, F.; Venet, F.; Cazalis, M.A.; Cerrato, E.; Peronnet, E.; Rimmelé, T.; et al. Identification of a sub-group of critically ill patients with high risk of intensive care unit-acquired infections and poor clinical course using a transcriptomic score. Crit. Care 2023, 27, 158. [Google Scholar] [CrossRef] [PubMed]
- Baghela, A.; Pena, O.M.; Lee, A.H.; Baquir, B.; Falsafi, R.; An, A.; Farmer, S.W.; Hurlburt, A.; Mondragon-Cardona, A.; Rivera, J.D.; et al. Predicting sepsis severity at first clinical presentation: The role of endotypes and mechanistic signatures. EBioMedicine 2022, 75, 103776. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, F.; Wu, Z.; Xie, J.; Yang, Y.; Qiu, H. Contribution of m6A subtype classification on heterogeneity of sepsis. Ann. Transl. Med. 2020, 8, 306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Antcliffe, D.B.; Burnham, K.L.; Al-Beidh, F.; Santhakumaran, S.; Brett, S.J.; Hinds, C.J.; Ashby, D.; Knight, J.C.; Gordon, A.C. Transcriptomic signatures in sepsis and a differential response to steroids from the VANISH randomized trial. Am. J. Respir. Crit. Care Med. 2019, 199, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Puskarich, M.A.; Jennaro, T.S.; Gillies, C.E.; Evans, C.R.; Karnovsky, A.; McHugh, C.E.; Flott, T.L.; Jones, A.E.; Stringer, K.A.; RACE Investigators. Pharmacometabolomics identifies candidate predictor metabolites of an L-carnitine treatment mortality benefit in septic shock. Clin. Transl. Sci. 2021, 14, 2288–2299. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Zhou, J.; Shou, S. Exploration of m6A methylation regulators as epigenetic targets for immunotherapy in advanced sepsis. BMC Bioinform. 2023, 24, 257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.; Jin, S.; Wang, C.; Jiang, R.; Wan, J. Histone deacetylase inhibitors attenuate acute lung injury during cecal ligation and puncture-induced polymicrobial sepsis. World J. Surg. 2010, 34, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Takebe, M.; Oishi, H.; Taguchi, K.; Aoki, Y.; Takashina, M.; Tomita, K.; Yokoo, H.; Takano, Y.; Yamazaki, M.; Hattori, Y. Inhibition of histone deacetylases protects septic mice from lung and splenic apoptosis. J. Surg. Res. 2014, 187, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.; Qu, D.; Yu, J.; Yang, J. Role of HDAC6 inhibition in sepsis-induced acute respiratory distress syndrome (Review). Exp. Ther. Med. 2021, 21, 422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williams, A.M.; Dennahy, I.S.; Bhatti, U.F.; Biesterveld, B.E.; Graham, N.J.; Li, Y.; Alam, H.B. Histone Deacetylase Inhibitors: A Novel Strategy in Trauma and Sepsis. Shock 2019, 52, 300–306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- von Knethen, A.; Brüne, B. Histone Deacetylation Inhibitors as Therapy Concept in Sepsis. Int. J. Mol. Sci. 2019, 20, 346. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, T.; Li, Y.; Liu, B.; Bronson, R.T.; Halaweish, I.; Alam, H.B. Histone deacetylase III as a potential therapeutic target for the treatment of lethal sepsis. J. Trauma Acute Care Surg. 2014, 77, 913–919; discussion 919. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, L.; Zhu, T.; Lang, X.; Jia, S.; Yang, Y.; Zhu, C.; Wang, Y.; Feng, S.; Wang, C.; Zhang, P.; et al. Inhibiting DNA Methylation Improves Survival in Severe Sepsis by Regulating NF-κB Pathway. Front. Immunol. 2020, 11, 1360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dragomir, M.P.; Fuentes-Mattei, E.; Winkle, M.; Okubo, K.; Bayraktar, R.; Knutsen, E.; Qdaisat, A.; Chen, M.; Li, Y.; Shimizu, M.; et al. Anti-miR-93-5p therapy prolongs sepsis survival by restoring the peripheral immune response. J. Clin. Investig. 2023, 133, e158348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Histone Modification | Mechanism | Target Genes/Pathways | Pathophysiological Effect |
|---|---|---|---|
| H3K27me3 | Catalyzed by EZH2; repressive mark | Sox9, IL-12 promoter | Suppresses gene expression; associated with immune cell dysfunction, AKI and reduced IL-12 in dendritic cells [42,50] |
| H3K4me3 | Activating mark at gene promoters | NF-κB target genes | Enhances transcription of inflammatory genes; its loss linked to long-term immune suppression post-sepsis [4,41] |
| H3K9ac/H4ac | Acetylation by HATs increases chromatin accessibility | Foxp3, cytokine promoters | Promotes transcription; H3K9ac linked to Treg polarization and immunosuppression [4] |
| H3K18la | Addition of lactyl group from lactate via p300 | EGR1, RhoA | Promotes transcription of genes involved in repair and inflammation; associated with ALI, AKI, and immunosuppression [46,47,48,51] |
| Citrullination | Converts arginine to citrulline | NET-related genes | Enables NET formation; contributes to hyperinflammation and tissue damage [6,38] |
| Deacetylation | Removes acetyl groups; represses gene transcription | TNF, IL-1β | Promotes immunosuppression; HDAC1/2 modulates TLR responses and contributes to endotoxin tolerance [6,39] |
| ncRNA Type/Example | Molecular Function/Target | Immune/Cellular Effects | Pathophysiological/Clinical Relevance |
|---|---|---|---|
| miR-146a | Targets IRAK1, TRAF6 in TLR/NF-κB pathway | Suppresses inflammatory signaling | Protects against cytokine storm and cardiac dysfunction [8,37,60,61] |
| miR-155 | Inhibits SOCS1 | Enhances NF-κB activation, promotes M1 macrophage polarization | Amplifies early inflammatory response [58] |
| miR-223 | Regulates NLRP3, RhoB | Promotes M2 macrophage phenotype; modulates neutrophil activation | Linked to improved survival in murine sepsis models [30,61,64] |
| miR-125b | Represses Bcl6, activates JNK pathway | Drives M1 polarization | Contributes to early inflammation [8,37] |
| miR-150 | General immunoregulatory role; diagnostic value | Reduced levels associated with worse outcome | Circulating biomarker correlating with SOFA score [8,58] |
| lncRNA NEAT1 | Sponges miR-125a-5p, derepresses TRAF6 | Promotes M1 polarization and NF-κB activation | Pro-inflammatory role; marker of immune dysregulation [37] |
| lncRNA MALAT1 | Sponges miR-150-5p | Upregulates ICAM-1, promotes endothelial activation | Aggravates sepsis-induced acute lung injury (ALI) [58] |
| lncRNA TUG1 | Activates SIRT1 via miR-9-5p axis | Promotes M2 polarization, stabilizes mitochondrial function | Protective in sepsis; enhances survival and tissue repair [65] |
| circRNA circPPM1F | Sponges miRNAs that inhibit NF-κB | Promotes M1 macrophage polarization | Exacerbates cytokine storm [60] |
| circRNA circ_0038644 | Inhibits IL-6 production | Supports anti-inflammatory profile | Protective effect during hyperinflammation [60] |
| Biomarker/Endotype | Molecular Characteristics | Associated Therapy Response |
|---|---|---|
| SRS1 (Sepsis Response Signature 1) | Suppression of HLA-DR and T-cell signaling; epigenetic repression of immune genes | No benefit or potential benefit from corticosteroids; potential target for immune-stimulatory therapies [69,76,80] |
| SRS2 (Sepsis Response Signature 2) | Preserved immune function; high expression of adaptive immune genes | Corticosteroids associated with significantly increased 28-day mortality [69,80] |
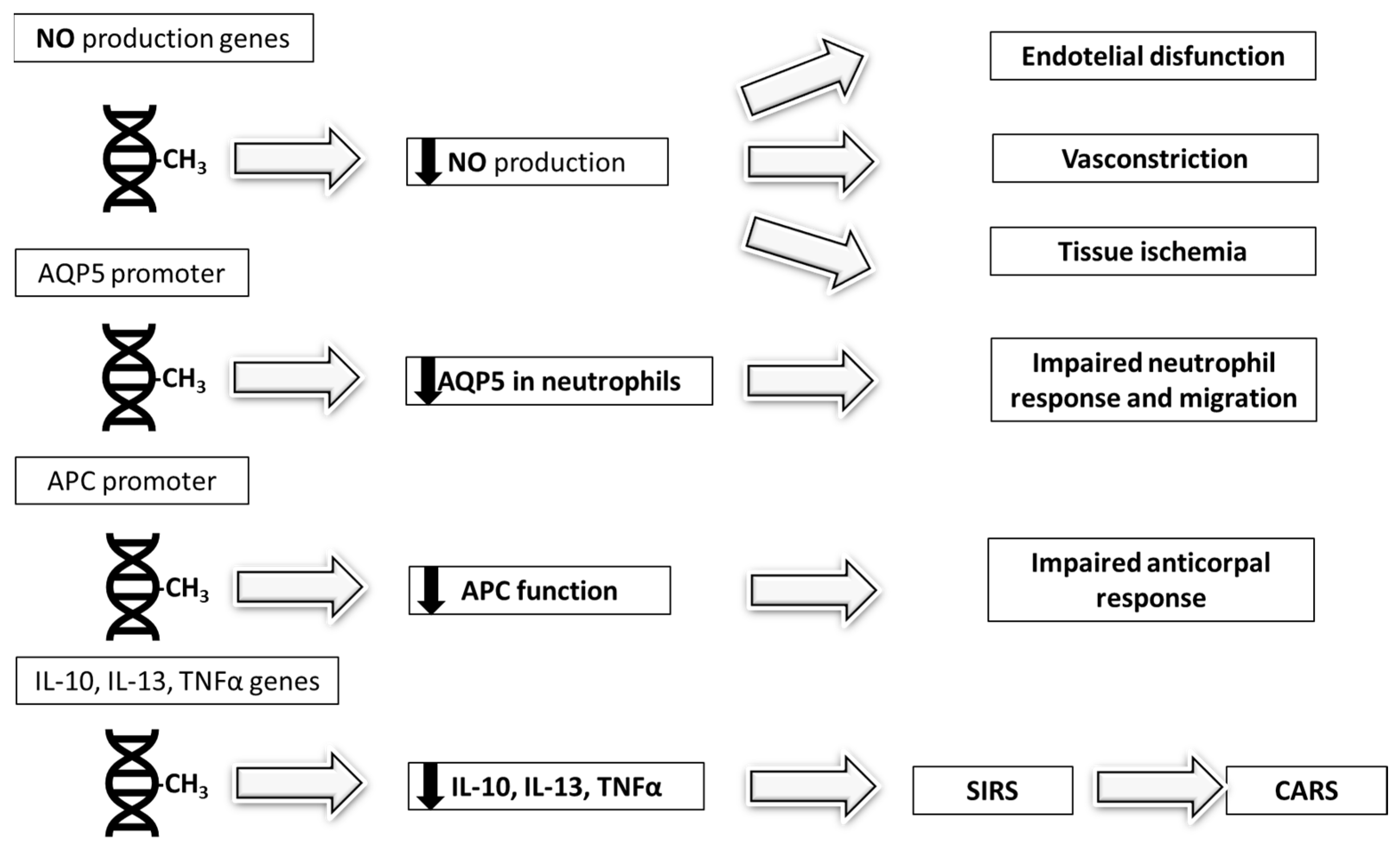
| AQP5 DNAMethylation | Hypermethylation of AQP5 promoter in neutrophils reduces gene expression and cell migration | Predicts poor outcome and reduced response to neutrophil-targeting therapies (e.g., G-CSF) [28] |
| Histone Acetylation/HDAC Activity | Altered levels of histone acetylation (e.g., reduced H3K9ac, increased HDAC activity) suppress immune gene transcription | Response to histone deacetylase inhibitors (HDACi) in preclinical models [6,80] |
| Acetyl carnitine Levels ≥ 35 µM | Reflects mitochondrial dysfunction and metabolic distress | Identifies responders to L-carnitine therapy; associated with reduced 90-day mortality in treated patients [81] |
| m6A Cluster A | High expression of METTL3, enrichment in Tregs and resting dendritic cells; immune-suppressive signature | May benefit from demethylase activators (e.g., FTO, ALKBH5) to restore immune competence [82] |
| m6A Cluster B | High YTHDF1, increased M1 macrophages and neutrophil activation; hyperinflammatory profile | Potential target for METTL3 or YTHDF1 inhibition to dampen cytokine storm [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pignataro, G.; Triunfo, C.; Piccioni, A.; Racco, S.; Fuorlo, M.; Forte, E.; Franceschi, F.; Candelli, M. The Epigenetics of Sepsis: How Gene Modulation Shapes Outcomes. Biomedicines 2025, 13, 1936. https://doi.org/10.3390/biomedicines13081936
Pignataro G, Triunfo C, Piccioni A, Racco S, Fuorlo M, Forte E, Franceschi F, Candelli M. The Epigenetics of Sepsis: How Gene Modulation Shapes Outcomes. Biomedicines. 2025; 13(8):1936. https://doi.org/10.3390/biomedicines13081936
Chicago/Turabian StylePignataro, Giulia, Cristina Triunfo, Andrea Piccioni, Simona Racco, Mariella Fuorlo, Evelina Forte, Francesco Franceschi, and Marcello Candelli. 2025. "The Epigenetics of Sepsis: How Gene Modulation Shapes Outcomes" Biomedicines 13, no. 8: 1936. https://doi.org/10.3390/biomedicines13081936
APA StylePignataro, G., Triunfo, C., Piccioni, A., Racco, S., Fuorlo, M., Forte, E., Franceschi, F., & Candelli, M. (2025). The Epigenetics of Sepsis: How Gene Modulation Shapes Outcomes. Biomedicines, 13(8), 1936. https://doi.org/10.3390/biomedicines13081936








