Non-Specific Pleuritis After Medical Thoracoscopy: The Portrait of an Open Issue and Practical Hints for Its Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Thoracoscopic Procedure
2.3. Diagnostic Criteria for NSP
2.4. Statistical Analysis
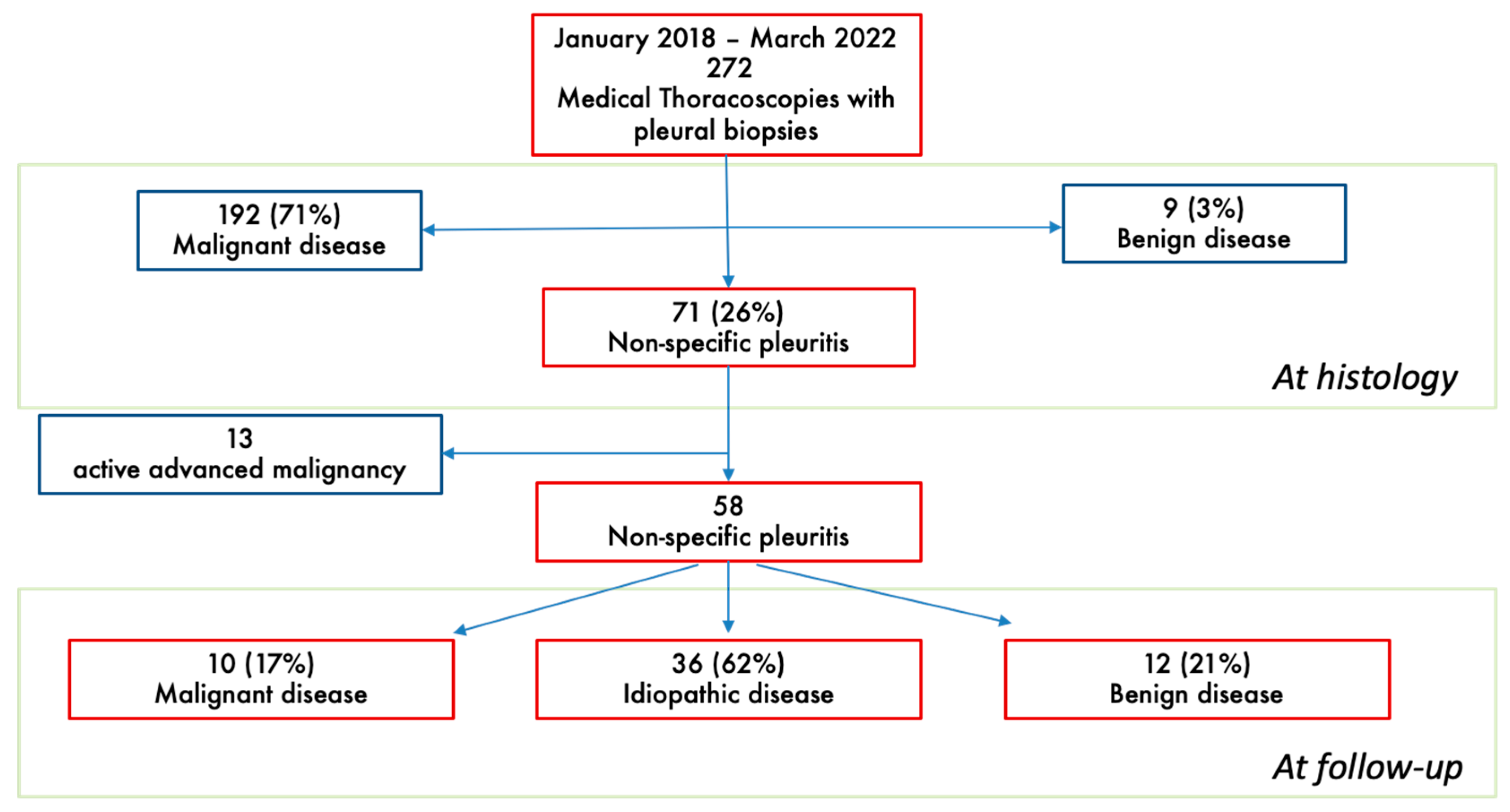
3. Results
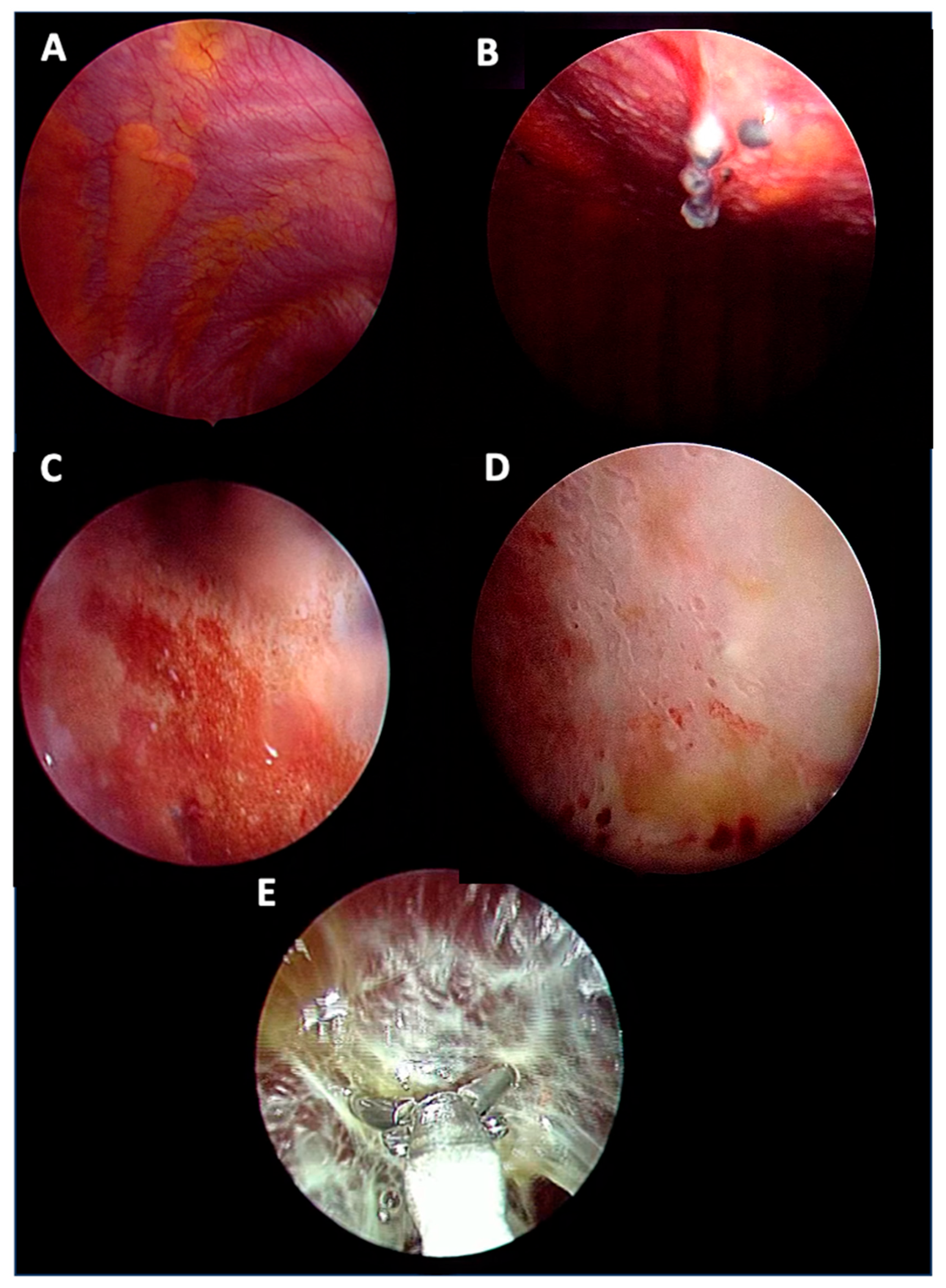
3.1. Clinical and Thoracoscopic Characteristics
3.2. Pleural Effusion Analysis
3.3. Follow-Up Evaluation at Pleural Clinic
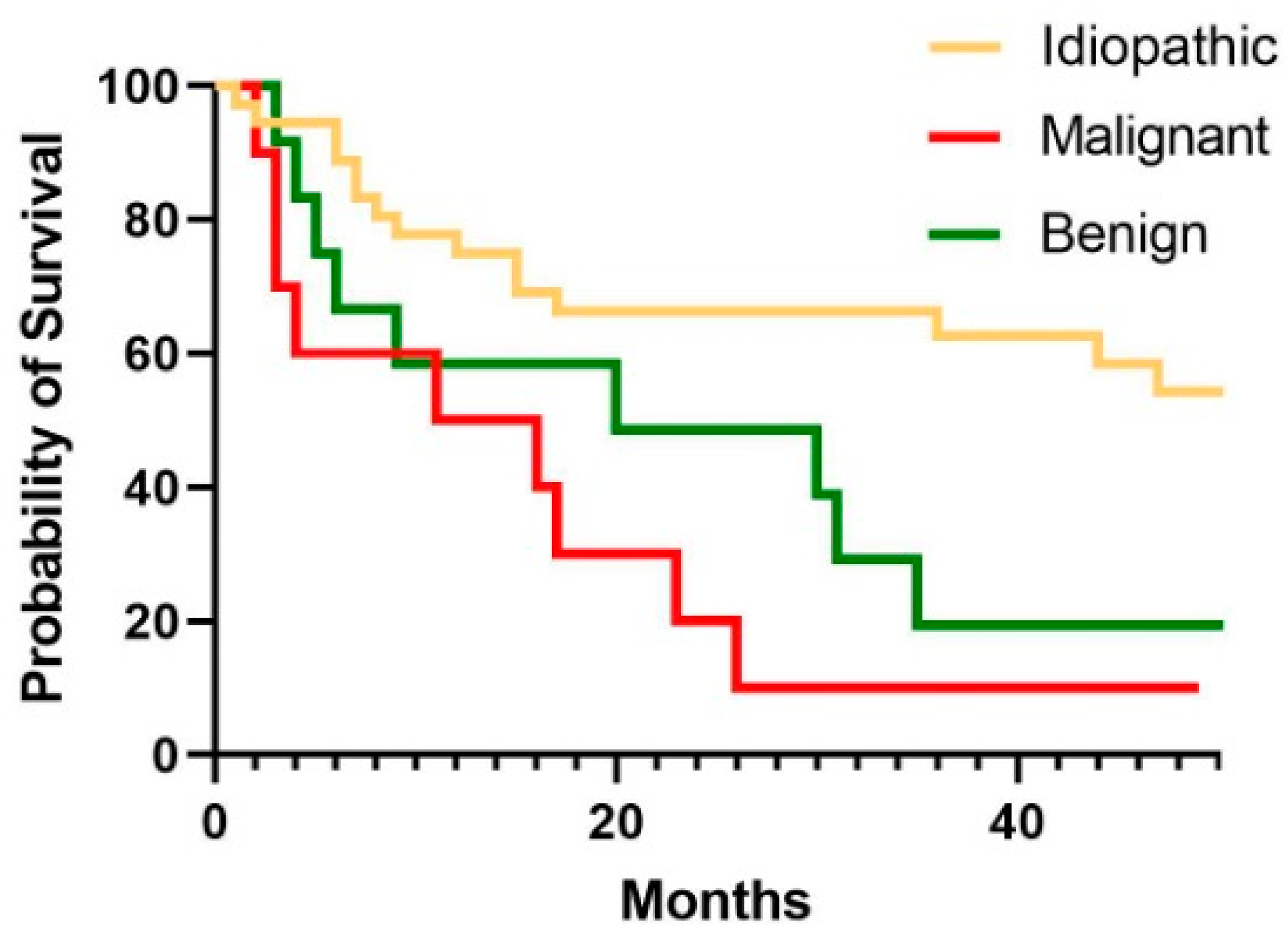
3.4. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NSP | Non-specific pleuritis |
| MPM | Malignant pleural mesothelioma |
| NSCLC-NOS | Non-small-cell lung cancer not otherwise specified |
| NTM | Non-tuberculous mycobacteria |
| GVHD | Graft-versus-host disease |
| VATS | Video-assisted thoracoscopic surgery |
| FU | Follow-up |
| PPV | Positive predictive value |
| NPV | Negative predictive value |
| HR | Hazard ratio |
References
- Rodríguez-Panadero, F. Medical thoracoscopy. Respiration 2008, 76, 363–372. [Google Scholar] [CrossRef] [PubMed]
- E Roberts, M.; Rahman, N.M.; A Maskell, N.; Bibby, A.C.; Blyth, K.G.; Corcoran, J.P.; Edey, A.; Evison, M.; de Fonseka, D.; Hallifax, R.; et al. British Thoracic Society Guideline for pleural disease. Thorax 2023, 78, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Bodtger, U.; Hallifax, R.J. Epidemiology: Why is pleural disease becoming more common? In Pleural Disease; Maskell, N.A., Laursen, C.B., Lee, Y.C.G., Rahman, N.M., Eds.; European Respiratory Society: Sheffield, UK, 2020; Chapter 1; pp. 1–12. [Google Scholar] [CrossRef]
- Metintas, M.; Ak, G.; Cadirci, O.; Yildirim, H.; Dundar, E.; Metintas, S. Outcome of patients diagnosed with fibrinous pleuritis after medical thoracoscopy. Respir. Med. 2012, 106, 1177–1183. [Google Scholar] [CrossRef]
- Davies, H.E.; Nicholson, J.E.; Rahman, N.M.; Wilkinson, E.M.; Davies, R.J.; Lee, Y.C.G. Outcome of patients with nonspecific pleuritis/fibrosis on thoracoscopic pleural biopsies. Eur. J. Cardio-Thoracic Surg. 2010, 38, 472–477. [Google Scholar] [CrossRef]
- Karpathiou, G.; Anevlavis, S.; Tiffet, O.; Casteillo, F.; Mobarki, M.; Mismetti, V.; Ntolios, P.; Koulelidis, A.; Trouillon, T.; Zadel, N.; et al. Clinical long-term outcome of non-specific pleuritis (NSP) after surgical or medical thoracoscopy. J. Thorac. Dis. 2020, 12, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Y.-B.; Wang, Z.; Wang, X.-J.; Xu, L.-L.; Tong, Z.-H.; Shi, H.-Z. Long-term outcome of patients with nonspecific pleurisy at medical thoracoscopy. Respir. Med. 2017, 124, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-X.; Yang, Y.; Wu, Y.-B.; Wang, X.-J.; Xu, L.-L.; Wang, Z.; Wang, F.; Tong, Z.-H.; Shi, H.-Z. An update of the long-term outcome of patients with nonspecific pleurisy at medical thoracoscopy. BMC Pulm. Med. 2021, 21, 226. [Google Scholar] [CrossRef] [PubMed]
- Seixas, E.; Ferreira, P.G.; Seixas, C.; Teixeira, G.; Rodrigues, B. Non-Specific Pleuritis after Medical Thoracoscopy: A Prospective Study. Acta Medica Port. 2023, 36, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Sundaralingam, A.; Aujayeb, A.; Jackson, K.A.; Pellas, E.I.; Khan, I.I.; Chohan, M.T.; Joosten, R.; Boersma, A.; Kerkhoff, J.; Bielsa, S.; et al. Investigation and outcomes in patients with nonspecific pleuritis: Results from the International Collaborative Effusion database. ERJ Open Res. 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- DePew, Z.S.; Verma, A.; Wigle, D.; Mullon, J.J.; Nichols, F.C.; Maldonado, F. Nonspecific Pleuritis: Optimal Duration of Follow-Up. Ann. Thorac. Surg. 2014, 97, 1867–1871. [Google Scholar] [CrossRef] [PubMed]
- Kapp, C.; Janssen, J.; Maldonado, F.; Yarmus, L. Nonspecific pleuritis. ERS Monograph. In Pleural Disease (ERS Monograph); Maskell, N.A., Laursen, C.B., Lee, Y.C.G., Rahman, N.M., Eds.; European Respiratory Society: Sheffield, UK, 2020; pp. 211–217. [Google Scholar] [CrossRef]
- Gunluoglu, G.; Olcmen, A.; Gunluoglu, M.Z.; Dincer, I.; Sayar, A.; Camsari, G.; Yilmaz, V.; Altin, S. Long-term Outcome of Patients with Undiagnosed Pleural Effusion. Arch. Bronc. (Engl. Ed.) 2015, 51, 632–636. [Google Scholar] [CrossRef]
- Tinè, M.; Daverio, M.; Semenzato, U.; Cocconcelli, E.; Bernardinello, N.; Damin, M.; Saetta, M.; Spagnolo, P.; Balestro, E. Pleural clinic: Where thoracic ultrasound meets respiratory medicine. Front. Med. 2023, 10, 1289221. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chan, S.C.; Pang, W.S.; Chow, S.H.; Lok, V.; Zhang, L.; Lin, X.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.-J.; et al. Global Incidence, Risk Factors, and Temporal Trends of Mesothelioma: A Population-Based Study. J. Thorac. Oncol. 2023, 18, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Gonnelli, F.; Hassan, W.; Bonifazi, M.; Pinelli, V.; O Bedawi, E.; Porcel, J.M.; Rahman, N.M.; Mei, F. Malignant pleural effusion: Current understanding and therapeutic approach. Respir. Res. 2024, 25, 47. [Google Scholar] [CrossRef] [PubMed]
- Ellayeh, M.; Bedawi, E.; Banka, R.; Sundaralingam, A.; George, V.; Kanellakis, N.; Hallifax, R.; Abdelwahab, H.; Rezk, N.; Hewidy, A.; et al. Objective Thoracoscopic Criteria in Differentiation between Benign and Malignant Pleural Effusions. Respiration 2022, 101, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Mercer, R.M.; Corcoran, J.P.; Porcel, J.M.; Rahman, N.M.; Psallidas, I. Interpreting pleural fluid results. Clin. Med. 2019, 19, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Vakil, E.; Ost, D.; Vial, M.R.; Stewart, J.; Sarkiss, M.G.; Morice, R.C.; Casal, R.F.; Eapen, G.A.; Grosu, H.B. Non-specific pleuritis in patients with active malignancy. Respirology 2017, 23, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.; Maldonado, F.; Metintas, M. What is the significance of non-specific pleuritis? A trick question. Clin. Respir. J. 2018, 12, 2407–2410. [Google Scholar] [CrossRef] [PubMed]
- Markatis, E.; Perlepe, G.; Afthinos, A.; Pagkratis, K.; Varsamas, C.; Chaini, E.; Papanikolaou, I.C.; Gourgoulianis, K.I. Mortality Among Hospitalized Patients With Pleural Effusions. A Multicenter, Observational, Prospective Study. Front. Med. 2022, 9, 828783. [Google Scholar] [CrossRef] [PubMed]
| Overall Population 58 | Malignant Disease 10 | Benign Disease 12 | Idiopathic Disease 36 | p Value | |
|---|---|---|---|---|---|
| Male, N (%) | 42 (72) | 7 (70) | 9 (75) | 26 (72) | n.s. |
| Age, years | 75 ± 10 | 75 ± 6 | 74 ± 9 | 74 ± 11 | n.s. |
| Smoking history, N (%) Former Current Never | 21 (44) 25 (52) 2 (4) | 5 (50) 5 (50) 0 | 3 (37) 5 (63) 0 | 13 (43) 15 (50) 2 (6) | n.s. |
| Pack years | 22 ± 17 | 20 ± 9 | 22 ± 10 | 22 ± 22 | n.s. |
| Professional exposure, N (%) Asbestos | 18 (40) 9 (20) | 4 (44) 0 | 1 (20) 1 (20) | 4 (13) 8 (26) | n.s. |
| Benign Effusion Aetiologies | N° (%) |
|---|---|
| Chronic heart failure | 4 (33%) |
| Asbestos-related pleuritis | 3 (25%) |
| Hepatic hydrothorax | 1 (8%) |
| NTM1 pleuritis | 1 (8%) |
| GVHD2 following stem cell transplantation | 1 (8%) |
| Serositis | 1 (8%) |
| Immunoglobulin G4-related disease | 1 (8%) |
| Overall Population 58 | Malignant Disease 10 | Benign Disease 12 | Idiopathic Disease 36 | |
|---|---|---|---|---|
| Fluid appearance Serous, N (%) Haemorrhagic, N (%) | 37 (64) 21 (36) | 7 (70) 3 (30) | 7 (58) 5 (42) | 23 (64) 13 (36) |
| Thoracoscopic appearance ^, N (%) Nodular Hyperaemia Septations Thickening Normal | 54 19 (35) 7 (13) 7 (13) 13 (24) 8 (15) | 9 5 (55) 2 (23) 1 (11) 0 (0) 1 (11) | 11 2 (18) 2 (18) 2 (18) 2 (18) 3 (28) | 34 12 (35) 3 (9) 4 (12) 11 (32) 4 (12) |
| Volume of drained effusion (L) | 1.8 ± 1.1 | 2.7 ± 1.6 * | 1.8 ± 1.1 | 1.6 ± 0.9 |
| Talc poudrage N (%) | 25 (43) | 7 (70) ** | 4 (33) | 14 (38) |
| Overall Population 53 | Malignant Disease 8 | Benign Disease 12 | Idiopathic Disease 33 | |
|---|---|---|---|---|
| Pleural Fluid Exudate (%) Lymphocytes > 50% (%) Eosinophils > 10% (%) | 44 (83) 27 (51) 2 (4) | 8 (100) 5 (63) 1 (13) | 7 (58) * 2 (16) ** 0 (0) | 29 (89) 20 (60) 1 (3) |
| Overall Population 37 | Malignant Disease 8 | Benign Disease 9 | Idiopathic Disease 20 | |
|---|---|---|---|---|
| Recurrency, N (%) Time from MT to recurrency ^ (days) Recurrency treatment ^ Medical therapy Thoracentesis Chest drain VATS No therapy | 19 (50) 197 (0–427) 10 (59) 2 (11) 2 (11) 3 (17) 2 (11) | 4 (50) 248 (67–429) 2 (50) 0 1 (25) 1 (25) 0 | 3 (33) 581 (393–769) 2(66) 0 1(33) 0 0 | 12 (60) 78 (17–214) 6 (60) 2 (20) 0 2 (20) 2 (20) |
| 1-year survival, N (%) | 40 (69) | 5 (50) | 7 (58) | 28 (78) ** |
| Survived at FU, N (%) | 21 (36) | 1 (10) | 2 (16) | 18 (50) * |
| Time from MT to death (days) | 371 (163–919) | 315 (83–591) | 434 (149–963) | 405 (195–1353) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daverio, M.; Tinè, M.; Semenzato, U.; Prevedello, R.; Dalla Libera, M.; Cocconcelli, E.; Balestro, E.; Damin, M.; Spagnolo, P.; Biondini, D. Non-Specific Pleuritis After Medical Thoracoscopy: The Portrait of an Open Issue and Practical Hints for Its Management. Biomedicines 2025, 13, 1934. https://doi.org/10.3390/biomedicines13081934
Daverio M, Tinè M, Semenzato U, Prevedello R, Dalla Libera M, Cocconcelli E, Balestro E, Damin M, Spagnolo P, Biondini D. Non-Specific Pleuritis After Medical Thoracoscopy: The Portrait of an Open Issue and Practical Hints for Its Management. Biomedicines. 2025; 13(8):1934. https://doi.org/10.3390/biomedicines13081934
Chicago/Turabian StyleDaverio, Matteo, Mariaenrica Tinè, Umberto Semenzato, Roberta Prevedello, Matteo Dalla Libera, Elisabetta Cocconcelli, Elisabetta Balestro, Marco Damin, Paolo Spagnolo, and Davide Biondini. 2025. "Non-Specific Pleuritis After Medical Thoracoscopy: The Portrait of an Open Issue and Practical Hints for Its Management" Biomedicines 13, no. 8: 1934. https://doi.org/10.3390/biomedicines13081934
APA StyleDaverio, M., Tinè, M., Semenzato, U., Prevedello, R., Dalla Libera, M., Cocconcelli, E., Balestro, E., Damin, M., Spagnolo, P., & Biondini, D. (2025). Non-Specific Pleuritis After Medical Thoracoscopy: The Portrait of an Open Issue and Practical Hints for Its Management. Biomedicines, 13(8), 1934. https://doi.org/10.3390/biomedicines13081934






