Preclinical Evidence of Curcuma longa Linn. as a Functional Food in the Management of Metabolic Syndrome: A Systematic Review and Meta-Analysis of Rodent Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Data Retrieval and Synthesis
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Risk of Bias Assessment
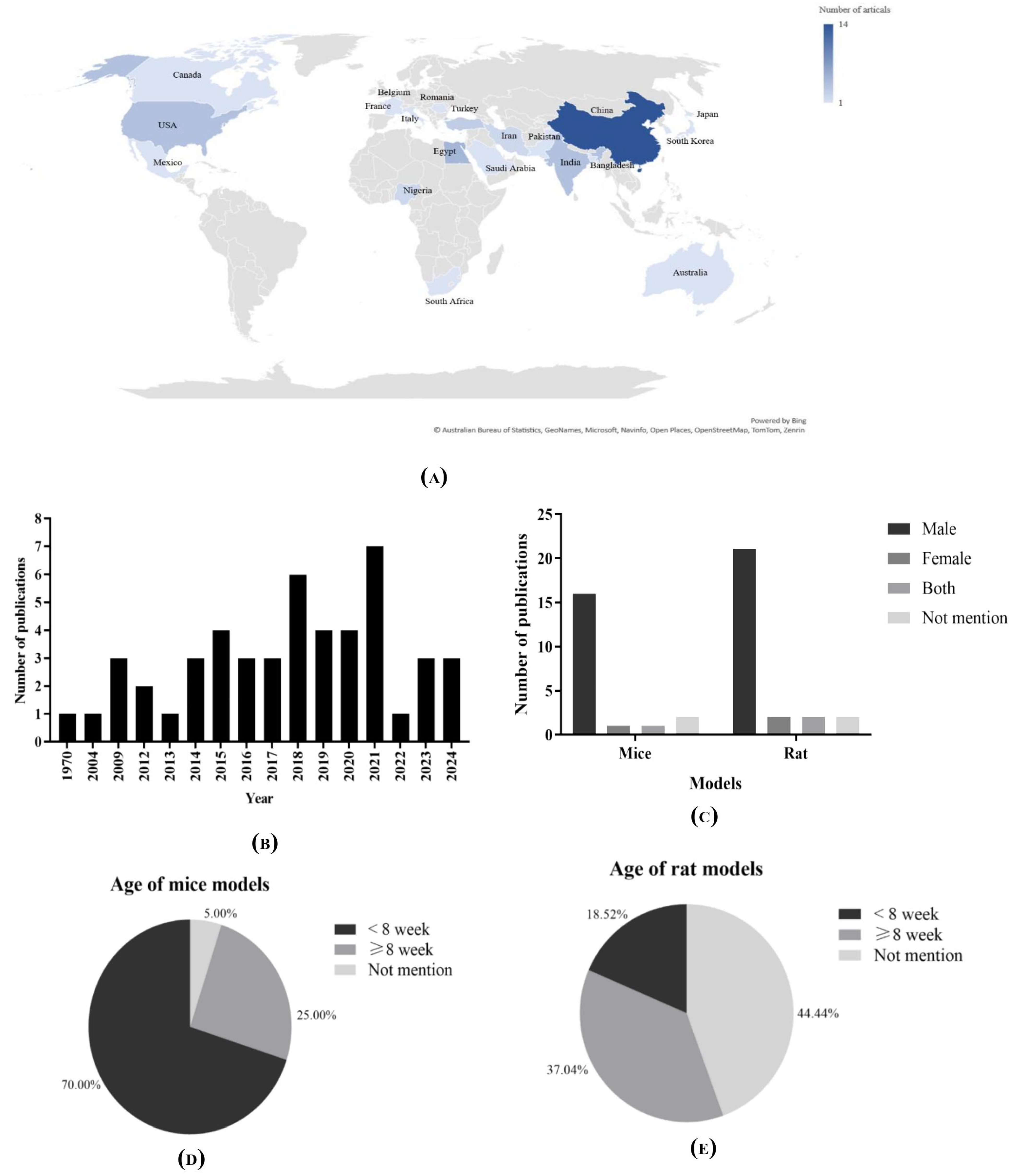
3.3. Study Characteristics
3.3.1. Demographic Data
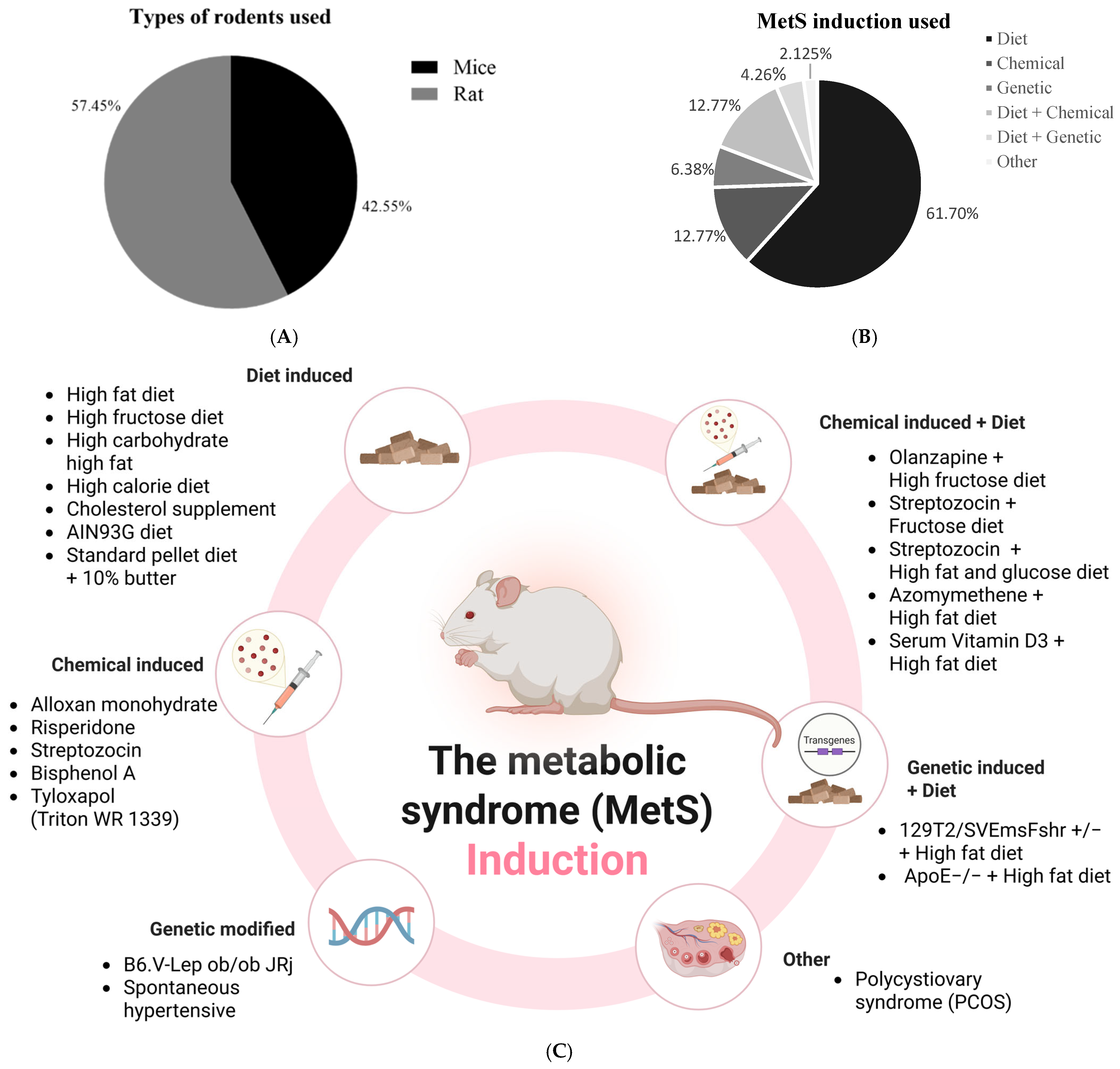
3.3.2. Animal Models of Included Studies
3.4. Induction Method of Metabolic Syndrome in Animal Models
3.4.1. Dietary Induction of Metabolic Syndrome Models
3.4.2. Chemical Induction of Metabolic Syndrome
3.4.3. Genetic Induction
3.4.4. Combined Diet and Genetic Induction of Metabolic Syndrome Model
3.4.5. Combined Diet and Chemical Induction of Metabolic Syndrome Model
3.4.6. Other Models of Metabolic Syndrome
3.5. Intervention Characteristics
3.5.1. Type of Intervention
3.5.2. Dosing Strategies
3.5.3. Treatment Duration
3.6. Effects of Curcumin and Curcuma longa Extracts on Metabolic-Syndrome-Related Parameters
3.6.1. Body Weight and Adiposity
3.6.2. Glycemic Control (Glucose and Insulin)
3.6.3. Lipid Profile (TG, TC, LDL, HDL)
3.6.4. Blood Pressure, Cardiovascular Parameters, and Other Metabolic Indicators
3.7. Effects of Curcumin and Its Derivatives on Inflammatory Markers in Rodent Models of Metabolic Syndrome
3.8. Effects of Curcumin and Its Derivatives on Oxidative Stress Markers in Rodent Models of Metabolic Syndrome
3.9. Meta-Analysis of Curcumin’s Effect on Metabolic Syndrome in Rodent Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| BP | Blood pressure |
| CAT | Catalase |
| CCNP | Curcumin nanoparticle |
| CRP | C-reactive protein |
| GLU | Glucose |
| GSH | Glutathione |
| GST | Glutathione S-Transferase |
| Hb1Ac | Hemoglobin A1C |
| HDL | High-density lipoprotein |
| HFD | High-fat diet |
| IL-10 | Interleukin-10 |
| IL-12 | Interleukin-12 |
| IL-1β | Interleukin-1beta |
| IL-6 | Interleukin-6 |
| INF-γ | Interferon-gamma |
| INS | Insulin |
| LDL | Low-density lipoprotein |
| MDA | Malondialdehyde |
| MMP-9 | Matrix metalloproteinase-9 |
| MPO | Myeloperoxidase |
| NAFLD | Non-alcoholic fatty liver disease |
| NCUR | Nano-curcumin |
| NFκB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| NOx | Total nitrites and nitrates |
| SOD | Superoxide dismutase |
| TAC | Total antioxidant capacity |
| TC | Total cholesterol |
| TG | Triglyceride |
| TNF-α | Tumor necrosis factor-alpha |
| TOS | Total oxidative status |
| WG | Weight gain |
References
- Laurindo, L.F.; Barbalho, S.M.; Joshi, R.K.; de Alvarez Rezende, B.; de Alvares Goulart, R.; Guiguer, E.L.; Araújo, A.C. Curcuma longa and curcumin on metabolic syndrome: A systematic review. Longhua Chin. Med. 2021, 4, 32. [Google Scholar] [CrossRef]
- Nsabimana, P.; Sombié, O.O.; Pauwels, N.S.; Boynito, W.G.; Tariku, E.Z.; Vasanthakaalam, H.; Abbeddou, S. Association between urbanization and metabolic syndrome in low- and middle-income countries: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The metabolic syndrome and cardiovascular risk: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef] [PubMed]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pract. 2022, 188, 109924. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rodrigues, C.F.; Sharopov, F.; Docea, A.O.; Can Karaca, A.; Sharifi-Rad, M.; Calina, D. Diet, lifestyle and cardiovascular diseases: Linking pathophysiology to cardioprotective effects of natural bioactive compounds. Int. J. Environ. Res. Public Health 2020, 17, 2326. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Hegde, M.; Parama, D.; Girisa, S.; Kumar, A.; Daimary, U.D.; Aggarwal, B.B. Role of turmeric and curcumin in prevention and treatment of chronic diseases: Lessons learned from clinical trials. ACS Pharmacol. Transl. Sci. 2023, 6, 447–518. [Google Scholar] [CrossRef]
- Hussain, Y.; Khan, H.; Alotaibi, G.; Khan, F.; Alam, W.; Aschner, M.; Jeandet, P.; Saso, L. How curcumin targets inflammatory mediators in diabetes: Therapeutic insights and possible solutions. Molecules 2022, 27, 4058. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Panzhinskiy, E.; Bashir, R.; Bagchi, D.; Nair, S. Effect of curcumin and α-lipoic acid in attenuating weight gain and adiposity. J. Am. Coll. Nutr. 2019, 38, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Rauf, A.; Akash, S.; Trisha, S.I.; Nasim, A.H.; Akter, M.; Thiruvengadam, M. Targeted therapies of curcumin: Focus on its therapeutic benefits in cancers and human health—Molecular signaling pathway-based approaches and future perspectives. Biomed. Pharmacother. 2024, 170, 116034. [Google Scholar] [CrossRef] [PubMed]
- Nabiuni, M.; Mohammadi, S.; Kayedpoor, P.; Karimzadeh, L. The effect of curcumin on the estradiol valerate-induced polycystic ovary in rats. Feyz Med. Sci. J. 2015, 18, 515–523. [Google Scholar]
- Wu, L.Y.; Chen, C.W.; Chen, L.K.; Chou, H.Y.; Chang, C.L.; Juan, C.C. Curcumin attenuates adipogenesis by inducing preadipocyte apoptosis and inhibiting adipocyte differentiation. Nutrients 2019, 11, 2307. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chou, W.L.; Ko, M.C.; Liao, J.C.; Huang, T.H. Curcumin mitigates obesity-driven dysbiosis and liver steatosis while promoting browning and thermogenesis in white adipose tissue of high-fat diet-fed mice. J. Nutr. Biochem. 2025, 143, 109920. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant potential of curcumin—A meta-analysis of randomized clinical trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef]
- Shao, W.; Yu, Z.; Chiang, Y.; Yang, Y.; Chai, T.; Foltz, W.; Lu, H.; Fantus, I.G.; Jin, T. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS ONE 2012, 7, e28784. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Fekete, J.T.; Győrffy, B. MetaAnalysisOnline.com: Web-based tool for the rapid meta-analysis of clinical and epidemiological studies. J. Med. Internet Res. 2025, 27, e64016. [Google Scholar] [CrossRef]
- Macleod, M.R.; O’Collins, T.; Howells, D.W.; Donnan, G.A. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 2004, 35, 203–208. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Abiodun, L.R.; Idowu, O.S.; Abiodun, I.R.; Raji, M.O.; Babatunde, A.; Kolawole, L.S. Meta-switch improved plasma lipid profile in male Wistar rats fed with a high-fat diet better than Curcuma longa ethanolic extract. Tanzan. J. Health Res. 2023, 24, 195. [Google Scholar]
- Afifi, A.H.M.; El-Bitar, H.I.; Ahmed, M.A. Evaluation of potential protective effects of curcumin versus metformin in experimentally-induced metabolic syndrome in rats. Bull. Pharm. Sci. Assiut Univ. 2014, 37, 77–90. [Google Scholar] [CrossRef]
- Ahmed, O.A.A.; El-Bassossy, H.M.; Azhar, A.S.; Tarkhan, M.M.; El-Mas, M.M. Interference with AGEs formation and AGEs-induced vascular injury mediates curcumin vascular protection in metabolic syndrome. Sci. Rep. 2020, 10, 10. [Google Scholar] [CrossRef]
- Akintunde, J.K.; Farouk, A.A.; Mogbojuri, O. Metabolic treatment of syndrome linked with Parkinson’s disease and hypothalamic–pituitary–gonadal hormones by turmeric curcumin in Bisphenol-A induced neuro-testicular dysfunction of Wistar rat. Biochem. Biophys. Rep. 2019, 17, 97–107. [Google Scholar] [CrossRef]
- Amin, F.; Gilani, A.-H.; Mehmood, M.H.; Siddiqui, B.S.; Khatoon, N. Coadministration of black seeds and turmeric shows enhanced efficacy in preventing metabolic syndrome in fructose-fed rats. J. Cardiovasc. Pharmacol. 2015, 65, 176–183. [Google Scholar] [CrossRef]
- Ariamoghaddam, A.R.; Ebrahimi-Hosseinzadeh, B.; Hatamian-Zarmi, A.; Sahraeian, R. Corrigendum to “In vivo anti-obesity efficacy of curcumin-loaded nanofibers transdermal patches in high-fat diet-induced obese rats”. Mater. Sci. Eng. C 2018, 92, 161–171. [Google Scholar] [CrossRef]
- Auger, F.; Martin, F.; Pétrault, O.; Samaillie, J.; Hennebelle, T.; Trabelsi, M.S.; Bailleul, F.; Staels, B.; Bordet, R.; Duriez, P. Risperidone-induced metabolic dysfunction is attenuated by Curcuma longa extract administration in mice. Metab. Brain Dis. 2018, 33, 63–77. [Google Scholar] [CrossRef]
- Bulboaca, A.; Bolboaca, S.D.; Suci, S. Protective effect of curcumin in fructose-induced metabolic syndrome and in streptozotocin-induced diabetes in rats. Iran. J. Basic Med. Sci. 2016, 19, 585–593. [Google Scholar]
- D’Antongiovanni, V.; Fornai, M.; Benvenuti, L.; Di Salvo, C.; Pellegrini, C.; Cappelli, F.; Masi, S.; Antonioli, L. Dietary supplement containing the dry extract of curcumin, emblica, and cassia counteracts intestinal inflammation and enteric dysmotility associated with obesity. Metabolites 2023, 13, 410. [Google Scholar] [CrossRef] [PubMed]
- Demir, E. Therapeutic effect of curcumin and C60 fullerene against hyperglycemia-mediated tissue damage in diabetic rat lungs. J. Bioenerg. Biomembr. 2021, 53, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.Q.; Gu, T.T.; Wang, W.; Song, L.; Chen, T.Y.; Xue, Q.C.; Zhou, F.; Li, J.M.; Kong, L.D. Curcumin protects against fructose-induced podocyte insulin signaling impairment through upregulation of miR-206. Mol. Nutr. Food Res. 2015, 59, 2355–2370. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, J.; Song, B.; Xiao, X.; Zhang, B.; Qi, M.; Huang, W.; Yang, L.; Wang, Z. Curcumin rescues high fat diet-induced obesity and insulin sensitivity in mice through regulating SREBP pathway. Toxicol. Appl. Pharmacol. 2016, 304, 99–109. [Google Scholar] [CrossRef]
- Eissa, A.A.; Abdelateef, A.S.; Elkotby, H.M. Potential effects of curcumin versus atorvastatin on hepatic and metabolic changes in rat model of metabolic syndrome. Int. J. Med. Arts 2021, 3, 1507–1515. [Google Scholar] [CrossRef]
- Ejaz, A.; Wu, D.; Kwan, P.; Meydani, M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J. Nutr. 2009, 139, 919–925. [Google Scholar] [CrossRef]
- Hong, T.; Zou, J.; Yang, J.; Liu, H.; Cao, Z.; He, Y.; Feng, D. Curcumin protects against bisphenol A-induced hepatic steatosis by inhibiting cholesterol absorption and synthesis in CD-1 mice. Food Sci. Nutr. 2023, 11, 5091–5101. [Google Scholar] [CrossRef]
- Hu, G.X.; Lin, H.; Lian, Q.Q.; Zhou, S.H.; Guo, J.J.; Zhou, H.Y.; Chu, Y.; Ge, R.S. Curcumin as a potent and selective inhibitor of 11β-hydroxysteroid dehydrogenase 1: Improving lipid profiles in high-fat-diet-treated rats. PLoS ONE 2013, 8, e49976. [Google Scholar] [CrossRef]
- Hu, J.; Shen, T.; Xie, J.; Wang, S.; He, Y.; Zhu, F. Curcumin modulates covalent histone modification and TIMP1 gene activation to protect against vascular injury in a hypertension rat model. Exp. Ther. Med. 2017, 14, 5896–5902. [Google Scholar] [CrossRef]
- Hussein, M.A.F.; Mandour, M.A.M.; AbdElghaffar, S.K.; Meki, A.-R.M.; Fakhry, M.E. Anti-obesity action of green tea extract and curcumin: Role of C1Q/TNF-related protein-12 (CTRP-12) and CASPASE-2. Bull. Pharm. Sci. 2024, 47, 345–362. [Google Scholar] [CrossRef]
- Ibrahim, K.G.; Wright, H.L.; Chivandi, E.; Madziva, M.T.; Erlwanger, K.H. The potential developmental programming effect of oral curcumin on bone health and plasma total osteocalcin of male and female rats fed a high-fructose diet during suckling and post-weaning. Gen. Physiol. Biophys. 2019, 38, 435–444. [Google Scholar] [CrossRef]
- Kapar, F.S.; Ciftci, G. The effects of curcumin and Lactobacillus acidophilus on certain hormones and insulin resistance in rats with metabolic syndrome. J. Diabetes Metab. Disord. 2020, 19, 907–914. [Google Scholar] [CrossRef]
- Kelany, M.E.; Hakami, T.M.; Omar, A.H. Curcumin improves the metabolic syndrome in high-fructose-diet-fed rats: Role of TNF-α, NF-κB, and oxidative stress. Can. J. Physiol. Pharmacol. 2016, 95, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Kobori, M.; Takahashi, Y.; Takeda, H.; Takahashi, M.; Izumi, Y.; Akimoto, Y.; Sakurai, M.; Oike, H.; Nakagawa, T.; Itoh, M.; et al. Dietary intake of curcumin improves EIF2 signaling and reduces lipid levels in the white adipose tissue of obese mice. Sci. Rep. 2018, 8, 27105. [Google Scholar] [CrossRef] [PubMed]
- Koboziev, I.; Scoggin, S.; Gong, X.; Mirzaei, P.; Zabet-Moghaddam, M.; Yosofvand, M.; Moussa, H.; Jones-Hall, Y.; Moustaid-Moussa, N. Effects of curcumin in a mouse model of very high-fat-diet-induced obesity. Biomolecules 2020, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Kwon, M.H.; Kim, H.M.; Woo, H.B.; Ahn, C.M.; Chung, C.H. Curcumin analog CUR5–8 ameliorates nonalcoholic fatty liver disease in mice with high-fat-diet-induced obesity. Metab. Clin. Exp. 2020, 103, 154015. [Google Scholar] [CrossRef]
- Li, H.B.; Xu, M.L.; Du, M.M.; Yu, X.J.; Bai, J.; Xia, W.J.; Dai, Z.M.; Li, C.X.; Li, Y.; Su, Q.; et al. Curcumin ameliorates hypertension via gut-brain communication in spontaneously hypertensive rat. Toxicol. Appl. Pharmacol. 2021, 429, 115701. [Google Scholar] [CrossRef]
- Li, S.; You, J.; Wang, Z.; Liu, Y.; Wang, B.; Du, M.; Zou, T. Curcumin alleviates high-fat-diet-induced hepatic steatosis and obesity in association with modulation of gut microbiota in mice. Food Res. Int. 2021, 143, 110270. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Sun, Y.; Zhang, Q. Effect of curcumin on permeability of coronary artery and expression of related proteins in rat coronary atherosclerosis heart disease model. Int. J. Clin. Exp. Pathol. 2015, 8, 7247–7254. [Google Scholar]
- Li, Z.Y.; Li, L.D.; Li, J.M.; Xu, B.L.; Li, Y.; Bi, K.S.; Wang, Z.T. 1H-NMR and MS based metabolomics study of the intervention effect of curcumin on hyperlipidemia mice induced by high-fat diet. PLoS ONE 2015, 10, e0120950. [Google Scholar] [CrossRef]
- Majithiya, J.B.; Parmar, A.N.; Balaraman, R. Effect of curcumin on Triton WR 1339-induced hypercholesterolemia in mice. Indian J. Pharmacol. 2004, 36, 382–383. [Google Scholar]
- Miyazawa, T.; Nakagawa, K.; Kim, S.H.; Thomas, M.J.; Paul, L.; Zingg, J.M.; Meydani, M. Curcumin and piperine supplementation of obese mice under caloric restriction modulates body fat and interleukin-1β. Nutr. Metab. 2018, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Karimzadeh Bardei, L.; Hojati, V.; Ghorbani, A.; Nabiuni, M. Anti-inflammatory effects of curcumin on insulin resistance index, levels of interleukin-6, C-reactive protein, and liver histology in polycystic ovary syndrome-induced rats. Cell J. 2017, 19, 425–433. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Robles Sánchez, C.; Rodriguez, J.; Cani, P.D.; Bindels, L.B.; Delzenne, N.M. Prebiotic effect of berberine and curcumin is associated with the improvement of obesity in mice. Nutrients 2021, 13, 1436. [Google Scholar] [CrossRef]
- Omaima, M.A.; Fouad, E.D. Curcumin/irbesartan combination improves insulin sensitivity and ameliorates diabetes-induced pro-inflammatory cytokines in type-2 diabetes rat model. Med. J. Cairo Univ. 2009, 77, 343–350. [Google Scholar]
- Pan, M.H.; Chen, J.W.; Kong, Z.L.; Wu, J.C.; Ho, C.T.; Lai, C.S. Attenuation by tetrahydrocurcumin of adiposity and hepatic steatosis in mice with high-fat-diet-induced obesity. J. Agric. Food Chem. 2018, 66, 12685–12695. [Google Scholar] [CrossRef]
- Preez, R.; Pahl, J.; Arora, M.; Rav Kumar, M.N.V.; Brown, L.; Panchal, S.K. Low-dose curcumin nanoparticles normalise blood pressure in male Wistar rats with diet-induced metabolic syndrome. Nutrients 2019, 11, 1542. [Google Scholar] [CrossRef]
- Ramesh, P.R.; Parasuraman, S.; Mohammad, S.K.; Devika, G.S. Effects of curcumin and telmisartan on olanzapine and high fructose diet induced metabolic syndrome in Sprague Dawley rats. Pharmacogn. J. 2012, 4, 25–29. [Google Scholar] [CrossRef]
- Rao, D.S.; Sekhara, N.C.; Satyanarayana, M.N.; Srinivasan, M. Effect of curcumin on serum and liver cholesterol levels in the rat. J. Nutr. 1970, 100, 1307–1315. [Google Scholar] [CrossRef]
- Rivero-Salgado, G.M.; Zamudio, S.R.; Fregoso-Aguilar, T.A.; Quevedo-Corona, L. Effects of a functional food made with Salvia hispanica L. (chia seed), Amaranthus hypochondriacus L. (amaranth), and an ethanolic extract of Curcuma longa L. (curcumin) in a rat model of childhood obesity. Foods 2024, 13, 1720. [Google Scholar] [CrossRef]
- Samadder, A.; Bhattacharjee, B.; Dey, S.; Chakrovorty, A.; Dey, R.; Sow, P.; Tarafdar, D.; Biswas, M.; Nandi, S. Enhanced drug carriage efficiency of curcumin-loaded PLGA nanoparticles in combating diabetic nephropathy via mitigation of renal apoptosis. J. Pharmacopunct. 2024, 27, 1–13. [Google Scholar] [CrossRef]
- Sarker, S.; Haque, M.I.; Sujan, K.M.; Talukder, M.I.; Miah, M.A. Curcumin attenuates butter fat–induced hyperlipidemia in mice. J. Bangladesh Agric. Univ. 2019, 17, 220–225. [Google Scholar] [CrossRef]
- Severcan, S.M.; Koca, G.; Severcan, Ç.; Yılmaz, C.; Paşaoğlu, Ö.; Paşaoğlu, H. An investigation of the effect of curcumin on fructose-induced metabolic syndrome rat models. Türkiye Diyabet Ve Obezite Derg. 2021, 5, 241–247. [Google Scholar] [CrossRef]
- Su, L.Q.; Wang, Y.D.; Chi, H.Y. Effect of curcumin on glucose and lipid metabolism, free fatty acids, and TNF-α in serum of type 2 diabetes mellitus rat models. Saudi J. Biol. Sci. 2017, 24, 1776–1780. [Google Scholar] [CrossRef] [PubMed]
- Tiwari-Pandey, R.; Sairam, M.R. Modulation of ovarian structure and abdominal obesity in curcumin- and flutamide-treated aging FSH-R haploinsufficient mice. Reprod. Sci. 2009, 16, 539–550. [Google Scholar] [CrossRef]
- Wu, X.; Ueland, P.M.; Roper, J.; Koh, G.Y.; Liang, X.; Crott, J.W.; Yilmaz, Ö.H.; Bronson, R.T.; Mason, J.B. Combined supplementation with vitamin B-6 and curcumin is superior to either agent alone in suppressing obesity-promoted colorectal tumorigenesis in mice. J. Nutr. 2021, 151, 3678–3688. [Google Scholar] [CrossRef]
- Zhang, D.M.; Li, Y.C.; Xu, D.; Ding, X.Q.; Kong, L.D. Protection of curcumin against fructose-induced hyperuricemia and renal endothelial dysfunction involves NO-mediated JAK–STAT signalling in rats. Food Chem. 2012, 134, 2184–2193. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, Y.; Gao, J.; Zheng, Z.; Zhang, Z.; Yao, L.; Li, D. Curcumin improves insulin sensitivity in high-fat diet-fed mice through gut microbiota. Nutr. Metab. 2022, 19, 1–11. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, S.; Li, P.; Zheng, X.; Feng, D. Supplementation with curcumin inhibits intestinal cholesterol absorption and prevents atherosclerosis in high-fat diet–fed apolipoprotein E knockout mice. Nutr. Res. 2018, 56, 32–40. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Sena, E.S.; van der Worp, H.B.; Bath, P.M.; Howells, D.W.; Macleod, M.R. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010, 8, e1000344. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune, and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed. Pharmacother. 2016, 82, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Soetikno, V.; Sari, F.R.; Lakshmanan, A.P.; Arumugam, S.; Harima, M.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the Nrf2–Keap1 pathway. Mol. Nutr. Food Res. 2013, 57, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of non-alcoholic fatty liver disease with curcumin: A randomized placebo-controlled trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Fazel Nabavi, S.; Thiagarajan, R.; Rastrelli, L.; Daglia, M.; Sobarzo-Sanchez, E.; Alinezhad, H.; Mohammad Nabavi, S. Curcumin: A natural product for diabetes and its complications. Curr. Top. Med. Chem. 2015, 15, 2445–2455. [Google Scholar] [CrossRef]
- Asai, A.; Miyazawa, T. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci. 2000, 67, 2785–2793. [Google Scholar] [CrossRef]
- Kim, T.; Davis, J.; Zhang, A.J.; He, X.; Mathews, S.T. Curcumin activates AMPK and suppresses gluconeogenic gene expression in hepatoma cells. Biochem. Biophys. Res. Commun. 2009, 388, 377–382. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Nakmareong, S.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Kongyingyoes, B.; Donpunha, W.; Prachaney, P.; Phisalaphong, C. Tetrahydrocurcumin alleviates hypertension, aortic stiffening and oxidative stress in rats with nitric oxide deficiency. Hypertens. Res. 2012, 35, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef]
- Sandur, S.K.; Ichikawa, H.; Pandey, M.K.; Kunnumakkara, A.B.; Sung, B.; Sethi, G.; Aggarwal, B.B. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radic. Biol. Med. 2007, 43, 568–580. [Google Scholar] [CrossRef]
- Karimian, M.; Pirro, M.; Johnston, T.P.; Majeed, M.; Sahebkar, A. Curcumin and endothelial function: Evidence and mechanisms of protective effects. Curr. Pharm. Des. 2017, 23, 2462–2473. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Suresh, D.; Srinivasan, K. Studies on the in vitro absorption of spice principles—Curcumin, capsaicin and piperine in rat intestines. Food Chem. Toxicol. 2007, 45, 1437–1442. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S.S.R. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Medica 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol. 2011, 214, 242–253. [Google Scholar] [CrossRef]
- Overton, J.M. Phenotyping small animals as models for the human metabolic syndrome: Thermoneutrality matters. Int. J. Obes. 2010, 34, S53–S58. [Google Scholar] [CrossRef]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Fonken, L.K.; Workman, J.L.; Walton, J.C.; Weil, Z.M.; Morris, J.S.; Haim, A.; Nelson, R.J. Light at night increases body mass by shifting the time of food intake. Proc. Natl. Acad. Sci. USA 2010, 107, 18664–18669. [Google Scholar] [CrossRef]
- Lombardi, N.; Crescioli, G.; Maggini, V.; Ippoliti, I.; Menniti-Ippolito, F.; Gallo, E.; Brilli, V.; Lanzi, C.; Mannaioni, G.; Firenzuoli, F.; et al. Acute liver injury following turmeric use in Tuscany: An analysis of the Italian Phytovigilance database and systematic review of case reports. Br. J. Clin. Pharmacol. 2021, 87, 741–753. [Google Scholar] [CrossRef]
- Halegoua-DeMarzio, D.; Navarro, V.; Ahmad, J.; Avula, B.; Barnhart, H.; Barritt, A.S.; Bonkovsky, H.L.; Fontana, R.J.; Ghabril, M.S.; Hoofnagle, J.H.; et al. Liver injury associated with turmeric—A growing problem: Ten cases from the Drug-Induced Liver Injury Network [DILIN]. Am. J. Med. 2023, 136, 200–206. [Google Scholar] [CrossRef]
- Smith, D.N.; Pungwe, P.; Comer, L.L.; Ajayi, T.A.; Suarez, M.G. Turmeric-associated liver injury: A rare case of drug-induced liver injury. Cureus 2023, 31, 15. [Google Scholar] [CrossRef]
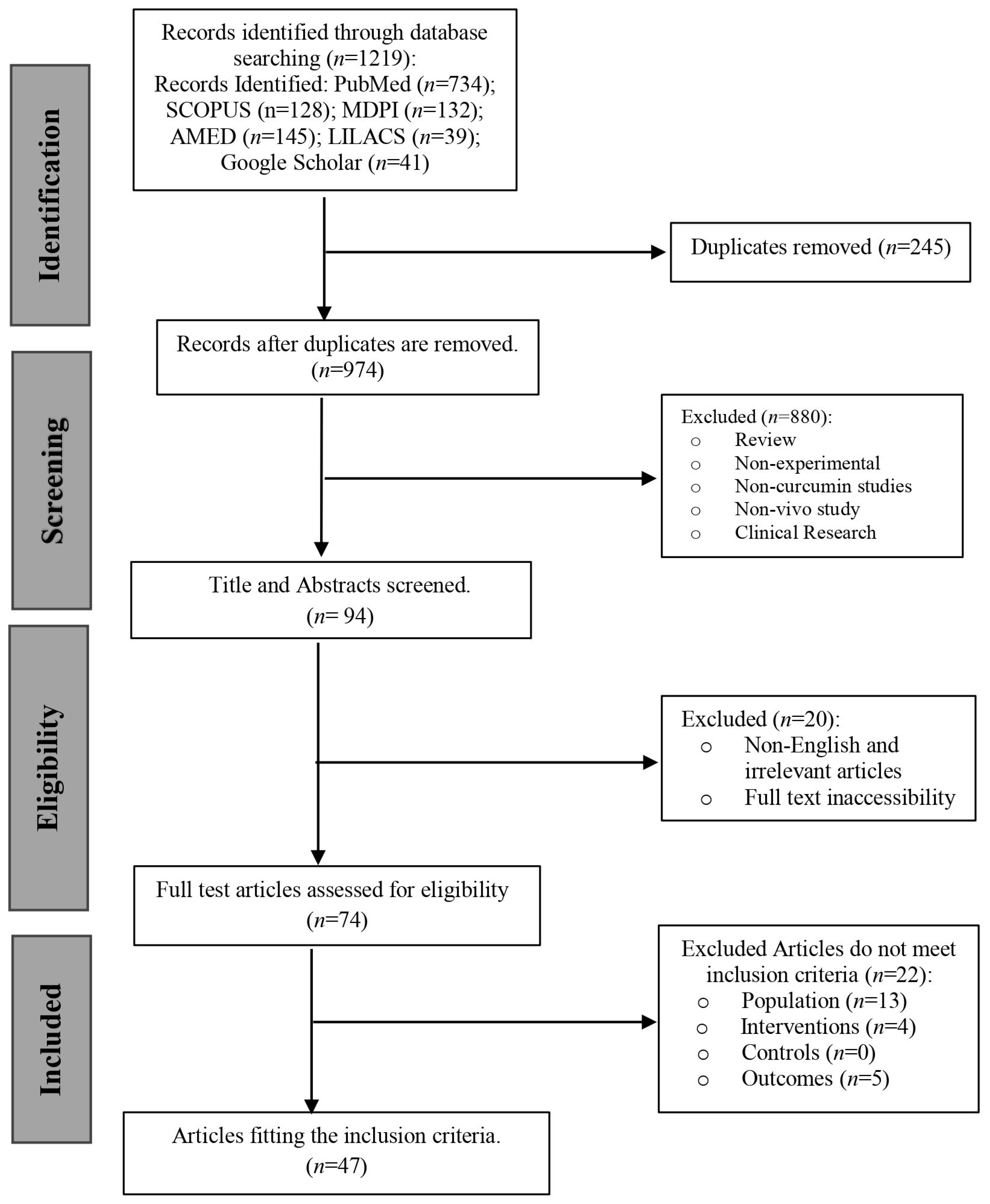



| Inclusion Criteria | |
|---|---|
| Patient/Population | Rodent models of metabolic syndrome. |
| Intervention | Curcumin, Curcuma longa, diferuloylmethane without any combination of other drugs or chemicals, and other types of intervention. |
| Comparison/Control | Both the effectiveness of curcumin or isolated compounds compared with placebo and/or control. |
| Outcomes |
|
| Exclusion Criteria | |
| |
| Author | Year | Country | No. of Animals (Total) | Per Group | Rodent Type | Sex | Age (Weeks) |
|---|---|---|---|---|---|---|---|
| Abiodun et al. [24] | 2023 | Nigeria | 30 | 5 | Wistar | Male | NM |
| Afifi et al. [25] | 2014 | Egypt | NM | 14–16 | Albino Wistar | Male | NM |
| Ahmed et al. [26] | 2020 | Saudi Arabia | NM | 7–8 | Wistar | Male | 6–8 |
| Akintunde et al. [27] | 2019 | Nigeria | 40 | 8 | Wistar | Male | 7 |
| Amin et al. [28] | 2015 | Pakistan | NM | 6–8 | Sprague Dawley | Male/ Female | NM |
| Ariamoghaddam et al. [29] | 2018 | Iran | 18 | 6 | Rat | NM | 8 |
| Auger et al. [30] | 2018 | France | 48 | 12 | C57BL/6 | Female | 7 |
| Bulboacă et al. [31] | 2016 | Romania | 50 | 10 | Wistar | Male | NM |
| D’Antongiovanni et al. [32] | 2023 | Italy | 100 | 10 | C57BL/6 | Male | 5 |
| Demir [33] | 2021 | Turkey | 63 | 7 | Wistar Albino | NM | 8–10 |
| Ding et al. [34] | 2015 | China | NM | 6–8 | Sprague Dawley | Male | 8–10 |
| Ding et al. [35] | 2016 | China | 40 | 8 | C57BL/6 | Male | 6 |
| Eissa et al. [36] | 2021 | Egypt | 50 | 10 | Sprague Dawley | Male | 8 |
| Ejaz et al. [37] | 2009 | USA | 18 | 6 | C57BL/6 | Male | 4 |
| Hong et al. [38] | 2023 | China | 24 | 8 | CD-1 | Male | 5 |
| Hu et al. [39] | 2013 | China | 30 | 10 | Sprague Dawley | Male | 12.9 |
| Hu et al. [40] | 2017 | China | 42 | 6 | SHRs | Male | 8–10 |
| Hussein et al. [41] | 2024 | Egypt | 60 | 7 and 10 | Sprague Dawley | Male | NM |
| Ibrahim et al. [42] | 2019 | South Africa | 128 | 31–32 | Sprague Dawley | Male/ Female | 0.9 |
| Kapar et al. [43] | 2020 | Turkey | 50 | 10 | Sprague Dawley | Male | 10–12 |
| Kelany et al. [44] | 2016 | Egypt | 30 | 10 | Sprague Dawley | Male | NM |
| Kobori et al. [45] | 2018 | Japan | 27 | 9 | C57BL/6J | Male | 5 |
| Koboziev et al. [46] | 2020 | USA | 20 | 10 | C57BL/6 | Male | 5 |
| Lee et al. [47] | 2020 | South Korea | 60 | 10 | C57BL/6 | Male | 8 |
| Li et al. [48] | 2021 | China | 30 | 6 | C57BL/6 Slac | Male | 4 |
| Li et al. [49] | 2021 | China | 45 | 15 | Wistar | Male | 9 |
| Li et al. [50] | 2015 | China | 30 | 10 | C57BL/6 | Male | 6 |
| Li et al. [51] | 2015 | China | 32 | 8 | SHRs, WKY | Male | 8–10 |
| Majithiya et al. [52] | 2004 | India | 36 | 6 | Swiss Albino | Male | NM |
| Miyazawa et al. [53] | 2018 | USA | 47 | 9–10 | C57BL/6 | Male | 8 |
| Mohammadi et al. [54] | 2017 | Iran | 90 | 18 | Wistar | Female | NM |
| Neyrinck et al. [55] | 2021 | Belgium | 36 | 9 | B6.V-Lep ob/ob JRj | Male | 6 |
| Omaima and Fouad [56] | 2009 | Egypt | 50 | 10 | Albino rat | Male | NM |
| Pan et al. [57] | 2018 | China | 40 | 8 | C57BL/6J | Male | 4 |
| Preez et al. [58] | 2019 | Australia | 120 | 12 | Wistar | Male | 8–9 |
| Ramesh et al. [59] | 2012 | India | 60 | 6 | Sprague Dawley | Male | NM |
| Rao et al. [60] | 1970 | India | NM | NM | Albino Wistar | Female | 6.4 |
| Rivero-Salgado et al. [61] | 2024 | Mexico | NM | 6–8 | Wistar | Male | 3 |
| Samadder et al. [62] | 2024 | India | 30 | 6 | Swiss Albino | NM | 6–8 |
| Sarker et al. [63] | 2019 | Bangladesh | 40 | 8 | Swiss Albino | NM | 7.5 |
| Severcan et al. [64] | 2021 | Turkey | 24 | 6 | Wistar Albino | Male | NM |
| Su et al. [65] | 2017 | China | 60 | 15 | Sprague Dawley | Male | NM |
| Tiwari-Pandey et al. [66] | 2009 | Canada | NM | 12–15 | 129T2/SV EmsJ Fshr+/−, WT | Male/ Female | NM |
| Wu et al. [67] | 2021 | USA | 110 | 21 | FVB | Male | 4 |
| Zhang et al. [68] | 2012 | China | 50 | 7 | Sprague Dawley | Male | NM |
| Zhong et al. [69] | 2022 | China | 16 | 8 | C57BL/6J | Male | 8 |
| Zou et al. [70] | 2018 | China | 20 | 10 | ApoE−/− | Male | 8 |
| Author, Year | Type of Treatment | Dose(s) of Treatment | Testing Duration (Weeks) |
|---|---|---|---|
| Abiodun et al. [24] | Curcuma longa ethanol extract | 1.5, 2, 2.5 g/kg | 2 |
| Afifi et al. [25] | Curcumin | 40, 80, 100, 200 mg/kg | 8 |
| Ahmed et al. [26] | Curcumin | 10, 30 µM | 12 |
| Akintunde et al. [27] | Curcumin dissolved in olive oil | 50, 100 mg/kg | 2 |
| Amin et al. [28] | Turmeric | 1.5, 3 g/kg | 6 |
| Ariamoghaddam et al. [29] | Curcumin (transdermal patch) | 4 cm2 patch loaded with 200–250 nM | 6 |
| Auger et al. [30] | Diet containing 0.05% (w/w) Biocurcuma™ (curcumin) | NM | 22 |
| Bulboacă et al. [31] | Curcumin | 1 g/kg | 2 (+3 days) |
| D’ Antongiovanni et al. [32] | Curcumin | 49 mg/kg/day | 4 |
| Demir [33] | Curcumin dissolved in olive oil | 1 mg/kg bw | 8 |
| Ding et al. [34] | Curcumin | 15, 30, 60 mg/kg | 6 |
| Ding et al. [35] | Curcumin | 40 mg/kg/day | 12 |
| Eissa et al. [36] | Curcumin | 200 mg/kg/day | 8 |
| Ejaz et al. [37] | Curcumin | 500 mg/kg | 12 |
| Hong et al. [38] | 0.5 mg/kg Bisphenol A + 0.1% (w/w) curcumin | 1000 mg/kg | 24 |
| Hu et al. [39] | Curcumin suspended in 0.1% cellulose | 200 mg/kg/day | 8 |
| Hu et al. [40] | Curcumin (200 µL) | 25, 50, 100, 200, 400 mg/kg | 8 (every 2 days) |
| Hussein et al. [41] | Curcumin | 80 mg/kg | 8 |
| Ibrahim et al. [42] | Curcumin | 500 mg/kg bm | 6 |
| Kapar et al. [43] | Curcumin | 100 mg/kg/day | 4 |
| Kelany et al. [44] | Curcumin | 200 mg/kg/day | 8 |
| Kobori et al. [45] | Curcumin | 0.1% w/w | 14 |
| Koboziew et al. [46] | Curcumin powder | 0.7% w/w | 13 |
| Lee et al. [47] | Curcumin | 100 mg/kg/day | 13 |
| Li et al. [48] | Curcumin | 2000 mg/kg | 10 |
| Li et al. [49] | Curcumin | 100, 300, 400 mg/kg | 12 (once every 2 days) |
| Li et al. [50] | Curcumin | 40, 80 mg/kg | 12 |
| Li et al. [51] | Curcumin | 100 mg/kg/day | 4 |
| Majithiya et al. [52] | Curcumin in 0.5% sodium carboxymethyl cellulose suspension | 100, 200, 400 mg/kg | 44 h |
| Miyazawa et al. [53] | Curcumin | 1 g/kg | 10 (phase 2) and 20 (phase 3) |
| Mohammadi et al. [54] | Curcumin prepared at 100 mmol/L in DMSO | 100, 300 mg/kg | 2 |
| Neyrinck et al. [55] | Curcumin | 0.3% curcumin | 4 |
| Omaima and Fouad [56] | Curcumin | 200 mg/kg | 6 |
| Pan et al. [57] | Tetrahydrocurcumin | 20, 100 mg/kg | 10 |
| Preez et al. [58] | Curcumin suspension | 5, 100 mg/kg/day | 8 |
| Curcumin nanoparticles | 5 mg/kg/day | 8 | |
| Ramesh et al. [59] | Curcumin | 50 mg/kg | 4 |
| Rao et al. [60] | Curcumin | 0.10%, 0.25%, 0.50% | 7 |
| Rivego-Sagado et al. [61] | Hypercaloric diet with functional food containing turmeric solution | NM | 8 |
| Samadder et al. [62] | Curcumin | 50 mg/kg | 1 |
| Nano-curcumin-1 | 25 mg/kg | 1 | |
| Nano-curcumin-2 | 12.5 mg/kg | 1 | |
| Sarker et al. [63] | Curcumin in drinking water | 1%, 2%, 3% w/v | 10 |
| Severcan et al. [64] | Curcumin dissolved in olive oil | 100, 200 mg/kg | 8 |
| Su et al. [65] | Curcumin | 250 mg/kg | 8 |
| Tiwari-Pandey et al. [66] | Curcumin | 25 mg/kg/day | 5 (+7 days) |
| Wu et al. [67] | Curcumin | 2000 mg/kg | 15 |
| Zhang et al. [68] | Curcumin | 15, 30, 60 mg/kg | 4 |
| Zhong et al. [69] | Curcumin in 0.5% carboxymethylcellulose | 100 mg/kg/day | 4 |
| Zou et al. [70] | Curcumin | 1000 mg/kg | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kehinde, S.A.; Qaisrani, Z.N.; Pattanayaiying, R.; Lin, W.P.; Lay, B.B.; Phyo, K.Y.; San, M.M.; Awaeloh, N.; Aunsorn, S.; Kitkangplu, R.; et al. Preclinical Evidence of Curcuma longa Linn. as a Functional Food in the Management of Metabolic Syndrome: A Systematic Review and Meta-Analysis of Rodent Studies. Biomedicines 2025, 13, 1911. https://doi.org/10.3390/biomedicines13081911
Kehinde SA, Qaisrani ZN, Pattanayaiying R, Lin WP, Lay BB, Phyo KY, San MM, Awaeloh N, Aunsorn S, Kitkangplu R, et al. Preclinical Evidence of Curcuma longa Linn. as a Functional Food in the Management of Metabolic Syndrome: A Systematic Review and Meta-Analysis of Rodent Studies. Biomedicines. 2025; 13(8):1911. https://doi.org/10.3390/biomedicines13081911
Chicago/Turabian StyleKehinde, Samuel Abiodun, Zahid Naeem Qaisrani, Rinrada Pattanayaiying, Wai Phyo Lin, Bo Bo Lay, Khin Yadanar Phyo, Myat Mon San, Nurulhusna Awaeloh, Sasithon Aunsorn, Ran Kitkangplu, and et al. 2025. "Preclinical Evidence of Curcuma longa Linn. as a Functional Food in the Management of Metabolic Syndrome: A Systematic Review and Meta-Analysis of Rodent Studies" Biomedicines 13, no. 8: 1911. https://doi.org/10.3390/biomedicines13081911
APA StyleKehinde, S. A., Qaisrani, Z. N., Pattanayaiying, R., Lin, W. P., Lay, B. B., Phyo, K. Y., San, M. M., Awaeloh, N., Aunsorn, S., Kitkangplu, R., & Chusri, S. (2025). Preclinical Evidence of Curcuma longa Linn. as a Functional Food in the Management of Metabolic Syndrome: A Systematic Review and Meta-Analysis of Rodent Studies. Biomedicines, 13(8), 1911. https://doi.org/10.3390/biomedicines13081911








