PCOS and the Genome: Is the Genetic Puzzle Still Worth Solving?
Abstract
1. Introduction
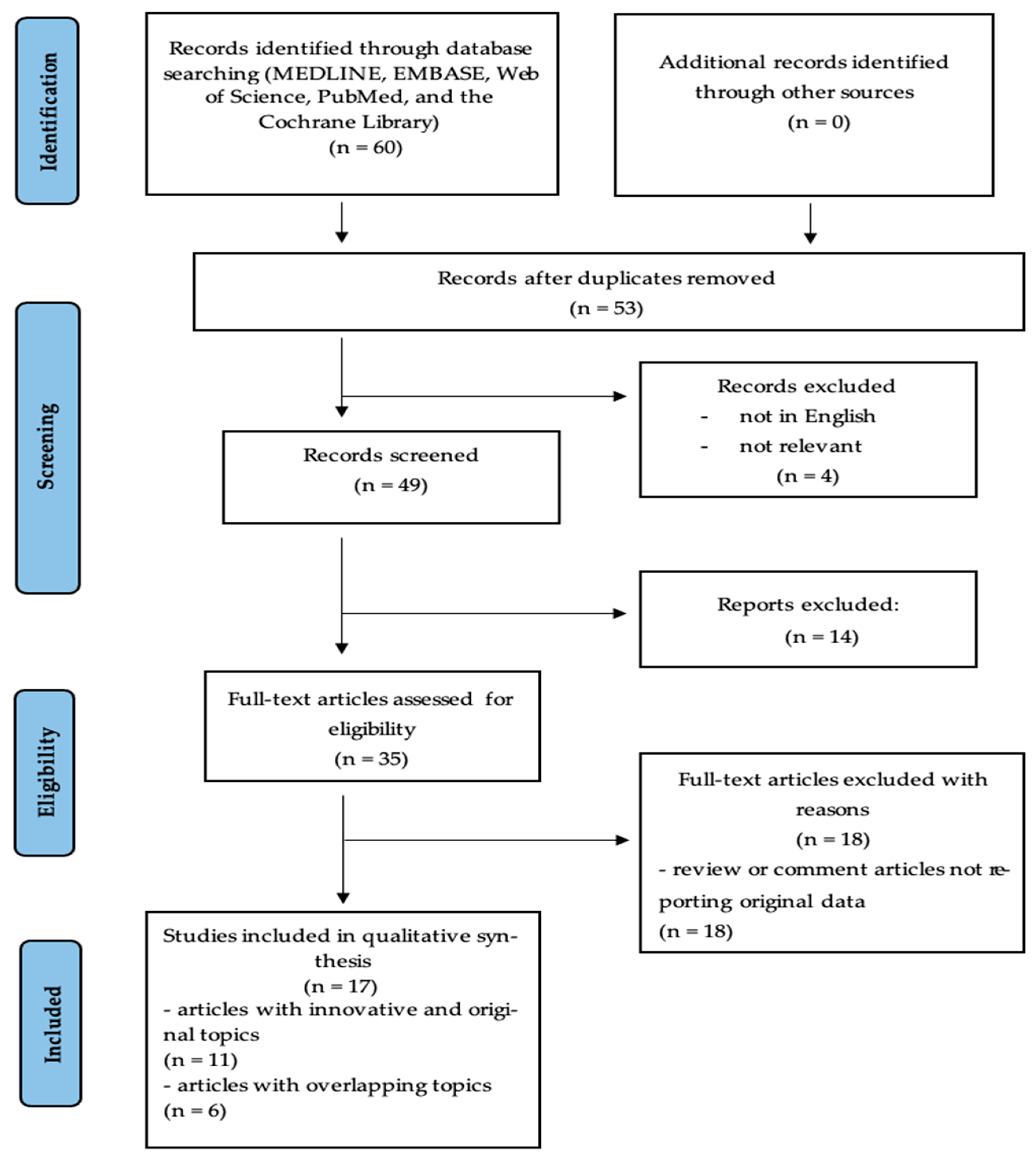
2. Materials and Methods
3. Advances in Genomic Research on PCOS
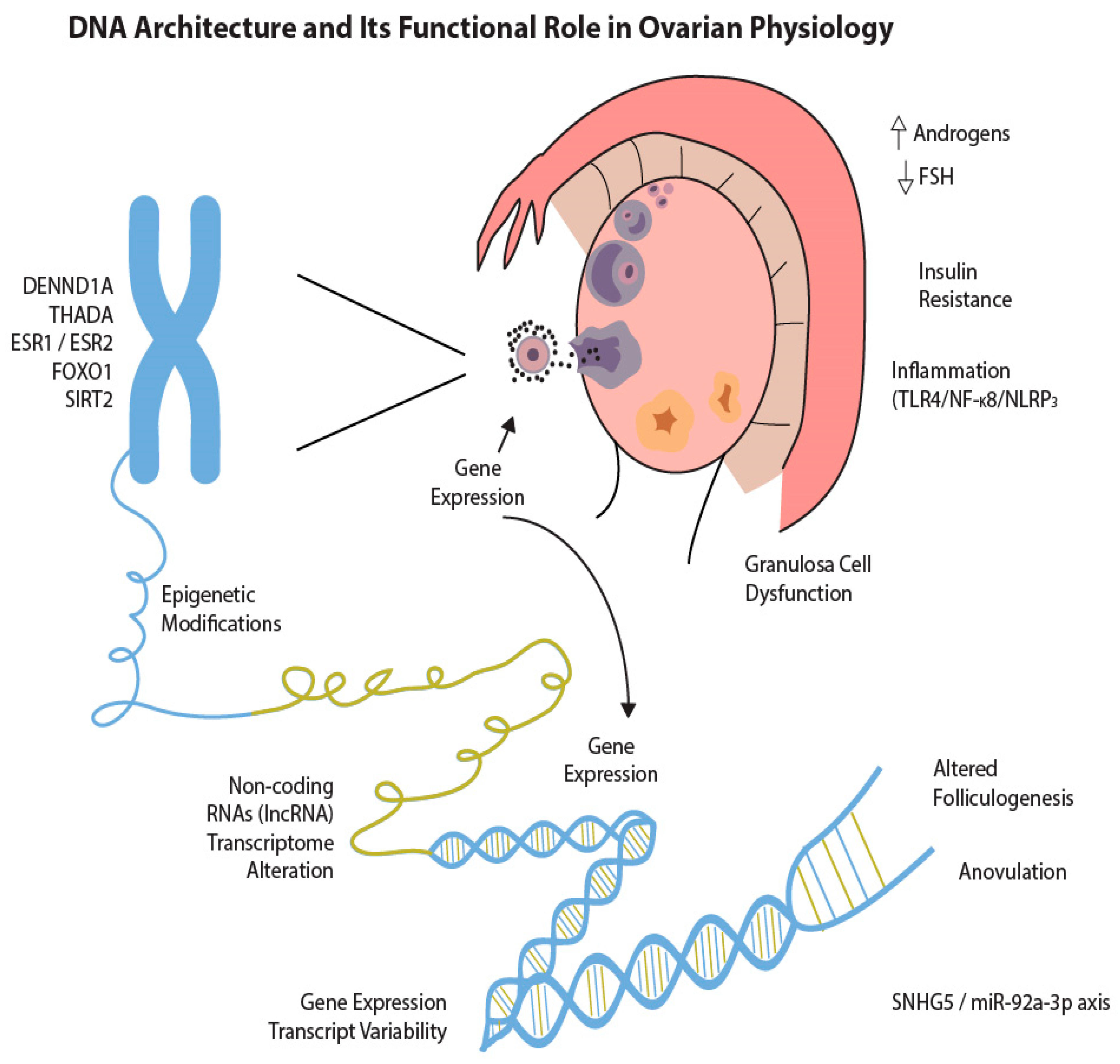
3.1. Understanding PCOS Through Genomic Approaches
3.2. Epigenetic Regulation and Environmental Interactions
3.3. Epigenetic Modifications in PCOS
3.4. Future Directions
- −
- Pharmacogenomics: Genetic profiling of patients—particularly those harboring variants in genes involved in insulin signaling or androgen biosynthesis—may inform the selection of therapeutic agents. For instance, the identification of insulin resistance (IR)-associated polymorphisms may help predict responsiveness to insulin sensitizers such as metformin or inositol-based therapies [34].
- −
- Epigenetic therapy: Although still in preclinical stages, targeting epigenetic regulators offers an exciting therapeutic frontier. Inhibitors of histone deacetylases (HDACs) and DNA methyltransferases (DNMTs) have shown potential in reversing abnormal gene expression patterns associated with PCOS in experimental models, suggesting their future utility in modulating disease-relevant pathways [35,36].
- −
- Microbiota modulation: Given the emerging role of gut dysbiosis in PCOS pathophysiology, interventions aimed at restoring eubiosis hold considerable promise. Dietary modification, prebiotic and probiotic supplementation, and fecal microbiota transplantation (FMT) are being investigated for their capacity to attenuate inflammation, improve metabolic function, and possibly restore hormonal balance [29,30].
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AK124742 | Annotated non-coding RNA (no standard name) |
| AMH | Anti-Müllerian Hormone |
| AMHR2 | Anti-Müllerian Hormone Receptor Type 2 |
| BAMBI | BMP and Activin Membrane-Bound Inhibitor |
| CCs | Cumulus Cells |
| CDKN1C | Cyclin-Dependent Kinase Inhibitor 1C |
| circRNA | Circular RNA |
| CXCR2 | C-X-C Motif Chemokine Receptor 2 |
| DENND1A | DENN Domain Containing 1A |
| DGKI | Diacylglycerol Kinase I |
| DNA | Deoxyribonucleic Acid |
| DNMT | DNA Methyltransferase |
| DOHaD | Developmental Origins of Health and Disease |
| ESR1 | Estrogen Receptor 1 |
| ESR2 | Estrogen Receptor 2 |
| FMT | Fecal Microbiota Transplantation |
| FOXO1 | Forkhead Box O1 |
| FSHR | Follicle-Stimulating Hormone Receptor |
| GC | Granulosa Cell |
| GV | Germinal Vesicle (oocyte stage) |
| GWAS | Genome-Wide Association Study |
| HDAC | Histone Deacetylase |
| HOXA10 | Homeobox A10 |
| INSR | Insulin Receptor |
| IR | Insulin Resistance |
| iPSC | Induced Pluripotent Stem Cell |
| LHCGR | Luteinizing Hormone/Choriogonadotropin Receptor |
| LIFR | Leukemia Inhibitory Factor Receptor |
| lncRNA | Long Non-Coding RNA |
| LMNB1 | Lamin B1 |
| miRNA | MicroRNA |
| MII | Metaphase II (oocyte stage) |
| MTNR1A | Melatonin Receptor 1A |
| MTNR1B | Melatonin Receptor 1B |
| NGS | Next-Generation Sequencing |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NLRP3 | NOD-, LRR- and Pyrin Domain-Containing Protein 3 |
| PBMCs | Peripheral Blood Mononuclear Cells |
| PCOS | Polycystic Ovary Syndrome |
| PSMD6 | Proteasome 26S Subunit, Non-ATPase 6 |
| RBX1 | Ring-Box 1 |
| SCFA | Short-Chain Fatty Acid |
| SHBG | Sex Hormone-Binding Globulin |
| SNP | Single Nucleotide Polymorphism |
| SNHG5 | Small Nucleolar RNA Host Gene 5 |
| SNPs | Single Nucleotide Polymorphisms |
| SIRT2 | Sirtuin 2 |
| THADA | Thyroid Adenoma Associated |
| TLR4 | Toll-Like Receptor 4 |
| TNF-α | Tumor Necrosis Factor Alpha |
| TOX3 | TOX High Mobility Group Box Family Member 3 |
| TGF-β1 | Transforming Growth Factor Beta 1 |
| UNC5C | Unc-5 Netrin Receptor C |
| YAP1 | Yes-Associated Protein 1 |
References
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic Ovary Syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef]
- Abbott, D.H.; Padmanabhan, V.; Dumesic, D.A. Contributions of androgen and estrogen to fetal programming of ovarian dysfunction. Reprod. Biol. Endocrinol. 2006, 4, 17. [Google Scholar] [CrossRef]
- Day, F.R.; Hinds, D.A.; Tung, J.Y.; Stolk, L.; Styrkarsdottir, U.; Saxena, R.; Bjonnes, A.; Broer, L.; Dunger, D.B.; Halldorsson, B.V.; et al. Causal Mechanisms and Balancing Selection Inferred from Genetic Associations with Polycystic Ovary Syndrome. Nat. Commun. 2018, 9, 8464. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qi, Y.; Yang, X.; Zhao, L.; Wen, S.; Liu, Y.; Tang, L. Association between Polycystic Ovary Syndrome and Gut Microbiota. PLoS ONE 2016, 11, e0153196. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Wang, L.; et al. Gut Microbiota–Bile Acid–Interleukin-22 Axis Orchestrates Polycystic Ovary Syndrome. Nat. Med. 2019, 25, 1225–1233. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- González-Fernández, R.; Martín-Ramírez, R.; Rotoli, D.; Hernández, J.; Naftolin, F.; Martín-Vasallo, P.; Palumbo, A.; Ávila, J. Granulosa-Lutein Cell Sirtuin Gene Expression Profiles Differ between Normal Donors and Infertile Women. Int. J. Mol. Sci. 2019, 21, 295. [Google Scholar] [CrossRef]
- Muccee, F.; Ashraf, N.M.; Razak, S.; Afsar, T.; Hussain, N.; Husain, F.M.; Shafique, H. Exploring the Association of ESR1 and ESR2 Gene SNPs with Polycystic Ovary Syndrome in Human Females: A Comprehensive Association Study. J. Ovarian Res. 2024, 17, 27. [Google Scholar] [CrossRef]
- Akbari, A.; Aboutorabi, R.; Kazemi, M.; Borzouie, Z.; Feizi, A.; Naghshineh, E.; Mostafavi, F. Differential Gene Expressions of CALM1, PSMD6, and AK124742 Long Noncoding RNA in Cumulus Cells from Polycystic Ovary Syndrome Patients versus Normal Control Women. Adv. Biomed. Res. 2023, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, Y.; Zhao, S.; Liu, F.; Zhao, H.; Chen, Z.J. Evidence of Positive Selection of Genetic Variants Associated with PCOS. Hum. Reprod. 2023, 38 (Suppl. 2), ii57–ii68. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, D.; Wang, N.; Xu, L.; Weng, Y.; Zhou, W.; Pan, Y. The Identification and Functional Analysis of CircRNAs in Endometrial Receptivity of Mice with Polycystic Ovary. Environ. Toxicol. 2024, 39, 1456–1470. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, Y.; Yang, L.; Li, P.; Han, X.; Li, Q.; Yang, Y.; Chao, L. Identification and Validation of Senescence-Related Genes in Polycystic Ovary Syndrome. J. Ovarian Res. 2024, 17, 7. [Google Scholar] [CrossRef]
- Huang, X.; Luo, X.; Huang, S.; Chen, X.; Qiu, L. Inhibition of FoxO1 Alleviates Polycystic Ovarian Syndrome by Reducing Inflammation and the Immune Response. Funct. Integr. Genom. 2024, 24, 6. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, X.; Gu, H.; Xu, H.; Zhong, W.; Wei, X.; Zhong, X. Association between Single Nucleotide Polymorphisms, TGF-β1 Promoter Methylation, and Polycystic Ovary Syndrome. BMC Pregnancy Childbirth 2024, 24, 5. [Google Scholar] [CrossRef]
- Monshizadeh, K.; Tajamolian, M.; Anbari, F.; Mehrjardi, M.Y.V.; Kalantar, S.M.; Dehghani, M. The Association of RBX1 and BAMBI Gene Expression with Oocyte Maturation in PCOS Women. BMC Med. Genom. 2024, 17, 24. [Google Scholar] [CrossRef]
- Postolache, T.T.; Al Tinawi, Q.M.; Gragnoli, C. The Melatonin Receptor Genes Are Linked and Associated with the Risk of Polycystic Ovary Syndrome. J. Ovarian Res. 2024, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Pan, J.; Zhou, C.; Yu, C.; Zhou, Z.; Ding, G.; Liu, X.; Sheng, J.; Jin, L.; Huang, H. LncRNA SNHG5 Adversely Governs Follicular Growth in PCOS via miR-92a-3p/CDKN1C Axis. iScience 2024, 27, 108522. [Google Scholar] [CrossRef]
- Vink, J.M.; Sadrzadeh, S.; Lambalk, C.B.; Boomsma, D.I. Heritability of Polycystic Ovary Syndrome in a Dutch Twin-Family Study. J. Clin. Endocrinol. Metab. 2006, 91, 2100–2104. [Google Scholar] [CrossRef]
- Alviggi, C.; Conforti, A.; Santi, D.; Esteves, S.C.; Andersen, C.Y.; Humaidan, P.; Chiodini, P.; De Placido, G.; Simoni, M. Clinical Relevance of Genetic Variants of Gonadotrophins and Their Receptors in Controlled Ovarian Stimulation: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2018, 24, 599–614. [Google Scholar] [CrossRef]
- Xu, N.; Chua, A.K.; Jiang, H.; Liu, N.A.; Goodarzi, M.O. DNA Methylation Profile Distinguishes Different Subtypes of Polycystic Ovary Syndrome. Fertil. Steril. 2016, 106, 1735–1743. [Google Scholar]
- Martinez-Arguelles, D.B.; Papadopoulos, V. Epigenetic Regulation of the Expression of Genes Involved in Steroid Hormone Biosynthesis and Action. Steroids 2010, 75, 467–476. [Google Scholar] [CrossRef]
- Abbott, D.H.; Barnett, D.K.; Bruns, C.M.; Dumesic, D.A. Androgen Excess Fetal Programming of Female Reproduction: A Developmental Aetiology for Polycystic Ovary Syndrome? Hum. Reprod. Update 2005, 11, 357–374. [Google Scholar] [CrossRef]
- Wadhwa, P.D.; Buss, C.; Entringer, S.; Swanson, J.M. Developmental Origins of Health and Disease: Brief History of the Approach and Current Focus on Epigenetic Mechanisms. Semin. Reprod. Med. 2009, 27, 358–368. [Google Scholar] [CrossRef]
- Bruni, V.; Capozzi, A.; Lello, S. The Role of Genetics, Epigenetics and Lifestyle in Polycystic Ovary Syndrome Development: The State of the Art. Reprod. Sci. 2022, 29, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Concha, C.F.; Sir, P.T.; Recabarren, S.E.; Pérez, B.F. Epigenética del síndrome de ovario poliquístico [Epigenetics of Polycystic Ovary Syndrome]. Rev. Med. Chile 2017, 145, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Hiam, D.; Simar, D.; Laker, R.; Altıntaş, A.; Gibson-Helm, M.; Fletcher, E.; Moreno-Asso, A.; Trewin, A.J.; Barres, R.; Stepto, N.K. Epigenetic Reprogramming of Immune Cells in Women with PCOS Impact Genes Controlling Reproductive Function. J. Clin. Endocrinol. Metab. 2019, 104, 6155–6170. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, V.V.; Beeraka, N.M.; Butko, D.Y.; Nikolenko, V.N.; Bondarev, S.A.; Achkasov, E.E.; Sinelnikov, M.Y.; Vikram, P.H. Updates on Molecular Targets and Epigenetic-Based Therapies for PCOS. Reprod. Sci. 2023, 30, 772–786. [Google Scholar] [CrossRef]
- Li, P.; Shuai, P.; Shen, S.; Zheng, H.; Sun, P.; Zhang, R.; Lan, S.; Lan, Z.; Jayawardana, T.; Yang, Y.; et al. Perturbations in gut microbiota composition in patients with polycystic ovary syndrome: A systematic review and meta-analysis. BMC Med. 2023, 21, 302. [Google Scholar] [CrossRef]
- Vatier, C.; Christin-Maitre, S. Epigenetic/Circadian Clocks and PCOS. Hum. Reprod. 2024, 39, 1167–1175. [Google Scholar] [CrossRef]
- Nilsson, E.; Benrick, A.; Kokosar, M.; Krook, A.; Lindgren, E.; Källman, T.; Martis, M.M.; Højlund, K.; Ling, C.; Stener-Victorin, E. Transcriptional and Epigenetic Changes Influencing Skeletal Muscle Metabolism in Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2018, 103, 4465–4477. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Deng, Q. Transmission of Polycystic Ovary Syndrome via Epigenetic Inheritance. Trends Mol. Med. 2021, 27, 723–724. [Google Scholar] [CrossRef]
- Qian, Y.; Tong, Y.; Zeng, Y.; Huang, J.; Liu, K.; Xie, Y.; Chen, J.; Gao, M.; Liu, L.; Zhao, J.; et al. Integrated lipid metabolomics and proteomics analysis reveal the pathogenesis of polycystic ovary syndrome. J. Transl. Med. 2024, 22, 364. [Google Scholar] [CrossRef]
- Goodarzi, M.O.; Dumesic, D.A.; Chazenbalk, G.; Azziz, R. Polycystic Ovary Syndrome: Etiology, Pathogenesis and Diagnosis. Nat. Rev. Endocrinol. 2011, 7, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, L. Aspekty genetyczne zespołu policystycznych jajników [Genetic Aspects of Polycystic Ovary Syndrome]. Endokrynol. Pol. 2005, 56, 285–293. [Google Scholar] [PubMed]
- Censin, J.C.; Bovijn, J.; Holmes, M.V.; Lindgren, C.M. Colocalization Analysis of Polycystic Ovary Syndrome to Identify Potential Disease-Mediating Genes and Proteins. Eur. J. Hum. Genet. 2021, 29, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.O.; Reiisi, S.; Parchami Barjui, S. Increased Risk of Polycystic Ovary Syndrome (PCOS) Associated with CC Genotype of miR-146a Gene Variation. Gynecol. Endocrinol. 2018, 34, 793–797. [Google Scholar] [CrossRef]
- Urbanek, M.; Legro, R.S.; Driscoll, D.A.; Azziz, R.; Ehrmann, D.A.; Norman, R.J.; Strauss, J.F., III; Spielman, R.S.; Dunaif, A. Thirty-Seven Candidate Genes for Polycystic Ovary Syndrome: Strongest Evidence for Linkage Is with Follistatin. Proc. Natl. Acad. Sci. USA 1999, 96, 8573–8578. [Google Scholar] [CrossRef]
- Lyle, S.M.; Ahmed, S.; Elliott, J.E.; Stener-Victorin, E.; Nachtigal, M.W.; Drögemöller, B.I. Transcriptome-wide association analyses identify an association between ARL14EP and polycystic ovary syndrome. J. Hum. Genet. 2023, 68, 347–353. [Google Scholar] [CrossRef]
- Dapas, M.; Dunaif, A. Deconstructing a Syndrome: Genomic Insights into PCOS Causal Mechanisms and Classification. Endocr. Rev. 2022, 43, 927–965. [Google Scholar] [CrossRef]
- Matos, M.R.; Ho, S.M.; Schrode, N.; Brennand, K.J. Integration of CRISPR-Engineering and hiPSC-Based Models of Psychiatric Genomics. Mol. Cell. Neurosci. 2020, 107, 103532. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Senapati, S.; Goyal, L.D.; Kaur, B.; Kamra, P.; Khetarpal, P. Genome-Wide Association Study (GWAS) Identified PCOS Susceptibility Variants and Replicates Reported Risk Variants. Arch. Gynecol. Obstet. 2024, 309, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Rawat, K.; Sandhu, A.; Gautam, V.; Saha, P.K.; Saha, L. Role of Genomic DNA Methylation in PCOS Pathogenesis: A Systematic Review and Meta-Analysis Involving Case-Controlled Clinical Studies. Mol. Hum. Reprod. 2022, 28, gaac024. [Google Scholar] [CrossRef]
- Della Corte, L.; Boccia, D.; Palumbo, M.; Mercorio, A.; Ronsini, C.; Bifulco, G.; Giampaolino, P. Is There Still a Place for Surgery in Patients with PCOS? A Review. Life 2023, 13, 1270. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, Y.; Zhao, H.; Yang, Z.; Kang, Y. Aberrant miRNA-mRNA Regulatory Network in Polycystic Ovary Syndrome Is Associated with Markers of Insulin Sensitivity and Inflammation. Ann. Transl. Med. 2021, 9, 1405. [Google Scholar] [CrossRef]
- Giampaolino, P.; Foreste, V.; Di Filippo, C.; Gallo, A.; Mercorio, A.; Serafino, P.; Improda, F.P.; Verrazzo, P.; Zara, G.; Buonfantino, C.; et al. Microbiome and PCOS: State-of-Art and Future Aspects. Int. J. Mol. Sci. 2021, 22, 2048. [Google Scholar] [CrossRef] [PubMed]
- Huang-Doran, I.; Franks, S. Genetic Rodent Models of Obesity-Associated Ovarian Dysfunction and Subfertility: Insights into Polycystic Ovary Syndrome. Front. Endocrinol. 2016, 7, 53. [Google Scholar] [CrossRef]
- McAllister, J.M.; Legro, R.S.; Modi, B.P.; Strauss, J.F., III. Functional Genomics of PCOS: From GWAS to Molecular Mechanisms. Trends Endocrinol. Metab. 2015, 26, 118–124. [Google Scholar] [CrossRef]
- Mykhalchenko, K.; Lizneva, D.; Trofimova, T.; Walker, W.; Suturina, L.; Diamond, M.P.; Azziz, R. Genetics of Polycystic Ovary Syndrome. Expert Rev. Mol. Diagn. 2017, 17, 723–733. [Google Scholar] [CrossRef]
- Hughes, C.; Elgasim, M.; Layfield, R.; Atiomo, W. Genomic and Post-Genomic Approaches to Polycystic Ovary Syndrome—Progress So Far: Mini Review. Hum. Reprod. 2006, 21, 2766–2775. [Google Scholar] [CrossRef] [PubMed]
- Giampaolino, P.; Della Corte, L.; De Rosa, N.; Mercorio, A.; Bruzzese, D.; Bifulco, G. Ovarian Volume and PCOS: A Controversial Issue. Gynecol. Endocrinol. 2018, 34, 229–232. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Population | Sample Size | Age (Years) | BMI/Weight | Gene(s) | Method | Type of Analysis | Main Outcome | Key Findings |
|---|---|---|---|---|---|---|---|---|---|
| González-Fernández et al., 2019 [9] | Human | 16 (case), 24 (control) | 27–39 | NR | SIRT1–7 | RT-PCR | Gene expression | Association between SIRT7 and PCOS | SIRT2 overexpression |
| Mucee et al., 2024 [10] | Human | 15 (ESR1), 15 (ESR2) | NR | NR | ESR1, ESR2 | Bioinformatics | SNP analysis | Correlation of SNPs with PCOS | 10 SNPs showed strong associations |
| Akbari et al., 2023 [11] | Human | 33 (case), 33 (control) | 31.1 ± 5.2 vs. 33.8 ± 5.3 | 66.4 ± 7.7 vs. 66.0 ± 6.4 | CALM1, PSMD6, AK124742 | RT-PCR | Gene expression | Expression in cumulus cells (CCs) of PCOS patients | ↑ CALM1, ↓ PSMD6 and AK124742 |
| Yu et al., 2023 [12] | Human | 2504 | NR | NR | 37 SNPs (incl. DENND1A, AOPEP, THADA, DGKI, UNC5C) | RT-PCR | SNP analysis | Genetic selection in PCOS | Positive selection for 5 genes |
| Zhao et al., 2024 [13] | Mouse | 12 (case), 12 (control) | NA | NA | 205 circRNAs | Microarray | Gene expression | Endometrial and ovarian dysfunction | 147 upregulated, 58 downregulated circRNAs |
| Jiang et al., 2024 [14] | Human | 15 (case), 15 (control) | 29.8 (25–33) vs. 28.3 (20–36) | 23.5 ± 3.4 vs. 26.3 ± 5.0 | ANXA3, CXCR2, IQGAP2, LMNB1 | RT-PCR | Gene expression | Aging-related DEGs in PCOS | 73 aging-related DEGs identified |
| Huang et al., 2024 [15] | Mouse | 20 (case), 20 (control) | NA | NA | FOXO1 | Western blot, ELISA, cytometry | Gene expression | FOXO1 and PCOS correlation | FOXO1 upregulation in PCOS |
| Gao et al., 2024 [16] | Human | PCOS “Han” families | NR | NR | TGF-β1 | qPCR | DNA methylation | CpG methylation and PCOS phenotype | Hypomethylation associated with PCOS |
| Monshizadeh et al., 2024 [17] | Human | 38 (case), 33 (control) | 32.58 ± 5.48 vs. 34.50 ± 4.00 | 28.16 ± 5.53 vs. 26.55 ± 3.94 | RBX1, BAMBI | RT-PCR | Gene expression | Expression in MII and GV cumulus cells | ↓ RBX1 and BAMBI in PCOS patients |
| Postolache et al., 2024 [18] | Human | 212 families | NR | NR | MTNR1A, MTNR1B | Genotyping | SNP analysis | MTNR variants and PCOS risk | 4 variants (MTNR1A), 2 variants (MTNR1B) linked to PCOS |
| Yang et al., 2024 [19] | Human, mouse, in vitro | Cohort 1 30 (case), 30 (control) Cohort 2 60 (case), 60 (control) | NR | NR | SNHG5 | RT-PCR | Gene expression | SNHG5 and follicular development | SNHG5 suppresses follicular growth via miR-92a-3p/CDKN1C axis |
| Gene | Gene Type | Biological Function | Associated Pathways/Gene Ontology Terms Annotations |
|---|---|---|---|
| SIRT [9] | Protein coding | Exhibits mono-ADP ribosyltransferase or deacylase activity; involved in oxidative stress, autophagy, ovulation disturbances, and insulin resistance. | Cellular response to oxidative stress, metabolic regulation. |
| ESR1/2 [10] | Protein coding | Encode estrogen receptors that bind and mediate the effects of estrogen. | Estrogen signaling pathway. |
| CALM1 [11] | Protein coding | Calmodulin regulates calcium signal transduction, ion channels, enzymes, and aquaporins. | Calcium signaling pathway. |
| PSMD6 [11] | Protein coding | Part of the 26S proteasome complex responsible for ATP-dependent degradation of ubiquitinated proteins. | Protein degradation via ubiquitin-proteasome pathway. |
| AK124742 [11] | Long non-coding RNA (lncRNA) | Regulates gene expression, associated with embryo quality and pregnancy outcomes. | Gene expression regulation (lncRNA-mediated). |
| DENND1A [12,20,21] | Protein coding | Guanine nucleotide exchange factor involved in vesicle-mediated transport. | Vesicle-mediated transport, Rab regulation of trafficking; GO: SH3 domain binding, GEF activity. |
| AOPEP [12] | Protein coding | Zinc-dependent aminopeptidase that removes N-terminal amino acids; involved in angiotensin IV generation. | Metallopeptidase activity, blood pressure regulation. |
| THADA [12] | Protein coding | Methylates the 2′-O-ribose of tRNA; involved in tRNA modification. | RNA methylation. |
| DGKI [12] | Protein coding | Diacylglycerol kinase involved in converting diacylglycerol to phosphatidic acid. | Lipid signaling pathways. |
| UNC5-family [12] | Protein coding | Netrin receptor involved in axon guidance and cell migration during neural development. | Netrin signaling, cell migration. |
| ANXA3 [14] | Protein coding | Calcium-dependent phospholipid-binding protein involved in inflammation and cancer. | Prostaglandin synthesis and regulation; GO: calcium ion binding. |
| CXCR2 [14] | Protein coding | Chemokine receptor involved in immune cell migration and inflammation. | GPCR signaling, chemokine-mediated signaling; GO: C-X-C chemokine receptor activity. |
| IQGAP [14] | Protein coding | Scaffold protein involved in cytoskeletal regulation, cell adhesion, signaling, and antiviral responses. | Cytoskeletal regulation, antiviral innate immunity. |
| LMNB1 [14] | Protein coding | Structural component of the nuclear lamina, involved in chromatin organization and apoptosis. | Apoptosis signaling, structural molecule activity; GO: phospholipase binding. |
| FOXO1 [15] | Protein coding | Transcription factor involved in metabolism, apoptosis, and cell cycle regulation. | FOXO-mediated transcription, IL-9 signaling; GO: DNA-binding transcription factor activity. |
| TGF-β1 [16] | Protein coding | Cytokine that controls cell growth, proliferation, differentiation, and apoptosis. | TGF-beta signaling pathway. |
| RBX1 [17] | Protein coding | Involved in ubiquitination and cell cycle progression; contains a RING finger domain. | Ubiquitin-proteasome pathway. |
| BAMBI [17] | Protein coding | Pseudoreceptor for TGF-β; modulates TGF-beta signaling. | TGF-beta signaling modulation. |
| MTNR1B [18] | Protein coding | G-protein-coupled receptor for melatonin, involved in circadian rhythm regulation. | Melatonin signaling, circadian regulation. |
| SNHG5 [19] | Long non-coding RNA (lncRNA) | Acts as a sponge for microRNAs and stabilizes mRNAs; involved in gene expression regulation. | lncRNA-mediated gene regulation, microRNA interaction. |
| Functional Category | Gene | Biological Role | Mechanistic Relevance in PCOS |
|---|---|---|---|
| Androgen Biosynthesis | CYP11A1 [12,20,21] | Initiates steroidogenesis by converting cholesterol to pregnenolone | Overexpression leads to androgen excess and theca cell hyperactivity |
| CYP17A1 [12,20,21] | Catalyzes 17α-hydroxylase and 17,20-lyase reactions in steroid biosynthesis | SNPs linked to hyperandrogenism and anovulation | |
| Insulin Signaling and Resistance | INSR [12,20,21,22,23] | Insulin receptor activating PI3K-Akt signaling | Mutations cause insulin resistance and contribute to metabolic PCOS phenotype |
| IRS1 [12,20,21] | Adapter protein transmitting insulin/IGF-1 signals | Variants linked to impaired glucose uptake and metabolic syndrome in PCOS | |
| Gonadotropin Response | FSHR [12,20,21] | FSH receptor regulating follicular maturation | Polymorphisms affect ovarian sensitivity and folliculogenesis |
| LHCGR [12,20,21] | LH receptor essential for ovulation and corpus luteum maintenance | Mutations impair ovulatory response and promote androgen production |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo, M.; Della Corte, L.; Colacurci, D.; Ascione, M.; D’Angelo, G.; Baldini, G.M.; Giampaolino, P.; Bifulco, G. PCOS and the Genome: Is the Genetic Puzzle Still Worth Solving? Biomedicines 2025, 13, 1912. https://doi.org/10.3390/biomedicines13081912
Palumbo M, Della Corte L, Colacurci D, Ascione M, D’Angelo G, Baldini GM, Giampaolino P, Bifulco G. PCOS and the Genome: Is the Genetic Puzzle Still Worth Solving? Biomedicines. 2025; 13(8):1912. https://doi.org/10.3390/biomedicines13081912
Chicago/Turabian StylePalumbo, Mario, Luigi Della Corte, Dario Colacurci, Mario Ascione, Giuseppe D’Angelo, Giorgio Maria Baldini, Pierluigi Giampaolino, and Giuseppe Bifulco. 2025. "PCOS and the Genome: Is the Genetic Puzzle Still Worth Solving?" Biomedicines 13, no. 8: 1912. https://doi.org/10.3390/biomedicines13081912
APA StylePalumbo, M., Della Corte, L., Colacurci, D., Ascione, M., D’Angelo, G., Baldini, G. M., Giampaolino, P., & Bifulco, G. (2025). PCOS and the Genome: Is the Genetic Puzzle Still Worth Solving? Biomedicines, 13(8), 1912. https://doi.org/10.3390/biomedicines13081912








