miRNAs in Pulmonary Hypertension: Mechanistic Insights and Therapeutic Potential
Abstract
1. Introduction
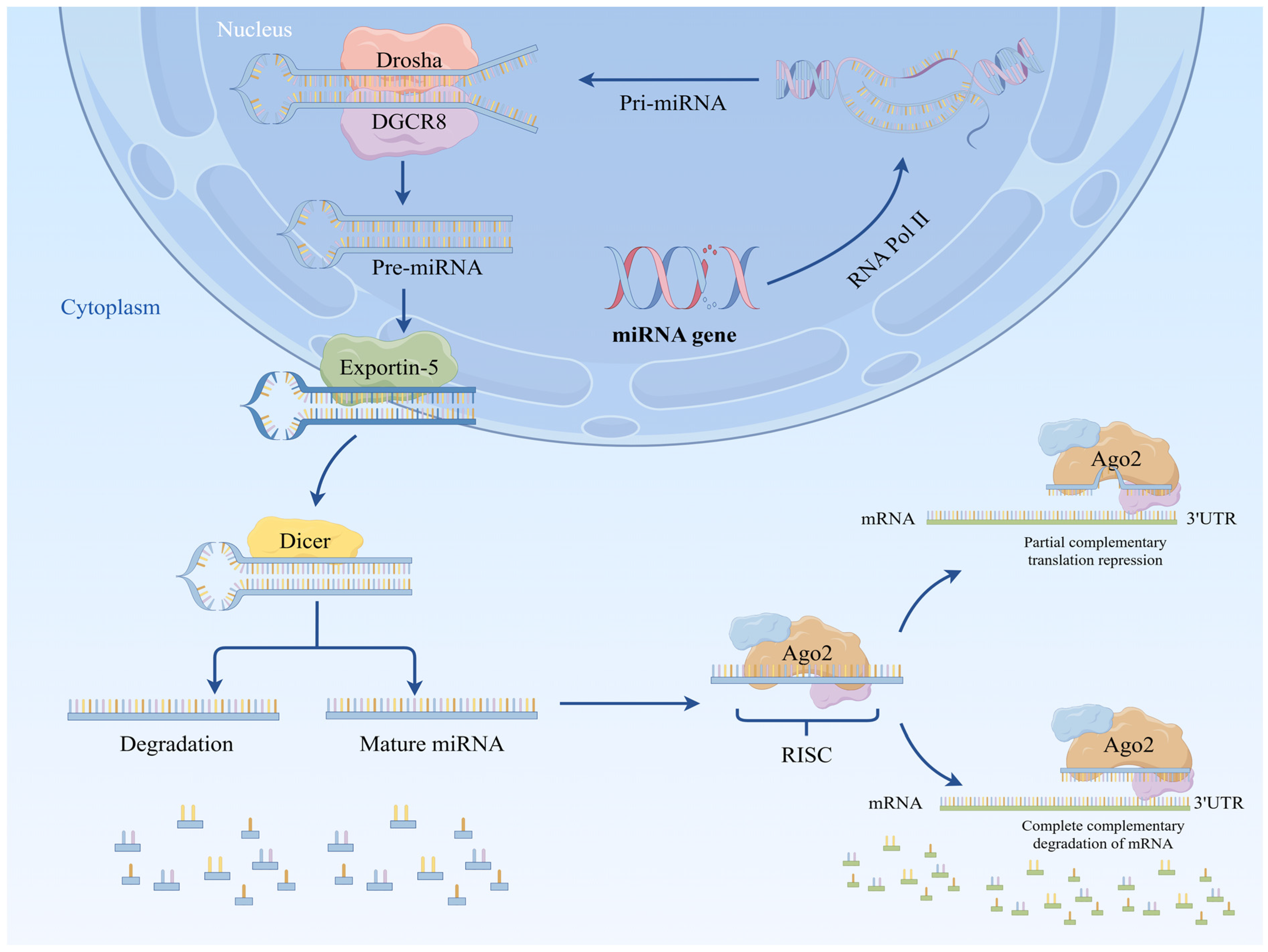
2. Biosynthesis of miRNA
2.1. Classical Biosynthetic Pathways
2.2. Non-Classical Biosynthetic Pathway
2.2.1. No Reliance on the Dicer Pathway
2.2.2. No Reliance on the Drosha/Dgcr8 Pathway in the Nucleus
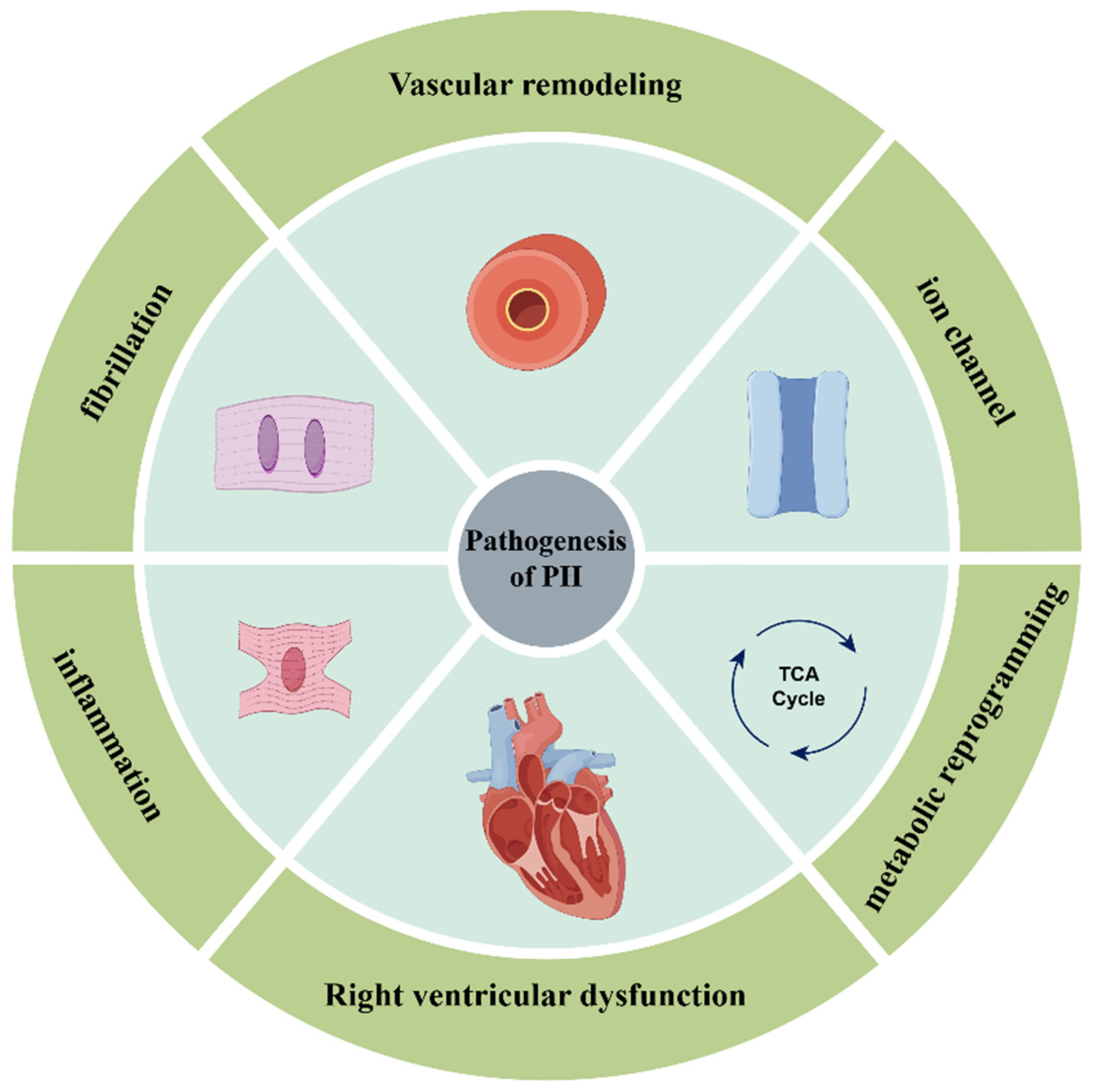
3. miRNAs Involved in the Pathogenesis of PH
3.1. miRNAs Involved in Vascular Remodeling
3.1.1. miRNAs Regulating Pulmonary Vascular Smooth Muscle Cells
3.1.2. miRNAs Regulating Pulmonary Vascular Endothelial Cells
3.1.3. miRNAs That Regulate Fibroblasts
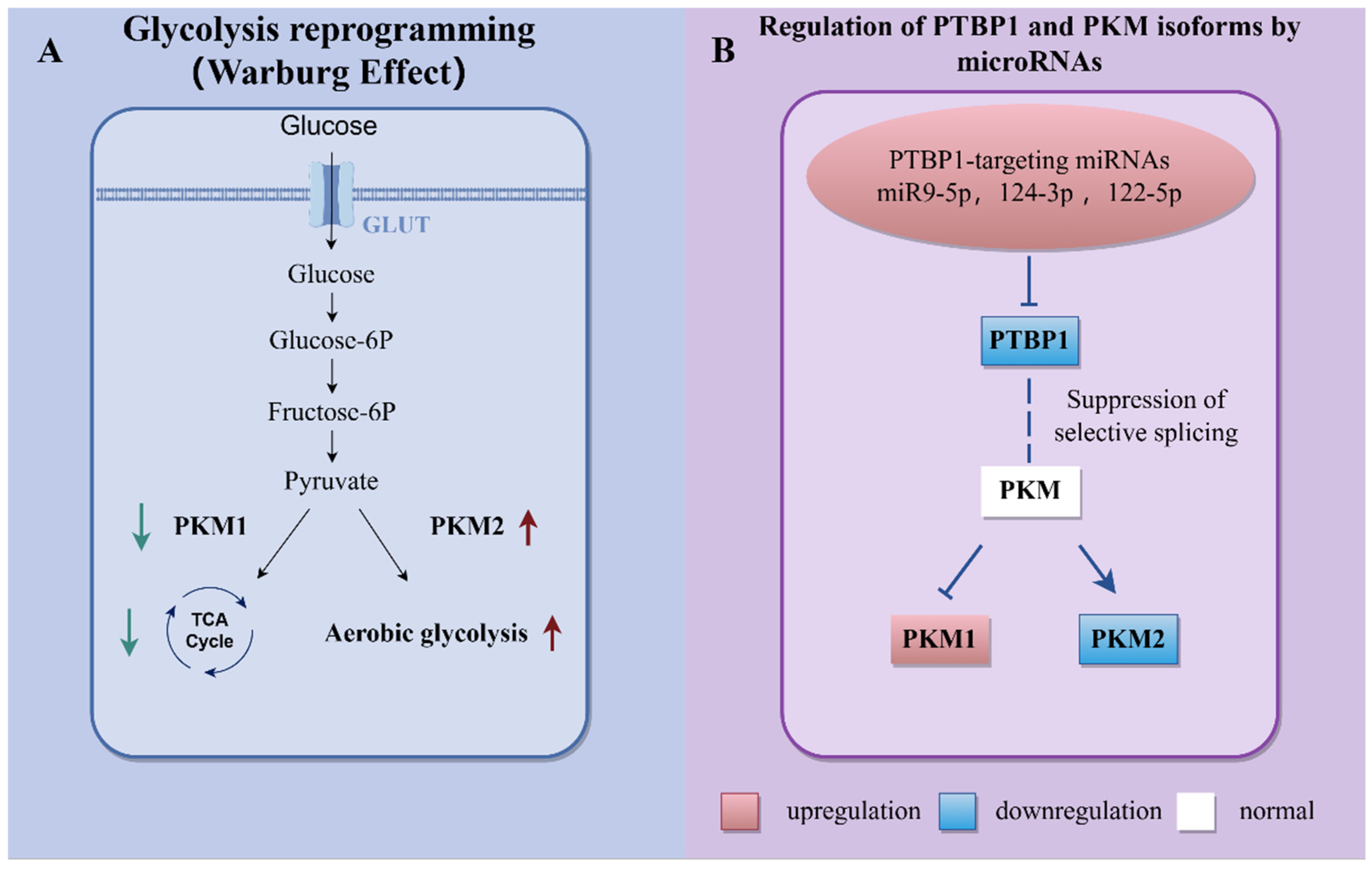
3.2. miRNAs Involved in Metabolic Reprogramming
3.3. miRNAs Involved in Ion Channels
3.4. miRNAs Involved in Inflammation and Fibrosis
3.5. miRNAs Involved in Right Ventricular Dysfunction
4. The Future of miRNAs in PH
4.1. miRNAs in PH as Diagnostic and Prognostic Biomarkers
4.2. miRNAs as Therapeutic Agents for PH
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mocumbi, A.; Humbert, M.; Saxena, A.; Jing, Z.C.; Sliwa, K.; Thienemann, F.; Archer, S.L.; Stewart, S. Pulmonary hypertension. Nat. Rev. Dis. Primers 2024, 10, 1. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2023, 61, 2200879. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Feinbaum, R.; Ambros, V. A short history of a short RNA. Cell 2004, 116 (Suppl. 2), S89–S92. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.; Yi, E.S.; Kim, J.M.; Jo, E.K.; Seo, S.; Kim, R.I.; Kim, K.L.; Sung, J.H.; Park, S.G.; Suh, W. FGF12 (Fibroblast Growth Factor 12) Inhibits Vascular Smooth Muscle Cell Remodeling in Pulmonary Arterial Hypertension. Hypertension 2020, 76, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Jose, A.; Eckman, M.H.; Elwing, J.M. Anticoagulation in pulmonary arterial hypertension: A decision analysis. Pulm. Circ. 2019, 9, 2045894019895451. [Google Scholar] [CrossRef]
- Xu, J.; Linneman, J.; Zhong, Y.; Yin, H.; Xia, Q.; Kang, K.; Gou, D. MicroRNAs in Pulmonary Hypertension, from Pathogenesis to Diagnosis and Treatment. Biomolecules 2022, 12, 496. [Google Scholar] [CrossRef]
- Gregory, R.I.; Yan, K.P.; Amuthan, G.; Chendrimada, T.; Doratotaj, B.; Cooch, N.; Shiekhattar, R. The Microprocessor complex mediates the genesis of microRNAs. Nature 2004, 432, 235–240. [Google Scholar] [CrossRef]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The biochemical basis of microRNA targeting efficacy. Science 2019, 366, eaav1741. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Cheloufi, S.; Dos Santos, C.O.; Chong, M.M.; Hannon, G.J. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature 2010, 465, 584–589. [Google Scholar] [CrossRef]
- Yang, J.S.; Maurin, T.; Robine, N.; Rasmussen, K.D.; Jeffrey, K.L.; Chandwani, R.; Papapetrou, E.P.; Sadelain, M.; O’Carroll, D.; Lai, E.C. Conserved vertebrate mir-451 provides a platform for Dicer-independent, Ago2-mediated microRNA biogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 15163–15168. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, D.; Xue, H.; Taylor, D.W.; Patnode, H.; Mishima, Y.; Cheloufi, S.; Ma, E.; Mane, S.; Hannon, G.J.; Lawson, N.D.; et al. A novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science 2010, 328, 1694–1698. [Google Scholar] [CrossRef]
- Wang, Y.; Sheng, G.; Juranek, S.; Tuschl, T.; Patel, D.J. Structure of the guide-strand-containing argonaute silencing complex. Nature 2008, 456, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Hagen, J.W.; Duan, H.; Tyler, D.M.; Lai, E.C. The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell 2007, 130, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Ruby, J.G.; Jan, C.H.; Bartel, D.P. Intronic microRNA precursors that bypass Drosha processing. Nature 2007, 448, 83–86. [Google Scholar] [CrossRef]
- Matera, A.G.; Terns, R.M.; Terns, M.P. Non-coding RNAs: Lessons from the small nuclear and small nucleolar RNAs. Nat. Rev. Mol. Cell Biol. 2007, 8, 209–220. [Google Scholar] [CrossRef]
- Ender, C.; Krek, A.; Friedländer, M.R.; Beitzinger, M.; Weinmann, L.; Chen, W.; Pfeffer, S.; Rajewsky, N.; Meister, G. A human snoRNA with microRNA-like functions. Mol. Cell 2008, 32, 519–528. [Google Scholar] [CrossRef]
- Babiarz, J.E.; Ruby, J.G.; Wang, Y.; Bartel, D.P.; Blelloch, R. Mouse ES cells express endogenous shRNAs, siRNAs, and other Microprocessor-independent, Dicer-dependent small RNAs. Genes. Dev. 2008, 22, 2773–2785. [Google Scholar] [CrossRef]
- Han, J.; Lee, Y.; Yeom, K.H.; Nam, J.W.; Heo, I.; Rhee, J.K.; Sohn, S.Y.; Cho, Y.; Zhang, B.T.; Kim, V.N. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell 2006, 125, 887–901. [Google Scholar] [CrossRef]
- Bogerd, H.P.; Karnowski, H.W.; Cai, X.; Shin, J.; Pohlers, M.; Cullen, B.R. A mammalian herpesvirus uses noncanonical expression and processing mechanisms to generate viral MicroRNAs. Mol. Cell 2010, 37, 135–142. [Google Scholar] [CrossRef]
- Inui, M.; Martello, G.; Piccolo, S. MicroRNA control of signal transduction. Nat. Rev. Mol. Cell Biol. 2010, 11, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gomes, J.; Mendes-Ferreira, P.; Adão, R.; Maia-Rocha, C.; Rego, B.; Poels, M.; Saint-Martin Willer, A.; Masson, B.; Provencher, S.; Bonnet, S.; et al. Unraveling the Impact of miR-146a in Pulmonary Arterial Hypertension Pathophysiology and Right Ventricular Function. Int. J. Mol. Sci. 2024, 25, 8054. [Google Scholar] [CrossRef] [PubMed]
- Düzgün, Z.; Kayıkçıoğlu, L.M.; Aktan, Ç.; Bara, B.; Eroğlu, F.Z.; Yağmur, B.; Çetintaş, V.B.; Bayındır, M.; Nalbantgil, S.; Vardarli, A.T. Decreased circulating microRNA-21 and microRNA-143 are associated to pulmonary hypertension. Turk. J. Med. Sci. 2023, 53, 130–141. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, J.; Wang, H.; Zhang, Z.; Yin, M.; Zhu, Y.; Li, L.; Chen, C.; Wei, M.; Hu, M.; et al. miR-30d Attenuates Pulmonary Arterial Hypertension via Targeting MTDH and PDE5A and Modulates the Beneficial Effect of Sildenafil. Adv. Sci. 2024, 11, e2407712. [Google Scholar] [CrossRef]
- Chen, T.; Sun, M.R.; Zhou, Q.; Guzman, A.M.; Ramchandran, R.; Chen, J.; Fraidenburg, D.R.; Ganesh, B.; Maienschein-Cline, M.; Obrietan, K.; et al. MicroRNA-212-5p, an anti-proliferative miRNA, attenuates hypoxia and sugen/hypoxia-induced pulmonary hypertension in rodents. Mol. Ther. Nucleic Acids 2022, 29, 204–216. [Google Scholar] [CrossRef]
- Huang, C.X.; Jiang, Z.X.; Du, D.Y.; Zhang, Z.M.; Liu, Y.; Li, Y.T. The MFF-SIRT1/3 axis, regulated by miR-340-5p, restores mitochondrial homeostasis of hypoxia-induced pulmonary artery smooth muscle cells. Lab Invest. 2022, 102, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, W.; Lu, Y.; Wang, N.; Kong, D.; Shan, L. MicroRNA-153 attenuates hypoxia-induced excessive proliferation and migration of pulmonary arterial smooth muscle cells by targeting ROCK1 and NFATc3. Mol. Med. Rep. 2021, 23, 194. [Google Scholar] [CrossRef]
- Bi, R.; Bao, C.; Jiang, L.; Liu, H.; Yang, Y.; Mei, J.; Ding, F. MicroRNA-27b plays a role in pulmonary arterial hypertension by modulating peroxisome proliferator-activated receptor γ dependent Hsp90-eNOS signaling and nitric oxide production. Biochem. Biophys. Res. Commun. 2015, 460, 469–475. [Google Scholar] [CrossRef]
- Yao, D.; He, Q.; Sun, J.; Cai, L.; Wei, J.; Cai, G.; Liu, J.; Lin, Y.; Wang, L.; Huang, X. FGF21 attenuates hypoxia-induced dysfunction and inflammation in HPAECs via the microRNA-27b-mediated PPARγ pathway. Int. J. Mol. Med. 2021, 47, 116. [Google Scholar] [CrossRef]
- Kang, B.Y.; Park, K.K.; Green, D.E.; Bijli, K.M.; Searles, C.D.; Sutliff, R.L.; Hart, C.M. Hypoxia mediates mutual repression between microRNA-27a and PPARγ in the pulmonary vasculature. PLoS ONE 2013, 8, e79503. [Google Scholar] [CrossRef]
- Tan, H.; Yao, H.; Lie, Z.; Chen, G.; Lin, S.; Zhang, Y. MicroRNA-30a-5p promotes proliferation and inhibits apoptosis of human pulmonary artery endothelial cells under hypoxia by targeting YKL-40. Mol. Med. Rep. 2019, 20, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Chen, J.; Chen, T.; Wang, Y.; Song, Y.; Dong, Y.; Zhao, S.; Machado, R.F. MicroRNA410 Inhibits Pulmonary Vascular Remodeling via Regulation of Nicotinamide Phosphoribosyltransferase. Sci. Rep. 2019, 9, 9949. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Nakamachi, Y. miR-124a as a key regulator of proliferation and MCP-1 secretion in synoviocytes from patients with rheumatoid arthritis. Ann. Rheum. Dis. 2011, 70 (Suppl. S1), i88–i91. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, D.; Li, M.; Plecitá-Hlavatá, L.; D’Alessandro, A.; Tauber, J.; Riddle, S.; Kumar, S.; Flockton, A.; McKeon, B.A.; et al. Metabolic and Proliferative State of Vascular Adventitial Fibroblasts in Pulmonary Hypertension Is Regulated Through a MicroRNA-124/PTBP1 (Polypyrimidine Tract Binding Protein 1)/Pyruvate Kinase Muscle Axis. Circulation 2017, 136, 2468–2485. [Google Scholar] [CrossRef]
- Niu, Z.; Fu, M.; Li, Y.; Ren, H.; Zhang, X.; Yao, L. Osthole alleviates pulmonary vascular remodeling by modulating microRNA-22–3p mediated lipid metabolic reprogramming. Phytomedicine 2022, 96, 153840. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.-F.; Geng, F.; Deng, L.P.; Lin, D.C.; Huang, Y.Z.; Lai, S.M.; Lin, Y.C.; Gui, L.X.; Sham, J.S.K.; Lin, M.J. Reduced CircSMOC1 Level Promotes Metabolic Reprogramming via PTBP1 (Polypyrimidine Tract-Binding Protein) and miR-329-3p in Pulmonary Arterial Hypertension Rats. Hypertension 2022, 79, 2465–2479. [Google Scholar] [CrossRef]
- Luo, L.; Xiao, L.; Lian, G.; Wang, H.; Xie, L. miR-125a-5p inhibits glycolysis by targeting hexokinase-II to improve pulmonary arterial hypertension. Aging 2020, 12, 9014–9030. [Google Scholar] [CrossRef]
- Mondejar-Parreño, G.; Callejo, M.; Barreira, B.; Morales-Cano, D.; Esquivel-Ruiz, S.; Moreno, L.; Cogolludo, A.; Perez-Vizcaino, F. miR-1 is increased in pulmonary hypertension and downregulates Kv1.5 channels in rat pulmonary arteries. J. Physiol. 2019, 597, 1185–1197. [Google Scholar] [CrossRef]
- Li, S.S.; Liang, S.; Li, L.; Yang, H.; Long, Y.; Zhuo, D.; Chen, X.; Jin, X. circNFXL1 Modulates the Kv2.1 Channel Function in Hypoxic Human Pulmonary Artery Smooth Muscle Cells via Sponging miR-29b-2-5p as a Competitive Endogenous RNA. J. Cardiovasc. Pharmacol. 2023, 81, 292–299. [Google Scholar] [CrossRef]
- Hong, Z.; Chen, K.H.; DasGupta, A.; Potus, F.; Dunham-Snary, K.; Bonnet, S.; Tian, L.; Fu, J.; Breuils-Bonnet, S.; Provencher, S.; et al. MicroRNA-138 and MicroRNA-25 Down-regulate Mitochondrial Calcium Uniporter, Causing the Pulmonary Arterial Hypertension Cancer Phenotype. Am. J. Respir. Crit. Care Med. 2017, 195, 515–529. [Google Scholar] [CrossRef]
- Zhang, J.; He, Y.; Yan, X.; Chen, S.; He, M.; Lei, Y.; Zhang, J.; Gongol, B.; Gu, M.; Miao, Y.; et al. MicroRNA-483 amelioration of experimental pulmonary hypertension. EMBO Mol. Med. 2020, 12, e11303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Guo, Y.; Sun, Y.; Zhang, N.; Wang, X. miR-181a/b-5p ameliorates inflammatory response in monocrotaline-induced pulmonary arterial hypertension by targeting endocan. J. Cell. Physiol. 2020, 235, 4422–4433. [Google Scholar] [CrossRef]
- Qi, R.; Zhang, Y.; Yan, F. Exosomes enriched by miR-429-3p derived from ITGB1 modified Telocytes alleviates hypoxia-induced pulmonary arterial hypertension through regulating Rac1 expression. Cell Biol. Toxicol. 2024, 40, 32. [Google Scholar] [CrossRef]
- Hsu, C.H.; Liu, I.F.; Kuo, H.F.; Li, C.Y.; Lian, W.S.; Chang, C.Y.; Chen, Y.H.; Liu, W.L.; Lu, C.Y.; Liu, Y.R.; et al. miR-29a-3p/THBS2 Axis Regulates PAH-Induced Cardiac Fibrosis. Int. J. Mol. Sci. 2021, 22, 10574. [Google Scholar] [CrossRef]
- Ma, H.; Ye, P.; Zhang, A.K.; Yu, W.D.; Lin, S.; Zheng, Y.G. Upregulation of miR-335-5p Contributes to Right Ventricular Remodeling via Calumenin in Pulmonary Arterial Hypertension. Biomed. Res. Int. 2022, 2022, 9294148. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Q.; Zhao, H.; Wu, W.; Zhao, Q.; Jiang, R.; Liu, J.; Wang, L.; Yuan, P. Role of miR-21-5p/FilGAP axis in estradiol alleviating the progression of monocrotaline-induced pulmonary hypertension. Anim. Model. Exp. Med. 2022, 5, 217–226. [Google Scholar] [CrossRef]
- Tang, Y.; Huo, X.; Liu, J.; Tang, Y.; Zhang, M.; Xie, W.; Zheng, Z.; He, J.; Lian, J. MicroRNA-325-3p Targets Human Epididymis Protein 4 to Relieve Right Ventricular Fibrosis in Rats with Pulmonary Arterial Hypertension. Cardiovasc. Ther. 2022, 2022, 4382999. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, S.; Yan, H.; Cao, Y.; Zhang, X.; Wang, L.; Zhang, Z.; Lin, S.; Wang, X.; Mao, J. Pulmonary Vascular Remodeling in Pulmonary Hypertension. J. Pers. Med. 2023, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Guignabert, C.; Dorfmüller, P. Pathology and Pathobiology of Pulmonary Hypertension. Semin. Respir. Crit. Care Med. 2017, 38, 571–584. [Google Scholar] [CrossRef]
- Fang, Y.C.; Yeh, C.H. Role of microRNAs in Vascular Remodeling. Curr. Mol. Med. 2015, 15, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Salvador, A.M.; Li, G.; Valkov, N.; Ziegler, O.; Yeri, A.; Yang Xiao, C.; Meechoovet, B.; Alsop, E.; Rodosthenous, R.S.; et al. Mir-30d Regulates Cardiac Remodeling by Intracellular and Paracrine Signaling. Circ. Res. 2021, 128, e1–e23. [Google Scholar] [CrossRef]
- Melman, Y.F.; Shah, R.; Danielson, K.; Xiao, J.; Simonson, B.; Barth, A.; Chakir, K.; Lewis, G.D.; Lavender, Z.; Truong, Q.A.; et al. Circulating MicroRNA-30d Is Associated With Response to Cardiac Resynchronization Therapy in Heart Failure and Regulates Cardiomyocyte Apoptosis: A Translational Pilot Study. Circulation 2015, 131, 2202–2216. [Google Scholar] [CrossRef]
- Yoshiyuki, R.; Tanaka, R.; Fukushima, R.; Machida, N. Preventive effect of sildenafil on right ventricular function in rats with monocrotaline-induced pulmonary arterial hypertension. Exp. Anim. 2016, 65, 215–222. [Google Scholar] [CrossRef]
- Wang, J.; Kuang, J.; Zhang, S.; Liu, Z.; Guo, Q.; Li, S.; Qiu, L.; Fu, G.; Lin, X.; Wu, J.; et al. Comprehensive characterization of small noncoding RNA profiles in hypoxia-induced pulmonary hypertension (HPH) rat tissues. iScience 2024, 27, 108815. [Google Scholar] [CrossRef]
- Yue, H.; Liu, L.; Song, Z. miR-212 regulated by HIF-1α promotes the progression of pancreatic cancer. Exp. Ther. Med. 2019, 17, 2359–2365. [Google Scholar]
- Remenyi, J.; Hunter, C.J.; Cole, C.; Ando, H.; Impey, S.; Monk, C.E.; Martin, K.J.; Barton, G.J.; Hutvagner, G.; Arthur, J.S. Regulation of the miR-212/132 locus by MSK1 and CREB in response to neurotrophins. Biochem. J. 2010, 428, 281–291. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, J.; Zhang, X.; Liu, Y.; Gu, S.; Zhang, X.; An, X.; Yan, J.; Xin, Y.; Su, P. microRNA-340-5p functions downstream of cardiotrophin-1 to regulate cardiac eccentric hypertrophy and heart failure via target gene dystrophin. Int. Heart J. 2015, 56, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Maston, L.D.; Jones, D.T.; Giermakowska, W.; Resta, T.C.; Ramiro-Diaz, J.; Howard, T.A.; Jernigan, N.L.; Herbert, L.; Maurice, A.A.; Gonzalez Bosc, L.V. Interleukin-6 trans-signaling contributes to chronic hypoxia-induced pulmonary hypertension. Pulm. Circ. 2018, 8, 2045894018780734. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lv, X.; Fu, X.; Su, L.; Yang, T.; Xu, P. MiR-153 inhibits the resistance of lung cancer to gefitinib via modulating expression of ABCE1. Cancer Biomark. 2019, 25, 361–369. [Google Scholar] [CrossRef]
- Liang, C.; Li, X.; Zhang, L.; Cui, D.; Quan, X.; Yang, W. The anti-fibrotic effects of microRNA-153 by targeting TGFBR-2 in pulmonary fibrosis. Exp. Mol. Pathol. 2015, 99, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Xiao, J.; Zhou, Z.; Wu, J.; Ge, F.; Li, Z.; Zhang, H.; Sun, J.; Li, F.; Liu, R.; et al. Hypoxia induces miR-153 through the IRE1α-XBP1 pathway to fine tune the HIF1α/VEGFA axis in breast cancer angiogenesis. Oncogene 2018, 37, 1961–1975. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, N.; Li, X.; Lin, L.; Chen, X. MiR-19a modulates hypoxia-mediated cell proliferation and migration via repressing PTEN in human pulmonary arterial smooth muscle. Life Sci. 2019, 239, 116928. [Google Scholar] [CrossRef] [PubMed]
- He, R.L.; Wu, Z.J.; Liu, X.R.; Gui, L.X.; Wang, R.X.; Lin, M.J. Calcineurin/NFAT Signaling Modulates Pulmonary Artery Smooth Muscle Cell Proliferation, Migration and Apoptosis in Monocrotaline-Induced Pulmonary Arterial Hypertension Rats. Cell Physiol. Biochem. 2018, 49, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Niu, S.; Xu, J.; Lu, W.; Zhou, L. miR-221-3p is upregulated in acute pulmonary embolism complicated with pulmonary hypertension and promotes pulmonary arterial smooth muscle cells proliferation and migration by inhibiting PTEN. Cytotechnology 2024, 76, 453–463. [Google Scholar] [CrossRef]
- Kang, K.; Sun, C.; Li, H.; Liu, X.; Deng, J.; Chen, S.; Zeng, L.; Chen, J.; Liu, X.; Kuang, J.; et al. N6-methyladenosine-driven miR-143/145-KLF4 circuit orchestrates the phenotypic switch of pulmonary artery smooth muscle cells. Cell Mol. Life Sci. 2024, 81, 256. [Google Scholar] [CrossRef]
- Sun, L.; Liu, L.; Liang, D.; Liu, L. SOCS5, targeted by miR-155-5p, plays a negative regulatory role in pulmonary hypertension through inhibiting JAK2/STAT3 signaling pathway. BMC Pulm. Med. 2024, 24, 52. [Google Scholar] [CrossRef]
- Meng, Q.; Song, L.; Wang, H.; Wang, G.; Zhou, G. Levosimendan mediates the BMP/Smad axis through upregulation of circUSP34-targeted miR-1298 to alleviate pulmonary hypertension. Respir. Res. 2024, 25, 316. [Google Scholar] [CrossRef]
- Su, H.; Zhu, H.; Wang, S.; Li, Y.; Yan, C.; Wang, J.; Ying, K. CircItgb5 promotes synthetic phenotype of pulmonary artery smooth muscle cells via interacting with miR-96-5p and Uba1 in monocrotaline-induced pulmonary arterial hypertension. Respir. Res. 2023, 24, 165. [Google Scholar] [CrossRef]
- Rong, X.; Ge, D.; Shen, D.; Chen, X.; Wang, X.; Zhang, L.; Jia, C.; Zeng, J.; He, Y.; Qiu, H.; et al. miR-27b Suppresses Endothelial Cell Proliferation and Migration by Targeting Smad7 in Kawasaki Disease. Cell Physiol. Biochem. 2018, 48, 1804–1814. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.Y.; Park, K.; Kleinhenz, J.M.; Murphy, T.C.; Sutliff, R.L.; Archer, D.; Hart, C.M. Peroxisome Proliferator-Activated Receptor γ Regulates the V-Ets Avian Erythroblastosis Virus E26 Oncogene Homolog 1/microRNA-27a Axis to Reduce Endothelin-1 and Endothelial Dysfunction in the Sickle Cell Mouse Lung. Am. J. Respir. Cell Mol. Biol. 2017, 56, 131–144. [Google Scholar] [CrossRef]
- Baraniskin, A.; Birkenkamp-Demtroder, K.; Maghnouj, A.; Zöllner, H.; Munding, J.; Klein-Scory, S.; Reinacher-Schick, A.; Schwarte-Waldhoff, I.; Schmiegel, W.; Hahn, S.A. MiR-30a-5p suppresses tumor growth in colon carcinoma by targeting DTL. Carcinogenesis 2012, 33, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.C.; Yang, B.L.; Chen, W.C.; Ho, A.S.; Sie, Z.L.; Lin, H.C.; Chang, C.C. STAT3 Mediated miR-30a-5p Inhibition Enhances Proliferation and Inhibits Apoptosis in Colorectal Cancer Cells. Int. J. Mol. Sci. 2020, 21, 7315. [Google Scholar] [CrossRef]
- Müssnich, P.; Raverot, G.; Jaffrain-Rea, M.L.; Fraggetta, F.; Wierinckx, A.; Trouillas, J.; Fusco, A.; D’Angelo, D. Downregulation of miR-410 targeting the cyclin B1 gene plays a role in pituitary gonadotroph tumors. Cell Cycle 2015, 14, 2590–2597. [Google Scholar] [CrossRef]
- Guo, R.; Gu, J.; Zhang, Z.; Wang, Y.; Gu, C. MicroRNA-410 functions as a tumor suppressor by targeting angiotensin II type 1 receptor in pancreatic cancer. IUBMB Life 2015, 67, 42–53. [Google Scholar] [CrossRef]
- Palumbo, T.; Poultsides, G.A.; Kouraklis, G.; Liakakos, T.; Drakaki, A.; Peros, G.; Hatziapostolou, M.; Iliopoulos, D. A functional microRNA library screen reveals miR-410 as a novel anti-apoptotic regulator of cholangiocarcinoma. BMC Cancer 2016, 16, 353. [Google Scholar] [CrossRef]
- Chen, J.; Sysol, J.R.; Singla, S.; Zhao, S.; Yamamura, A.; Valdez-Jasso, D.; Abbasi, T.; Shioura, K.M.; Sahni, S.; Reddy, V.; et al. Nicotinamide Phosphoribosyltransferase Promotes Pulmonary Vascular Remodeling and Is a Therapeutic Target in Pulmonary Arterial Hypertension. Circulation 2017, 135, 1532–1546. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, X.; Wang, Y.; Hu, Y. LncRNA GAS5 promotes spermidine-induced autophagy through the miRNA-31-5p/NAT8L axis in pulmonary artery endothelial cells of patients with CTEPH. Mol. Med. Rep. 2022, 26, 297. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Kang, H. Platelet-derived growth factor-stimulated pulmonary artery smooth muscle cells regulate pulmonary artery endothelial cell dysfunction through extracellular vesicle miR-409-5p. Biol. Chem. 2024, 405, 203–215. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, X.; Cui, G.; Wang, S. Mir-150-5p distinguishes acute pulmonary embolism, predicts the occurrence of pulmonary arterial hypertension, and regulates ox-LDL-induced endothelial cell injury. Hereditas 2024, 161, 33. [Google Scholar] [CrossRef]
- Zhao, W.H.; Wu, S.Q.; Zhang, Y.D. Downregulation of miR-124 promotes the growth and invasiveness of glioblastoma cells involving upregulation of PPP1R13L. Int. J. Mol. Med. 2013, 32, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, H.; Li, M.; Frid, M.G.; Flockton, A.R.; McKeon, B.A.; Yeager, M.E.; Fini, M.A.; Morrell, N.W.; Pullamsetti, S.S.; et al. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circ. Res. 2014, 114, 67–78. [Google Scholar] [CrossRef]
- Hanahan, D.; Robert, A. Weinberg, Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Willson, C.; Watanabe, M.; Tsuji-Hosokawa, A.; Makino, A. Pulmonary vascular dysfunction in metabolic syndrome. J. Physiol. 2019, 597, 1121–1141. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol. Life Sci. 2016, 73, 377–392. [Google Scholar] [CrossRef]
- Te Slaa, T.; Ralser, M.; Fan, J.; Rabinowitz, J.D. The pentose phosphate pathway in health and disease. Nat. Metab. 2023, 5, 1275–1289. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; El Kasmi, K.C.; Plecitá-Hlavatá, L.; Ježek, P.; Li, M.; Zhang, H.; Gupte, S.A.; Stenmark, K.R. Hallmarks of Pulmonary Hypertension: Mesenchymal and Inflammatory Cell Metabolic Reprogramming. Antioxid. Redox Signal 2018, 28, 230–250. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Lemons, J.M.; Feng, X.J.; Bennett, B.D.; Legesse-Miller, A.; Johnson, E.L.; Raitman, I.; Pollina, E.A.; Rabitz, H.A.; Rabinowitz, J.D.; Coller, H.A. Quiescent fibroblasts exhibit high metabolic activity. PLoS Biol. 2010, 8, e1000514. [Google Scholar] [CrossRef]
- Silva, T.d.O.; Lino, C.A.; Buzatto, V.C.; Asprino, P.F.; Lu, Y.W.; Lima, V.M.; Fonseca, R.I.B.; Jensen, L.; Murata, G.M.; Filho, S.V.; et al. Deletion of miRNA-22 induces cardiac hypertrophy in females but attenuates obesogenic diet-mediated metabolic disorders. Cell Physiol. Biochem. 2020, 54, 1199–1217. [Google Scholar] [CrossRef]
- Aufiero, S.; Reckman, Y.J.; Pinto, Y.M.; Creemers, E.E. Circular RNAs open a new chapter in cardiovascular biology. Nat. Rev. Cardiol. 2019, 16, 503–514. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Cui, Y.; Tang, L. miR-125a-5p-targeted regulation of TRA2β expression inhibits proliferation and metastasis of hepatocellular carcinoma cells. Am. J. Transl. Res. 2021, 13, 14074–14080. [Google Scholar]
- Wang, Q.; Tan, L.; Lv, Y.; Yu, T.; Chang, Y.; Liu, J.; Sun, Y. MiR-125a-5p regulates the radiosensitivity of laryngeal squamous cell carcinoma via HK2 targeting through the DDR pathway. Front. Oncol. 2024, 14, 1438722. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hu, Z.; Wang, Z.; Zhu, T.; Wang, G.; Gao, B.; Wang, J.; Deng, X. miR-125a-5p promotes gastric cancer growth and invasion by regulating the Hippo pathway. J. Clin. Lab. Anal. 2021, 35, e24078. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Patti, G.J. The Warburg effect: A signature of mitochondrial overload. Trends Cell Biol. 2023, 33, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhou, Q.; Chen, J.; Rexius-Hall, M.L.; Rehman, J.; Zhou, G. Alpha-enolase regulates the malignant phenotype of pulmonary artery smooth muscle cells via the AMPK-Akt pathway. Nat. Commun. 2018, 9, 3850. [Google Scholar] [CrossRef]
- Makino, A.; Firth, A.L.; Yuan, J.X. Endothelial and smooth muscle cell ion channels in pulmonary vasoconstriction and vascular remodeling. Compr. Physiol. 2011, 1, 1555–1602. [Google Scholar] [CrossRef]
- Mondéjar-Parreño, G.; Cogolludo, A.; Perez-Vizcaino, F. Potassium (K+) channels in the pulmonary vasculature: Implications in pulmonary hypertension Physiological, pathophysiological and pharmacological regulation. Pharmacol. Ther. 2021, 225, 107835. [Google Scholar] [CrossRef]
- Boucherat, O.; Chabot, S.; Antigny, F.; Perros, F.; Provencher, S.; Bonnet, S. Potassium channels in pulmonary arterial hypertension. Eur. Respir. J. 2015, 46, 1167–1177. [Google Scholar] [CrossRef]
- Jernigan, N.L.; Resta, T.C. Calcium homeostasis and sensitization in pulmonary arterial smooth muscle. Microcirculation 2014, 21, 259–271. [Google Scholar] [CrossRef]
- Babicheva, A.; Ayon, R.J.; Zhao, T.; Ek Vitorin, J.F.; Pohl, N.M.; Yamamura, A.; Yamamura, H.; Quinton, B.A.; Ba, M.; Wu, L.; et al. MicroRNA-mediated downregulation of K+ channels in pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L10–L26. [Google Scholar] [CrossRef]
- Kirichok, Y.; Krapivinsky, G.; Clapham, D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 2004, 427, 360–364. [Google Scholar] [CrossRef]
- Conte, E. Targeting monocytes/macrophages in fibrosis and cancer diseases: Therapeutic approaches. Pharmacol. Ther. 2022, 234, 108031. [Google Scholar] [CrossRef]
- Wang, R.-R.; Yuan, T.Y.; Wang, J.M.; Chen, Y.C.; Zhao, J.L.; Li, M.T.; Fang, L.H.; Du, G.H. Immunity and inflammation in pulmonary arterial hypertension: From pathophysiology mechanisms to treatment perspective. Pharmacol. Res. 2022, 180, 106238. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Esmerats, J.; Villa-Roel, N.; Kumar, S.; Gu, L.; Salim, M.T.; Ohh, M.; Taylor, W.R.; Nerem, R.M.; Yoganathan, A.P.; Jo, H. Disturbed Flow Increases UBE2C (Ubiquitin E2 Ligase C) via Loss of miR-483-3p, Inducing Aortic Valve Calcification by the pVHL (von Hippel-Lindau Protein) and HIF-1α (Hypoxia-Inducible Factor-1α) Pathway in Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 467–481. [Google Scholar] [CrossRef]
- Carrella, S.; Di Guida, M.; Brillante, S.; Piccolo, D.; Ciampi, L.; Guadagnino, I.; Garcia Piqueras, J.; Pizzo, M.; Marrocco, E.; Molinari, M.; et al. miR-181a/b downregulation: A mutation-independent therapeutic approach for inherited retinal diseases. EMBO Mol. Med. 2022, 14, e15941. [Google Scholar] [CrossRef]
- Hu, W.; Yan, F.; Ru, Y.; Xia, M.; Yan, G.; Zhang, M.; Wang, H.; Wu, G.; Yao, L.; Shen, L.; et al. MIIP inhibits EMT and cell invasion in prostate cancer through miR-181a/b-5p-KLF17 axis. Am. J. Cancer Res. 2020, 10, 630–647. [Google Scholar] [PubMed]
- Kasprzak, A. Prognostic Biomarkers of Cell Proliferation in Colorectal Cancer (CRC): From Immunohistochemistry to Molecular Biology Techniques. Cancers 2023, 15, 4570. [Google Scholar] [CrossRef]
- Jiang, K.; Guo, S.; Zhang, T.; Yang, Y.; Zhao, G.; Shaukat, A.; Wu, H.; Deng, G. Downregulation of TLR4 by miR-181a Provides Negative Feedback Regulation to Lipopolysaccharide-Induced Inflammation. Front. Pharmacol. 2018, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, H.; Li, K.; Wu, H.; Zhan, X.; Fang, F.; Qin, Y.; Wei, Y. ESM-1 promotes adhesion between monocytes and endothelial cells under intermittent hypoxia. J. Cell Physiol. 2019, 234, 1512–1521. [Google Scholar] [CrossRef]
- Yang, L.; Li, H.; Huang, A. MiR-429 and MiR-143-3p Function as Diagnostic and Prognostic Markers for Osteosarcoma. Clin. Lab. 2020, 66, 1954. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Hao, X. Swainsonine protects H9c2 cells against lipopolysaccharide-induced apoptosis and inflammatory injury via down-regulating miR-429. Cell Cycle 2020, 19, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qin, F.; Xue, M.; Lei, Y.; Hu, F.; Xu, H.; Sun, G.; Wang, T.; Guo, M. miR-429 and miR-424-5p inhibit cell proliferation and Ca2+ influx by downregulating CaSR in pulmonary artery smooth muscle cells. Am. J. Physiol. Cell Physiol. 2019, 316, C111–C120. [Google Scholar] [CrossRef]
- Li, Y.; Sun, C.; Tan, Y.; Zhang, H.; Li, Y.; Zou, H. ITGB1 enhances the Radioresistance of human Non-small Cell Lung Cancer Cells by modulating the DNA damage response and YAP1-induced Epithelial-mesenchymal Transition. Int. J. Biol. Sci. 2021, 17, 635–650. [Google Scholar] [CrossRef]
- Nowak, M.; Górczyńska, J.; Kołodzińska, K.; Rubin, J.; Choromańska, A. Extracellular Vesicles as Drug Transporters. Int. J. Mol. Sci. 2023, 24, 10267. [Google Scholar] [CrossRef]
- Russomanno, G.; Jo, K.B.; Abdul-Salam, V.B.; Morgan, C.; Endruschat, J.; Schaeper, U.; Osman, A.H.; Alzaydi, M.M.; Wilkins, M.R.; Wojciak-Stothard, B. miR-150-PTPMT1-cardiolipin signaling in pulmonary arterial hypertension. Mol. Ther. Nucleic Acids 2021, 23, 142–153. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Xi, X.; Zhu, G.; Wang, S.; Liu, Y.; Song, M. MicroRNA-15a-5p induces pulmonary artery smooth muscle cell apoptosis in a pulmonary arterial hypertension model via the VEGF/p38/MMP-2 signaling pathway. Int. J. Mol. Med. 2020, 45, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yu, Y.; Mo, L.; Chen, Q.; Dong, H.; Xu, Y.; Zhuan, B. Exosomal miR-663b from “M1” macrophages promotes pulmonary artery vascular smooth muscle cell dysfunction through inhibiting the AMPK/Sirt1 axis. Aging 2023, 15, 3549–3571. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Soc. Cardiol. 2021, 42, 3599–3726. [Google Scholar]
- Wen, S.; Pislaru, C.; Ommen, S.R.; Ackerman, M.J.; Pislaru, S.V.; Geske, J.B. Right Ventricular Enlargement and Dysfunction Are Associated With Increased All-Cause Mortality in Hypertrophic Cardiomyopathy. Mayo Clin. Proc. 2022, 97, 1123–1133. [Google Scholar] [CrossRef]
- Santens, B.; Van De Bruaene, A.; De Meester, P.; D’Alto, M.; Reddy, S.; Bernstein, D.; Koestenberger, M.; Hansmann, G.; Budts, W. Diagnosis and treatment of right ventricular dysfunction in congenital heart disease. Cardiovasc. Diagn. Ther. 2020, 10, 1625–1645. [Google Scholar] [CrossRef]
- Guo, H.M.; Liu, Z.P. Up-regulation of circRNA_0068481 promotes right ventricular hypertrophy in PAH patients via regulating miR-646/miR-570/miR-885. J. Cell Mol. Med. 2021, 25, 3735–3743. [Google Scholar] [CrossRef]
- Kong, H.; Song, Q.; Hu, W.; Guo, S.; Xiang, D.; Huang, S.; Xu, X.; He, J.; Pan, L.; Tao, R.; et al. MicroRNA-29a-3p prevents Schistosoma japonicum-induced liver fibrosis by targeting Roundabout homolog 1 in hepatic stellate cells. Parasit. Vectors 2023, 16, 184. [Google Scholar] [CrossRef]
- Shi, S.; Song, L.; Yu, H.; Feng, S.; He, J.; Liu, Y.; He, Y. Knockdown of LncRNA-H19 Ameliorates Kidney Fibrosis in Diabetic Mice by Suppressing miR-29a-Mediated EndMT. Front. Pharmacol. 2020, 11, 586895. [Google Scholar] [CrossRef]
- Calabro, N.E.; Barrett, A.; Chamorro-Jorganes, A.; Tam, S.; Kristofik, N.J.; Xing, H.; Loye, A.M.; Sessa, W.C.; Hansen, K.; Kyriakides, T.R. Thrombospondin-2 regulates extracellular matrix production, LOX levels, and cross-linking via downregulation of miR-29. Matrix Biol. 2019, 82, 71–85. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Wang, X.; Zhao, C.; Wang, F.; Du, J.; Zhang, H.; Shi, H.; Feng, Y.; Li, D.; et al. miR-335-5p suppresses gastric cancer progression by targeting MAPK10. Cancer Cell Int. 2021, 21, 71. [Google Scholar] [CrossRef]
- Song, G.; Ma, Y.; Ma, Y.; Liu, P.; Hou, L.; Xu, Z.; Jiang, J.; Shen, Y.; Cao, Y.; Zhao, Y. miR-335-5p Targets SDC1 to Regulate the Progression of Breast Cancer. Crit. Rev. Eukaryot. Gene Expr. 2022, 32, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.Y.; Song, Y.; Wang, A.N. MiR-335-5p inhibits proliferation of Huh-7 liver cancer cells via targeting the Oct4/Akt pathway. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1853–1860. [Google Scholar]
- Deng, H.; Cui, M.; Liu, L.; Yang, F. Circ-Marc2 Silencing Protects Human Cardiomyocytes from Hypoxia/Reoxygenation-Induced Injury by Modulating Mir-335-5p/Trpm7 Axis. Shock 2024, 61, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Surina, S.; Fontanella, R.A.; Scisciola, L.; Marfella, R.; Paolisso, G.; Barbieri, M. miR-21 in Human Cardiomyopathies. Front. Cardiovasc. Med. 2021, 8, 767064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, B.; Xu, Y.; Zhou, N.; Zhang, R.; Lu, L.; Feng, Z. MiR-208b/miR-21 Promotes the Progression of Cardiac Fibrosis Through the Activation of the TGF-β1/Smad-3 Signaling Pathway: An in vitro and in vivo Study. Front. Cardiovasc. Med. 2022, 9, 924629. [Google Scholar] [CrossRef]
- Chang, W.-T.; Fisch, S.; Dangwal, S.; Mohebali, J.; Fiedler, A.G.; Chen, M.; Hsu, C.H.; Yang, Y.; Qiu, Y.; Alexander, K.M.; et al. MicroRNA-21 regulates right ventricular remodeling secondary to pulmonary arterial pressure overload. J. Mol. Cell. Cardiol. 2021, 154, 106–114. [Google Scholar] [CrossRef]
- Chang, W.T.; Wu, C.C.; Lin, Y.W.; Shih, J.Y.; Chen, Z.C.; Wu, S.N.; Wu, C.C.; Hsu, C.H. Dynamic Changes in miR-21 Regulate Right Ventricular Dysfunction in Congenital Heart Disease-Related Pulmonary Arterial Hypertension. Cells 2022, 11, 564. [Google Scholar] [CrossRef]
- Powers, J.C.; Sabri, A.; Al-Bataineh, D.; Chotalia, D.; Guo, X.; Tsipenyuk, F.; Berretta, R.; Kavitha, P.; Gopi, H.; Houser, S.R.; et al. Differential microRNA-21 and microRNA-221 Upregulation in the Biventricular Failing Heart Reveals Distinct Stress Responses of Right Versus Left Ventricular Fibroblasts. Circ. Heart Fail. 2020, 13, e006426. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; Lu, W.; Xie, L.; Lv, J.; Li, H.; Yang, S. MiR-325-3p inhibits renal inflammation and fibrosis by targeting CCL19 in diabetic nephropathy. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1850–1860. [Google Scholar] [CrossRef]
- Wang, C.C.; Shang, B.B.; Yang, C.W.; Liu, Y.F.; Li, X.D.; Wang, S.Y. MicroRNA-325 alleviates myocardial fibrosis after myocardial infarction via downregulating GLI1. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5339–5346. [Google Scholar]
- Yamamoto, M.; Hanatani, S.; Araki, S.; Izumiya, Y.; Yamada, T.; Nakanishi, N.; Ishida, T.; Yamamura, S.; Kimura, Y.; Arima, Y.; et al. HE4 Predicts Progressive Fibrosis and Cardiovascular Events in Patients With Dilated Cardiomyopathy. J. Am. Heart Assoc. 2021, 10, e021069. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Song, J.; Du, H.; Lu, Y.; Dong, S.; Zhou, S.; Guo, Z.; Wu, H.; Zhao, X.; Qin, Y.; et al. MicroRNA-663 prevents monocrotaline-induced pulmonary arterial hypertension by targeting TGF-β1/smad2/3 signaling. J. Mol. Cell. Cardiol. 2021, 161, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Correale, M.; Tricarico, L.; Bevere, E.M.L.; Chirivì, F.; Croella, F.; Severino, P.; Mercurio, V.; Magrì, D.; Dini, F.; Licordari, R.; et al. Circulating Biomarkers in Pulmonary Arterial Hypertension: An Update. Biomolecules 2024, 14, 552. [Google Scholar] [CrossRef]
- Shen, Y.; Liao, D.; Shangguan, W.; Chen, L. Variation and significance of serum microRNA-21 level in pediatric pulmonary artery hypertension associated with congenital heart disease. Front. Cardiovasc. Med. 2024, 11, 1424679. [Google Scholar] [CrossRef]
- Yang, Y.; Li, R.; Cao, Y.; Dai, S.; Luo, S.; Guo, Q.; Wang, E. Plasma MIR-212-3p as a biomarker for acute right heart failure with pulmonary artery hypertension. Ann. Transl. Med. 2020, 8, 1571. [Google Scholar] [CrossRef] [PubMed]
- Ameri, A.; Ahmed, H.M.; Pecho, R.D.C.; Arabnozari, H.; Sarabadani, H.; Esbati, R.; Mirabdali, S.; Yazdani, O. Diverse activity of miR-150 in Tumor development: Shedding light on the potential mechanisms. Cancer Cell Int. 2023, 23, 261. [Google Scholar] [CrossRef]
- Gourishetti, K.; Balaji Easwaran, V.; Mostakim, Y.; Ranganath Pai, K.S.; Bhere, D. MicroRNA (miR)-124: A Promising Therapeutic Gateway for Oncology. Biology 2023, 12, 922. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, Q.; Pang, W.; Hou, L.; Liang, Y.; Han, X.; Luo, X.; Wang, P.; Zhang, X.; Li, L.; et al. YTHDC1-mediated augmentation of miR-30d in repressing pancreatic tumorigenesis via attenuation of RUNX1-induced transcriptional activation of Warburg effect. Cell Death Differ. 2021, 28, 3105–3124. [Google Scholar] [CrossRef]
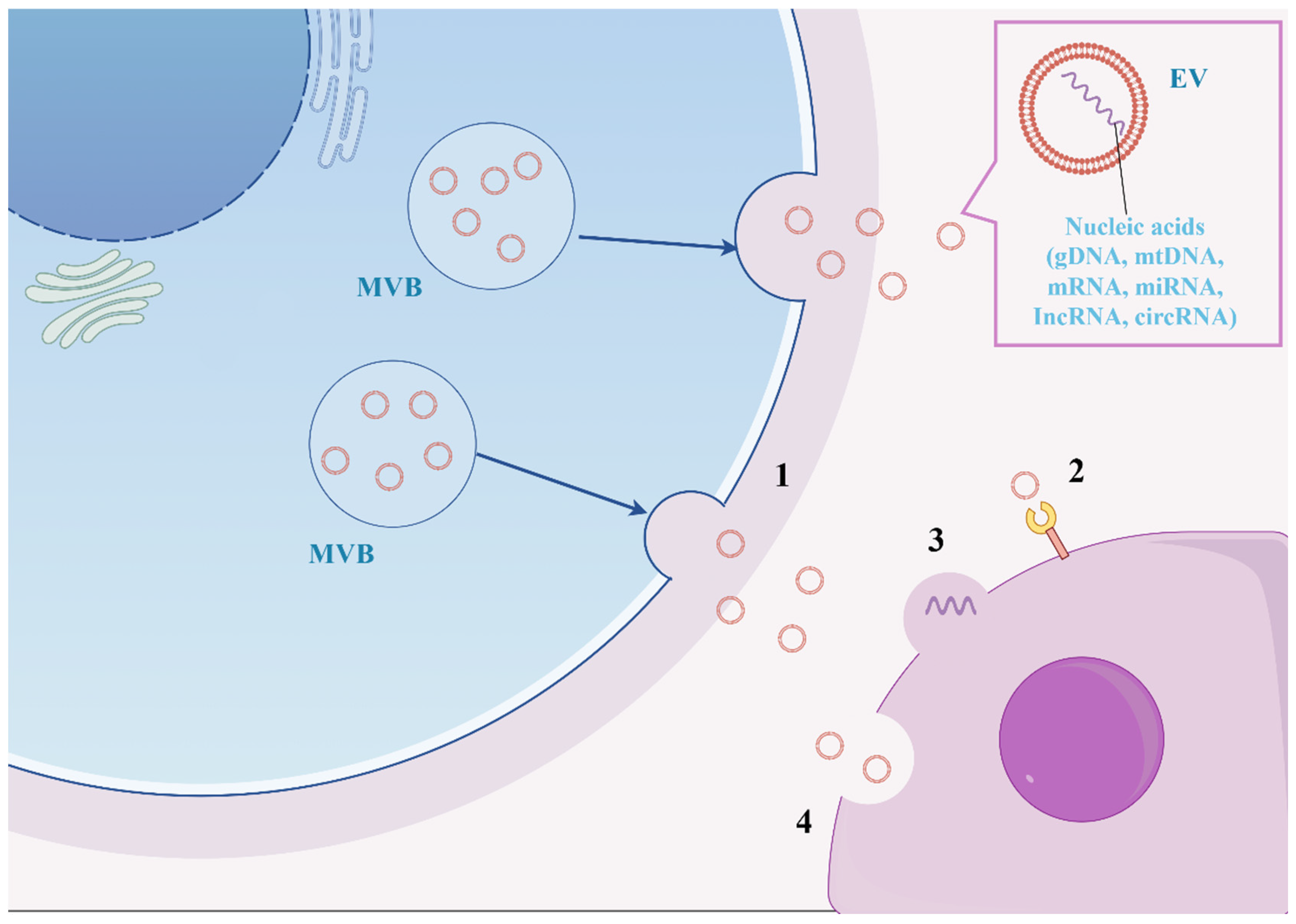
- Huang, C.; Neupane, Y.R.; Lim, X.C.; Shekhani, R.; Czarny, B.; Wacker, M.G.; Pastorin, G.; Wang, J.W. Extracellular vesicles in cardiovascular disease. Adv. Clin. Chem. 2021, 103, 47–95. [Google Scholar]
- Cai, J.; Wu, J.; Wang, J.; Li, Y.; Hu, X.; Luo, S.; Xiang, D. Extracellular vesicles derived from different sources of mesenchymal stem cells: Therapeutic effects and translational potential. Cell Biosci. 2020, 10, 69. [Google Scholar] [CrossRef]
- Mohan, A.; Agarwal, S.; Clauss, M.; Britt, N.S.; Dhillon, N.K. Extracellular vesicles: Novel communicators in lung diseases. Respir. Res. 2020, 21, 175. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Lu, C.; Liu, Y.; Luo, F.; Zhou, J.; Xu, F. Mesenchymal stem cell-derived extracellular vesicles prevent the formation of pulmonary arterial hypertension through a microRNA-200b-dependent mechanism. Respir. Res. 2023, 24, 233. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, E.; Neveu, B.; Kostantin, E.; Tsongalis, G.J.; De Guire, V. How close are miRNAs from clinical practice? A perspective on the diagnostic and therapeutic market. Ejifcc 2019, 30, 114–127. [Google Scholar] [PubMed]
- Haussecker, D. Current issues of RNAi therapeutics delivery and development. J. Control. Release 2014, 195, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Rogula, S.; Pomirski, B.; Czyżak, N.; Eyileten, C.; Postuła, M.; Szarpak, Ł.; Filipiak, K.J.; Kurzyna, M.; Jaguszewski, M.; Mazurek, T.; et al. Biomarker-based approach to determine etiology and severity of pulmonary hypertension: Focus on microRNA. Front. Cardiovasc. Med. 2022, 9, 980718. [Google Scholar] [CrossRef] [PubMed]
| miRNA | Target | Disease | Reference(s) |
|---|---|---|---|
| miR-30d | MTDH PDE5A | Pulmonary hypertension | [24] |
| miR-212-5p | Unknown | Pulmonary hypertension | [25] |
| miR-340-5p | MFF | Pulmonary hypertension | [26] |
| miR-153 | ROCK1 NFATc3 | Pulmonary hypertension | [27] |
| miR-27 | PPARγ | Pulmonary hypertension | [28,29,30] |
| miR-30a-5p | YKL-40 | Pulmonary hypertension | [31] |
| miR-410 | NAMPT | Pulmonary hypertension | [32] |
| miR-124 | PTBP1 | Pulmonary hypertension Idiopathic pulmonary arterial hypertension | [33,34] |
| miR-22-3p | C10:2 | Pulmonary hypertension | [35] |
| miR-329-3p | PDHB | Pulmonary hypertension | [36] |
| miR-125a-5p | HK-II | Pulmonary hypertension | [37] |
| miR-1 | Kv1.5 channel | Pulmonary hypertension | [38] |
| miR-29b, miR-138 and miR-222 | Kv2.1 channel | Pulmonary hypertension | [39] |
| miR-25 and miR-138 | MCU | Pulmonary hypertension | [40] |
| miR-483 | TGF-β TGFBR2 β-catenin CTGF IL-1β ET-1 | Pulmonary hypertension | [41] |
| miR-181a/b | Endocan | Pulmonary hypertension | [42] |
| miR-429-3p | Rac1 | Pulmonary hypertension | [43] |
| miR-29a-3p | THBS2 | Pulmonary hypertension | [44] |
| miR-335-5p | CALU | Pulmonary hypertension | [45] |
| miR-21 | FilGAP | Pulmonary hypertension | [46] |
| miR-325-3p | HE4 | Pulmonary hypertension | [47] |
| miRNA Drug Name | Targeted miRNA | Disease/Condition | Clinical Phase |
|---|---|---|---|
| INT-1B3 | miR-193a-3p | Advanced solid tumors | Phase I |
| RC.012/lademirsen/SAR339375 | miR-21 | Alport syndrome | Phase II |
| RGLS4326 | miR-17 | Autosomal dominant polycystic kidney disease | Phase I |
| RG-125/AZD4076 | miR-103/107 | T2DM with NAFLD | Phase I |
| Remlarsen/MRG201 | miR-29 | Keloid disorder | Phase II |
| Miravirsen/SPC3649 | miR-122 | Chronic hepatitis Cvirus | Phase II |
| MRX34 | miR-34a | Primary liver cancer, SCLC, lymphoma, multiple myeloma, renal cell carcinoma, NSCLC | Phase I |
| Cobomarsen/MRG-106 | miR-155 | Mycosis fungoides (MF), cutaneous T-cell lymphoma (CTCL), chronic lymphocytic leukemia (CLL), diffuse large B-cell lymphoma (DLBCL), ABC subtype adult T-cell leukemia/lymphoma (ATLL) | Phase II |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, J.; Chen, H.; Jia, Z.; Dai, J.; Ma, F. miRNAs in Pulmonary Hypertension: Mechanistic Insights and Therapeutic Potential. Biomedicines 2025, 13, 1910. https://doi.org/10.3390/biomedicines13081910
Fang J, Chen H, Jia Z, Dai J, Ma F. miRNAs in Pulmonary Hypertension: Mechanistic Insights and Therapeutic Potential. Biomedicines. 2025; 13(8):1910. https://doi.org/10.3390/biomedicines13081910
Chicago/Turabian StyleFang, Jindong, Hongyang Chen, Zhuangzhuang Jia, Jinjin Dai, and Fengli Ma. 2025. "miRNAs in Pulmonary Hypertension: Mechanistic Insights and Therapeutic Potential" Biomedicines 13, no. 8: 1910. https://doi.org/10.3390/biomedicines13081910
APA StyleFang, J., Chen, H., Jia, Z., Dai, J., & Ma, F. (2025). miRNAs in Pulmonary Hypertension: Mechanistic Insights and Therapeutic Potential. Biomedicines, 13(8), 1910. https://doi.org/10.3390/biomedicines13081910






