From Survival to Parenthood: The Fertility Journey After Childhood Cancer
Abstract
1. Introduction
2. Materials and Methods
- Discussed the gonadotoxic effects of cancer therapies in pediatric patients;
- Described or evaluated fertility preservation methods applicable to children and adolescents;
- Provided data or recommendations on post-treatment fertility surveillance and reproductive health outcomes.
3. Fertility Preservation
3.1. Pediatric Female Patients
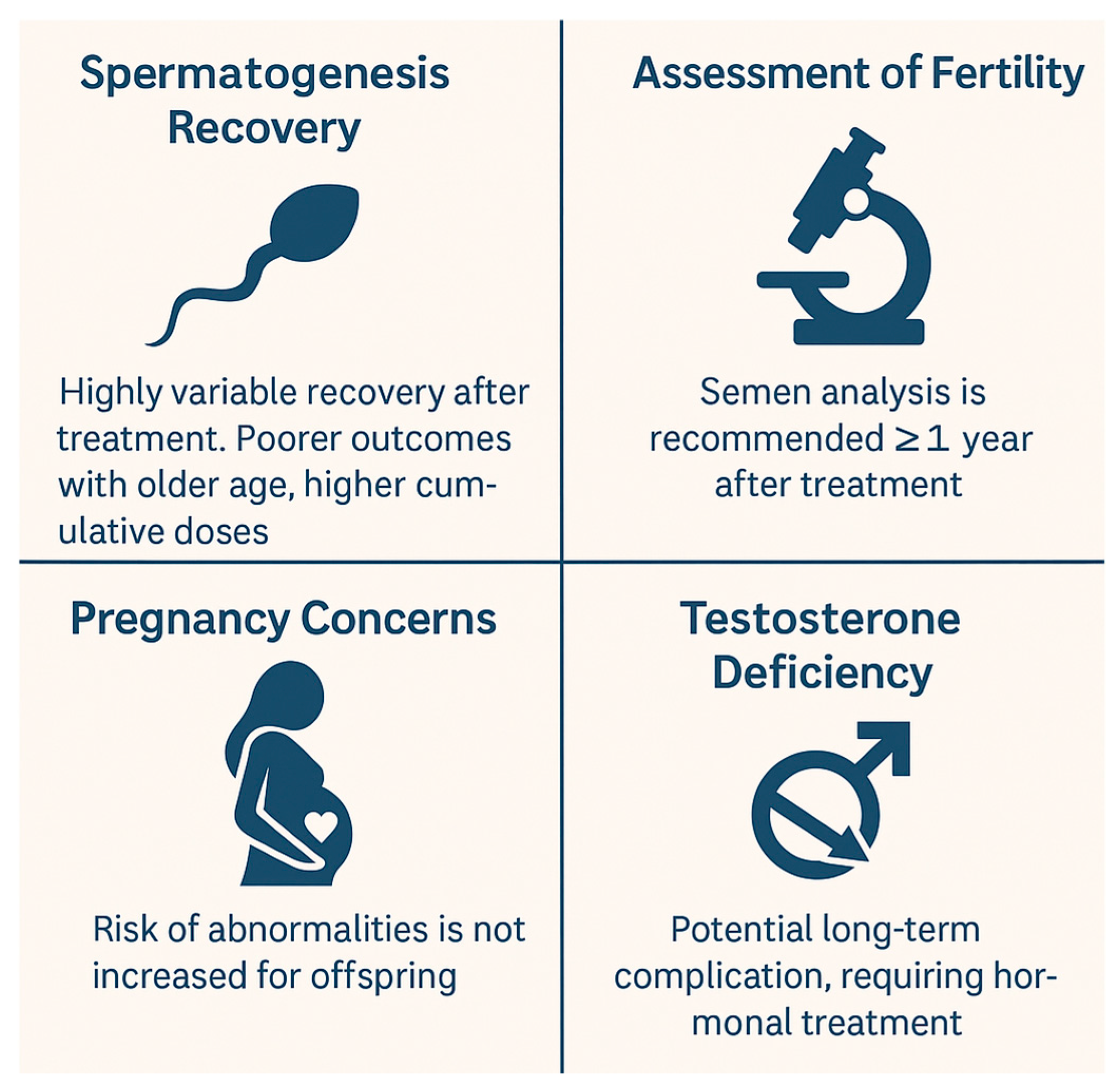
3.2. Pediatric Male Patients
4. Fertility After Cancer Treatment
4.1. Pediatric Female Patients
- -
- “Acute ovarian failure (AOF)” refers to the immediate and complete loss of ovarian function occurring during or shortly after cancer treatment. It is characterized by amenorrhea and elevated gonadotropins soon after therapy initiation.
- -
- “Premature ovarian insufficiency (POI)” is defined as the cessation of ovarian activity before the age of 40, with amenorrhea lasting at least four months and elevated follicle-stimulating hormone (FSH) levels on two separate occasions. Unlike AOF, ovarian activity in POI can be intermittent and may allow spontaneous ovulation and even pregnancy.
- -
- “Premature menopause” is often used interchangeably with POI in common language but technically denotes the permanent and irreversible cessation of ovarian function before age 40, leading to definitive infertility and hypoestrogenism. While all premature menopause is POI, not all POI necessarily progresses to premature menopause.
4.2. Pediatric Male Patients
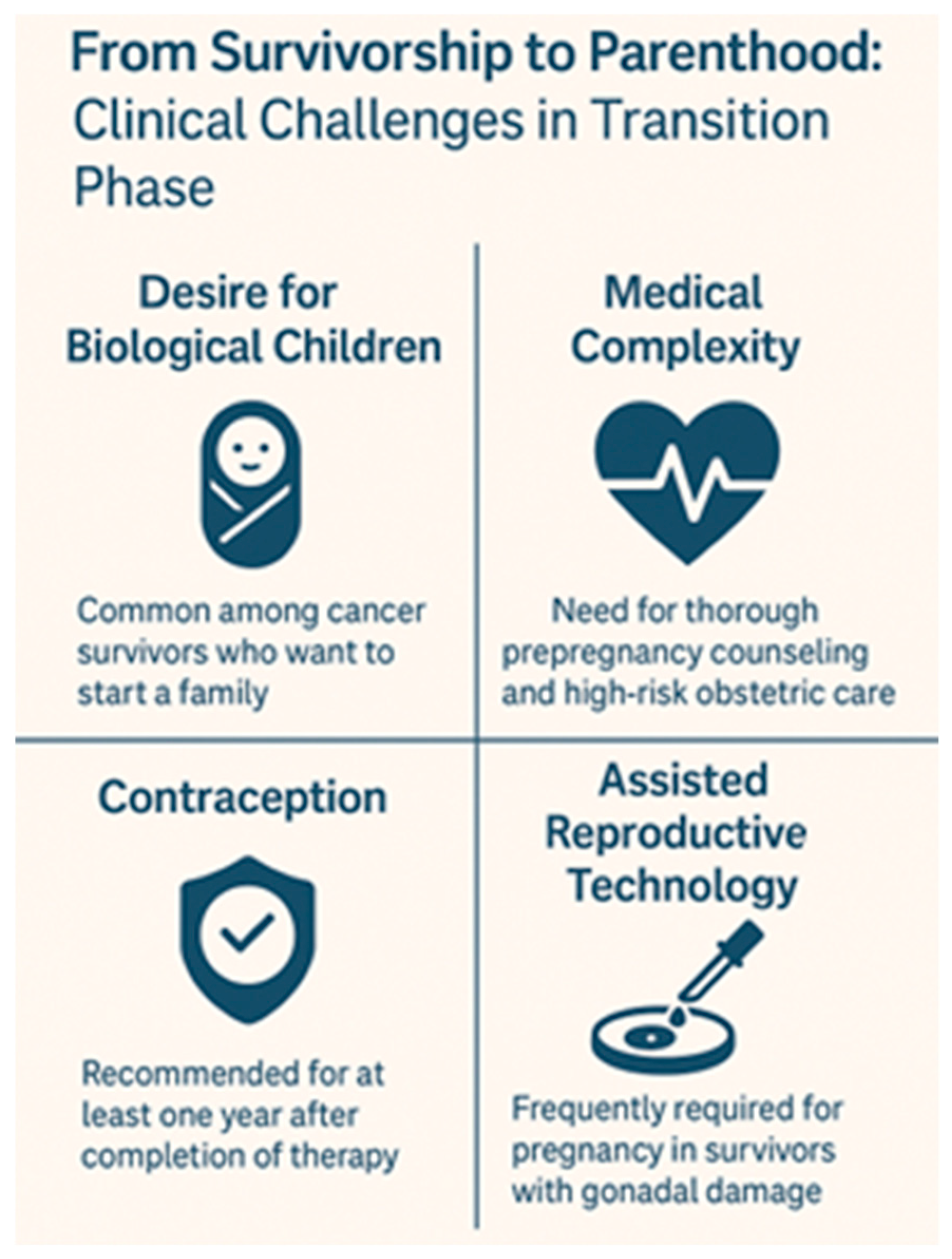
4.3. From Survivorship to Parenthood: Clinical Challenges in the Transition Phase
5. Psychosocial Impact of Fertility Loss in Childhood Cancer Survivors
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erdmann, F.; Frederiksen, L.E.; Bonaventure, A.; Mader, L.; Hasle, H.; Robison, L.L.; Winther, J.F. Childhood cancer: Survival, treatment modalities, late effects and improvements over time. Cancer Epidemiol. 2021, 71 Pt B, 101733. [Google Scholar] [CrossRef] [PubMed]
- Robison, L.L. The Childhood Cancer Survivor Study: A resource for research of long-term outcomes among adult survivors of childhood cancer. Minn Med. 2005, 88, 45–49. [Google Scholar] [PubMed]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Childhood Cancer Survivor Study. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.; Shalet, S. Gonadal damage from chemotherapy and radiotherapy. Endocrinol. Metab. Clin. N. Am. 1998, 27, 927–943. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.B.; Campbell, A.J.; Irvine, D.C.; Anderson, R.A.; Kelnar, C.J.; Wallace, W.H. Semen quality and spermatozoal DNA integrity in survivors of childhood cancer: A case-control study. Lancet 2002, 360, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Meirow, D.; Biederman, H.; Anderson, R.A.; Wallace, W.H. Toxicity of chemotherapy and radiation on female reproduction. Clin. Obs. Gynecol. 2010, 53, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Meistrich, M.L. Male gonadal toxicity. Pediatr. Blood Cancer 2009, 53, 261–266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Dorp, W.; van der Geest, I.M.; Laven, J.S.; Hop, W.C.; Neggers, S.J.; de Vries, A.C.; Pieters, R.; van den Heuvel-Eibrink, M.M. Gonadal function recovery in very long-term male survivors of childhood cancer. Eur. J. Cancer 2013, 49, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Levine, J. Gonadotoxicity of cancer therapies in pediatric and reproductive-age females. In Oncofertility Medical Practice: Clinical Issues and Implementation; Gracia, C., Woodruff, T.K., Eds.; Springer: New York, NY, USA, 2012; pp. 3–14. [Google Scholar] [CrossRef]
- Kort, J.D.; Eisenberg, M.L.; Millheiser, L.S.; Westphal, L.M. Fertility issues in cancer survivorship. CA Cancer J. Clin. 2014, 64, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Brungardt, J.G.; Burns, K.C.; Dasgupta, R. Fertility preservation in children and young adults with cancer. Curr. Opin. Pediatr. 2022, 34, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients with Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.; Ku, S.Y. Fertility preservation in pediatric and young adult female cancer patients. Ann. Pediatr. Endocrinol. Metab. 2018, 23, 70–74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Waimey, K.E.; Smith, B.M.; Confino, R.; Jeruss, J.S.; Pavone, M.E. Understanding Fertility in Young Female Cancer Patients. J. Womens Health 2015, 24, 812–818. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guida, L. Preservazione della Fertilità nei Pazienti Oncologici; Linee Guida; Associazione Italiana di Oncologia Medica (AIOM): Roma, Italy, 2020. [Google Scholar]
- Wheeler, S.B.; Roberts, M.C.; Bloom, D.; Reeder-Hayes, K.E.; Espada, M.; Peppercorn, J.; Golin, C.E.; Earp, J.A. Oncology providers’ perspectives on endocrine therapy prescribing and management. Patient Prefer. Adherence 2016, 10, 2007–2019. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El Alaoui-Lasmaili, K.; Nguyen-Thi, P.L.; Demogeot, N.; Lighezzolo-Alnot, J.; Gross, M.J.; Mansuy, L.; Chastagner, P.; Koscinski, I. Fertility discussions and concerns in childhood cancer survivors, a systematic review for updated practice. Cancer Med. 2023, 12, 6023–6039. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ginsberg, J.P. New advances in fertility preservation for pediatric cancer patients. Curr. Opin. Pediatr. 2011, 23, 9–13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suzuki, N. Clinical Practice Guidelines for Fertility Preservation in Pediatric, Adolescent, and Young Adults with Cancer. Int. J. Clin. Oncol. 2019, 24, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Resetkova, N.; Hayashi, M.; Kolp, L.A.; Christianson, M.S. Fertility Preservation for Prepubertal Girls: Update and Current Challenges. Curr. Obs. Gynecol. Rep. 2013, 2, 218–225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nelson, M.; Levine, J. Current Issues in Fertility Preservation Among Pediatric and Adolescent Cancer Patients. Curr. Oncol. Rep. 2023, 25, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.; Canada, A.; Stern, C.J. Fertility preservation in adolescents and young adults with cancer. J. Clin. Oncol. 2010, 28, 4831–4841. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, S.; Cervelló, I. New insights for fertility preservation by ovarian tissue cryopreservation and transplantation in pediatric cancer patients. Fertil. Steril. 2020, 114, 1191. [Google Scholar] [CrossRef] [PubMed]
- Chian, R.C.; Wang, Y.; Li, Y.R. Oocyte vitrification: Advances, progress and future goals. J. Assist. Reprod. Genet. 2014, 31, 411–420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Donnell, L.; Stanton, P.; de Kretser, D.M. Endocrinology of the Male Reproductive System and Spermatogenesis. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar] [PubMed]
- Romerius, P.; Ståhl, O.; Moëll, C.; Relander, T.; Cavallin-Ståhl, E.; Wiebe, T.; Giwercman, Y.L.; Giwercman, A. High risk of azoospermia in men treated for childhood cancer. Int. J. Androl. 2011, 34, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.L.; Choi, A.W.; Fitzgerald Keeter, M.K.; Brannigan, R.E. Male adolescent fertility preservation. Fertil. Steril. 2016, 105, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.H., IV. Fertility preservation in the male with cancer. Curr. Urol. Rep. 2013, 14, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Clouthier, D.E.; Avarbock, M.R.; Maika, S.D.; Hammer, R.E.; Brinster, R.L. Rat spermatogenesis in mouse testis. Nature 1996, 381, 418–421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pendergraft, S.S.; Sadri-Ardekani, H.; Atala, A.; Bishop, C.E. Three-dimensional testicular organoid: A novel tool for the study of human spermatogenesis and gonadotoxicity in vitro. Biol. Reprod. 2017, 96, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Fayomi, A.P.; Peters, K.; Sukhwani, M.; Valli-Pulaski, H.; Shetty, G.; Meistrich, M.L.; Houser, L.; Robertson, N.; Roberts, V.; Ramsey, C.; et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 2019, 363, 1314–1319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sung, Z.Y.; Liao, Y.Q.; Hou, J.H.; Lai, H.H.; Weng, S.M.; Jao, H.W.; Lu, B.J.; Chen, C.H. Advancements in fertility preservation strategies for pediatric male cancer patients: A review of cryopreservation and transplantation of immature testicular tissue. Reprod. Biol. Endocrinol. 2024, 22, 47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wistuba, J.; Luetjens, C.M.; Wesselmann, R.; Nieschlag, E.; Simoni, M.; Schlatt, S. Meiosis in autologous ectopic transplants of immature testicular tissue grafted to Callithrix jacchus. Biol. Reprod. 2006, 74, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Luetjens, C.M.; Stukenborg, J.B.; Nieschlag, E.; Simoni, M.; Wistuba, J. Complete spermatogenesis in orthotopic but not in ectopic transplants of autologously grafted marmoset testicular tissue. Endocrinology 2008, 149, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Jahnukainen, K.; Ehmcke, J.; Nurmio, M.; Schlatt, S. Autologous ectopic grafting of cryopreserved testicular tissue preserves the fertility of prepubescent monkeys that receive sterilizing cytotoxic therapy. Cancer Res. 2012, 72, 5174–5178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mieusset, R.; Bujan, L.; Mansat, A.; Pontonnier, F.; Grandjean, H. Hyperthermia and human spermatogenesis: Enhancement of the inhibitory effect obtained by ‘artificial cryptorchidism’. Int. J. Androl. 1987, 10, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Masliukaite, I.; Ntemou, E.; Feijen, E.A.M.; van de Wetering, M.; Meissner, A.; Soufan, A.T.; Repping, S.; Kremer, L.M.C.; Jahnukainen, K.; Goossens, E.; et al. Childhood cancer and hematological disorders negatively affect spermatogonial quantity at diagnosis: A retrospective study of a male fertility preservation cohort. Hum. Reprod. 2023, 38, 359–370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Long, C.J.; Ginsberg, J.P.; Kolon, T.F. Fertility Preservation in Children and Adolescents With Cancer. Urology 2016, 91, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Jahnukainen, K.; Hou, M.; Petersen, C.; Setchell, B.; Söder, O. Intratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemia. Cancer Res. 2001, 61, 706–710. [Google Scholar] [PubMed]
- Letourneau, J.M.; Ebbel, E.E.; Katz, P.P.; Katz, A.; Ai, W.Z.; Chien, A.J.; Melisko, M.E.; Cedars, M.I.; Rosen, M.P. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer 2012, 118, 1710–1717. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lobo, R.A. Potential options for preservation of fertility in women. N. Engl. J. Med. 2005, 353, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Webber, L.; Davies, M.C.; Anderson, R.A.; Braat, D.; Cartwright, B.; Cifkova, R.; Hogervorst, E.; Janse, F.; Liao, L.; Vlaisavljevic, V.; et al. ESHRE Guideline: Management of women with premature ovarian insufficiency. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Sklar, C.A.; Mertens, A.C.; Mitby, P.; Whitton, J.; Stovall, M.; Kasper, C.; Mulder, J.; Green, D.; Nicholson, H.S.; Yasui, Y.; et al. Premature menopause in survivors of childhood cancer: A report from the childhood cancer survivor study. J. Natl. Cancer Inst. 2006, 98, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Sklar, C. Maintenance of ovarian function and risk of premature menopause related to cancer treatment. J. Natl. Cancer Inst. Monogr. 2005, 2005, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.; Wallace, W.H. Impact of cancer treatment on uterine function. J. Natl. Cancer Inst. Monogr. 2005, 2005, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Wo, J.Y.; Viswanathan, A.N. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1304–1312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burke, P.J. Human oocyte radiosensitivity. Radiol. Technol. 2004, 75, 419–424. [Google Scholar] [PubMed]
- Adriaens, I.; Smitz, J.; Jacquet, P. The current knowledge on radiosensitivity of ovarian follicle development stages. Hum. Reprod. Update 2009, 15, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Jaroudi, S.; Kakourou, G.; Cawood, S.; Doshi, A.; Ranieri, D.M.; Serhal, P.; Harper, J.C.; SenGupta, S.B. Expression profiling of DNA repair genes in human oocytes and blastocysts using microarrays. Hum. Reprod. 2009, 24, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Stillman, R.J.; Schinfeld, J.S.; Schiff, I.; Gelber, R.D.; Greenberger, J.; Larson, M.; Jaffe, N.; Li, F.P. Ovarian failure in long-term survivors of childhood malignancy. Am. J. Obs. Gynecol. 1981, 139, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Mitchell, R.T.; Kelsey, T.W.; Spears, N.; Telfer, E.E.; Wallace, W.H. Cancer treatment and gonadal function: Experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol. 2015, 3, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Sudour, H.; Chastagner, P.; Claude, L.; Desandes, E.; Klein, M.; Carrie, C.; Bernier, V. Fertility and pregnancy outcome after abdominal irradiation that included or excluded the pelvis in childhood tumor survivors. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Knopman, J.M.; Papadopoulos, E.B.; Grifo, J.A.; Fino, M.E.; Noyes, N. Surviving childhood and reproductive-age malignancy: Effects on fertility and future parenthood. Lancet Oncol. 2010, 11, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Barton, S.E.; Najita, J.S.; Ginsburg, E.S.; Leisenring, W.M.; Stovall, M.; Weathers, R.E.; Sklar, C.A.; Robison, L.L.; Diller, L. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2013, 14, 873–881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wallace, W.H.; Anderson, R.A.; Irvine, D.S. Fertility preservation for young patients with cancer: Who is at risk and what can be offered? Lancet Oncol. 2005, 6, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Green, D.M.; Sklar, C.A.; Boice, J.D., Jr.; Mulvihill, J.J.; Whitton, J.A.; Stovall, M.; Yasui, Y. Ovarian failure and reproductive outcomes after childhood cancer treatment: Results from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2009, 27, 2374–2381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Byrne, J.; Fears, T.R.; Gail, M.H.; Pee, D.; Connelly, R.R.; Austin, D.F.; Holmes, G.F.; Holmes, F.F.; Latourette, H.B.; Meigs, J.W.; et al. Early menopause in long-term survivors of cancer during adolescence. Am. J. Obs. Gynecol. 1992, 166, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, A.M.; Marrett, L.D.; Darlington, G. Early menopause and infertility in females after treatment for childhood cancer diagnosed in 1964–1988 in Ontario, Canada. Am. J. Epidemiol. 1999, 150, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Stern, C.; Hickey, M.; Goldblatt, F.; Anazodo, A.; Stevenson, W.S.; Phillips, K.A. Preventing ovarian failure associated with chemotherapy. Med. J. Aust. 2018, 209, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Chemaitilly, W.; Mertens, A.C.; Mitby, P.; Whitton, J.; Stovall, M.; Yasui, Y.; Robison, L.L.; Sklar, C.A. Acute ovarian failure in the childhood cancer survivor study. J. Clin. Endocrinol. Metab. 2006, 91, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- Bjornard, K.; Close, A.; Burns, K.; Chavez, J.; Chow, E.J.; Meacham, L.R. Fertility preservation in pediatric solid tumors: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2024, 71, e30960. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Imai, A.; Ichigo, S.; Matsunami, K.; Takagi, H.; Kawabata, I. Ovarian function following targeted anti-angiogenic therapy with bevacizumab. Mol. Clin. Oncol. 2017, 6, 807–810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jones, W.C.; Parry, C.; Devine, S.; Main, D.S.; Okuyama, S. Understanding distress in posttreatment adult leukemia and lymphoma survivors: A lifespan perspective. J. Psychosoc. Oncol. 2015, 33, 142–162. [Google Scholar] [CrossRef] [PubMed]
- Geue, K.; Sender, A.; Schmidt, R.; Richter, D.; Hinz, A.; Schulte, T.; Brähler, E.; Stöbel-Richter, Y. Gender-specific quality of life after cancer in young adulthood: A comparison with the general population. Qual. Life Res. 2014, 23, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, K.; Cymbaluk-Płoska, A. Fertility Preservation and Long-Term Monitoring of Gonadotoxicity in Girls, Adolescents and Young Adults Undergoing Cancer Treatment. Cancers 2021, 13, 202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quinn, G.P.; Knapp, C.; Murphy, D.; Sawczyn, K.; Sender, L. Congruence of reproductive concerns among adolescents with cancer and parents: Pilot testing an adapted instrument. Pediatrics 2012, 129, e930–e936. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lehmann, V.; Grönqvist, H.; Engvall, G.; Ander, M.; Tuinman, M.A.; Hagedoorn, M.; Sanderman, R.; Mattsson, E.; von Essen, L. Negative and positive consequences of adolescent cancer 10 years after diagnosis: An interview-based longitudinal study in Sweden. Psychooncology 2014, 23, 1229–1235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kenney, L.B.; Cohen, L.E.; Shnorhavorian, M.; Metzger, M.L.; Lockart, B.; Hijiya, N.; Duffey-Lind, E.; Constine, L.; Green, D.; Meacham, L. Male reproductive health after childhood, adolescent, and young adult cancers: A report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 3408–3416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, C.; Crowne, E. The impact of childhood cancer and its treatment on puberty and subsequent hypothalamic pituitary and gonadal function, in both boys and girls. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101291. [Google Scholar] [CrossRef] [PubMed]
- Skinner, R.; Mulder, R.L.; Kremer, L.C.; Hudson, M.M.; Constine, L.S.; Bardi, E.; Boekhout, A.; Borgmann-Staudt, A.; Brown, M.C.; Cohn, R.; et al. Recommendations for gonadotoxicity surveillance in male childhood, adolescent, and young adult cancer survivors: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Lancet Oncol. 2017, 18, e75–e90, Erratum in Lancet Oncol. 2017, 18, e196. https://doi.org/10.1016/S1470-2045(17)30204-8. [Google Scholar] [CrossRef] [PubMed]
- van Santen, H.M.; van de Wetering, M.D.; Bos, A.M.E.; Vd Heuvel-Eibrink, M.M.; van der Pal, H.J.; Wallace, W.H. Reproductive Complications in Childhood Cancer Survivors. Pediatr. Clin. N. Am. 2020, 67, 1187–1202. [Google Scholar] [CrossRef] [PubMed]
- Santaballa, A.; Márquez-Vega, C.; Rodríguez-Lescure, Á.; Rovirosa, Á.; Vázquez, L.; Zeberio-Etxetxipia, I.; Andrés, M.; Bassas, L.; Ceballos-Garcia, E.; Domingo, J.; et al. Multidisciplinary consensus on the criteria for fertility preservation in cancer patients. Clin. Transl. Oncol. 2022, 24, 227–243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chemes, H.E. Infancy is not a quiescent period of testicular development. Int. J. Androl. 2001, 24, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Delessard, M.; Saulnier, J.; Rives, A.; Dumont, L.; Rondanino, C.; Rives, N. Exposure to Chemotherapy During Childhood or Adulthood and Consequences on Spermatogenesis and Male Fertility. Int. J. Mol. Sci. 2020, 21, 1454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Himpe, J.; Lammerant, S.; Van den Bergh, L.; Lapeire, L.; De Roo, C. The Impact of Systemic Oncological Treatments on the Fertility of Adolescents and Young Adults-A Systematic Review. Life 2023, 13, 1209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marmor, D.; Duyck, F. Male reproductive potential after MOPP therapy for Hodgkin’s disease: A long-term survey. Andrologia 1995, 27, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Pedrick, T.J.; Hoppe, R.T. Recovery of spermatogenesis following pelvic irradiation for Hodgkin’s disease. Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Chemaitilly, W.; Liu, Q.; van Iersel, L.; Ness, K.K.; Li, Z.; Wilson, C.L.; Brinkman, T.M.; Klosky, J.L.; Barnes, N.; Clark, K.L.; et al. Leydig Cell Function in Male Survivors of Childhood Cancer: A Report From the St Jude Lifetime Cohort Study. J. Clin. Oncol. 2019, 37, 3018–3031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gilleland Marchak, J.; Seidel, K.D.; Mertens, A.C.; Ritenour, C.W.M.; Wasilewski-Masker, K.; Leisenring, W.M.; Sklar, C.A.; Ford, J.S.; Krull, K.R.; Stovall, M.; et al. Perceptions of risk of infertility among male survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2018, 124, 2447–2455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Madanat, L.M.; Malila, N.; Dyba, T.; Hakulinen, T.; Sankila, R.; Boice, J.D., Jr.; Lähteenmäki, P.M. Probability of parenthood after early onset cancer: A population-based study. Int. J. Cancer 2008, 123, 2891–2898. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Claessens, J.J.M.; Penson, A.; Bronkhorst, E.M.; Kremer, L.C.M.; van Dulmen-den Broeder, E.; van der Heiden-van der Loo, M.; Tissing, W.J.E.; van der Pal, H.J.H.; Blijlevens, N.M.A.; van den Heuvel-Eibrink, M.M.; et al. Desire for children among male survivors of childhood cancer: A DCCSS LATER study. Cancer 2023, 129, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Claessens, J.J.M.; Penson, A.; Bronkhorst, E.M.; Kremer, L.C.M.; van Dulmen-den Broeder, E.; van der Heiden-van der Loo, M.; Tissing, W.J.E.; van der Pal, H.J.H.; Blijlevens, N.M.A.; van den Heuvel-Eibrink, M.M.; et al. Reproductive outcomes and reproductive health care utilization among male survivors of childhood cancer: A DCCSS-LATER study. Cancer 2024, 130, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Torella, M.; Riemma, G.; De Franciscis, P.; La Verde, M.; Colacurci, N. Serum Anti-Müllerian Hormone Levels and Risk of Premature Ovarian Insufficiency in Female Childhood Cancer Survivors: Systematic Review and Network Meta-Analysis. Cancers 2021, 13, 6331. [Google Scholar] [CrossRef] [PubMed]
- Nyström, A.; Mörse, H.; Øra, I.; Henic, E.; Engellau, J.; Wieslander, E.; Tomaszewicz, A.; Elfving, M. Anti-Müllerian hormone and fertility in women after childhood cancer treatment: Association with current infertility risk classifications. PLoS ONE 2024, 19, e0308827. [Google Scholar] [CrossRef] [PubMed]




| Type of Therapy | Effects on Females | Effects on Males |
|---|---|---|
| Alkylating agents | High risk of premature ovarian insufficiency (POI) [57,60] | Risk of azoospermia or oligospermia [70] |
| Non-alkylating agents | Moderate or low gonadotoxicity (e.g., taxanes, anthracyclines) [59] | Generally reduced gonadotoxic effects (e.g., vincristine, methotrexate) [70] |
| Radiotherapy directed on the gonads | Direct ovarian damage, with POI risk even at low doses (>1 Gy) [43,46] | Azoospermia almost certain with doses >2–3 Gy [70] |
| Total Body Irradiation (TBI) | Nearly certain ovarian insufficiency with high doses (>4 Gy) [70] | Damage to germ cells and Leydig cells with exposure >12 Gy [70] |
| Stem Cell Transplantation | Very high impact on fertility, especially if associated with preparatory regimens based on high doses of cyclophosphamide or busulfan [61] | Severe impact on spermatogenesis and Leydig cell function [78] |
| Technique | Gender | Target Age | Current Status | Notes |
|---|---|---|---|---|
| Ovarian Tissue Cryopreservation | Female | Prepubertal and postpubertal | Experimental |
|
| Oocyte Cryopreservation | Female | Postpubertal | Standardized |
|
| Embryo Cryopreservation | Female | Postpubertal | Standardized |
|
| Testicular Tissue Cryopreservation | Male | Prepubertal | Experimental |
|
| Sperm Cryopreservation | Male | Postpubertal | Standardized |
|
| Domain | Unmet Needs | Research Priorities |
|---|---|---|
| Long-term efficacy of preservation | Limited data on live birth rates post cryopreservation; uncertainty about graft longevity | Prospective studies on ovarian tissue transplantation outcomes; monitoring of spermatogenic recovery post TTC |
| Psychosocial impact | Insufficient integration of psychosocial care into fertility pathways; gaps in counseling | Studies on survivor satisfaction and mental health after fertility preservation; development of tailored interventions |
| Impact of novel therapies | Sparse data on fertility effects of TKIs, CAR-T, immunotherapies | Preclinical and clinical studies to determine gonadotoxicity profiles; fertility monitoring guidelines for new drugs |
| Access and equity | Socioeconomic disparities in fertility care | Policies to improve funding and universal access to preservation services |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, S.; Sesenna, V.; Osorio Arce, D.; Maugeri, E.; Esposito, S. From Survival to Parenthood: The Fertility Journey After Childhood Cancer. Biomedicines 2025, 13, 1859. https://doi.org/10.3390/biomedicines13081859
Rahman S, Sesenna V, Osorio Arce D, Maugeri E, Esposito S. From Survival to Parenthood: The Fertility Journey After Childhood Cancer. Biomedicines. 2025; 13(8):1859. https://doi.org/10.3390/biomedicines13081859
Chicago/Turabian StyleRahman, Sofia, Veronica Sesenna, Diana Osorio Arce, Erika Maugeri, and Susanna Esposito. 2025. "From Survival to Parenthood: The Fertility Journey After Childhood Cancer" Biomedicines 13, no. 8: 1859. https://doi.org/10.3390/biomedicines13081859
APA StyleRahman, S., Sesenna, V., Osorio Arce, D., Maugeri, E., & Esposito, S. (2025). From Survival to Parenthood: The Fertility Journey After Childhood Cancer. Biomedicines, 13(8), 1859. https://doi.org/10.3390/biomedicines13081859







