New Neonatal and Prenatal Approach to Home Therapy with Amoxicillin, Rifaximin, and Anti-Inflammatory Drugs for Pregnant Women with COVID-19 Infections—Monitoring of Fetal Growth as a Prognostic Factor: A Triple Case Series (N.A.T.H.A.N.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Mother Not Vaccinated Against COVID-19
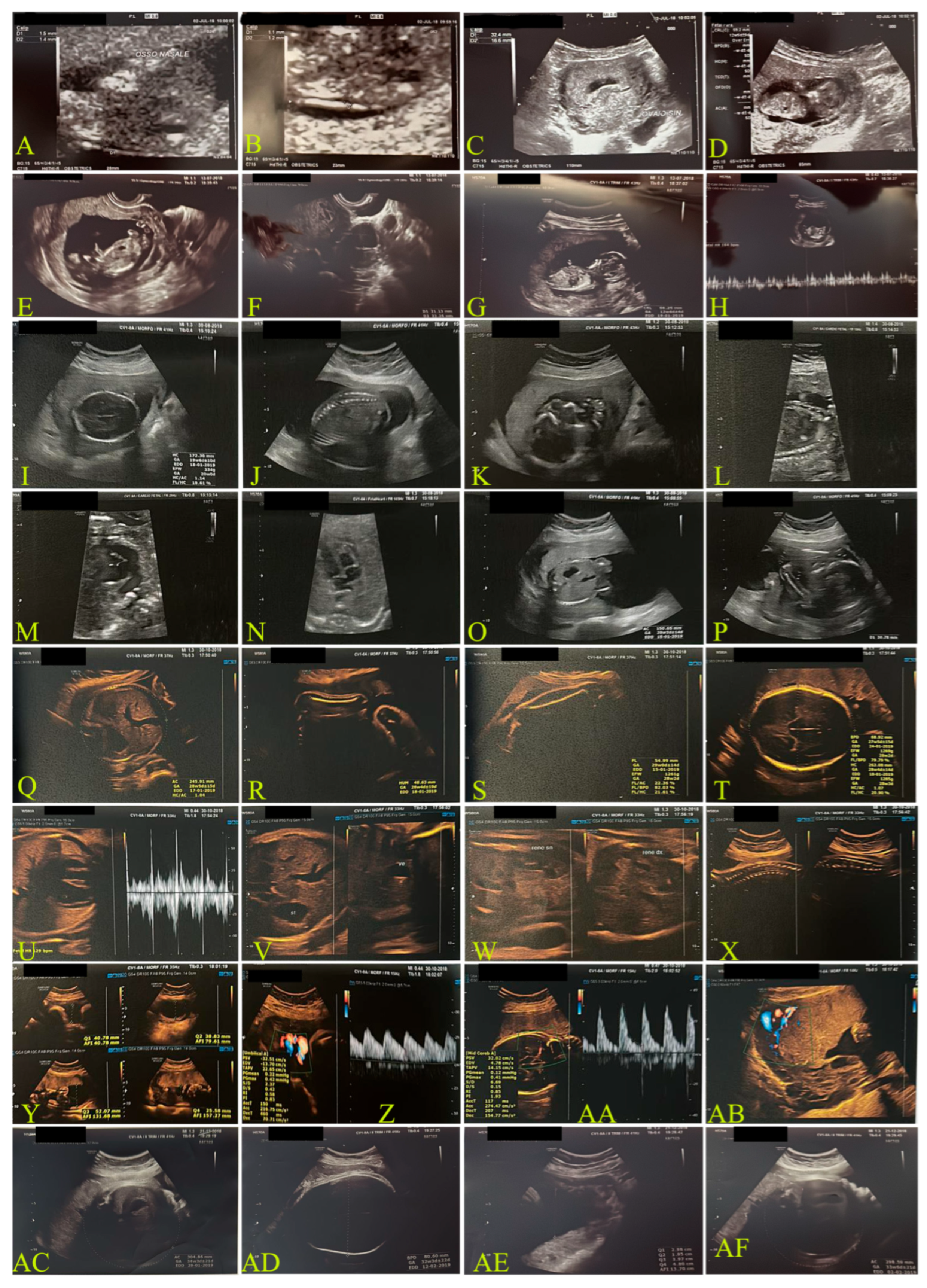
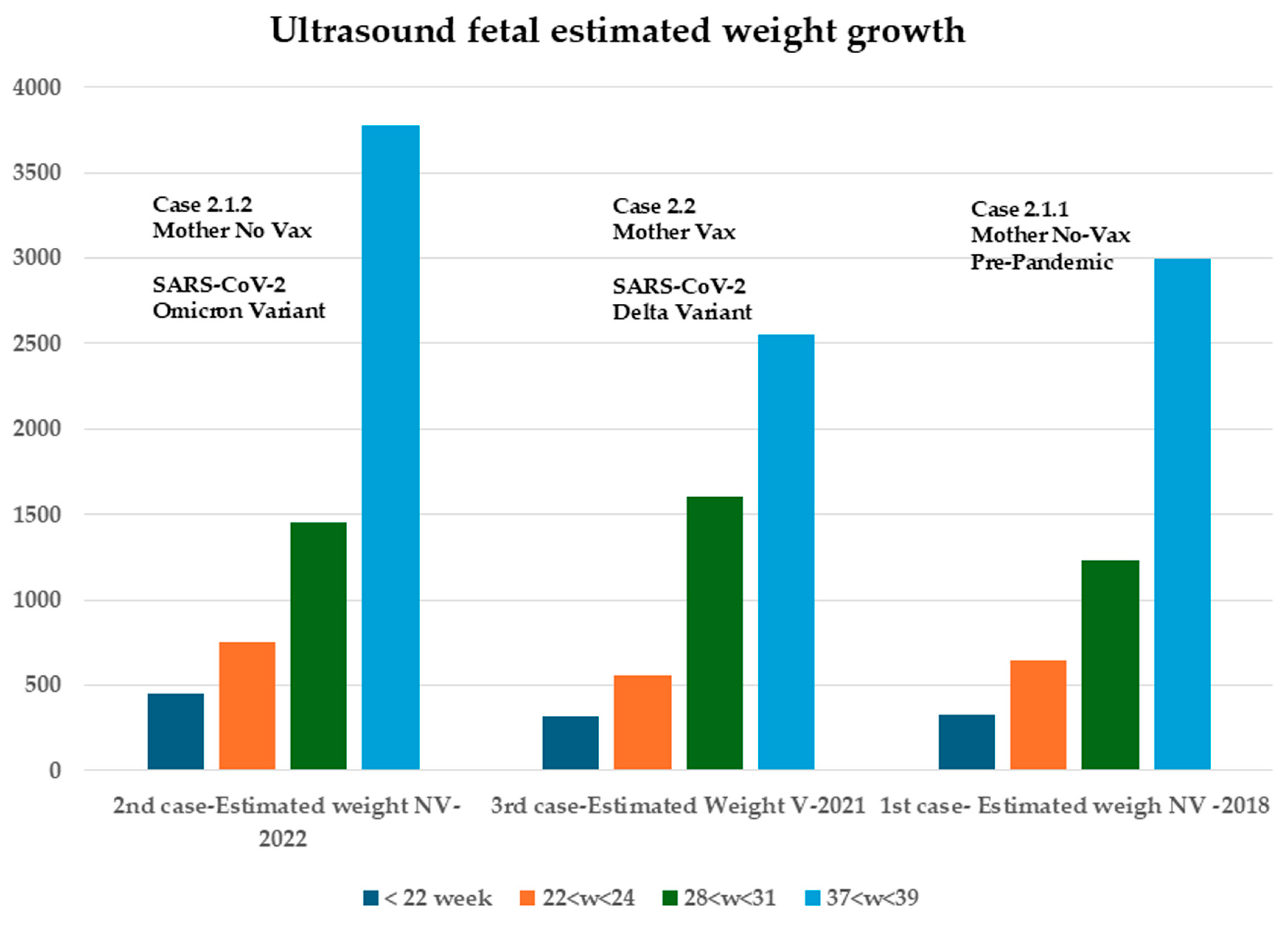
2.1.1. First Case: Pre-Pandemic COVID-19, 2018–2019 (Figure 1)
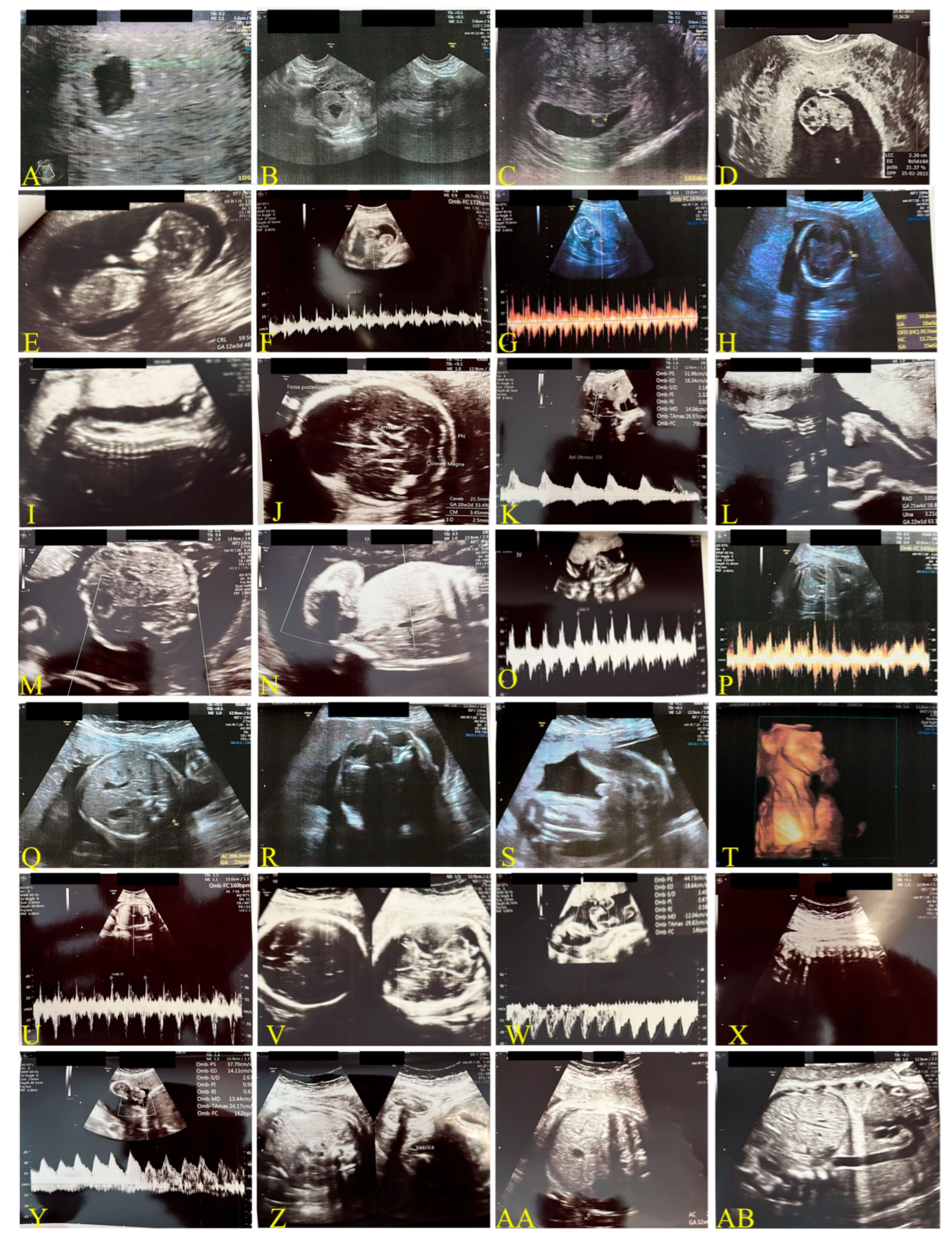
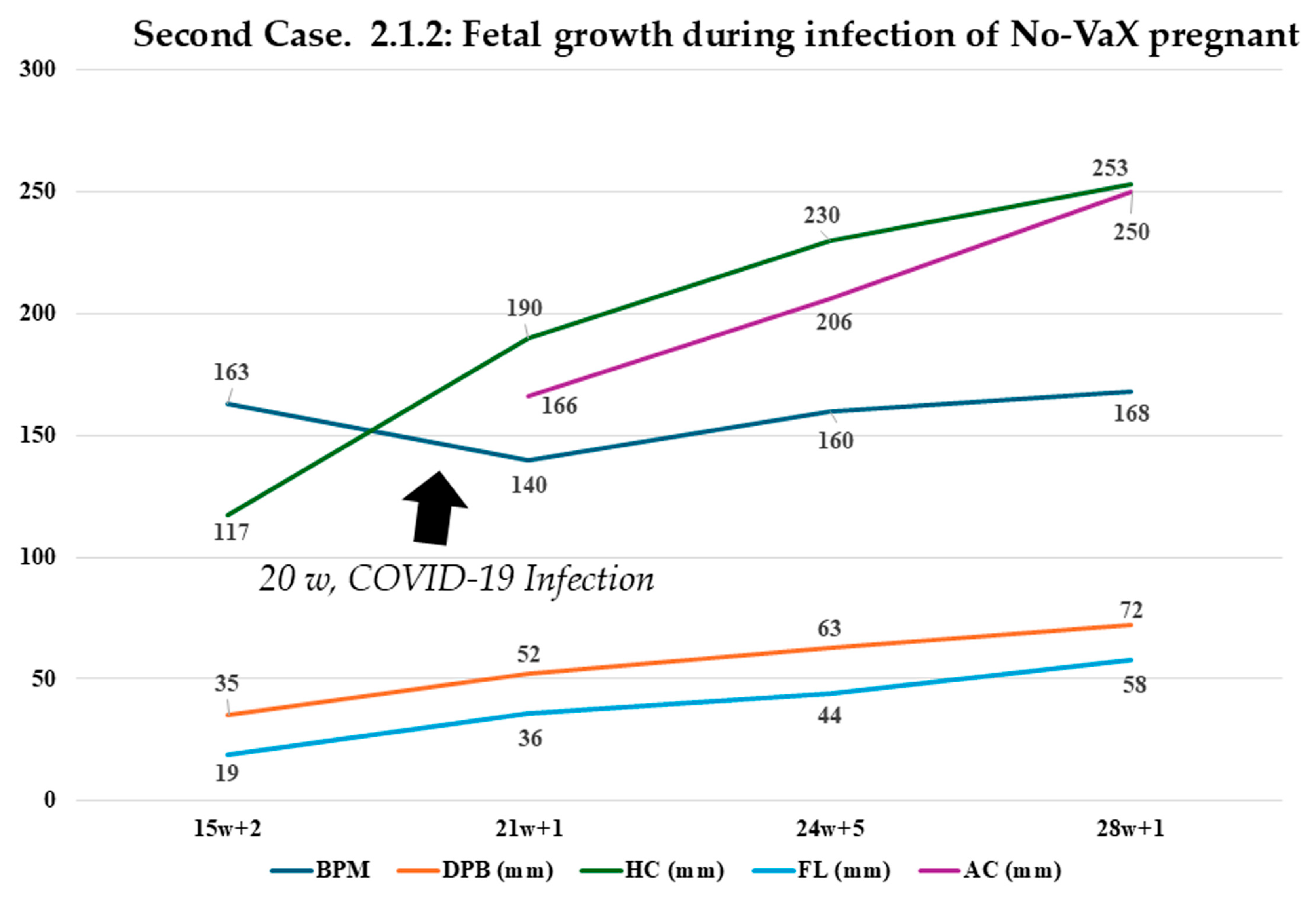
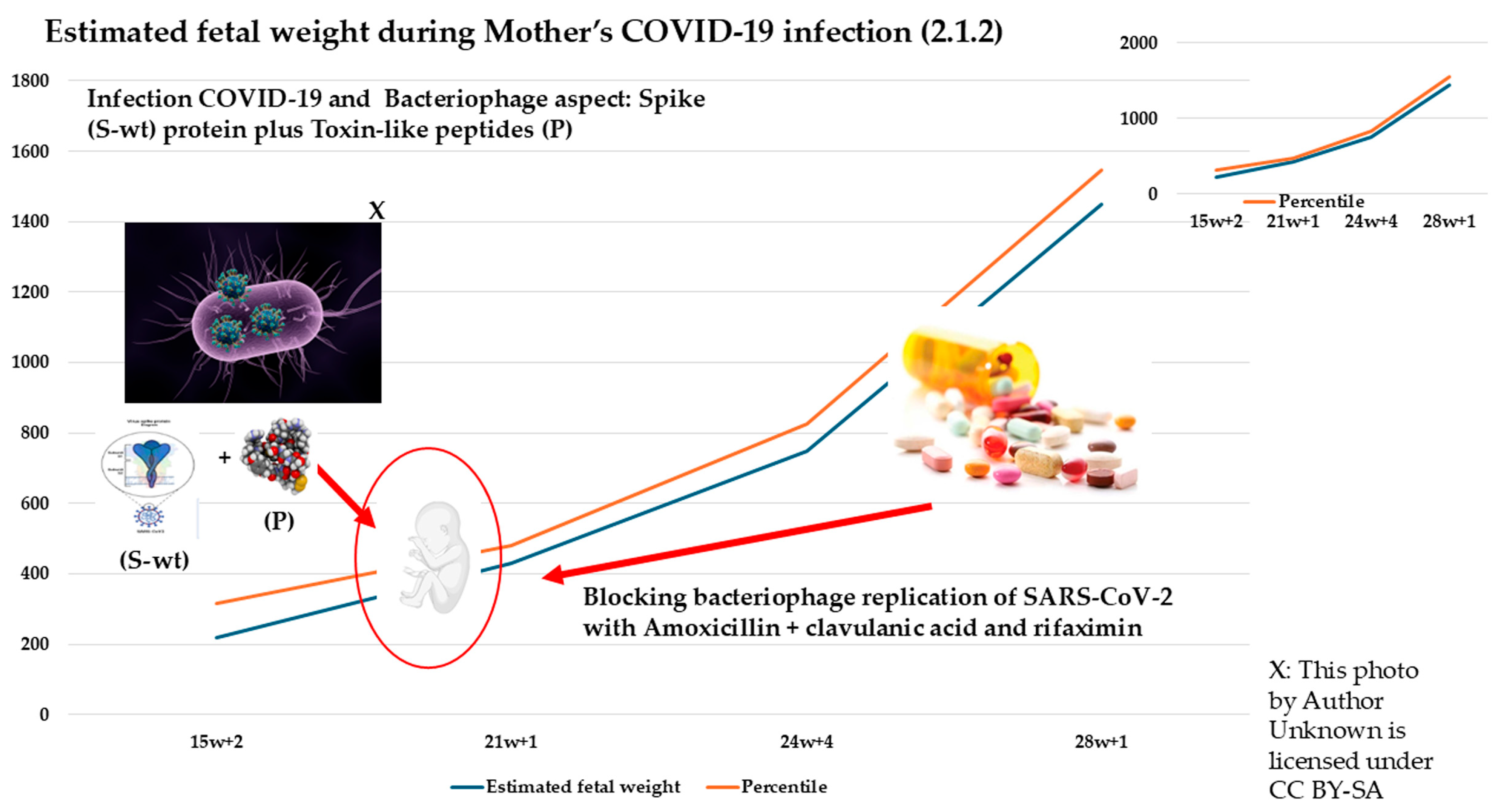
2.1.2. Second Case: COVID-19 Pandemic, 2022–2023, Omicron Variant, Same Mother as the Above-Mentioned Case (Figure 2)
2.2. Mother Vaccinated Against COVID-19
Third Case: Pandemic Period, December 2021, Delta Variant (Figure 3)
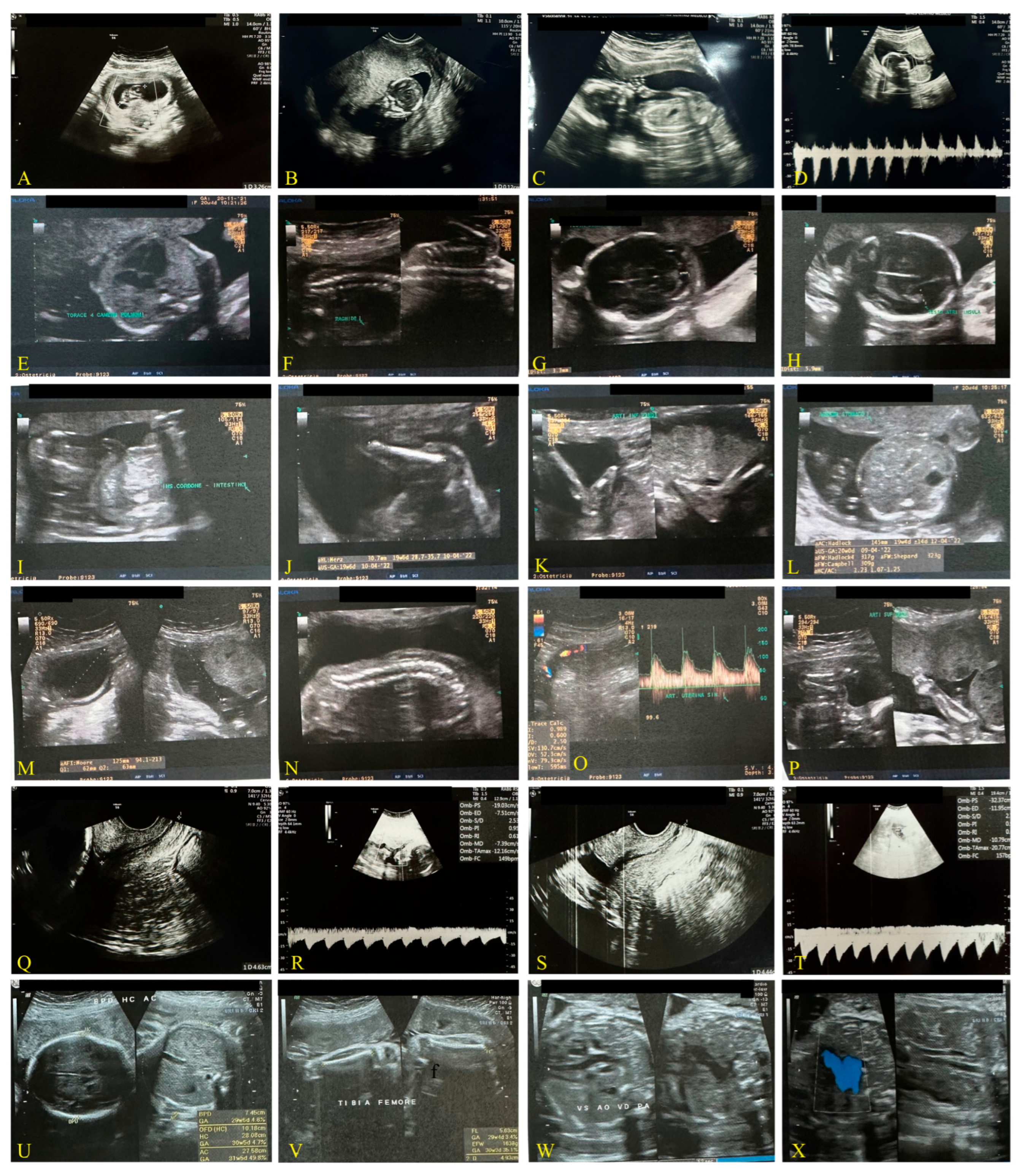
2.3. COVID-19 Reinfection of the Mother Not Vaccinated During the Breastfeeding
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasan, T.; Beardsley, J.; Marais, B.J.; Nguyen, T.A.; Fox, G.J. The Implementation of Mass-Vaccination against SARS-CoV-2: A Systematic Review of Existing Strategies and Guidelines. Vaccines 2021, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Bangaru, S.; Ozorowski, G.; Turner, H.L.; Antanasijevic, A.; Huang, D.; Wang, X.; Torres, J.L.; Diedrich, J.K.; Tian, J.-H.; Portnoff, A.D.; et al. Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate. Science 2020, 370, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Xiaojie, S.; Yu, L.; Lei, Y.; Guang, Y.; Min, Q. Neutralizing antibodies targeting SARS-CoV-2 spike protein. Stem Cell Res. 2021, 50, 102125. [Google Scholar] [CrossRef]
- Premkumar, L.; Segovia-Chumbez, B.; Jadi, R.; Martinez, D.R.; Raut, R.; Markmann, A.; Cornaby, C.; Bartelt, L.; Weiss, S.; Park, Y.; et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 2020, 5, eabc8413. [Google Scholar] [CrossRef]
- Huang, J.; Huang, H.; Wang, D.; Wang, C.; Wang, Y. Immunological strategies against spike protein: Neutralizing antibodies and vaccine development for COVID-19. Clin. Transl. Med. 2020, 10, e184. [Google Scholar] [CrossRef]
- Riva, L.; Yuan, S.; Yin, X.; Martin-Sancho, L.; Matsunaga, N.; Pache, L.; Burgstaller-Muehlbacher, S.; De Jesus, P.D.; Teriete, P.; Hull, M.V.; et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature 2020, 586, 113–119. [Google Scholar] [CrossRef]
- Garcia, G.; Sharma, A.; Ramaiah, A.; Sen, C.; Purkayastha, A.; Kohn, D.B.; Parcells, M.S.; Beck, S.; Kim, H.; Bakowski, M.A.; et al. Antiviral drug screen identifies DNA-damage response inhibitor as potent blocker of SARS-CoV-2 replication. Cell Rep. 2021, 35, 108940. [Google Scholar] [CrossRef]
- Pickard, A.; Calverley, B.C.; Chang, J.; Garva, R.; Gago, S.; Lu, Y.; Kadler, K.E.; Pekosz, A. Discovery of re-purposed drugs that slow SARS-CoV-2 replication in human cells. PLoS Pathog. 2021, 17, e1009840. [Google Scholar] [CrossRef]
- Pizzorno, A.; Padey, B.; Dubois, J.; Julien, T.; Traversier, A.; Dulière, V.; Brun, P.; Lina, B.; Rosa-Calatrava, M.; Terrier, O. In vitro evaluation of antiviral activity of single and combined repurposable drugs against SARS-CoV-2. Antivir. Res. 2020, 181, 104878. [Google Scholar] [CrossRef]
- Zhou, N.E.; Tang, S.; Bian, X.; Parai, M.K.; Krieger, I.V.; Flores, A.; Jaiswal, P.K.; Bam, R.; Wood, J.L.; Shi, Z.; et al. An oral non-covalent non-peptidic inhibitor of SARS-CoV-2 Mpro ameliorates viral replication and pathogenesis in vivo. Cell Rep. 2024, 43, 114929. [Google Scholar] [CrossRef] [PubMed]
- Rhodin, M.H.J.; Reyes, A.C.; Balakrishnan, A.; Bisht, N.; Kelly, N.M.; Gibbons, J.S.; Lloyd, J.; Vaine, M.; Cressey, T.; Crepeau, M.; et al. The small molecule inhibitor of SARS-CoV-2 3CLpro EDP-235 prevents viral replication and transmission in vivo. Nat. Commun. 2024, 15, 6503. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gao, X.; Liang, J.; Wu, Q.; Shen, L.; Zheng, Y.; Ma, Y.; Peng, Y.; He, Y.; Yin, J.; et al. Association between gut microbiota dysbiosis and poor functional outcomes in acute ischemic stroke patients with COVID-19 infection. mSystems 2024, 9, e0018524. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e948. [Google Scholar] [CrossRef]
- Zuo, T.; Liu, Q.; Zhang, F.; Lui, G.; Tso, E.; Yeoh, Y.K.; Chen, Z.; Boon, S.; Chan, F.K.L.; Chan, P.; et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut 2020, 70, 276–284. [Google Scholar] [CrossRef]
- Tang, L.; Gu, S.; Gong, Y.; Li, B.; Lu, H.; Li, Q.; Zhang, R.; Gao, X.; Wu, Z.; Zhang, J.; et al. Clinical Significance of the Correlation between Changes in the Major Intestinal Bacteria Species and COVID-19 Severity. Engineering 2020, 6, 1178–1184. [Google Scholar] [CrossRef]
- Sun, Z.; Song, Z.-G.; Liu, C.; Tan, S.; Lin, S.; Zhu, J.; Dai, F.-H.; Gao, J.; She, J.-L.; Mei, Z.; et al. Gut microbiome alterations and gut barrier dysfunction are associated with host immune homeostasis in COVID-19 patients. BMC Med. 2022, 20, 24. [Google Scholar] [CrossRef]
- Hazan, S.; Stollman, N.; Bozkurt, H.S.; Dave, S.; Papoutsis, A.J.; Daniels, J.; Barrows, B.D.; Quigley, E.M.; Borody, T.J. Lost microbes of COVID-19: Bifidobacterium, Faecalibacterium depletion and decreased microbiome diversity associated with SARS-CoV-2 infection severity. BMJ Open Gastroenterol. 2022, 9, e000871. [Google Scholar] [CrossRef]
- Reinold, J.; Farahpour, F.; Fehring, C.; Dolff, S.; Konik, M.; Korth, J.; van Baal, L.; Hoffmann, D.; Buer, J.; Witzke, O.; et al. A Pro-Inflammatory Gut Microbiome Characterizes SARS-CoV-2 Infected Patients and a Reduction in the Connectivity of an Anti-Inflammatory Bacterial Network Associates With Severe COVID-19. Front. Cell. Infect. Microbiol. 2021, 11, 747816. [Google Scholar] [CrossRef]
- Schult, D.; Reitmeier, S.; Koyumdzhieva, P.; Lahmer, T.; Middelhoff, M.; Erber, J.; Schneider, J.; Kager, J.; Frolova, M.; Horstmann, J.; et al. Gut bacterial dysbiosis and instability is associated with the onset of complications and mortality in COVID-19. Gut Microbes 2022, 14, 2031840. [Google Scholar] [CrossRef] [PubMed]
- Gaibani, P.; D’aMico, F.; Bartoletti, M.; Lombardo, D.; Rampelli, S.; Fornaro, G.; Coladonato, S.; Siniscalchi, A.; Re, M.C.; Viale, P.; et al. The Gut Microbiota of Critically Ill Patients With COVID-19. Front. Cell. Infect. Microbiol. 2021, 11, 670424. [Google Scholar] [CrossRef]
- Moreira-Rosário, A.; Marques, C.; Pinheiro, H.; Araújo, J.R.; Ribeiro, P.; Rocha, R.; Mota, I.; Pestana, D.; Ribeiro, R.; Pereira, A.; et al. Gut Microbiota Diversity and C-Reactive Protein Are Predictors of Disease Severity in COVID-19 Patients. Front. Microbiol. 2021, 12, 705020. [Google Scholar] [CrossRef]
- Brogna, C.; Bisaccia, D.R.; Costanzo, V.; Lettieri, G.; Montano, L.; Viduto, V.; Fabrowski, M.; Cristoni, S.; Prisco, M.; Piscopo, M. Who Is the Intermediate Host of RNA Viruses? A Study Focusing on SARS-CoV-2 and Poliovirus. Microorganisms 2024, 12, 643. [Google Scholar] [CrossRef]
- Brogna, C.; Cristoni, S. A new absolute quantitative method for peptide and metabolite detection. J. Mass Spectrom. 2023, 59, e4991. [Google Scholar] [CrossRef] [PubMed]
- Brogna, C.; Cristoni, S.; Brogna, B.; Bisaccia, D.R.; Marino, G.; Viduto, V.; Montano, L.; Piscopo, M. Toxin-like Peptides from the Bacterial Cultures Derived from Gut Microbiome Infected by SARS-CoV-2—New Data for a Possible Role in the Long COVID Pattern. Biomedicines 2022, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Brogna, C.; Brogna, B.; Bisaccia, D.R.; Lauritano, F.; Marino, G.; Montano, L.; Cristoni, S.; Prisco, M.; Piscopo, M. Could SARS-CoV-2 Have Bacteriophage Behavior or Induce the Activity of Other Bacteriophages? Vaccines 2022, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Zajac, V.; Matelova, L.; Liskova, A.; Mego, M.; Holec, V.; Adamcikova, Z.; Stevurkova, V.; Shahum, A.; Krcmery, V. Confirmation of HIV-like sequences in respiratory tract bacteria of Cambodian and Kenyan HIV-positive pediatric patients. Med. Sci. Monit. 2011, 17, CR154–CR158. [Google Scholar] [CrossRef]
- Petrillo, M.; Querci, M.; Brogna, C.; Ponti, J.; Cristoni, S.; Markov, P.V.; Valsesia, A.; Leoni, G.; Benedetti, A.; Wiss, T.; et al. Evidence of SARS-CoV-2 Bacteriophage Potential in Human Gut Microbiota. F1000Research 2025, 11, 292. [Google Scholar] [CrossRef]
- Brogna, C.; Costanzo, V.; Brogna, B.; Bisaccia, D.R.; Brogna, G.; Giuliano, M.; Montano, L.; Viduto, V.; Cristoni, S.; Fabrowski, M.; et al. Analysis of Bacteriophage Behavior of a Human RNA Virus, SARS-CoV-2, through the Integrated Approach of Immunofluorescence Microscopy, Proteomics and D-Amino Acid Quantification. Int. J. Mol. Sci. 2023, 24, 3929. [Google Scholar] [CrossRef]
- Brogna, C.; Cristoni, S.; Petrillo, M.; Bisaccia, D.R.; Lauritano, F.; Montano, L.; Prisco, M.; Piscopo, M. The first report on detecting SARS-CoV-2 inside bacteria of the human gut microbiome: A case series on asymptomatic family members and a child with COVID-19. F1000Research 2022, 11, 135. [Google Scholar] [CrossRef]
- Petrillo, M.; Brogna, C.; Cristoni, S.; Querci, M.; Piazza, O.; Eede, G.V.D. Increase of SARS-CoV-2 RNA load in faecal samples prompts for rethinking of SARS-CoV-2 biology and COVID-19 epidemiology. F1000Research 2021, 10, 370. [Google Scholar] [CrossRef]
- Brogna, C.; Montano, L.; Zanolin, M.E.; Bisaccia, D.R.; Ciammetti, G.; Viduto, V.; Fabrowski, M.; Baig, A.M.; Gerlach, J.; Gennaro, I.; et al. A retrospective cohort study on early antibiotic use in vaccinated and unvaccinated COVID-19 patients. J. Med. Virol. 2024, 96, e29507. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.-W.; Yuan, S.; Chu, H.; Sridhar, S.; Yuen, K.-Y. COVID-19 drug discovery and treatment options. Nat. Rev. Microbiol. 2024, 22, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Manciulli, T.; Modi, G.; Campolmi, I.; Borchi, B.; Trotta, M.; Spinicci, M.; Lagi, F.; Bartoloni, A.; Zammarchi, L. Treatment with anti-SARS-CoV-2 monoclonal antibodies in pregnant and postpartum women: First experiences in Florence, Italy. Infection 2022, 50, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Blakeway, H.; Prasad, S.; Kalafat, E.; Heath, P.T.; Ladhani, S.N.; Le Doare, K.; Magee, L.A.; O’bRien, P.; Rezvani, A.; von Dadelszen, P.; et al. COVID-19 vaccination during pregnancy: Coverage and safety. Am. J. Obstet. Gynecol. 2022, 226, 236.e1–236.e14. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, L.; Ming, L.; Wei, M.; Li, J.; Hu, R.; Yang, J. Severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) infection during late pregnancy: A report of 18 patients from Wuhan, China. BMC Pregnancy Childbirth 2020, 20, 394. [Google Scholar] [CrossRef]
- Hsu, A.L.; Guan, M.; Johannesen, E.; Stephens, A.J.; Khaleel, N.; Kagan, N.; Tuhlei, B.C.; Wan, X. Placental SARS-CoV-2 in a pregnant woman with mild COVID-19 disease. J. Med. Virol. 2020, 93, 1038–1044. [Google Scholar] [CrossRef]
- Menter, T.; Tzankov, A.; Bruder, E. SARS-CoV-2/COVID-19-Auswirkungen auf die Plazenta. Der Pathol. 2021, 42, 591–597. [Google Scholar] [CrossRef]
- Bellos, I.; Pandita, A.; Panza, R. Maternal and perinatal outcomes in pregnant women infected by SARS-CoV-2: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 194–204. [Google Scholar] [CrossRef]
- de Vasconcelos Gaspar, A.; Santos Silva, I. SARS-CoV-2 in Pregnancy—The First Wave. Medicina 2021, 57, 241. [Google Scholar] [CrossRef]
- Donati, S.; Corsi, E.; Maraschini, A.; Salvatore, M.A. The first SARS-CoV-2 wave among pregnant women in Italy: Results from a prospective population-based study. Annali dell’Istituto Superiore di Sanita 2021, 57, 272–285. [Google Scholar] [CrossRef]
- Di Guardo, F.; Di Grazia, F.M.; Di Gregorio, L.M.; Zambrotta, E.; Carrara, G.; Gulino, F.A.; Tuscano, A.; Palumbo, M. Poor maternal–neonatal outcomes in pregnant patients with confirmed SARS-Cov-2 infection: Analysis of 145 cases. Arch. Gynecol. Obstet. 2021, 303, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Dong, S.; Shah, N.K.; Liang, Y.; Wang, J.; Shan, Y.-H.; He, J. Peripartum outcomes and immune responses after SARS-CoV-2 infection in the third trimester of pregnancy. BMC Pregnancy Childbirth 2024, 24, 498. [Google Scholar] [CrossRef]
- Brogna, C.; Cristoni, S.; Petrillo, M.; Querci, M.; Piazza, O.; Eede, G.V.D. Toxin-like peptides in plasma, urine and faecal samples from COVID-19 patients. F1000Research 2021, 10, 550. [Google Scholar] [CrossRef]
- Individuals Birth Weight Centile Calculator. Available online: https://timms.le.ac.uk/birth-weight-centiles/individuals-calculator.html (accessed on 27 June 2025).
- Fetal Growth Calculator. Available online: https://srhr.org/fetalgrowthcalculator/#/ (accessed on 27 June 2025).
- Fahmi, A.; Brügger, M.; Démoulins, T.; Zumkehr, B.; Esteves, B.I.O.; Bracher, L.; Wotzkow, C.; Blank, F.; Thiel, V.; Baud, D.; et al. SARS-CoV-2 can infect and propagate in human placenta explants. Cell Rep. Med. 2021, 2, 100456. [Google Scholar] [CrossRef]
- Pique-Regi, R.; Romero, R.; Tarca, A.L.; Luca, F.; Xu, Y.; Alazizi, A.; Leng, Y.; Hsu, C.-D.; Gomez-Lopez, N. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? eLife 2020, 9, e58716. [Google Scholar] [CrossRef]
- Ashary, N.; Bhide, A.; Chakraborty, P.; Colaco, S.; Mishra, A.; Chhabria, K.; Jolly, M.K.; Modi, D. Single-Cell RNA-seq Identifies Cell Subsets in Human Placenta That Highly Expresses Factors Driving Pathogenesis of SARS-CoV-2. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, L.; Zhang, J.; Xiong, C.; Li, X.; Chan, R.W. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS ONE 2020, 15, e0230295. [Google Scholar] [CrossRef]
- Pistollato, F.; Petrillo, M.; Clerbaux, L.-A.; Leoni, G.; Ponti, J.; Bogni, A.; Brogna, C.; Cristoni, S.; Sanges, R.; Gyves, E.M.-D.; et al. Effects of spike protein and toxin-like peptides found in COVID-19 patients on human 3D neuronal/glial model undergoing differentiation: Possible implications for SARS-CoV-2 impact on brain development. Reprod. Toxicol. 2022, 111, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Silasi, M.; Cardenas, I.; Kwon, J.; Racicot, K.; Aldo, P.; Mor, G. Viral Infections During Pregnancy. Am. J. Reprod. Immunol. 2015, 73, 199–213. [Google Scholar] [CrossRef]
- Auriti, C.; De Rose, D.U.; Santisi, A.; Martini, L.; Piersigilli, F.; Bersani, I.; Ronchetti, M.P.; Caforio, L. Pregnancy and viral infections: Mechanisms of fetal damage, diagnosis and prevention of neonatal adverse outcomes from cytomegalovirus to SARS-CoV-2 and Zika virus. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166198. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Casillas, A.; Cowley, D.; Raszek, M.; Uversky, V.N.; Redwan, E.M. Corrigendum to “Review: N1-methyl-pseudouridine (m1Ψ): Friend or foe of cancer?”. Int. J. Biol. Macromol. 2024, 270, 132447. [Google Scholar] [CrossRef]
- Fernández-Buhigas, I.; Rayo, N.; Silos, J.C.; Serrano, B.; Ocón-Hernández, O.; Leung, B.W.; Delgado, J.L.; Fernández, D.S.-N.; Valle, S.; De Miguel, L.; et al. Anti-SARS-CoV-2-specific antibodies in human breast milk following SARS-CoV-2 infection during pregnancy: A prospective cohort study. Int. Breastfeed. J. 2024, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Zeballos, R.S.; da Silva Helbingen, M.F.; Melo, P.M.P.; Alves, F.E.C.; Salvino, C.R.; Seródio, E.P.; de Carvalho, E.R.M. Post spike syndrome (PSS): Simple solution leading to resolving results, five cases report. IDCases 2025, 41, e02278. [Google Scholar] [CrossRef] [PubMed]
| Period | 2018–2019 Mother UnVax | 2022–2023 Mother UnVax Omicron Variant | 2021–2022 Mother Vax Delta Variant |
|---|---|---|---|
| Vaccination mRNA | No | No | 2 doses—second during pregnancy, 1 month before infection |
| Newborn gender | Female | Male | Female |
| Birth weight | 3100 g (32.3° pc) | 3775 g (79.1° pc) | 2550 g (22.8° pc) |
| Birth length | 50 cm | 53 cm | 46 cm |
| Cranial circumference | 32 cm | 35 cm | 33.5 cm |
| Apgar first-minute index | 9 | 9 | 8 |
| Apgar index at 5 min | 9 | 9 | 9 |
| Surgery | Cesarean section under general anesthesia | Cesarean section under general anesthesia | Cesarean section under locoregional anesthesia |
| Mother’s weight at term | 78 kg | 78 kg | 78 kg |
| Mother’s BMI at term pregnancy | 27.97 | 27.97 | 31.6 |
| Infections during pregnancy | None | COVID-19 | COVID-19 |
| Therapy for infections | - | Amoxicillin plus clavulanic acid + rifaximin | Paracetamol plus ibuprofen |
| Comorbidity 1 Comorbidity 2 | Pervious foramen ovale; interatrial septal aneurysm | Pervious Foramen ovale; interatrial septal aneurysm | Migraine |
| Comorbidity 3 | MGUS | ||
| Standard therapy | Cardioaspirin | Cardioaspirin | Cardioaspirin |
| Pregnancy weeks | 39 weeks | 39 weeks | 37 weeks + 4 days |
| Weeks of gestation and Ultrasound data | 20 + 2 days | 21 + 1 days | 20 + 4 days |
| Biparietal diameter (BPD) | 47 mm | 52 mm | 46.4 mm |
| Occipital-frontal diameter (OFD) | 61 mm | 67 mm | - |
| Head circumference (HC) | - | 190 mm | 178 mm |
| Thermal index for bone (TIB) | - | 31 mm | - |
| Fetal corpus callosum (CC) | 172 mm | 190 mm | - |
| Cerebellum transverse diameter | 20.5 mm | 21.5 mm | - |
| Lateral trigon diameter (Lateral Ventriculum) | 6.8 mm | 6 mm | - |
| Abdominal circumference (AC) | 150 mm | 166 mm | 145 mm |
| Femur length (FL) | 32 mm | 36 mm | 32.3 |
| Occiput lateral (head down, facing your side) (OL) | - | 33 mm | - |
| Heart rate | 151 bpm | 140 bpm | 140 bpm |
| Estimated weight | 330 g | 430 g | 320 g |
| Weeks of gestation and US data | 29 days | 28 + 1 days | 31 days |
| Biparietal diameter (BPD) | 69 mm | 72 mm | 79 mm |
| CC | 258 mm | 253 mm | 280 mm |
| Cerebellum transverse diameter | 30 mm | 21.5 mm | - |
| AC | 246 mm | 250 mm | 273 mm |
| LF | 49 mm | 58 mm | 56 mm |
| Estimated weight | 1230 g | 1451 g | 1600 g |
| Week | 15 + 2 Days | 21 + 1 Day | 24 + 5 Days | 28 + 1 Day |
|---|---|---|---|---|
| MAF | YES | YES | YES | YES |
| Heart rate | 163 bpm | 140 bpm | 160 bpm | 168 bpm |
| Biparietal Diameter (DPB) | 35 mm (68° pc) | 52 mm | 63 mm | 72 mm |
| Head circumference (HC) | 117 mm (31° pc) | 190 mm | 230 mm | 253 mm |
| Femur length (FL) | 19 mm (45° pc) | 36 mm | 44 mm | 58 mm |
| Abdominal circumference (AC) | - | 166 mm | 206 mm | 250 mm |
| Estimated fetal weight | 218 g | 430 g | 750 g | 1451 g |
| Percentile of weight | 97.5th | 67.3th | 52.5th | 97.5th |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brogna, C.; Castellucci, G.; Redwan, E.M.; Rubio-Casillas, A.; Montano, L.; Ciammetti, G.; Giuliano, M.; Viduto, V.; Fabrowski, M.; Lettieri, G.; et al. New Neonatal and Prenatal Approach to Home Therapy with Amoxicillin, Rifaximin, and Anti-Inflammatory Drugs for Pregnant Women with COVID-19 Infections—Monitoring of Fetal Growth as a Prognostic Factor: A Triple Case Series (N.A.T.H.A.N.). Biomedicines 2025, 13, 1858. https://doi.org/10.3390/biomedicines13081858
Brogna C, Castellucci G, Redwan EM, Rubio-Casillas A, Montano L, Ciammetti G, Giuliano M, Viduto V, Fabrowski M, Lettieri G, et al. New Neonatal and Prenatal Approach to Home Therapy with Amoxicillin, Rifaximin, and Anti-Inflammatory Drugs for Pregnant Women with COVID-19 Infections—Monitoring of Fetal Growth as a Prognostic Factor: A Triple Case Series (N.A.T.H.A.N.). Biomedicines. 2025; 13(8):1858. https://doi.org/10.3390/biomedicines13081858
Chicago/Turabian StyleBrogna, Carlo, Grazia Castellucci, Elrashdy M. Redwan, Alberto Rubio-Casillas, Luigi Montano, Gianluca Ciammetti, Marino Giuliano, Valentina Viduto, Mark Fabrowski, Gennaro Lettieri, and et al. 2025. "New Neonatal and Prenatal Approach to Home Therapy with Amoxicillin, Rifaximin, and Anti-Inflammatory Drugs for Pregnant Women with COVID-19 Infections—Monitoring of Fetal Growth as a Prognostic Factor: A Triple Case Series (N.A.T.H.A.N.)" Biomedicines 13, no. 8: 1858. https://doi.org/10.3390/biomedicines13081858
APA StyleBrogna, C., Castellucci, G., Redwan, E. M., Rubio-Casillas, A., Montano, L., Ciammetti, G., Giuliano, M., Viduto, V., Fabrowski, M., Lettieri, G., Marinaro, C., & Piscopo, M. (2025). New Neonatal and Prenatal Approach to Home Therapy with Amoxicillin, Rifaximin, and Anti-Inflammatory Drugs for Pregnant Women with COVID-19 Infections—Monitoring of Fetal Growth as a Prognostic Factor: A Triple Case Series (N.A.T.H.A.N.). Biomedicines, 13(8), 1858. https://doi.org/10.3390/biomedicines13081858










