Low-Frequency rTMS and Diazepam Exert Synergistic Effects on the Excitability of an SH-SY5Y Model of Epileptiform Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. Differentiation Protocol
2.4. Calcium Imaging
2.5. ΔF/F
2.6. Diazepam
2.7. rTMS
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| rTMS | Repetitive transcranial magnetic stimulation |
| DRE | Drug-resistant epilepsy |
| GABA | Gamma-aminobutyric acid |
| MS | Multiple sclerosis |
| SLEs | Seizure-like events |
| EMEM | Eagle’s Minimum Essential Medium |
| FBS | Fetal bovine serum |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMSO | Dimethylsulfoxide |
| BDNF | Brain-derived neurotrophic factor |
| NB | Neurobasal medium |
| LTP | Long-term potentiation |
| LTD | Long-term depression |
| NMDA | N-methyl-D-aspartate |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| cAMP | Cyclic adenosine monophosphate |
References
- Fiest, K.M.; Sauro, K.M.; Wiebe, S.; Patten, S.B.; Kwon, C.S.; Dykeman, J.; Pringsheim, T.; Lorenzetti, D.L.; Jetté, N. Prevalence and incidence of epilepsy: A systematic review and meta-analysis of international studies. Neurology 2017, 88, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Beghi, E.; Giussani, G.; Sander, J.W. The natural history and prognosis of epilepsy. Epileptic Disord. 2015, 17, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Manford, M. Recent advances in epilepsy. J. Neurol. 2017, 264, 1811–1824. [Google Scholar] [CrossRef] [PubMed]
- Sultana, B.; Panzini, M.A.; Veilleux Carpentier, A.; Comtois, J.; Rioux, B.; Gore, G.; Bauer, P.R.; Kwon, C.S.; Jetté, N.; Josephson, C.B.; et al. Incidence and prevalence of drug-resistant epilepsy: A systematic review and meta-analysis. Neurology 2021, 96, 805–817. [Google Scholar] [CrossRef]
- Nimmaanrat, S.; Charuenporn, B.; Jensen, M.P.; Geater, A.F.; Tanasansuttiporn, J.; Chanchayanon, T. The anxiolytic effects of preoperative administration of pregabalin in comparison to diazepam and placebo. Sci. Rep. 2023, 13, 9680. [Google Scholar] [CrossRef]
- Abou-Khalil, B.; Wheless, J.; Rogin, J.; Wolter, K.D.; Pixton, G.C.; Shukla, R.B.; Sherman, N.A.; Sommerville, K.; Goli, V.; Roland, C.L. A double-blind, randomized, placebo-controlled trial of a diazepam auto-injector administered by caregivers to patients with epilepsy who require intermittent intervention for acute repetitive seizures. Epilepsia 2013, 54, 1968–1976. [Google Scholar] [CrossRef]
- National Guideline Centre (UK). Evidence Review: Antiseizure Medication for Status Epilepticus; National Institute for Health and Care Excellence: London, UK, 2022. [Google Scholar]
- Taghizadehghalehjoughi, A.; Naldan, M.E. The study of diazepam, pregabalin and glucose effect on glutamate toxicity: In vitro study. Medicine 2018, 7, 797–801. [Google Scholar] [CrossRef]
- Penovich, P.E.; Rao, V.R.; Long, L.; Carrazana, E.; Rabinowicz, A.L. Benzodiazepines for the treatment of seizure clusters. CNS Drugs 2024, 38, 125–140. [Google Scholar] [CrossRef]
- Misra, S.N.; Jarrar, R.; Stern, J.M.; Becker, D.A.; Carrazana, E.; Rabinowicz, A.L. Rapid rescue treatment with diazepam nasal spray leads to faster seizure cluster termination in epilepsy: An exploratory post hoc cohort analysis. Neurol. Ther. 2024, 13, 221–231. [Google Scholar] [CrossRef]
- Pellock, J.M. Safety of Diastat, a rectal gel formulation of diazepam for acute seizure treatment. Drug Saf. 2004, 27, 383–392. [Google Scholar] [CrossRef]
- Klomjai, W.; Katz, R.; Lackmy-Vallée, A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann. Phys. Rehabil. Med. 2015, 58, 208–213. [Google Scholar] [CrossRef]
- Pourzitaki, C.; Dardalas, I.; Poutoglidou, F.; Kouvelas, D.; Kimiskidis, V.K. The combination of rTMS and pharmacotherapy on in vitro models: A mini-review. CNS Neurol. Disord. Drug Targets 2020, 19, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Şahin, M.; Öncü, G.; Yılmaz, M.A.; Özkan, D.; Saybaşılı, H. Transformation of SH-SY5Y cell line into neuron-like cells: Investigation of electrophysiological and biomechanical changes. Neurosci. Lett. 2021, 745, 135628. [Google Scholar] [CrossRef] [PubMed]
- Nagarkatti, N.; Deshpande, L.S.; DeLorenzo, R.J. Development of the calcium plateau following status epilepticus: Role of calcium in epileptogenesis. Expert Rev. Neurother. 2009, 9, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Grainger, A.I.; King, M.C.; Nagel, D.A.; Parri, H.R.; Coleman, M.D.; Hill, E.J. In vitro models for seizure-liability testing using induced pluripotent stem cells. Front. Neurosci. 2018, 12, 590. [Google Scholar] [CrossRef]
- Albowitz, B.; König, P.; Kuhnt, U. Spatiotemporal distribution of intracellular calcium transients during epileptiform activity in guinea pig hippocampal slices. J. Neurophysiol. 1997, 77, 491–501. [Google Scholar] [CrossRef]
- Pisani, A.; Bonsi, P.; Martella, G.; Cacciari, S.; Brugnoli, A.; Calabresi, P.; Bernardi, G. Intracellular calcium increase in epileptiform activity: Modulation by levetiracetam and lamotrigine. Epilepsia 2004, 45, 719–728. [Google Scholar] [CrossRef]
- Gao, W.J.; Goldman-Rakic, P.S. NMDA receptor-mediated epileptiform persistent activity requires calcium release from intracellular stores in prefrontal neurons. Exp. Neurol. 2006, 197, 495–504. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, L.Y. A simple and fast method to image calcium activity of neurons from intact dorsal root ganglia using fluorescent chemical Ca2+ indicators. Mol. Pain 2017, 13, 1744806917748051. [Google Scholar] [CrossRef]
- Forster, J.I.; Köglsberger, S.; Trefois, C.; Boyd, O.; Baumuratov, A.S.; Buck, L.; Balling, R.; Antony, P.M. Characterization of differentiated SH-SY5Y as neuronal screening model reveals increased oxidative vulnerability. J. Biomol. Screen. 2016, 21, 496–509. [Google Scholar] [CrossRef]
- Fluo Calcium Indicators—Thermo Fisher Scientific. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/mp01240.pdf (accessed on 6 August 2024).
- Van Acker, K.; Bautmans, B.; Bultynck, G.; Maes, K.; Weidema, A.F.; De Smet, P.; Parys, J.B.; De Smedt, H.; Missiaen, L.; Callewaert, G. Mapping of IP3-mediated Ca2+ signals in single human neuroblastoma SH-SY5Y cells: Cell volume shaping the Ca2+ signal. J. Neurophysiol. 2000, 83, 1052–1057. [Google Scholar] [CrossRef]
- Buhner, S.; Barki, N.; Greiter, W.; Giesbertz, P.; Demir, I.E.; Ceyhan, G.O.; Zeller, F.; Daniel, H.; Schemann, M. Calcium imaging of nerve-mast cell signaling in the human intestine. Front. Physiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed]
- Bruun, D.A.; Cao, Z.; Inceoglu, B.; Vito, S.T.; Austin, A.T.; Hulsizer, S.; Hammock, B.D.; Tancredi, D.J.; Rogawski, M.A.; Pessah, I.N.; et al. Combined treatment with diazepam and allopregnanolone reverses tetramethylenedisulfotetramine (TETS)-induced calcium dysregulation in cultured neurons and protects TETS-intoxicated mice against lethal seizures. Neuropharmacology 2015, 95, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Kreuzer, M.; García, P.S.; Brucklacher-Waldert, V.; Claassen, R.; Schneider, G.; Antkowiak, B.; Drexler, B. Diazepam and ethanol differently modulate neuronal activity in organotypic cortical cultures. BMC Neurosci. 2019, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Khire, T.S.; Nehilla, B.J.; Getpreecharsawas, J.; Gracheva, M.E.; Waugh, R.E.; McGrath, J.L. Finite element modeling to analyze TEER values across silicon nanomembranes. Biomed. Microdevices 2018, 20, 11. [Google Scholar] [CrossRef]
- Patil, I. Visualizations with statistical details: The ‘ggstatsplot’ approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Shipley, M.M.; Mangold, C.A.; Szpara, M.L. Differentiation of the SH-SY5Y human neuroblastoma cell line. J. Visualized Exp. 2016, 108, 53193. [Google Scholar] [CrossRef]
- Reyes, A.P.; Martinez Torres, A.; Carreon Castro, M.D.; Rodríguez Talavera, J.R.; Muñoz, S.V.; Aguilar, V.M.; Torres, M.G. Novel poly(3-hydroxybutyrate-g-vinyl alcohol) polyurethane scaffold for tissue engineering. Sci. Rep. 2016, 6, 31140. [Google Scholar] [CrossRef]
- Sigel, E.; Ernst, M. The benzodiazepine binding sites of GABAA receptors. Trends Pharmacol. Sci. 2018, 39, 659–671. [Google Scholar] [CrossRef]
- Rudolph, U.; Knoflach, F. Beyond classical benzodiazepines: Novel therapeutic potential of GABAA receptor subtypes. Nat. Rev. Drug Discov. 2011, 10, 685–697. [Google Scholar] [CrossRef]
- Vetter, I.; Mozar, C.A.; Durek, T.; Wingerd, J.S.; Alewood, P.F.; Christie, M.J.; Lewis, R.J. Characterisation of Nav types endogenously expressed in human SH-SY5Y neuroblastoma cells. Biochem. Pharmacol. 2012, 83, 1562–1571. [Google Scholar] [CrossRef]
- Andersson, H.; Björnström, K.; Eintrei, C.; Sundqvist, T. Orexin A phosphorylates the γ-aminobutyric acid type A receptor β2 subunit on a serine residue and changes the surface expression of the receptor in SH-SY5Y cells exposed to propofol. J. Neurosci. Res. 2015, 93, 1748–1755. [Google Scholar] [CrossRef]
- Tsuboyama, M.; Lee Kaye, H.; Rotenberg, A. Biomarkers obtained by transcranial magnetic stimulation of the motor cortex in epilepsy. Front. Integr. Neurosci. 2019, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Cullen, C.L.; Young, K.M. How does transcranial magnetic stimulation influence glial cells in the central nervous system? Front. Neural Circuits 2016, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Kamalova, A.; Nakagawa, T. AMPA Receptor Structure and Auxiliary Subunits. J. Physiol. 2021, 599, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, K.D.; Sharma, G.; Prakash, M.M.; Parihar, M.S.; Dawane, V. Effects of high glutamate concentrations on mitochondria of human neuroblastoma SH-SY5Y cells. Ann. Pharm. Fr. 2023, 81, 457–465. [Google Scholar] [CrossRef]
- Pacico, N.; Mingorance-Le Meur, A. New in vitro phenotypic assay for epilepsy: Fluorescent measurement of synchronized neuronal calcium oscillations. PLoS ONE 2014, 9, e84755. [Google Scholar] [CrossRef]
- Lage, C.; Wiles, K.; Shergill, S.S.; Tracy, D.K. A systematic review of the effects of low-frequency repetitive transcranial magnetic stimulation on cognition. J. Neural Transm. 2016, 123, 1479–1490. [Google Scholar] [CrossRef]
- Kinoshita, M.; Ikeda, A.; Begum, T.; Yamamoto, J.; Hitomi, T.; Shibasaki, H. Low-frequency repetitive transcranial magnetic stimulation for seizure suppression in patients with extratemporal lobe epilepsy—A pilot study. Seizure 2005, 14, 387–392. [Google Scholar] [CrossRef][Green Version]
- Chen, R.M.; Classen, J.; Gerloff, C.; Celnik, P.; Wassermann, E.M.; Hallett, M.; Cohen, L.G. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 1997, 48, 1398–1403. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chang, Y. Study the impact of the SH-SY5Y cells under the magnetic stimulation with different frequency and duration. In Proceedings of the 1st Global Conference on Biomedical Engineering & 9th Asian-Pacific Conference on Medical and Biological Engineering, Tainan, Taiwan, 9–12 October 2014; Springer International Publishing: Cham, Switzerland, 2015; pp. 241–243. [Google Scholar] [CrossRef]
- Shaul, U.; Ben-Shachar, D.; Karry, R.; Klein, E. Modulation of frequency and duration of repetitive magnetic stimulation affects catecholamine levels and tyrosine hydroxylase activity in human neuroblastoma cells: Implication for the antidepressant effect of rTMS. Int. J. Neuropsychopharmacol. 2003, 6, 233–241. [Google Scholar] [CrossRef]
- Hellmann, J.; Jüttner, R.; Roth, C.; Bajbouj, M.; Kirste, I.; Heuser, I.; Gertz, K.; Endres, M.; Kronenberg, G. Repetitive magnetic stimulation of human-derived neuron-like cells activates cAMP-CREB pathway. Eur. Arch. Psychiatry Clin. Neurosci. 2012, 262, 87–91. [Google Scholar] [CrossRef]
- Che, L.Q.; Qu, Z.Z.; Mao, Z.F.; Qiao, Q.; Zhou, K.P.; Jia, L.J.; Wang, W.P. Low-frequency rTMS exerts neuroprotective effects in pilocarpine-induced status epilepticus rat models via the AMPAR GluA1-STIM-Ca2+ pathway. Mol. Neurobiol. 2025, 62, 4042–4054. [Google Scholar] [CrossRef]
- Kano, T.; Inaba, Y.; D’Antuono, M.; Biagini, G.; Levésque, M.; Avoli, M. Blockade of in vitro ictogenesis by low-frequency stimulation coincides with increased epileptiform response latency. J. Neurophysiol. 2015, 114, 21–28. [Google Scholar] [CrossRef]
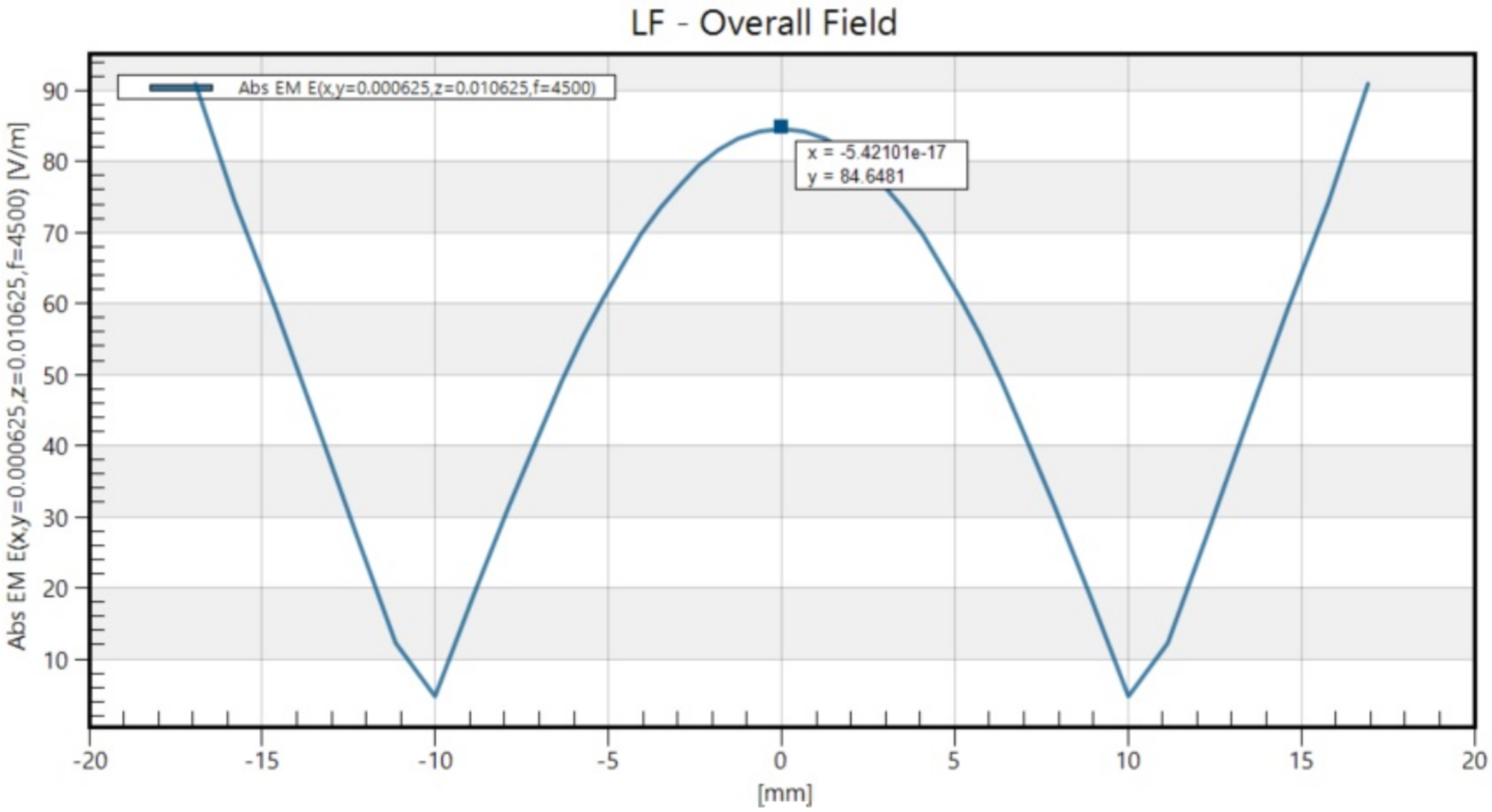
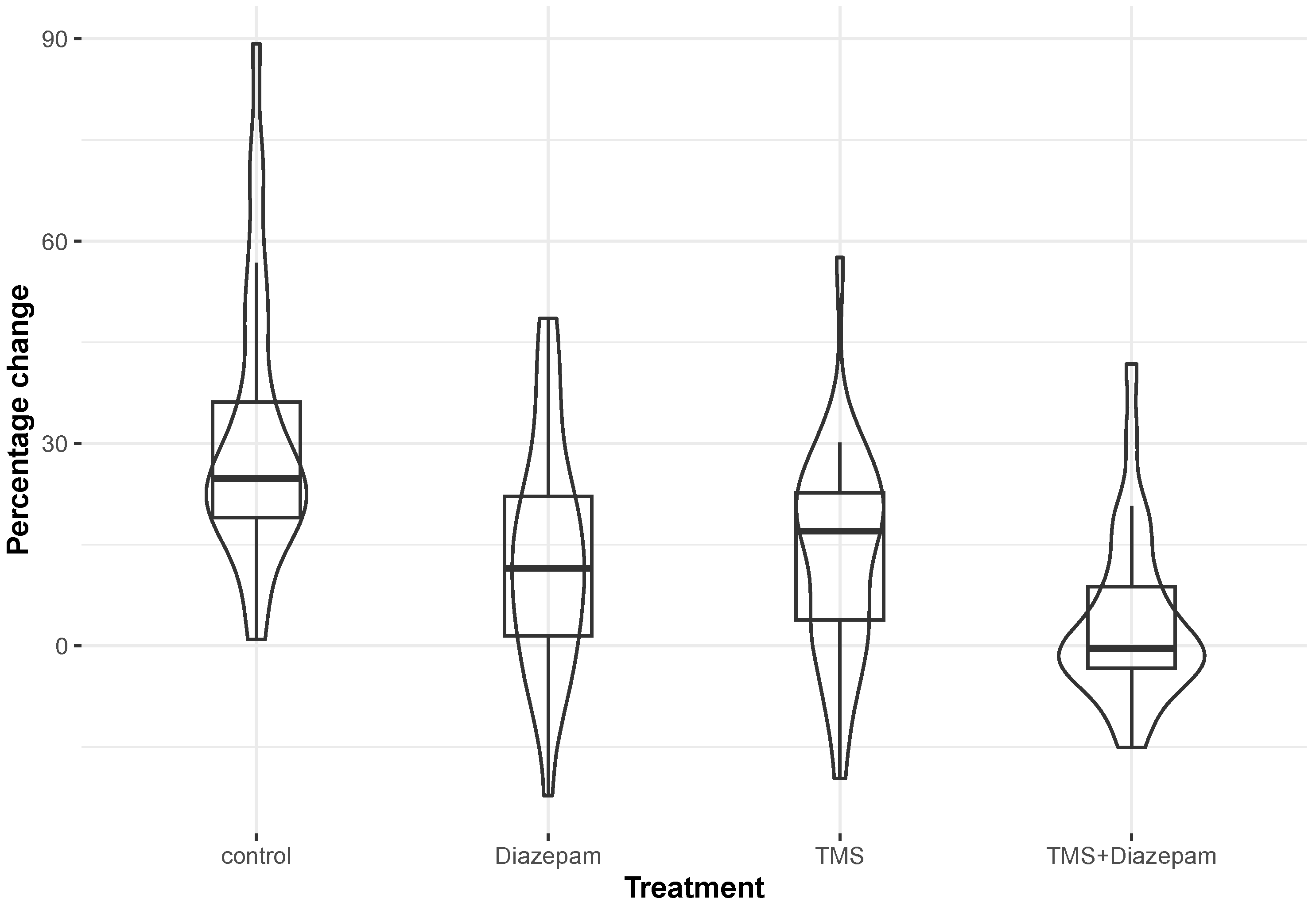
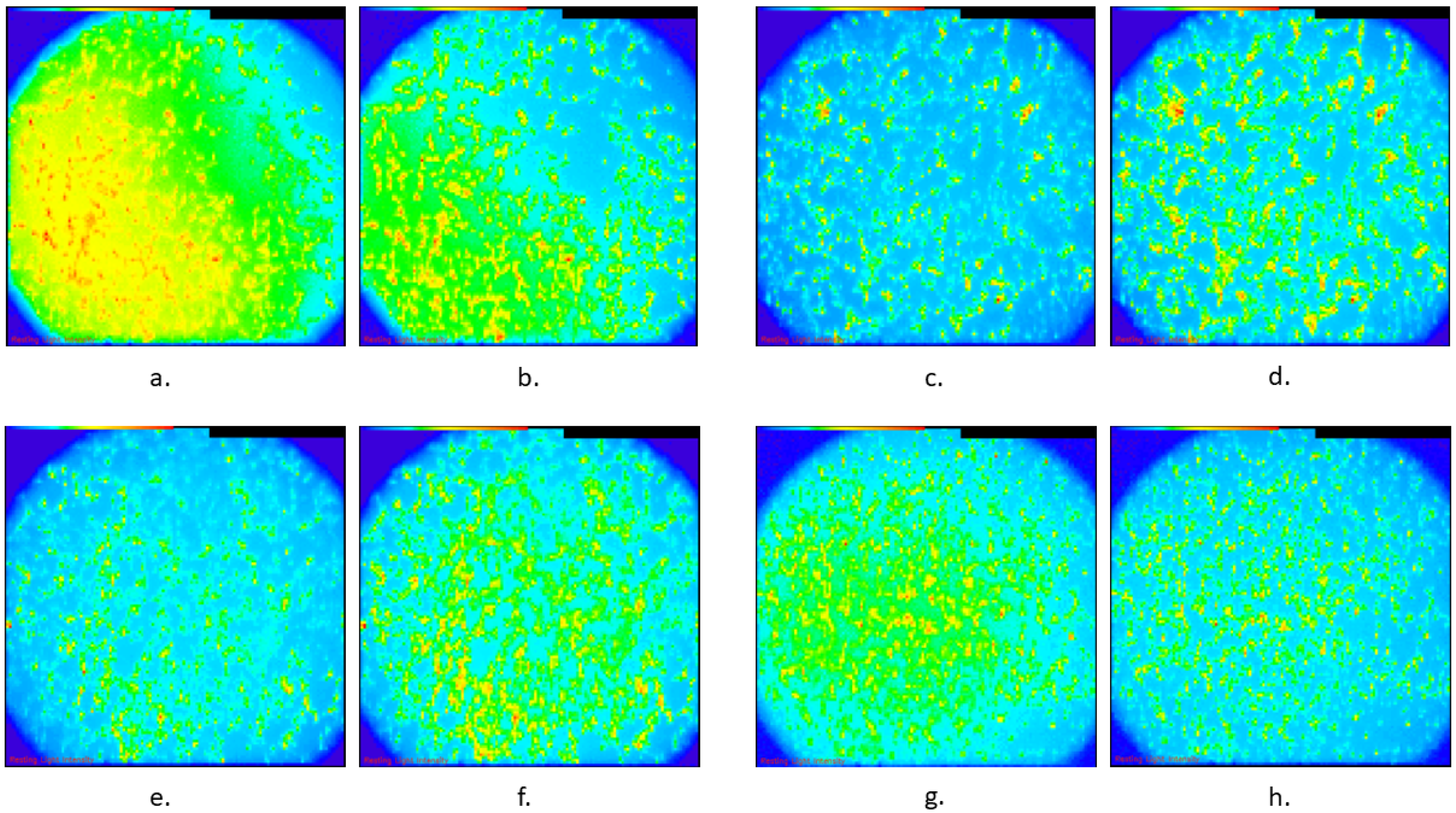
| (A) | ||||
| Group | Median | Mean | Std. Deviation | |
| Control | 24.80 | 30.98 | 20.37 | |
| TMS | 16.96 | 13.55 | 15.55 | |
| Diazepam | 11.46 | 13.35 | 17.14 | |
| TMS + diazepam | −0.44 | 3.70 | 12.48 | |
| (B) | ||||
| Group 1 vs. Group 2 | Dunn’s Test Statistic | Unadjusted p-Value | Adjusted p-Value | |
| Control | TMS | 28.433 | 0.002 | 0.003 |
| Diazepam | 30.800 | 0.001 | 0.002 | |
| TMS + diazepam | 51.967 | <0.001 | <0.001 | |
| TMS | Diazepam | 2.367 | 0.792 | 0.792 |
| TMS + diazepam | 23.533 | 0.009 | 0.013 | |
| Diazepam | TMS + diazepam | 21.167 | 0.018 | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dardalas, I.; Kosmidis, E.K.; Lagoudaki, R.; Kimiskidis, V.K.; Samaras, T.; Moysiadis, T.; Kouvelas, D.; Pourzitaki, C. Low-Frequency rTMS and Diazepam Exert Synergistic Effects on the Excitability of an SH-SY5Y Model of Epileptiform Activity. Biomedicines 2025, 13, 1857. https://doi.org/10.3390/biomedicines13081857
Dardalas I, Kosmidis EK, Lagoudaki R, Kimiskidis VK, Samaras T, Moysiadis T, Kouvelas D, Pourzitaki C. Low-Frequency rTMS and Diazepam Exert Synergistic Effects on the Excitability of an SH-SY5Y Model of Epileptiform Activity. Biomedicines. 2025; 13(8):1857. https://doi.org/10.3390/biomedicines13081857
Chicago/Turabian StyleDardalas, Ioannis, Efstratios K. Kosmidis, Roza Lagoudaki, Vasilios K. Kimiskidis, Theodoros Samaras, Theodoros Moysiadis, Dimitrios Kouvelas, and Chryssa Pourzitaki. 2025. "Low-Frequency rTMS and Diazepam Exert Synergistic Effects on the Excitability of an SH-SY5Y Model of Epileptiform Activity" Biomedicines 13, no. 8: 1857. https://doi.org/10.3390/biomedicines13081857
APA StyleDardalas, I., Kosmidis, E. K., Lagoudaki, R., Kimiskidis, V. K., Samaras, T., Moysiadis, T., Kouvelas, D., & Pourzitaki, C. (2025). Low-Frequency rTMS and Diazepam Exert Synergistic Effects on the Excitability of an SH-SY5Y Model of Epileptiform Activity. Biomedicines, 13(8), 1857. https://doi.org/10.3390/biomedicines13081857








