Herpes Zoster Reactivation Following COVID-19 and the Risk of Renal, Infectious, and Autoimmune Complications: A Global Propensity-Matched Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Framework and Data Source
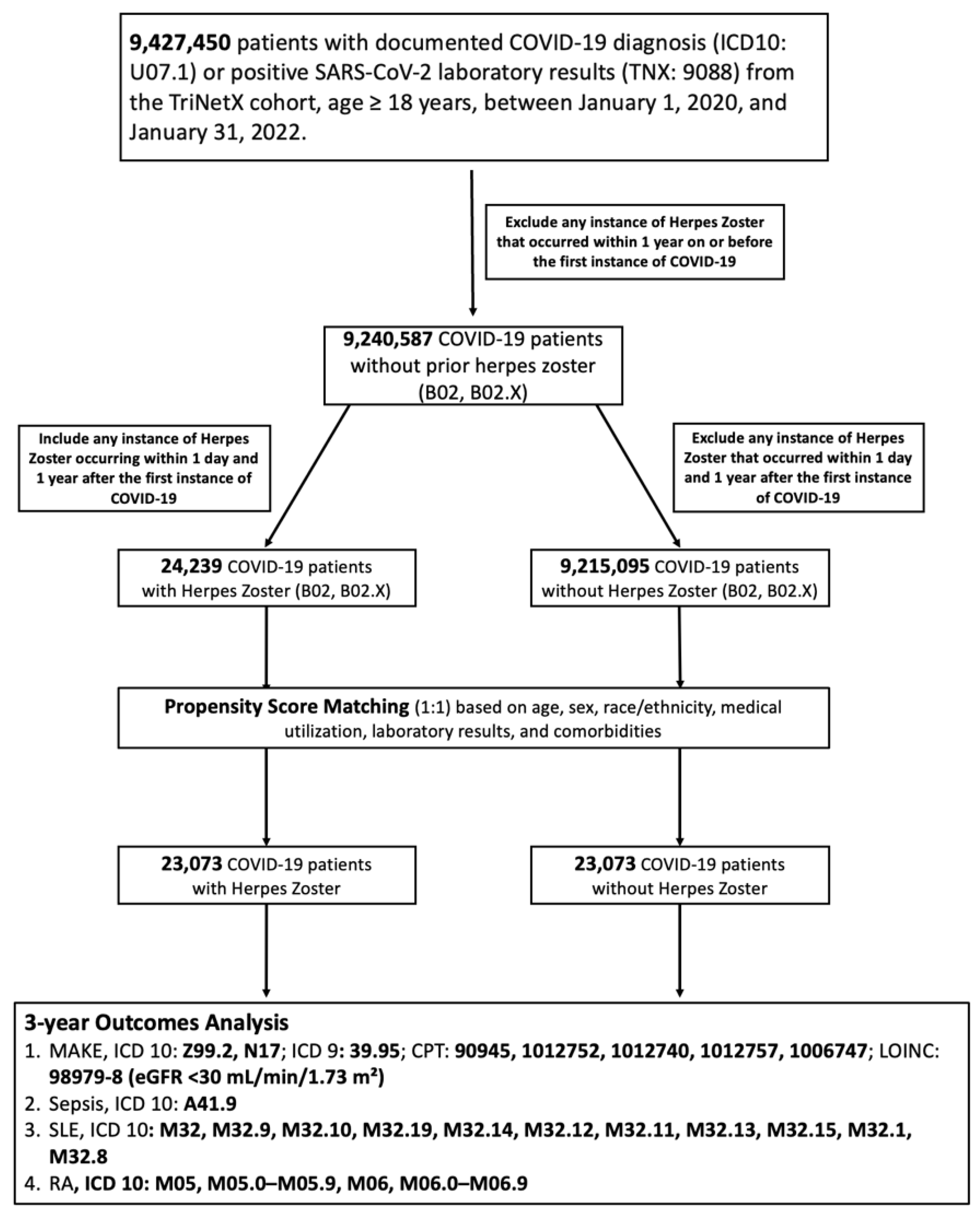
2.2. Cohort Assembly and Exposure Definition
2.3. Index Date and Outcome Observation Period
2.4. Propensity Score Matching
2.5. Study Outcomes
2.6. Statistical Analysis
2.7. Sensitivity Analyses
3. Results
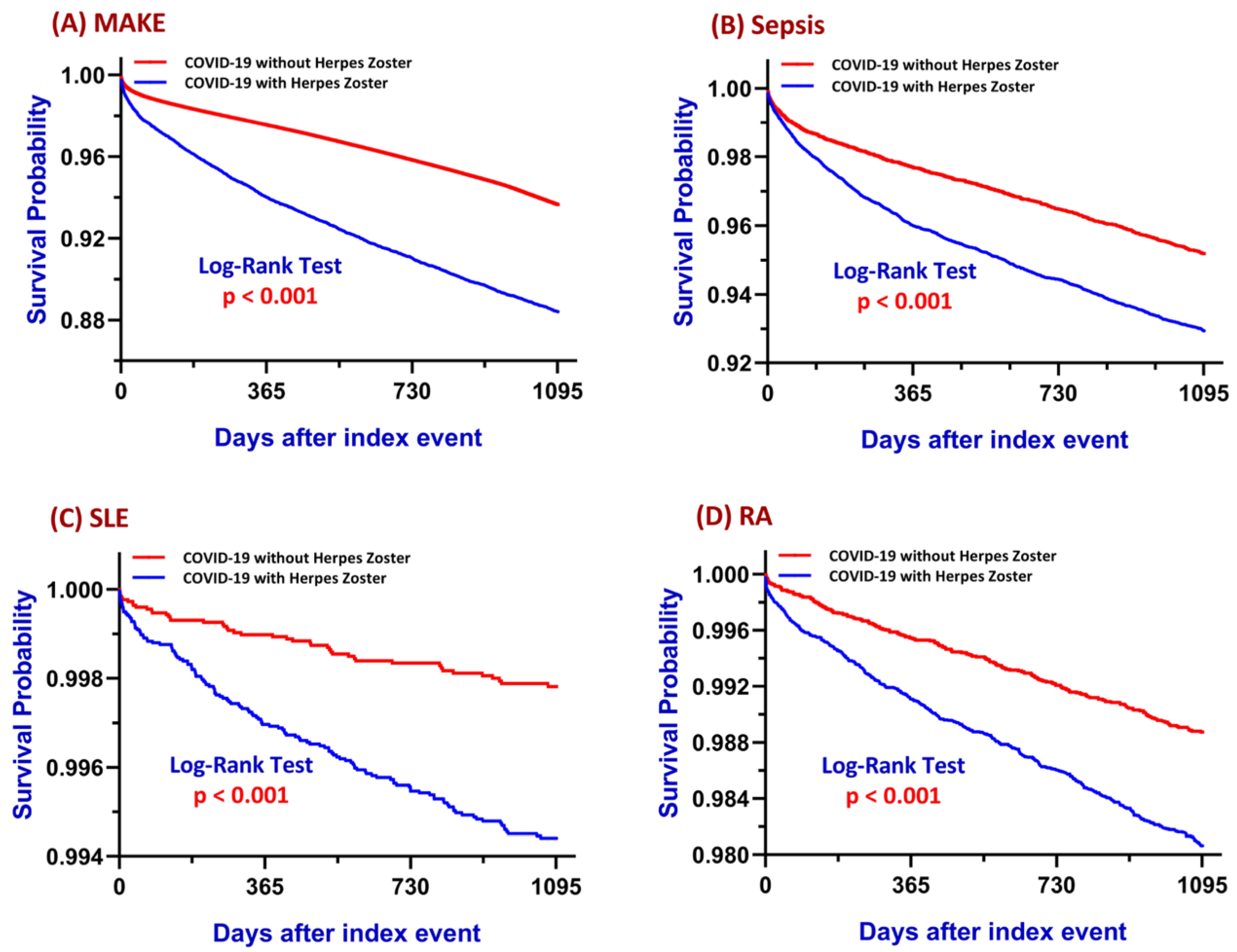
3.1. Major Adverse Kidney Events (MAKE)
3.2. Sepsis
3.3. Systemic Lupus Erythematosus
3.4. Rheumatoid Arthritis
3.5. Timing and Prevalence of COVID-19 Vaccination Relative to HZ
3.6. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKI | Acute Kidney Injury |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| BUN | Blood Urea Nitrogen |
| BMI | Body Mass Index |
| CKD | Chronic Kidney Disease |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration |
| CMV | Cytomegalovirus |
| COPD | Chronic Obstructive Pulmonary Disease |
| COVID-19 | Coronavirus Disease 2019 |
| CRP | C-Reactive Protein |
| CPT | Current Procedural Terminology |
| EBV | Epstein–Barr Virus |
| eGFR | Estimated Glomerular Filtration Rate |
| ESR | Erythrocyte Sedimentation Rate |
| HbA1c | Hemoglobin A1c |
| HDL | High-Density Lipoprotein |
| HSV | Herpes Simplex Virus |
| HZ | Herpes Zoster |
| IL-6 | Interleukin-6 |
| LDL | Low-Density Lipoprotein |
| MAKE | Major Adverse Kidney Events |
| PSM | Propensity Score Matching |
| RA | Rheumatoid Arthritis |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SLE | Systemic Lupus Erythematosus |
| TNF-α | Tumor Necrosis Factor Alpha |
| VZV | Varicella-Zoster Virus |
References
- Higgins, V.; Sohaei, D.; Diamandis, E.P.; Prassas, I. COVID-19: From an acute to chronic disease? Potential long-term health consequences. Crit. Rev. Clin. Lab. Sci. 2021, 58, 297–310. [Google Scholar] [CrossRef]
- Ramasamy, A.; Wang, C.; Brode, W.M.; Verduzco-Gutierrez, M.; Melamed, E. Immunologic and Autoimmune-Related Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection: Clinical Symptoms and Mechanisms of Disease. Phys. Med. Rehabil. Clin. N. Am. 2023, 34, 623–642. [Google Scholar] [CrossRef]
- Adhikari, A.; Maddumage, J.; Eriksson, E.M.; Annesley, S.J.; Lawson, V.A.; Bryant, V.L.; Gras, S. Beyond acute infection: Mechanisms underlying post-acute sequelae of COVID-19 (PASC). Med. J. Aust. 2024, 221 (Suppl. 9), S40–S48. [Google Scholar] [CrossRef]
- Narasimhan, M.; Ramakrishnan, R.; Durai, P.C.T.; Sneha, B. Association between COVID-19 infection and herpes zoster: A case series. J. Family Med. Prim. Care 2023, 12, 2516–2519. [Google Scholar] [CrossRef]
- Pona, A.; Jiwani, R.A.; Afriyie, F.; Labbe, J.; Cook, P.P.; Mao, Y. Herpes zoster as a potential complication of coronavirus disease 2019. Dermatol. Ther. 2020, 33, e13930. [Google Scholar] [CrossRef]
- Saati, A.; Al-Husayni, F.; Malibari, A.A.; Bogari, A.A.; Alharbi, M. Herpes Zoster Co-Infection in an Immunocompetent Patient With COVID-19. Cureus 2020, 12, e8998. [Google Scholar] [CrossRef]
- Puri, P.; Parnami, P.; Athwal, P.S.S.; Kumari, S.; Kumar, C.; Suri, Y. COVID-19 Rekindling Herpes Zoster in an Immunocompetent Patient. Cureus 2021, 13, e18049. [Google Scholar] [CrossRef]
- Oh, J.; Lee, M.; Kim, M.; Kim, H.J.; Lee, S.W.; Rhee, S.Y.; Koyanagi, A.; Smith, L.; Kim, M.S.; Lee, H.; et al. Incident allergic diseases in post-COVID-19 condition: Multinational cohort studies from South Korea, Japan and the UK. Nat. Commun. 2024, 15, 2830. [Google Scholar] [CrossRef]
- Muñoz-Quiles, C.; López-Lacort, M.; Díez-Domingo, J.; Orrico-Sánchez, A. Herpes zoster risk and burden of disease in immunocompromised populations: A population-based study using health system integrated databases, 2009–2014. BMC Infect. Dis. 2020, 20, 905. [Google Scholar] [CrossRef]
- Piazza, M.F.; Paganino, C.; Amicizia, D.; Trucchi, C.; Orsi, A.; Astengo, M.; Romairone, P.; Simonetti, S.; Icardi, G.; Ansaldi, F. The Unknown Health Burden of Herpes Zoster Hospitalizations: The Effect on Chronic Disease Course in Adult Patients ≥50 Years. Vaccines 2020, 8, 20. [Google Scholar] [CrossRef]
- Erskine, N.; Tran, H.; Levin, L.; Ulbricht, C.; Fingeroth, J.; Kiefe, C.; Goldberg, R.J.; Singh, S. A systematic review and meta-analysis on herpes zoster and the risk of cardiac and cerebrovascular events. PLoS ONE 2017, 12, e0181565. [Google Scholar] [CrossRef]
- Jin, Y.; Ji, W.; Yang, H.; Chen, S.; Zhang, W.; Duan, G. Endothelial activation and dysfunction in COVID-19: From basic mechanisms to potential therapeutic approaches. Signal Transduct. Target. Ther. 2020, 5, 293. [Google Scholar] [CrossRef]
- Nägele, M.P.; Haubner, B.; Tanner, F.C.; Ruschitzka, F.; Flammer, A.J. Endothelial dysfunction in COVID-19: Current findings and therapeutic implications. Atherosclerosis 2020, 314, 58–62. [Google Scholar] [CrossRef]
- Xu, S.W.; Ilyas, I.; Weng, J.P. Endothelial dysfunction in COVID-19: An overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol. Sin. 2023, 44, 695–709. [Google Scholar] [CrossRef]
- Simonnet, A.; Engelmann, I.; Moreau, A.S.; Garcia, B.; Six, S.; El Kalioubie, A.; Robriquet, L.; Hober, D.; Jourdain, M. High incidence of Epstein-Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infect. Dis. Now 2021, 51, 296–299. [Google Scholar] [CrossRef]
- Libert, N.; Bigaillon, C.; Chargari, C.; Bensalah, M.; Muller, V.; Merat, S.; de Rudnicki, S. Epstein-Barr virus reactivation in critically ill immunocompetent patients. Biomed. J. 2015, 38, 70–76. [Google Scholar] [CrossRef]
- Mattei, A.; Schiavoni, L.; Riva, E.; Ciccozzi, M.; Veralli, R.; Urselli, A.; Citriniti, V.; Nenna, A.; Pascarella, G.; Costa, F.; et al. Epstein-Barr virus, Cytomegalovirus, and Herpes Simplex-1/2 reactivations in critically ill patients with COVID-19. Intensive Care Med. Exp. 2024, 12, 40. [Google Scholar] [CrossRef]
- Lachance, P.; Chen, J.; Featherstone, R.; Sligl, W.I. Association Between Cytomegalovirus Reactivation and Clinical Outcomes in Immunocompetent Critically Ill Patients: A Systematic Review and Meta-Analysis. Open Forum Infect. Dis. 2017, 4, ofx029. [Google Scholar] [CrossRef]
- Kernan, K.F.; Carcillo, J.A. Hyperferritinemia and inflammation. Int. Immunol. 2017, 29, 401–409. [Google Scholar] [CrossRef]
- Giemza-Stokłosa, J.; Islam, M.A.; Kotyla, P.J. Hyperferritinaemia: An Iron Sword of Autoimmunity. Curr. Pharm. Des. 2019, 25, 2909–2918. [Google Scholar] [CrossRef]
- Dartiguelongue, J.B. Biological significance and clinical utility of lactate in sepsis. Arch. Argent. Pediatr. 2024, 122, e202310149. [Google Scholar] [CrossRef]
- Loiacono, L.A.; Shapiro, D.S. Detection of hypoxia at the cellular level. Crit. Care Clin. 2010, 26, 409–421. [Google Scholar] [CrossRef]
- Dupuis, M.L.; Maselli, A.; Pagano, M.T.; Pierdominici, M.; Ortona, E. Immune response and autoimmune diseases: A matter of sex. Ital. J. Gend. Specif. Med. 2019, 5, 11–20. [Google Scholar] [CrossRef]
- Straub, R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef]
- Miquel, C.H.; Faz-Lopez, B.; Guéry, J.C. Influence of X chromosome in sex-biased autoimmune diseases. J. Autoimmun. 2023, 137, 102992. [Google Scholar] [CrossRef]
- Syrett, C.M.; Anguera, M.C. When the balance is broken: X-linked gene dosage from two X chromosomes and female-biased autoimmunity. J. Leukoc. Biol. 2019, 106, 919–932. [Google Scholar] [CrossRef]
- Fairweather, D.; Rose, N.R. Women and autoimmune diseases. Emerg. Infect. Dis. 2004, 10, 2005–2011. [Google Scholar] [CrossRef]
- Fairweather, D.; Frisancho-Kiss, S.; Rose, N.R. Sex differences in autoimmune disease from a pathological perspective. Am. J. Pathol. 2008, 173, 600–609. [Google Scholar] [CrossRef]
- Savel, R.H.; Simon, R.J.; Kupfer, Y. Unraveling the Mysterious Relationship Between Obesity and Outcomes in Patients With Sepsis. Crit. Care Med. 2016, 44, 2104–2105. [Google Scholar] [CrossRef]
- Roth, J.; Sahota, N.; Patel, P.; Mehdi, S.F.; Wiese, M.M.; Mahboob, H.B.; Bravo, M.; Eden, D.J.; Bashir, M.A.; Kumar, A.; et al. Obesity paradox, obesity orthodox, and the metabolic syndrome: An approach to unity. Mol. Med. 2017, 22, 873–885. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Raimondo, A.; Lembo, S.; Fausti, F.; Dini, V.; Costanzo, A.; Monfrecola, G.; Balato, N.; Ayala, F.; Romanelli, M.; et al. Crosstalk between skin inflammation and adipose tissue-derived products: Pathogenic evidence linking psoriasis to increased adiposity. Expert Rev. Clin. Immunol. 2016, 12, 1299–1308. [Google Scholar] [CrossRef]
- Toprak, O.; Turan, O.F.; Bozyel, E.A.; Alp, B.; Ucdu, G.Z. Dialysis-requiring acute kidney injury and electrolyte imbalances as a result of prodromal herpes zoster in a kidney transplant recipient. Transpl. Infect. Dis. 2021, 23, e13578. [Google Scholar] [CrossRef]
- Ayus, J.C.; Negri, A.L.; Moritz, M.L.; Lee, K.M.; Caputo, D.; Borda, M.E.; Go, A.S.; Eghi, C. Hyponatremia, Inflammation at Admission, and Mortality in Hospitalized COVID-19 Patients: A Prospective Cohort Study. Front. Med. 2021, 8, 748364. [Google Scholar] [CrossRef]
- Al-Alawi, A.M.; Al-Maqbali, J.S.; Al-Adawi, M.; Al-Jabri, A.; Falhammar, H. Incidence, patterns, risk factors and clinical outcomes of intravenous acyclovir induced nephrotoxicity. Saudi. Pharm. J. 2022, 30, 874–877. [Google Scholar] [CrossRef]
- Ronco, C.; Reis, T.; Husain-Syed, F. Management of acute kidney injury in patients with COVID-19. Lancet. Respir. Med. 2020, 8, 738–742. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Shen, C.-H.; Wang, S.-H.; Wei, K.-Y.; Huang, Y.-H. Varicella-Zoster Virus-Induced Rhabdomyolysis: A Case Report and Literature Review. J. Med. Sci. 2020, 40, 141–144. [Google Scholar] [CrossRef]
- Pirounaki, M.; Liatsos, G.; Elefsiniotis, I.; Skounakis, M.; Moulakakis, A. Unusual onset of varicella zoster reactivation with meningoencephalitis, followed by rhabdomyolysis and renal failure in a young, immunocompetent patient. Scand. J. Infect. Dis. 2007, 39, 90–93. [Google Scholar] [CrossRef]
- Chen, J.; Song, J.; Dai, L.; Post, S.R.; Qin, Z. SARS-CoV-2 infection and lytic reactivation of herpesviruses: A potential threat in the postpandemic era? J. Med. Virol. 2022, 94, 5103–5111. [Google Scholar] [CrossRef]
- Xu, R.; Zhou, Y.; Cai, L.; Wang, L.; Han, J.; Yang, X.; Chen, J.; Chen, J.; Ma, C.; Shen, L. Co-reactivation of the human herpesvirus alpha subfamily (herpes simplex virus-1 and varicella zoster virus) in a critically ill patient with COVID-19. Br. J. Dermatol. 2020, 183, 1145–1147. [Google Scholar] [CrossRef]
- Mallet, F.; Diouf, L.; Meunier, B.; Perret, M.; Reynier, F.; Leissner, P.; Quemeneur, L.; Griffiths, A.D.; Moucadel, V.; Pachot, A.; et al. Herpes DNAemia and TTV Viraemia in Intensive Care Unit Critically Ill Patients: A Single-Centre Prospective Longitudinal Study. Front. Immunol. 2021, 12, 698808. [Google Scholar] [CrossRef]
- Doughty, L. Adaptive immune function in critical illness. Curr. Opin. Pediatr. 2016, 28, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Jensen, I.J.; Sjaastad, F.V.; Griffith, T.S.; Badovinac, V.P. Sepsis-Induced T Cell Immunoparalysis: The Ins and Outs of Impaired T Cell Immunity. J. Immunol. 2018, 200, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Inthasot, V.; Goushchi, A.; Lazzaroni, S.; Papaleo, A.; Galdon, M.G.; Chochrad, D. Fatal Septic Shock Associated with Herpes Simplex Virus Hepatitis: A Case Report. Eur. J. Case Rep. Intern. Med. 2018, 5, 000982. [Google Scholar] [CrossRef] [PubMed]
- Kahlenberg, J.M.; Kaplan, M.J. Mechanisms of premature atherosclerosis in rheumatoid arthritis and lupus. Annu. Rev. Med. 2013, 64, 249–263. [Google Scholar] [CrossRef]
- Sumichika, Y.; Temmoku, J.; Saito, K.; Yoshida, S.; Matsumoto, H.; Watanabe, G.; Utsumi, A.; Fujita, Y.; Matsuoka, N.; Asano, T.; et al. New-onset Systemic Lupus Erythematosus Manifestation Following COVID-19: A Case Report and Literature Review. Intern. Med. 2024, 63, 1491–1498. [Google Scholar] [CrossRef]
- Ramachandran, L.; Dontaraju, V.S.; Troyer, J.; Sahota, J. New onset systemic lupus erythematosus after COVID-19 infection: A case report. AME Case Rep. 2022, 6, 14. [Google Scholar] [CrossRef]
- Crow, M.K. Type I interferon in the pathogenesis of lupus. J. Immunol. 2014, 192, 5459–5468. [Google Scholar] [CrossRef]
- Crow, M.K.; Olferiev, M.; Kirou, K.A. Type I Interferons in Autoimmune Disease. Annu. Rev. Pathol. 2019, 14, 369–393. [Google Scholar] [CrossRef]
- Pope, J.E.; Krizova, A.; Ouimet, J.M.; Goodwin, J.L.; Lankin, M. Close association of herpes zoster reactivation and systemic lupus erythematosus (SLE) diagnosis: Case-control study of patients with SLE or noninflammatory musculoskeletal disorders. J. Rheumatol. 2004, 31, 274–279. [Google Scholar]
- Hu, S.C.; Lin, C.L.; Lu, Y.W.; Chen, G.S.; Yu, H.S.; Wu, C.S.; Lan, C.C. Lymphopaenia, anti-Ro/anti-RNP autoantibodies, renal involvement and cyclophosphamide use correlate with increased risk of herpes zoster in patients with systemic lupus erythematosus. Acta Derm. Venereol. 2013, 93, 314–318. [Google Scholar] [CrossRef]
- Kondo, N.; Kuroda, T.; Kobayashi, D. Cytokine Networks in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 10922. [Google Scholar] [CrossRef] [PubMed]
- Bokarewa, M.; Tarkowski, A.; Magnusson, M. Pathological survivin expression links viral infections with pathogenesis of erosive rheumatoid arthritis. Scand. J. Immunol. 2007, 66, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Caielli, S.; Wan, Z.; Pascual, V. Systemic Lupus Erythematosus Pathogenesis: Interferon and Beyond. Annu. Rev. Immunol. 2023, 41, 533–560. [Google Scholar] [CrossRef] [PubMed]
- Rose, T.; Dörner, T. Drivers of the immunopathogenesis in systemic lupus erythematosus. Best Pr. Res. Clin. Rheumatol. 2017, 31, 321–333. [Google Scholar] [CrossRef]
- Eloranta, M.L.; Rönnblom, L. Cause and consequences of the activated type I interferon system in SLE. J. Mol. Med. 2016, 94, 1103–1110. [Google Scholar] [CrossRef]
- El-Banna, H.S.; Gado, S.E. Vitamin D: Does it help Tregs in active rheumatoid arthritis patients. Expert Rev. Clin. Immunol. 2020, 16, 847–853. [Google Scholar] [CrossRef]
- Dankers, W.; Davelaar, N.; van Hamburg, J.P.; van de Peppel, J.; Colin, E.M.; Lubberts, E. Human Memory Th17 Cell Populations Change Into Anti-inflammatory Cells With Regulatory Capacity Upon Exposure to Active Vitamin D. Front. Immunol. 2019, 10, 1504. [Google Scholar] [CrossRef]
- Sen, D.; Ranganathan, P. Vitamin D in rheumatoid arthritis: Panacea or placebo? Discov. Med. 2012, 14, 311–319. [Google Scholar]
- Shafiee, A.; Amini, M.J.; Arabzadeh Bahri, R.; Jafarabady, K.; Salehi, S.A.; Hajishah, H.; Mozhgani, S.H. Herpesviruses reactivation following COVID-19 vaccination: A systematic review and meta-analysis. Eur. J. Med. Res. 2023, 28, 278. [Google Scholar] [CrossRef]
- Munasinghe, B.M.; Fernando, U.; Mathurageethan, M.; Sritharan, D. Reactivation of varicella-zoster virus following mRNA COVID-19 vaccination in a patient with moderately differentiated adenocarcinoma of rectum: A case report. SAGE Open Med. Case Rep. 2022, 10, 2050313x221077737. [Google Scholar] [CrossRef]
- Diesel, J.; Sterrett, N.; Dasgupta, S.; Kriss, J.L.; Barry, V.; Vanden Esschert, K.; Whiteman, A.; Cadwell, B.L.; Weller, D.; Qualters, J.R.; et al. COVID-19 Vaccination Coverage Among Adults—United States, December 14, 2020–May 22, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Carbone, T.; Picerno, V.; Pafundi, V.; Esposito, E.; Leccese, P.; Padula, A.A.; D’Angelo, S. Impact of the COVID-19 Pandemic on the Appropriateness of Diagnostic Pathways of Autoimmune Rheumatic Diseases. J. Rheumatol. 2022, 49, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Sloan, M.; Gordon, C.; Harwood, R.; Lever, E.; Wincup, C.; Bosley, M.; Brimicombe, J.; Pilling, M.; Sutton, S.; Holloway, L.; et al. The impact of the COVID-19 pandemic on the medical care and health-care behaviour of patients with lupus and other systemic autoimmune diseases: A mixed methods longitudinal study. Rheumatol. Adv. Pract. 2021, 5, rkaa072. [Google Scholar] [CrossRef] [PubMed]

| Before Matching | After Matching b | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics a | COVID-19 with HZ (n = 5209) | COVID-19 Without HZ (n = 1,848,644) | p Value | SMD | COVID-19 with HZ (n = 5208) | COVID-19 Without HZ (n = 5208) | p Value | SMD |
| Demographics | ||||||||
| Age at Index, mean ± SD | 51.2 ± 3.2 | 50.9 ± 3.2 | <0.01 * | 0.09 | 51.2 ± 3.2 | 51.2 ± 3.2 | 0.60 | 0.01 * |
| Female (%) | 63.1% | 53.9% | <0.01 * | 0.19 | 63.1% | 62.3% | 0.38 | 0.02 |
| Male (%) | 33.8% | 44.4% | <0.01 * | 0.22 | 33.8% | 34.7% | 0.38 | 0.02 |
| White (%) | 67.2% | 55.3% | <0.01 * | 0.25 | 67.3% | 69.1% | 0.05 * | 0.04 |
| Black or African American (%) | 10.4% | 14.2% | <0.01 * | 0.12 | 10.4% | 10.3% | 0.87 | 0.01 |
| Asian (%) | 5.4% | 4.2% | <0.01 * | 0.06 | 5.4% | 5.0% | 0.29 | 0.02 |
| Diagnosis (%) | ||||||||
| Diabetes mellitus | 12.8% | 6.5% | <0.01 * | 0.22 | 12.7% | 12.4% | 0.55 | 0.01 |
| Hypertensive diseases | 24.1% | 12.9% | <0.01 * | 0.29 | 24.1% | 23.6% | 0.55 | 0.01 |
| Cerebrovascular diseases | 2.1% | 1.0% | <0.01 * | 0.09 | 2.1% | 2.0% | 0.68 | 0.01 |
| Medication (%) | ||||||||
| Blood glucose regulation agents | 11.6% | 6.0% | <0.01 * | 0.20 | 11.6% | 11.3% | 0.58 | 0.01 |
| Beta blockers | 9.3% | 4.9% | <0.01 * | 0.17 | 9.3% | 8.9% | 0.50 | 0.01 |
| Antilipidemic agents | 11.9% | 6.8% | <0.01 * | 0.18 | 11.9% | 12.3% | 0.49 | 0.01 |
| Angiotensin II inhibitor | 5.0% | 3.0% | <0.01 * | 0.10 | 5.0% | 4.6% | 0.39 | 0.02 |
| Diuretics | 9.6% | 5.4% | <0.01 * | 0.16 | 9.6% | 9.5% | 0.84 | 0.01 |
| Laboratory (mean ± SD) | ||||||||
| Sodium, mmol/L | 138.8 ± 3.0 | 138.9 ± 3.0 | 0.74 | 0.01 | 138.8 ± 3.0 | 138.8 ± 3.1 | 0.41 | 0.03 |
| Potassium, mmol/L | 4.1 ± 0.5 | 4.1 ± 0.5 | 0.44 | 0.02 | 4.1 ± 0.5 | 4.2 ± 0.5 | 0.20 | 0.04 |
| Hemoglobin, g/dL | 13.2 ± 2.0 | 13.5 ± 2.0 | <0.01 * | 0.13 | 13.2 ± 2.0 | 13.4 ±1.9 | 0.01 * | 0.09 |
| Hematocrit, % | 39.8 ± 6.7 | 39.7 ± 8.6 | 0.37 | 0.02 | 39.8 ± 6.7 | 40.0 ± 7.2 | 0.52 | 0.02 |
| Leukocytes, 103/uL | 60.9 ± 462.2 | 20.2 ± 229.9 | <0.01 * | 0.11 | 58.9 ± 454.4 | 25.8 ± 274.4 | 0.01 * | 0.09 |
| Platelets, 103/uL | 257.2 ± 87.5 | 260.5 ± 84.5 | 0.08 | 0.04 | 257.2 ± 87.5 | 258.0 ± 83.4 | 0.75 | 0.01 |
| Lymphocytes/100 WBC, % | 27.2 ± 11.4 | 27.1 ± 10.9 | 0.81 | 0.01 | 27.2 ± 11.4 | 27.3 ± 10.9 | 0.75 | 0.01 |
| Iron, ug/dL | 71.0 ± 43.2 | 73.4 ± 44.7 | 0.41 | 0.06 | 71.0 ± 43.2 | 71.4 ± 40.9 | 0.93 | 0.01 |
| Ferritin, ng/mL | 317.4 ± 682.6 | 271.4 ± 868.7 | 0.40 | 0.06 | 317.0 ± 684.0 | 235.0 ± 482.5 | 0.13 | 0.14 |
| Creatinine, mg/dL | 1.1 ± 1.3 | 1.0 ± 1.5 | 0.16 | 0.03 | 1.1 ± 1.3 | 1.0 ± 1.0 | 0.01 * | 0.08 |
| BUN, mg/dL | 16.7 ± 11.4 | 15.7 ± 9.4 | <0.01 * | 0.10 | 16.7 ± 11.4 | 15.9 ± 8.6 | 0.01 * | 0.09 |
| Bicarbonate, mmol/L | 25.7 ± 3.1 | 25.8 ± 3.2 | 0.19 | 0.03 | 25.7 ± 3.1 | 25.8 ± 3.3 | 0.33 | 0.03 |
| Glucose, mg/dL | 118.2 ± 58.2 | 116.9 ± 55.2 | 0.26 | 0.02 | 118.2 ± 58.2 | 120.8 ± 63.6 | 0.17 | 0.04 |
| ALT, U/L | 28.2 ± 40.2 | 31.3 ± 69.9 | 0.05 * | 0.06 | 28.1 ± 39.9 | 31.7 ± 51.3 | 0.01 * | 0.08 |
| AST, U/L | 27.2 ± 28.4 | 30.4 ± 96.0 | 0.14 | 0.05 | 27.1± 28.2 | 29.4 ± 37.1 | 0.03 * | 0.07 |
| Alk phosphatase, U/L | 89.3 ± 45.6 | 88.1 ± 61.0 | 0.41 | 0.02 | 89.3 ± 45.6 | 89.0 ± 71.4 | 0.88 | 0.01 |
| Albumin, g/dL | 4.1 ± 0.5 | 4.1 ± 0.5 | 0.33 | 0.02 | 4.1 ± 0.5 | 4.1 ± 0.5 | 0.54 | 0.02 |
| Protein, g/dL | 7.1 ± 0.7 | 7.2 ± 0.8 | 0.01 * | 0.09 | 7.1 ± 0.7 | 7.1 ± 0.7 | 0.20 | 0.04 |
| Cholesterol, mg/dL | 192.2 ± 43.9 | 190.4 ± 44.7 | 0.19 | 0.04 | 192.2 ± 43.9 | 189.3 ± 44.6 | 0.13 | 0.065 |
| LDL Cholesterol, mg/dL | 111.2 ± 36.3 | 111.0 ± 37.3 | 0.81 | 0.01 | 111.2 ± 36.3 | 109.7 ± 38.3 | 0.34 | 0.04 |
| HDL Cholesterol, mg/dL | 49.1 ± 20.7 | 48.5 ± 20.1 | 0.37 | 0.03 | 49.1 ± 20.7 | 48.1 ± 20.5 | 0.27 | 0.05 |
| Triglyceride, mg/dL | 151.8 ± 128.5 | 150.2 ± 145.5 | 0.72 | 0.01 | 151.8 ± 128.5 | 155.6 ± 153.0 | 0.53 | 0.03 |
| HbA1c, % | 6.7 ± 2.0 | 6.6 ±1.9 | 0.13 | 0.05 | 6.7 ± 2.0 | 6.7 ± 2.1 | 0.46 | 0.03 |
| Calcidiol, ng/mL | 34.2 ± 16.8 | 32.7 ± 16.7 | 0.15 | 0.09 | 34.2 ± 16.8 | 34.7 ± 18.1 | 0.79 | 0.03 |
| CRP, mg/L | 17.7 ± 49.4 | 24.6 ± 51.3 | 0.02 * | 0.14 | 17.7 ± 49.4 | 19.2 ± 40.2 | 0.69 | 0.04 |
| ESR, mm/h | 22.8 ± 23.9 | 25.0 ± 25.5 | 0.17 | 0.09 | 22.8 ± 23.9 | 22.4 ± 22.2 | 0.87 | 0.02 |
| Lactate, mmol/L | 1.4 ± 0.7 | 1.6 ± 1.5 | 0.32 | 0.12 | 1.4 ± 0.7 | 1.5± 0.7 | 0.40 | 0.12 |
| Urate, mg/dL | 5.6 ± 1.8 | 5.8 ± 2.1 | 0.52 | 0.06 | 5.6 ± 1.8 | 5.6 ± 1.8 | 0.98 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chien, M.-H.; Wang, J.; Lu, K.-C.; Lu, C.-L. Herpes Zoster Reactivation Following COVID-19 and the Risk of Renal, Infectious, and Autoimmune Complications: A Global Propensity-Matched Cohort Study. Biomedicines 2025, 13, 1628. https://doi.org/10.3390/biomedicines13071628
Chien M-H, Wang J, Lu K-C, Lu C-L. Herpes Zoster Reactivation Following COVID-19 and the Risk of Renal, Infectious, and Autoimmune Complications: A Global Propensity-Matched Cohort Study. Biomedicines. 2025; 13(7):1628. https://doi.org/10.3390/biomedicines13071628
Chicago/Turabian StyleChien, Ming-Hung, Joshua Wang, Kuo-Cheng Lu, and Chien-Lin Lu. 2025. "Herpes Zoster Reactivation Following COVID-19 and the Risk of Renal, Infectious, and Autoimmune Complications: A Global Propensity-Matched Cohort Study" Biomedicines 13, no. 7: 1628. https://doi.org/10.3390/biomedicines13071628
APA StyleChien, M.-H., Wang, J., Lu, K.-C., & Lu, C.-L. (2025). Herpes Zoster Reactivation Following COVID-19 and the Risk of Renal, Infectious, and Autoimmune Complications: A Global Propensity-Matched Cohort Study. Biomedicines, 13(7), 1628. https://doi.org/10.3390/biomedicines13071628






