Zinc Ions Inactivate Influenza Virus Hemagglutinin and Prevent Receptor Binding
Abstract
1. Introduction
2. Materials and Methods
2.1. Influenza Viruses and Cells
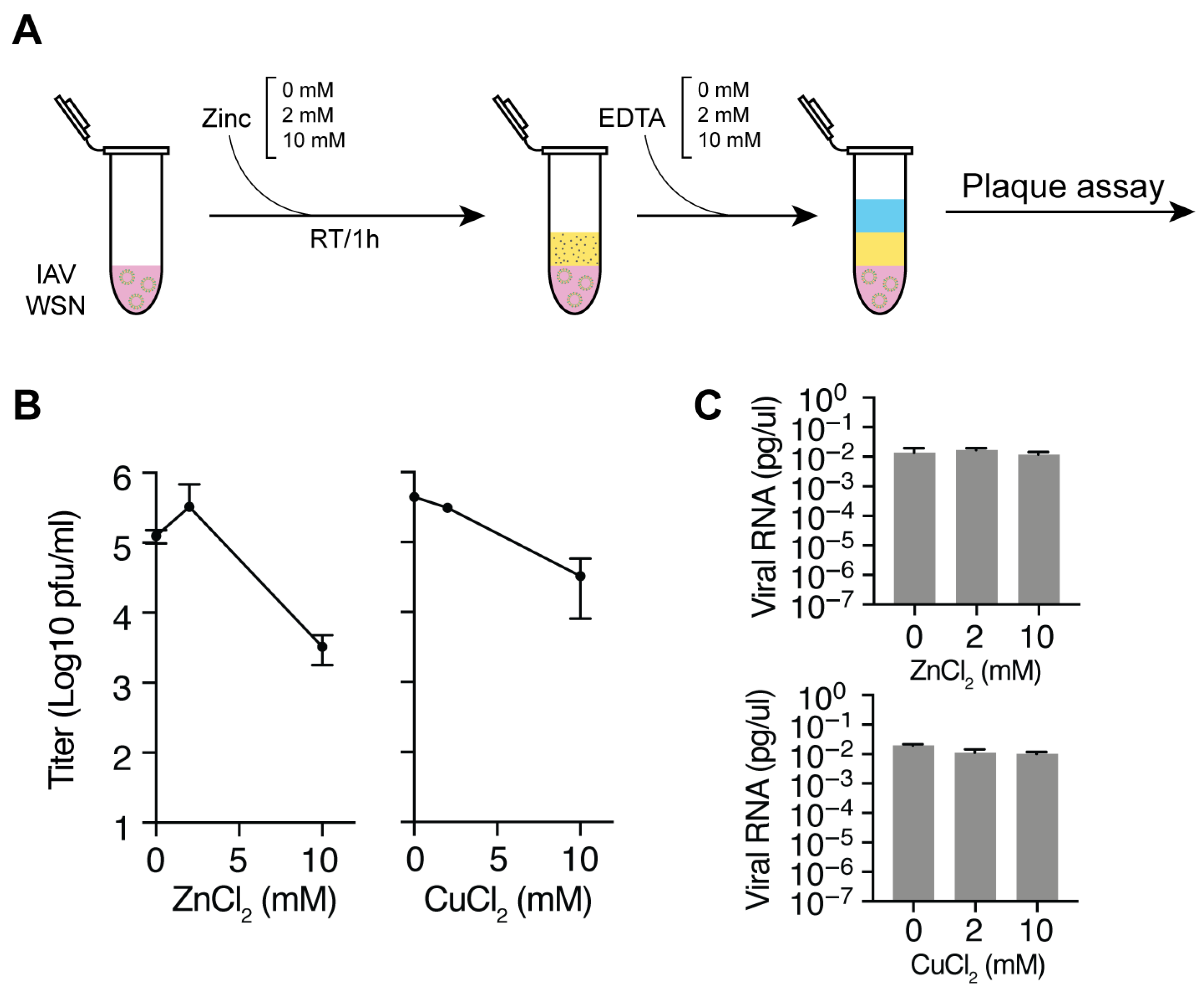
2.2. Zinc Incubation and Neutralization with EDTA
2.3. Hemagglutination Assays
2.4. Passaging Assays
2.5. Reverse-Transcription PCR
2.6. Data Analysis and Statistics
3. Results
3.1. Zinc and Copper Ions Reduce IAV Titers
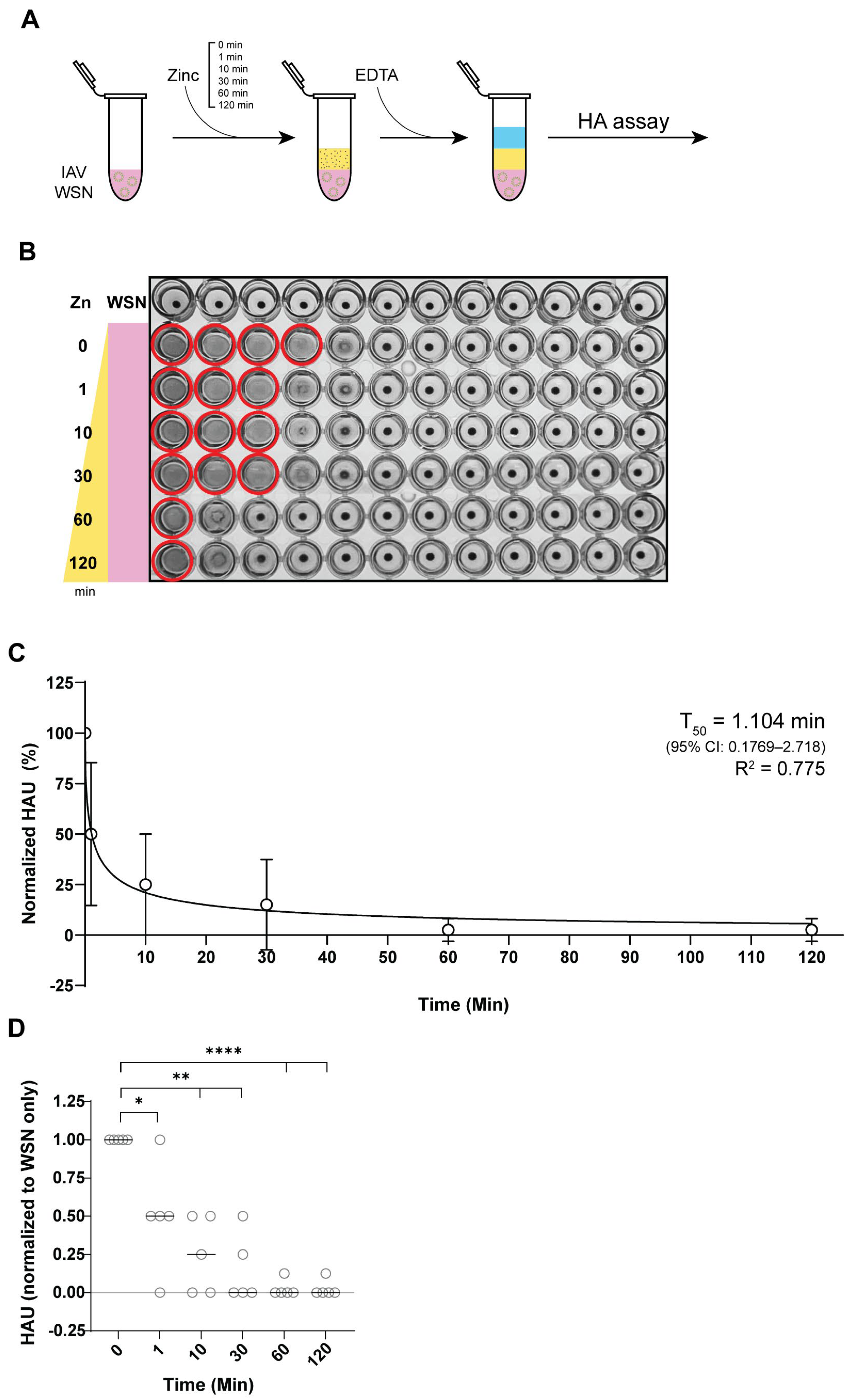
3.2. Zinc-Mediated Inhibition of IAV Hemagglutination
3.3. Zinc Reduces IAV Hemagglutination Within 1 Minute of Incubation
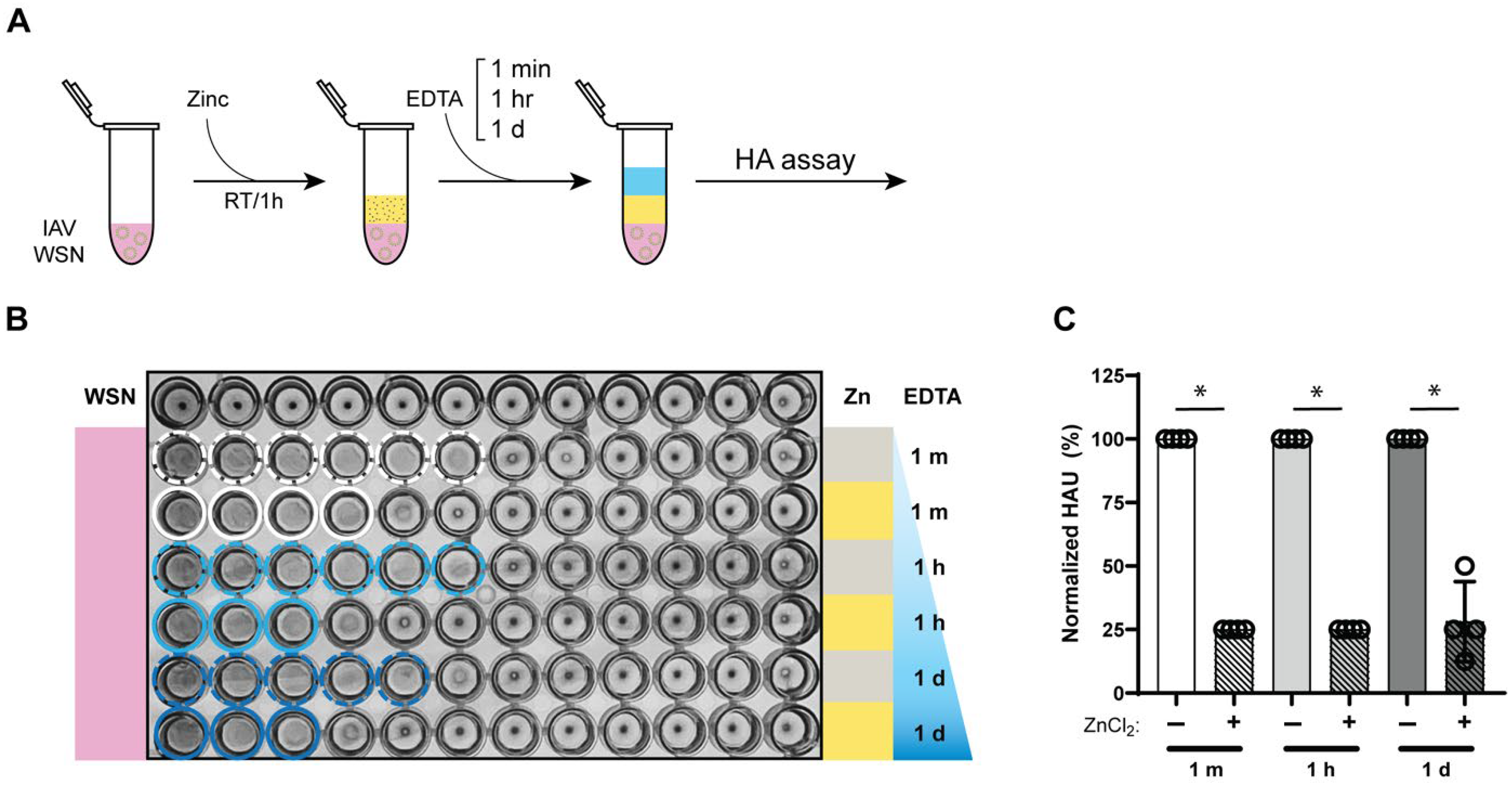
3.4. Zinc-Mediated Reduction in Influenza a Virus Hemagglutination Retained for 24 h
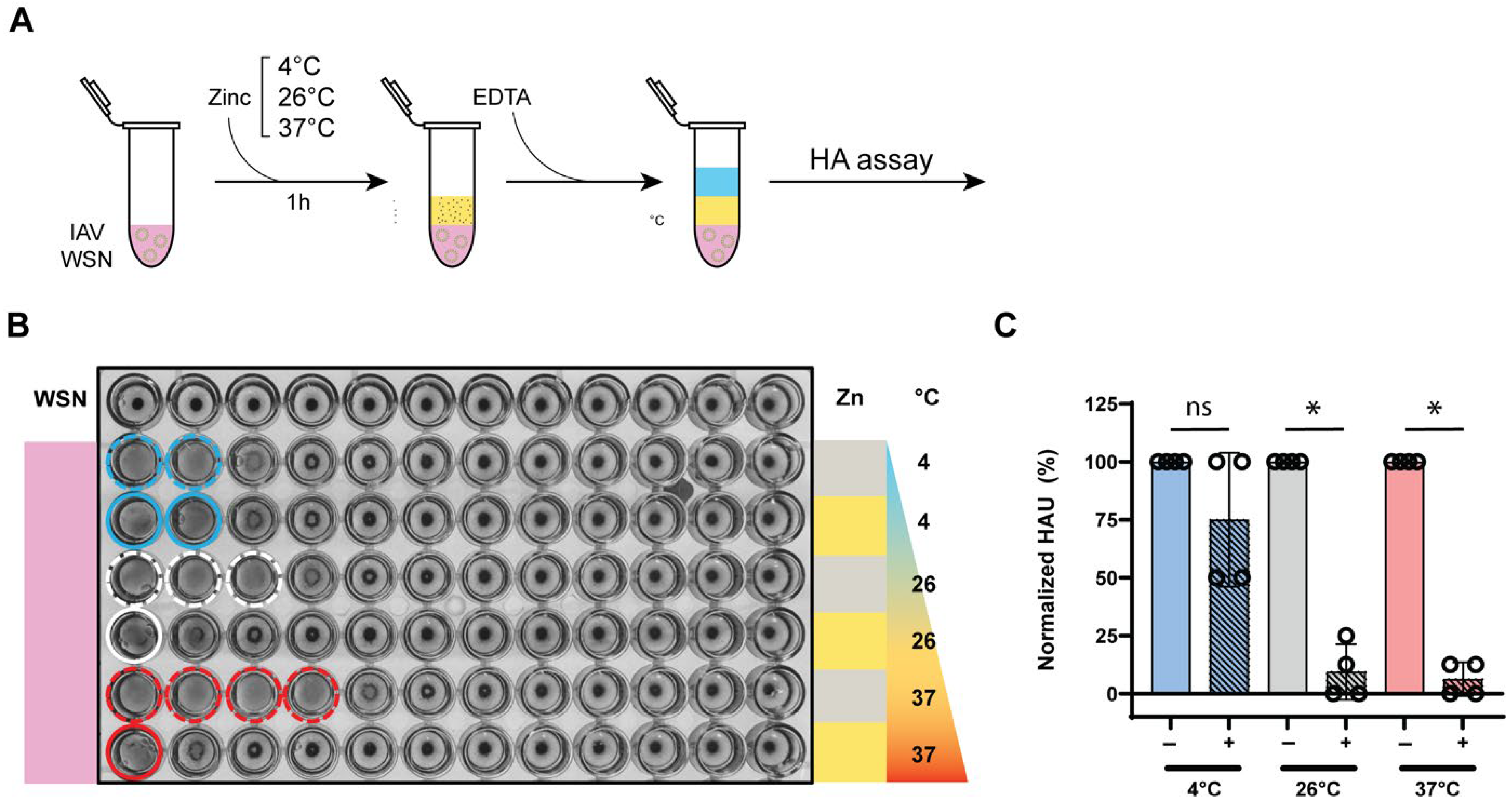
3.5. Zinc-Mediated Hemagglutination Inhibition Is Maintained Across Different Temperatures
3.6. Virus Passaging After Zinc Exposure Does Not Lead to Resistance Emergence
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korneta, P.; Rostek, K. The Impact of the SARS-CoV-19 Pandemic on the Global Gross Domestic Product. Int. J. Environ. Res. Public. Health 2021, 18, 5246. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K.; Kash, J.C. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe 2010, 7, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Telford, C.T.; Amman, B.R.; Towner, J.S.; Montgomery, J.M.; Lessler, J.; Shoemaker, T. Predictive Model for Estimating Annual Ebolavirus Spillover Potential. Emerg. Infect. Dis. 2025, 31, 689–698. [Google Scholar] [CrossRef]
- Horman, W.S.J.; Nguyen, T.H.O.; Kedzierska, K.; Bean, A.G.D.; Layton, D.S. The Drivers of Pathology in Zoonotic Avian Influenza: The Interplay Between Host and Pathogen. Front. Immunol. 2018, 9, 1812. [Google Scholar] [CrossRef]
- Wille, M.; Holmes, E.C. The Ecology and Evolution of Influenza Viruses. Cold Spring Harb. Perspect. Med. 2019, 10, a038489. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, X.-R.; Zhang, Y.-L.; Jiang, R.; Wang, X.; Guan, Z.-Q.; Li, Q.; Yao, Y.-L.; Gong, Q.-C.; Geng, R.; et al. Strategy To Assess Zoonotic Potential Reveals Low Risk Posed by SARS-Related Coronaviruses from Bat and Pangolin. mBio 2023, 14, e0328522. [Google Scholar] [CrossRef]
- Grange, Z.L.; Goldstein, T.; Johnson, C.K.; Anthony, S.; Gilardi, K.; Daszak, P.; Olival, K.J.; O’Rourke, T.; Murray, S.; Olson, S.H.; et al. Ranking the risk of animal-to-human spillover for newly discovered viruses. Proc. Natl. Acad. Sci. USA 2021, 118, e2002324118. [Google Scholar] [CrossRef]
- Keusch, G.T.; Amuasi, J.H.; Anderson, D.E.; Daszak, P.; Eckerle, I.; Field, H.; Koopmans, M.; Lam, S.K.; Das Neves, C.G.; Peiris, M.; et al. Pandemic origins and a One Health approach to preparedness and prevention: Solutions based on SARS-CoV-2 and other RNA viruses. Proc. Natl. Acad. Sci. USA 2022, 119, e2202871119. [Google Scholar] [CrossRef]
- Lipsitch, M.; Barclay, W.; Raman, R.; Russell, C.J.; Belser, J.A.; Cobey, S.; Kasson, P.M.; Lloyd-Smith, J.O.; Maurer-Stroh, S.; Riley, S.; et al. Viral factors in influenza pandemic risk assessment. Elife 2016, 5, e18491. [Google Scholar] [CrossRef]
- Tada, T.; Zhou, H.; Samanovic, M.I.; Dcosta, B.M.; Cornelius, A.; Herati, R.S.; Mulligan, M.J.; Landau, N.R. Neutralization of SARS-CoV-2 Variants by mRNA and Adenoviral Vector Vaccine-Elicited Antibodies. Front. Immunol. 2022, 13, 797589. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, C.M.; Kistner, O.; Montomoli, E.; Viviani, S.; Marchi, S. Influenza Viruses and Vaccines: The Role of Vaccine Effectiveness Studies for Evaluation of the Benefits of Influenza Vaccines. Vaccines 2022, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jana, S.; Patra, S.; Haldar, P.K.; Bhowmik, R.; Mandal, A.; Anand, K.; Mazumdar, H.; Shaharyar, M.A.; Karmakar, S. Chapter 20—Insights into the mechanism of action of antiviral drugs. In How Synthetic Drugs Work; Kazmi, I., Karmakar, S., Shaharyar, M.d.A., Afzal, M., Al-Abbasi, F.A., Eds.; Academic Press: New York, NY, USA, 2023; pp. 447–475. ISBN 978-0-323-99855-0. [Google Scholar]
- de Jong, M.D.; Simmons, C.P.; Thanh, T.T.; Hien, V.M.; Smith, G.J.; Chau, T.N.; Hoang, D.M.; Chau, N.V.; Khanh, T.H.; Dong, V.C.; et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 2006, 12, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef]
- Zaraket, H.; Hurt, A.C.; Clinch, B.; Barr, I.; Lee, N. Burden of influenza B virus infection and considerations for clinical management. Antiviral Res. 2021, 185, 104970. [Google Scholar] [CrossRef]
- Kauppila, J.; Rönkkö, E.; Juvonen, R.; Saukkoriipi, A.; Saikku, P.; Bloigu, A.; Vainio, O.; Ziegler, T. Influenza C virus infection in military recruits--symptoms and clinical manifestation. J. Med. Virol. 2014, 86, 879–885. [Google Scholar] [CrossRef]
- Gupta, D.; Mohan, S. Influenza vaccine: A review on current scenario and future prospects. J. Genet. Eng. Biotechnol. 2023, 21, 154. [Google Scholar] [CrossRef]
- World Health Organization. Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 6 April 2025).
- Taylor, K.Y.; Agu, I.; José, I.; Mäntynen, S.; Campbell, A.J.; Mattson, C.; Chou, T.-W.; Zhou, B.; Gresham, D.; Ghedin, E.; et al. Influenza A virus reassortment is strain dependent. PLoS Pathog. 2023, 19, e1011155. [Google Scholar] [CrossRef]
- Vijaykrishna, D.; Poon, L.L.; Zhu, H.C.; Ma, S.K.; Li, O.T.; Cheung, C.L.; Smith, G.J.; Peiris, J.S.; Guan, Y. Reassortment of pandemic H1N1/2009 influenza A virus in swine. Science 2010, 328, 1529. [Google Scholar] [CrossRef]
- Poon, L.L.; Mak, P.W.; Li, O.T.; Chan, K.H.; Cheung, C.L.; Ma, E.S.; Yen, H.L.; Vijaykrishna, D.; Guan, Y.; Peiris, J.S. Rapid detection of reassortment of pandemic H1N1/2009 influenza virus. Clin. Chem. 2010, 56, 1340–1344. [Google Scholar] [CrossRef]
- Joseph, U.; Linster, M.; Suzuki, Y.; Krauss, S.; Halpin, R.A.; Vijaykrishna, D.; Fabrizio, T.P.; Bestebroer, T.M.; Maurer-Stroh, S.; Webby, R.J.; et al. Adaptation of pandemic H2N2 influenza A viruses in humans. J. Virol. 2015, 89, 2442–2447. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Watanabe, T.; Hatta, M.; Das, S.C.; Ozawa, M.; Shinya, K.; Zhong, G.; Hanson, A.; Katsura, H.; Watanabe, S.; et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 2012, 486, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Watanabe, T.; Kiso, M.; Nakajima, N.; Yamayoshi, S.; Iwatsuki-Horimoto, K.; Hatta, M.; Yamada, S.; Ito, M.; Sakai-Tagawa, Y.; et al. A Highly Pathogenic Avian H7N9 Influenza Virus Isolated from A Human Is Lethal in Some Ferrets Infected via Respiratory Droplets. Cell Host Microbe 2017, 22, 615–626.e8. [Google Scholar] [CrossRef] [PubMed]
- Herfst, S.; Schrauwen, E.J.; Linster, M.; Chutinimitkul, S.; de Wit, E.; Munster, V.J.; Sorrell, E.M.; Bestebroer, T.M.; Burke, D.F.; Smith, D.J.; et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 2012, 336, 1534–1541. [Google Scholar] [CrossRef]
- Linster, M.; van Boheemen, S.; de Graaf, M.; Schrauwen, E.J.A.; Lexmond, P.; Manz, B.; Bestebroer, T.M.; Baumann, J.; van Riel, D.; Rimmelzwaan, G.F.; et al. Identification, characterization, and natural selection of mutations driving airborne transmission of A/H5N1 virus. Cell 2014, 157, 329–339. [Google Scholar] [CrossRef]
- Al-Ghadeer, H.; Chu, D.K.W.; Rihan, E.M.A.; Abd-Allah, E.A.; Gu, H.; Chin, A.W.H.; Qasim, I.A.; Aldoweriej, A.; Alharbi, S.S.; Al-Aqil, M.A.; et al. Circulation of Influenza A(H5N8) Virus, Saudi Arabia. Emerg. Infect. Dis. 2018, 24, 1961–1964. [Google Scholar] [CrossRef]
- Stencel-Baerenwald, J.E.; Reiss, K.; Reiter, D.M.; Stehle, T.; Dermody, T.S. The sweet spot: Defining virus-sialic acid interactions. Nat. Rev. Microbiol. 2014, 12, 739–749. [Google Scholar] [CrossRef]
- Harrison, S.C. Viral membrane fusion. Virology 2015, 479–480, 498–507. [Google Scholar] [CrossRef]
- Bullough, P.A.; Hughson, F.M.; Skehel, J.J.; Wiley, D.C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature 1994, 371, 37–43. [Google Scholar] [CrossRef]
- Te Velthuis, A.J.; Fodor, E. Influenza virus RNA polymerase: Insights into the mechanisms of viral RNA synthesis. Nat. Rev. Microbiol. 2016, 14, 479–493. [Google Scholar] [CrossRef]
- Gopal, V.; Nilsson-Payant, B.E.; French, H.; Siegers, J.Y.; Yung, W.S.; Hardwick, M.; Te Velthuis, A.J.W. Zinc-Embedded Polyamide Fabrics Inactivate SARS-CoV-2 and Influenza A Virus. ACS Appl. Mater. Interfaces 2021, 13, 30317–30325. [Google Scholar] [CrossRef]
- Sousa, B.C.; Cote, D.L. Antimicrobial Copper Cold Spray Coatings and SARS-CoV-2 Surface Inactivation. MRS Adv. 2020, 5, 2873–2880. [Google Scholar] [CrossRef] [PubMed]
- Budowsky, E.I.; Bresler, S.E.; Friedman, E.A.; Zheleznova, N.V. Principles of selective inactivation of viral genome. I. UV-induced inactivation of influenza virus. Arch. Virol. 1981, 68, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Bormann, M.; Alt, M.; Schipper, L.; van de Sand, L.; Otte, M.; Meister, T.L.; Dittmer, U.; Witzke, O.; Steinmann, E.; Krawczyk, A. Disinfection of SARS-CoV-2 Contaminated Surfaces of Personal Items with UVC-LED Disinfection Boxes. Viruses 2021, 13, 598. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Gabbay, J. Copper as a biocidal tool. Curr. Med. Chem. 2005, 12, 2163–2175. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Putting copper into action: Copper-impregnated products with potent biocidal activities. FASEB J. 2004, 18, 1728–1730. [Google Scholar] [CrossRef]
- Chaturvedi, U.C.; Shrivastava, R. Interaction of viral proteins with metal ions: Role in maintaining the structure and functions of viruses. FEMS Immunol. Med. Microbiol. 2005, 43, 105–114. [Google Scholar] [CrossRef]
- Seok, J.H.; Kim, H.; Lee, D.B.; An, J.S.; Kim, E.J.; Lee, J.-H.; Chung, M.S.; Kim, K.H. Divalent cation-induced conformational changes of influenza virus hemagglutinin. Sci. Rep. 2020, 10, 15457. [Google Scholar] [CrossRef]
- Goldhill, D.H.; Te Velthuis, A.J.W.; Fletcher, R.A.; Langat, P.; Zambon, M.; Lackenby, A.; Barclay, W.S. The mechanism of resistance to favipiravir in influenza. Proc. Natl. Acad. Sci. USA 2018, 115, 11613–11618. [Google Scholar] [CrossRef]
- Yoshihara, A.; Dierickx, E.E.; Brewer, G.J.; Sekiguchi, Y.; Stearns, R.L.; Casa, D.J. Effects of Face Mask Use on Objective and Subjective Measures of Thermoregulation During Exercise in the Heat. Sports Health Multidiscip. Approach 2021, 13, 463–470. [Google Scholar] [CrossRef]
- Bénézeth, P. Potentiometric Studies of Zinc Oxide Solubility to High Temperatures. Mineral. Mag. 1998, 62A, 145–146. [Google Scholar] [CrossRef]
- Reed, R.B.; Ladner, D.A.; Higgins, C.P.; Westerhoff, P.; Ranville, J.F. Solubility of nano-zinc oxide in environmentally and biologically important matrices. Environ. Toxicol. Chem. 2012, 31, 93–99. [Google Scholar] [CrossRef]
- Hussain, M.; Galvin, H.D.; Haw, T.Y.; Nutsford, A.N.; Husain, M. Drug resistance in influenza A virus: The epidemiology and management. Infect. Drug Resist. 2017, 10, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Abulikemu, M.; Tabrizi, B.E.A.; Ghobadloo, S.M.; Mofarah, H.M.; Jabbour, G.E. Silver Nanoparticle-Decorated Personal Protective Equipment for Inhibiting Human Coronavirus Infectivity. ACS Appl. Nano Mater. 2022, 5, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Carmona, L.; Sepúlveda-Robles, O.A.; Almaguer-Flores, A.; Bello-Lopez, J.M.; Ramos-Vilchis, C.; Rodil, S.E. Antimicrobial activity of silver-copper coating against aerosols containing surrogate respiratory viruses and bacteria. PLoS ONE 2023, 18, e0294972. [Google Scholar] [CrossRef]
- Ueki, H.; Furusawa, Y.; Iwatsuki-Horimoto, K.; Imai, M.; Kabata, H.; Nishimura, H.; Kawaoka, Y. Effectiveness of Face Masks in Preventing Airborne Transmission of SARS-CoV-2. mSphere 2020, 5, e00637-20. [Google Scholar] [CrossRef]
- Tabatabaeizadeh, S.-A. Airborne transmission of COVID-19 and the role of face mask to prevent it: A systematic review and meta-analysis. Eur. J. Med. Res. 2021, 26, 1. [Google Scholar] [CrossRef]
- Haegdorens, F.; Franck, E.; Smith, P.; Bruyneel, A.; Monsieurs, K.G.; Van Bogaert, P. Sufficient personal protective equipment training can reduce COVID-19 related symptoms in healthcare workers: A prospective cohort study. Int. J. Nurs. Stud. 2022, 126, 104132. [Google Scholar] [CrossRef]

| Passage | Mean Titer After 2 mM Zinc Passage (pfu/mL) | Titer After 10 mM Zinc Passage (pfu/mL) | Titer of 2 mM Passage After 10 mM Zinc Challenge (pfu/mL) | Mean Titer of 10 mM Passage After 10 mM Zinc Challenge (pfu/mL) |
|---|---|---|---|---|
| Untreated | 7.26 × 107 | 7.26 × 107 | 1.00 × 105 (input) | 1.00 × 105 (input) |
| 1 | 7.83 × 106 | 8.91 × 105 | 7.29 × 104 | 5.13 × 103 |
| 2 | 6.37 × 107 | 3.11 × 106 | 8.98 × 104 | 4.96 × 103 |
| 3 | 9.41 × 106 | 2.83 × 106 | 4.73 × 104 | 1.58 × 103 |
| 4 | 7.17 × 106 | 6.97 × 105 | 9.33 × 104 | 5.73 × 103 |
| 5 | 4.52 × 107 | 8.22 × 105 | 7.11 × 104 | 8.92 × 102 |
| 6 | 2.92 × 107 | 2.09 × 106 | 5.54 × 104 | 4.66 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, A.Y.; Gopal, V.; te Velthuis, A.J.W. Zinc Ions Inactivate Influenza Virus Hemagglutinin and Prevent Receptor Binding. Biomedicines 2025, 13, 1843. https://doi.org/10.3390/biomedicines13081843
Jeong AY, Gopal V, te Velthuis AJW. Zinc Ions Inactivate Influenza Virus Hemagglutinin and Prevent Receptor Binding. Biomedicines. 2025; 13(8):1843. https://doi.org/10.3390/biomedicines13081843
Chicago/Turabian StyleJeong, Ahn Young, Vikram Gopal, and Aartjan J. W. te Velthuis. 2025. "Zinc Ions Inactivate Influenza Virus Hemagglutinin and Prevent Receptor Binding" Biomedicines 13, no. 8: 1843. https://doi.org/10.3390/biomedicines13081843
APA StyleJeong, A. Y., Gopal, V., & te Velthuis, A. J. W. (2025). Zinc Ions Inactivate Influenza Virus Hemagglutinin and Prevent Receptor Binding. Biomedicines, 13(8), 1843. https://doi.org/10.3390/biomedicines13081843






