The Prognostic Value of Non-Invasive Ventilation in Patients with Acute Heart Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Patients
2.2. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADHF | acute decompensation of chronic heart failure |
| AHF | acute heart failure |
| BiPAP | bilevel positive airway pressure |
| BIVA | bioimpedance vector analysis |
| BNP | brain natriuretic peptide |
| BUN | blood urea nitrogen |
| CI | confidence interval |
| eGFR | estimated glomerular filtration rate |
| ePVS | estimated plasma volume status |
| CPAP | continuous positive airway pressure |
| HI | hydration index |
| HR | hazard ratio |
| hsCRP | high-sensitivity C-reactive protein |
| ICU | cardiac intensive care unit |
| LOS | hospital length of stay |
| LVEF | left ventricular ejection fraction |
| NIV | non-invasive ventilation |
| NPPV | non-invasive positive pressure ventilation |
| NYHA | New York Heart Association |
| OR | odds ratio |
| PaCO2 | partial pressure of arterial carbon dioxide |
| PaO2 | partial pressure of arterial oxygen |
References
- Farmakis, D.; Parissis, J.; Lekakis, J.; Filippatos, G. Acute heart failure: Epidemiology, risk factors, and prevention. Rev. Esp. Cardiol. 2015, 68, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar]
- Miller, P.E.; Van Diepen, S.; Metkus, T.S.; Alviar, C.L.; Rayner-Hartley, E.; Rathwell, S.; Katz, J.N.; Ezekowitz, J.; Desai, N.R.; Ahmad, T. Association between Respiratory Failure and Clinical Outcomes in Patients with Acute Heart Failure: Analysis of 5 Pooled Clinical Trials. J. Card. Fail. 2021, 27, 602–606. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar]
- Alviar, C.L.; Miller, P.E.; McAreavey, D.; Katz, J.N.; Lee, B.; Moriyama, B.; Soble, J.; van Diepen, S.; Solomon, M.A.; Morrow, D.A.; et al. Positive Pressure Ventilation in the Cardiac Intensive Care Unit. J. Am. Coll. Cardiol. 2018, 72, 1532–1553. [Google Scholar] [CrossRef]
- Metkus, T.S.; Stephens, R.S.; Schulman, S.; Hsu, S.; Morrow, D.A.; Eid, S.M. Utilization and outcomes of early respiratory support in 6.5 million acute heart failure hospitalizations. Eur. Heart J. Qual. Care Clin. Outcomes 2020, 6, 72–80. [Google Scholar] [PubMed]
- Gray, A.; Goodacre, S.; Newby, D.E.; Masson, M.; Sampson, F.; Nicholl, J.; 3CPO Trialists. Noninvasive ventilation in acute cardiogenic pulmonary edema. N. Engl. J. Med. 2008, 359, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Berbenetz, N.; Wang, Y.; Brown, J.; Godfrey, C.; Ahmad, M.; Vital, F.M.; Lambiase, P.; Banerjee, A.; Bakhai, A.; Chong, M. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst. Rev. 2019, 4, CD005351. [Google Scholar] [CrossRef]
- Miró, Ò.; Martínez, G.; Masip, J.; Gil, V.; Martín-Sánchez, F.J.; Llorens, P.; Herrero-Puente, P.; Sánchez, C.; Richard, F.; Lucas-Invernón, J.; et al. Effects on short term outcome of non-invasive ventilation use in the emergency department to treat patients with acute heart failure: A propensity score-based analysis of the EAHFE Registry. Eur. J. Intern. Med. 2018, 53, 45–51. [Google Scholar] [CrossRef]
- Cameli, M.; Pastore, M.C.; De Carli, G.; Henein, M.Y.; Mandoli, G.E.; Lisi, E.; Cameli, P.; Lunghetti, S.; D’Ascenzi, F.; Nannelli, C.; et al. ACUTE HF score, a multiparametric prognostic tool for acute heart failure: A real-life study. Int. J. Cardiol. 2019, 296, 103–108. [Google Scholar] [CrossRef]
- Massari, F.; Scicchitano, P.; Iacoviello, M.; Passantino, A.; Guida, P.; Sanasi, M.; Piscopo, A.; Romito, R.; Valle, R.; Caldarola, P.; et al. Multiparametric approach to congestion for predicting long-term survival in heart failure. J. Cardiol. 2020, 75, 47–52. [Google Scholar] [CrossRef]
- Barach, A.L.; Martin, J.; Eckman, M. Positive pressure respiration and its application to the treatment of acute pulmonary edema. Annals of Internal Medicine. Ann. Intern. Med. 1938, 12, 754–795. [Google Scholar] [CrossRef]
- Masip, J.; Peacock, W.F.; Price, S.; Cullen, L.; Martin-Sanchez, F.J.; Seferovic, P.; Maisel, A.S.; Miro, O.; Filippatos, G.; Vrints, C.; et al. Indications and practical approach to non-invasive ventilation in acute heart failure. Eur. Heart J. 2018, 39, 17–25. [Google Scholar] [CrossRef]
- Marjanovic, N.; Couvreur, R.; Lamarre, J.; Piton, M.; Guenezan, J.; Mimoz, O. High-flow nasal cannula oxygen therapy versus noninvasive ventilation in acute respiratory failure related to suspected or confirmed acute heart failure: A systematic review with meta-analysis. Eur. J. Emerg. Med. 2024, 31, 388–397. [Google Scholar] [CrossRef]
- Vital, F.M.; Ladeira, M.T.; Atallah, A.N. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst. Rev. 2013, 5, CD005351. [Google Scholar] [CrossRef] [PubMed]
- Yukino, M.; Nagatomo, Y.; Goda, A.; Kohno, T.; Takei, M.; Nishihata, Y.; Saji, M.; Toyosaki, Y.; Nakano, S.; Ikegami, Y.; et al. Association of Non-Invasive Positive Pressure Ventilation with Short-Term Clinical Outcomes in Patients Hospitalized for Acute Decompensated Heart Failure. J. Clin. Med. 2021, 10, 5092. [Google Scholar] [CrossRef]
- Scicchitano, P.; Ciccone, M.M.; Iacoviello, M.; Guida, P.; De Palo, M.; Potenza, A.; Basile, M.; Sasanelli, P.; Trotta, F.; Sanasi, M.; et al. Respiratory failure and bioelectrical phase angle are independent predictors for long-term survival in acute heart failure. Scand. Cardiovasc. J. 2022, 56, 28–34. [Google Scholar] [CrossRef]
- Gorlicki, J.; Masip, J.; Gil, V.; Llorens, P.; Jacob, J.; Alquézar-Arbé, A.; Domingo Baldrich, E.; Fortuny, M.J.; Romero, M.; Esquivias, M.A.; et al. Effect of early initiation of noninvasive ventilation in patients transported by emergency medical service for acute heart failure. Eur. J. Emerg. Med. 2024, 31, 339–346. [Google Scholar] [CrossRef]
- Carrillo-Aleman, L.; Agamez-Luengas, A.A.; Guia, M.; Renedo-Villarroya, A.; Alonso-Fernández, N.; Lopez-Gomez, L.; Bayoumy-Delis, P.; Sanchez-Nieto, J.M.; Pascual-Figal, D.; Carrillo-Alcaraz, A. Effectiveness and safety of non-invasive ventilation in the management of cardiogenic shock. Rev. Port. Cardiol. 2024, 43, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, H.; Kohno, T.; Kohsaka, S.; Shiraishi, Y.; Fukuoka, R.; Nagatomo, Y.; Goda, A.; Mizuno, A.; Fukuda, K.; Yoshikawa, T.; et al. Length of hospital stay and its impact on subsequent early readmission in patients with acute heart failure: A report from the WET-HF Registry. Heart Vessel. 2019, 34, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Metkus, T.S.; Stephens, R.S.; Schulman, S.; Hsu, S.; Morrow, D.A.; Eid, S.M. Respiratory support in acute heart failure with preserved vs reduced ejection fraction. Clin. Cardiol. 2020, 43, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Kagiyama, N.; Nakamura, Y.; Dotare, T.; Sunayama, T.; Ishiwata, S.; Maeda, D.; Iso, T.; Kato, T.; Suda, S.; et al. External validation of the ACUTE HF score for risk stratification in acute heart failure. Int. J. Cardiol. 2023, 370, 396–401. [Google Scholar] [CrossRef] [PubMed]
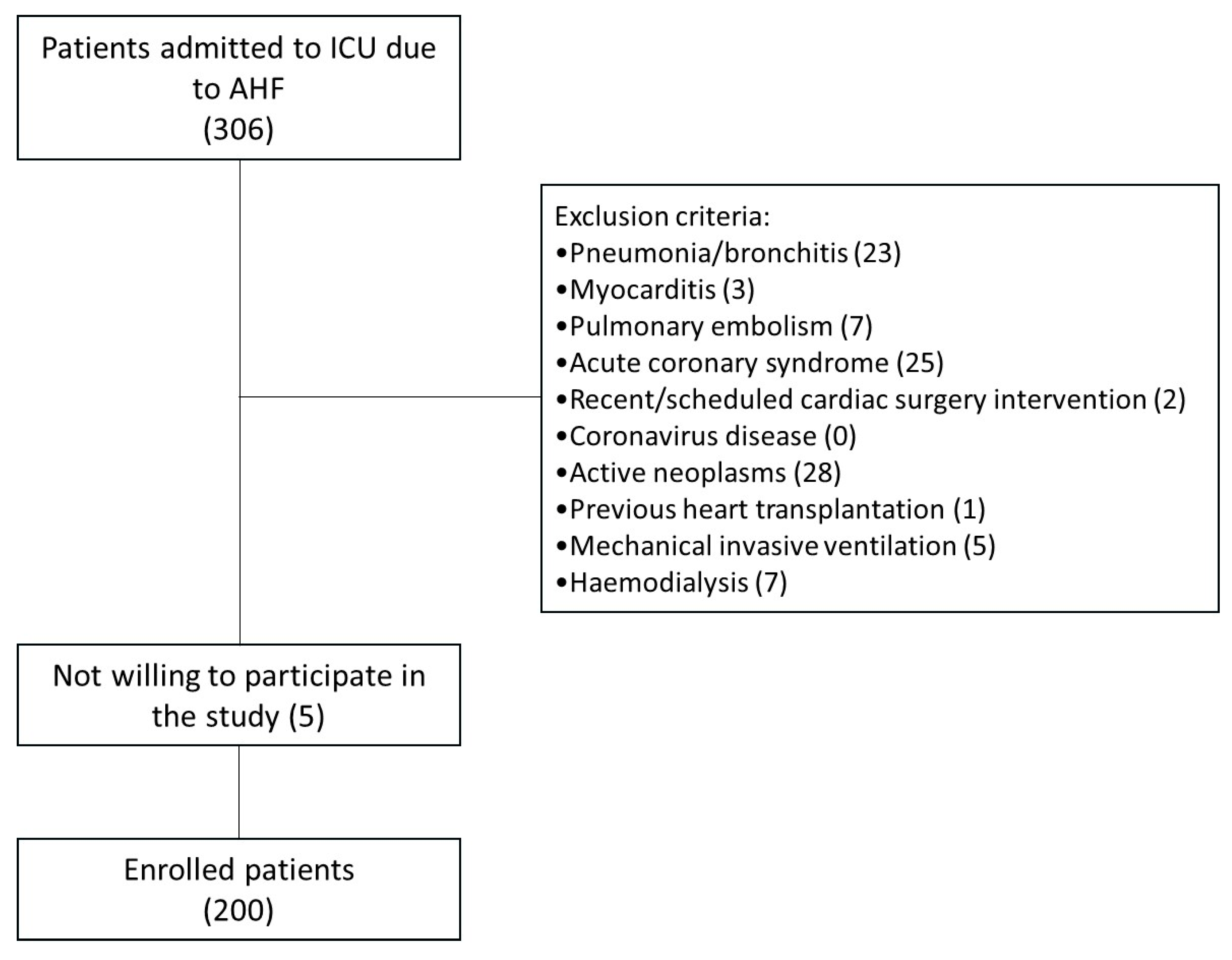
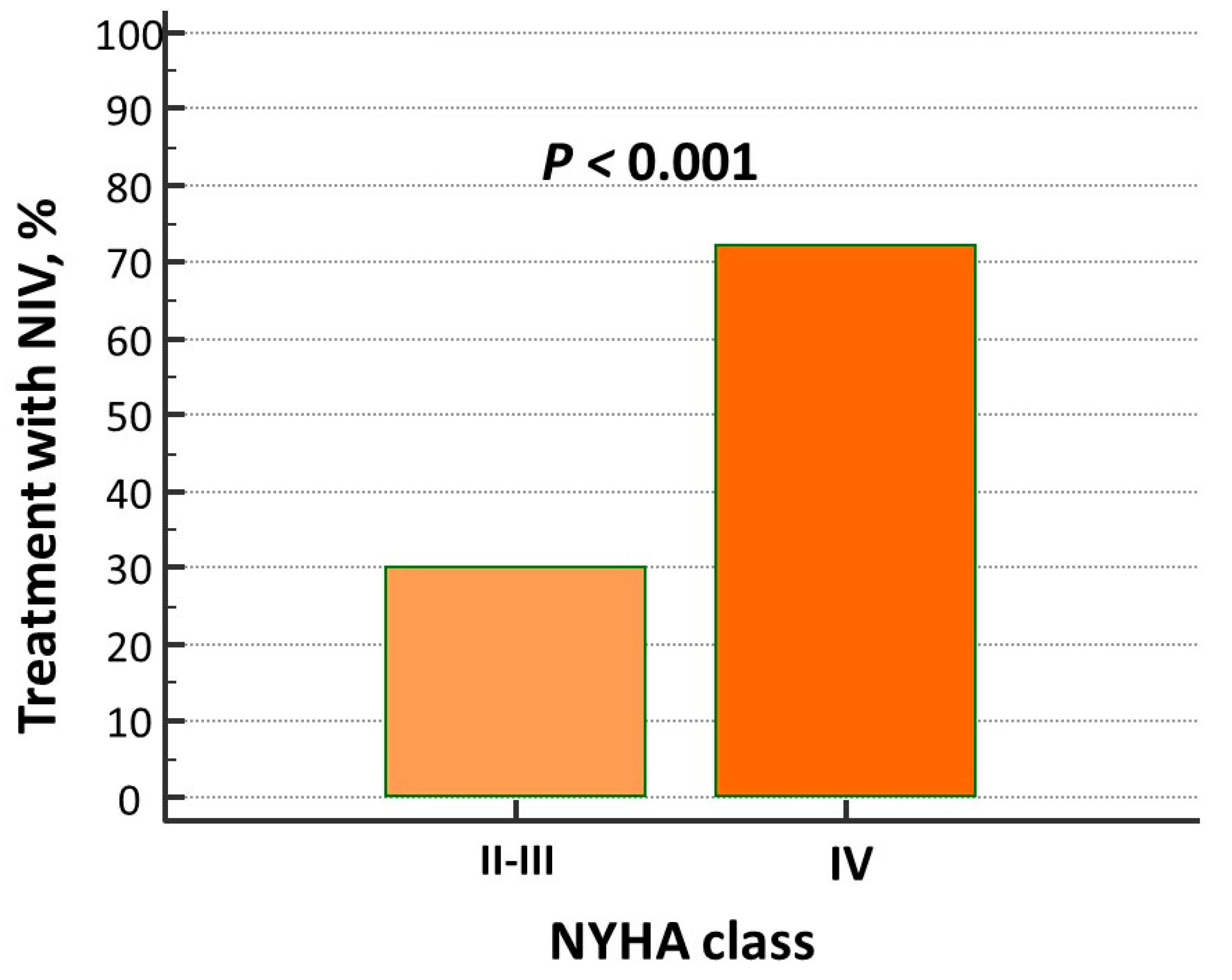

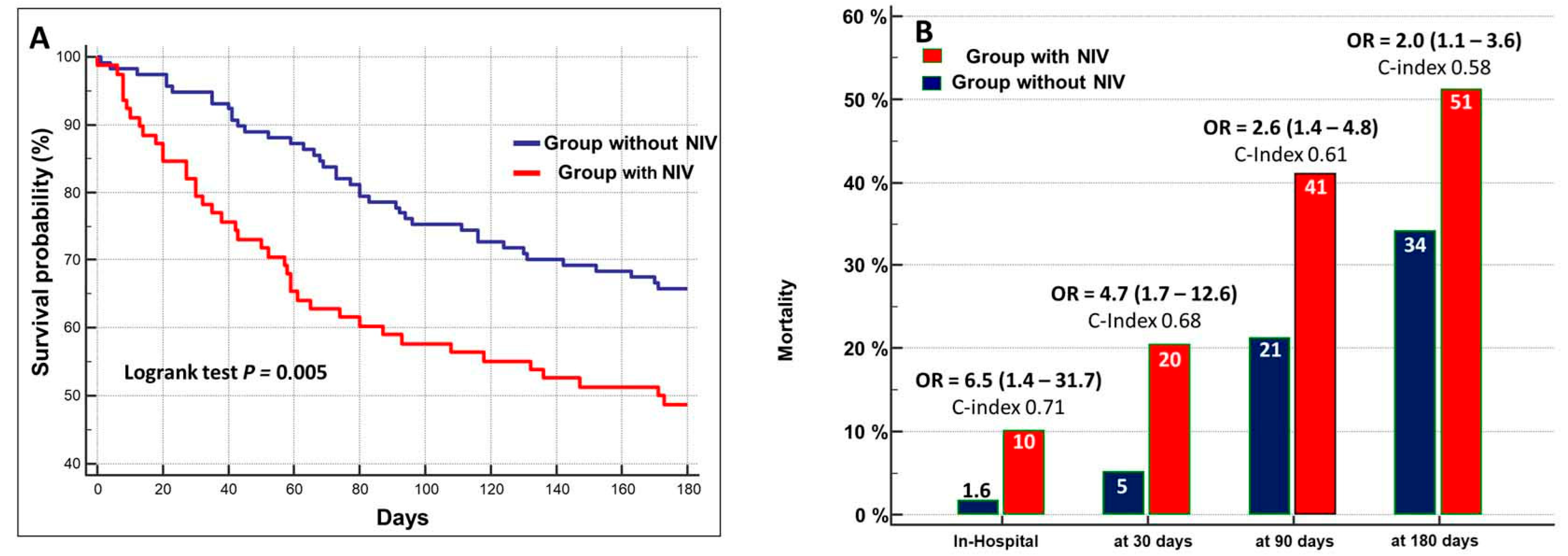
| Overall (n = 200) | Non-NIV Group (n = 120) | NIV Group (n = 80) | p Level | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, yrs | 81 ± 9 | 80 ± 9 | 81 ± 9 | 0.3 |
| Male, % | 52 | 55 | 47 | 0.3 |
| Body mass index, kg/m2 | 27 ± 6 | 27 ± 5 | 28 ± 6 | |
| De novo % | 57 | 50 | 67 | 0.01 |
| Peripheral edema, % | 62 | 62 | 63 | 0.7 |
| Pulmonary crackles, % | 63 | 55 | 72 | 0.02 |
| Jugular venous distention, % | 8 | 6 | 10 | 0.4 |
| Systolic blood pressure, mmHg | 127 ± 21 | 126 ± 22 | 127 ± 18 | 0.8 |
| Diastolic blood pressure, mmHg | 72 ± 15 | 72 ± 16 | 7 1± 14 | 0.6 |
| Heart rate, bpm | 93 ± 27 | 91 ± 26 | 94 ± 27 | 0.3 |
| Medical history, % | ||||
| Coronary artery disease | 33 | 29 | 36 | 0.3 |
| Diabetes | 33 | 30 | 36 | 0.4 |
| Atrial fibrillation | 43 | 45 | 40 | 0.1 |
| COPD | 41 | 40 | 44 | 0.6 |
| PM | 12 | 10 | 8 | 0.5 |
| ICD | 17 | 18 | 7 | 0.06 |
| Laboratory values | ||||
| LVEF, % | 40 ± 12 | 40 ± 12 | 41 ± 12 | 0.5 |
| Preserved EF | 36 | 35 | 36 | 0.9 |
| s-PAP, mmHg | 43 ± 16 | 42 ± 15 | 45 ± 16 | 0.2 |
| BNP, pg/ml | 1119 (916–1230) | 1132 (900–1307) | 1115 (849–1302) | 0.8 |
| Hemoglobin, g/dL | 12 ± 2 | 12 ± 2 | 12 ± 2 | 0.2 |
| BUN, mg/dL | 43 ± 28 | 39 ± 21 | 49 ± 35 | 0.02 |
| Creatinine, mg/dL | 1.7 ± 2.2 | 1.5 ± 0.8 | 1.7 ± 1.1 | 0.07 |
| eGFR, ml/min/1.73 m2 | 52 ± 25 | 54 ± 22 | 50 ± 28 | 0.3 |
| Sodium, mmol/L | 139 ± 5 | 139 ± 8 | 139 ± 6 | 0.8 |
| Potassium, mmol/L | 4.1 ± 0.6 | 4.1 ± 0.6 | 4.2 ± 0.7 | 0.1 |
| hsCRP, mg/L | 13 (11–14) | 11 (8–13) | 19 (11–40) | 0.001 |
| Oxygen blood saturation, % | 90 ± 5 | 92 ± 4 | 88 ±11 | <0.001 |
| pO2, mmHg | 64 ± 12 | 67 ± 12 | 61 ±11 | <0.001 |
| pCO2, mmHg | 38 ± 10 | 35 ± 6 | 41 ±11 | <0.001 |
| Hydration index, % | 81 ± 6 | 80 ± 5 | 82 ± 8 | 0.08 |
| e PVS, dL/g | 5.4 ± 1.7 | 5.3 ± 1.7 | 5.6 ± 1.7 | 0.2 |
| Therapies | ||||
| IV Furosemide, mg | 120 (60–150) | 80 (40–125) | 205 (80–250) | 0.001 |
| Beta-blockers, % | 78 | 77 | 80 | 0.6 |
| ACE inhibitors, % | 21 | 45 | 44 | 0.5 |
| ARB, % | 24 | 25 | 22 | 0.7 |
| ARNI, % | 7 | 8 | 5 | 0.4 |
| MRA, % | 77 | 75 | 78 | 0.6 |
| SGLT2i, % | 10 | 9 | 10 | 0.8 |
| Digitalis, % | 11 | 10 | 11 | 0.7 |
| Ivabradine, % | 1 | 1 | 1 | 0.8 |
| IV inotropes, % | 35 | 32 | 40 | 0.2 |
| Variables | Odds Ratio (95% CI) | p | B Coefficient | SE | Wald |
|---|---|---|---|---|---|
| De novo vs. CHF decompensated | 2.8 (1.2–6.6) | 0.02 | 1.03 | 0.4 | 5.6 |
| Pulmonary crackles, yes vs. no | 1.1 (0.5–2.5) | 0.8 | 0.1 | 0.4 | 0.05 |
| NYHA, II–III vs. IV | 2.3 (1.1–4.6) | 0.01 | 0.8 | 0.4 | 6.1 |
| BUN, × 10 mg/dL | 1.01 (0.99–1.02) | 0.1 | 0.01 | 0.01 | 2.4 |
| hsCRP, × 10 mg/L BNP, pg/mL× 100 eGFR, mL/min | 1.05 (0.99–1.01) | 0.1 | 0.05 | 0.03 | 2.4 |
| Oxygen blood saturation, % | 0.8 (0.7–0.9) | 0.01 | −0.2 | 0.08 | 4.8 |
| pO2, mmHg | 1.01 (0.95–1.07) | 0.8 | 0.03 | 0.03 | 0.2 |
| pCO2, mmHg | 1.09 (1.04–1.14) | 0.0001 | 0.09 | 0.02 | 14.9 |
| IV Furosemide, × 10 mg | 1.03 (1.01–1.06) | 0.02 | 0.03 | 0.01 | 5.6 |
| Variables | r | p | VIF |
|---|---|---|---|
| De novo AHF vs. ADCHF | 0.03 | 0.7 | 1.06 |
| NYHA, II–III vs. IV | −0.02 | 0.7 | 1.12 |
| Oxygen blood saturation, % | 0.11 | 0.1 | 1.20 |
| pCO2, mmHg | −0.004 | 0.9 | 1.22 |
| IV Furosemide, × 10 mg | 0.21 | 0.005 | 1.14 |
| NIV group vs. No NIV group | 0.26 | 0.0007 | 1.4 |
| Univariate Cox Regression Analysis | Adjusted Cox Regression Analysis | ||||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | Wald | |
| NIV+ vs. NIV− | 1.84 (1.18–2.85) | 0.006 | 1.7 (1.1–2.9) | 0.01 | 6.0 |
| Age, year | 1.01 (0.98–1.03) | 0.3 | |||
| LVEF, % | 0.98 (0.97–1.01) | 0.2 | |||
| NYHA II–III vs. IV | 1.21 (0.77–1.9) | 0.4 | |||
| eGFR, ml/min | 0.98 (0.97–0.99) | 0.005 | |||
| BUN, × 10 mg/dL | 1.06 (1.03–1.08) | 0.0001 | 1.05 (1.02–1.08) | 0.002 | 9.8 |
| BNP, × 100 pg/ml | 1.11 (1.07–1.15) | <0.0001 | 1.02 (1.0–1.04) | 0.007 | 7.3 |
| hsCRP, × 10 mg/L | 1.04 (1.01–1.07) | 0.009 | |||
| pO2, mmHg | 1.00 (0.98–1.01) | 0.9 | |||
| pCO2, mmHg | 1.00 (0.98–1.02) | 0.4 | |||
| Oxygen saturation, % | 0.97 (0.93–1.00) | 0.1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scicchitano, P.; Cinelli, A.; Citarelli, G.; Livrieri, A.; Campanella, C.; De Palo, M.; Caldarola, P.; Ciccone, M.M.; Massari, F. The Prognostic Value of Non-Invasive Ventilation in Patients with Acute Heart Failure. Biomedicines 2025, 13, 1844. https://doi.org/10.3390/biomedicines13081844
Scicchitano P, Cinelli A, Citarelli G, Livrieri A, Campanella C, De Palo M, Caldarola P, Ciccone MM, Massari F. The Prognostic Value of Non-Invasive Ventilation in Patients with Acute Heart Failure. Biomedicines. 2025; 13(8):1844. https://doi.org/10.3390/biomedicines13081844
Chicago/Turabian StyleScicchitano, Pietro, Assunta Cinelli, Gaetano Citarelli, Anna Livrieri, Cosimo Campanella, Micaela De Palo, Pasquale Caldarola, Marco Matteo Ciccone, and Francesco Massari. 2025. "The Prognostic Value of Non-Invasive Ventilation in Patients with Acute Heart Failure" Biomedicines 13, no. 8: 1844. https://doi.org/10.3390/biomedicines13081844
APA StyleScicchitano, P., Cinelli, A., Citarelli, G., Livrieri, A., Campanella, C., De Palo, M., Caldarola, P., Ciccone, M. M., & Massari, F. (2025). The Prognostic Value of Non-Invasive Ventilation in Patients with Acute Heart Failure. Biomedicines, 13(8), 1844. https://doi.org/10.3390/biomedicines13081844









