The Small Intestinal Microbiota and the Gut–Brain Axis in Parkinson’s Disease: A Narrative Review
Abstract
1. Introduction
2. From Composition to Clinical Relevance: Understanding the Gut Microbiota
2.1. Segmental Dysbiosis in the Small Intestine of PD Patients
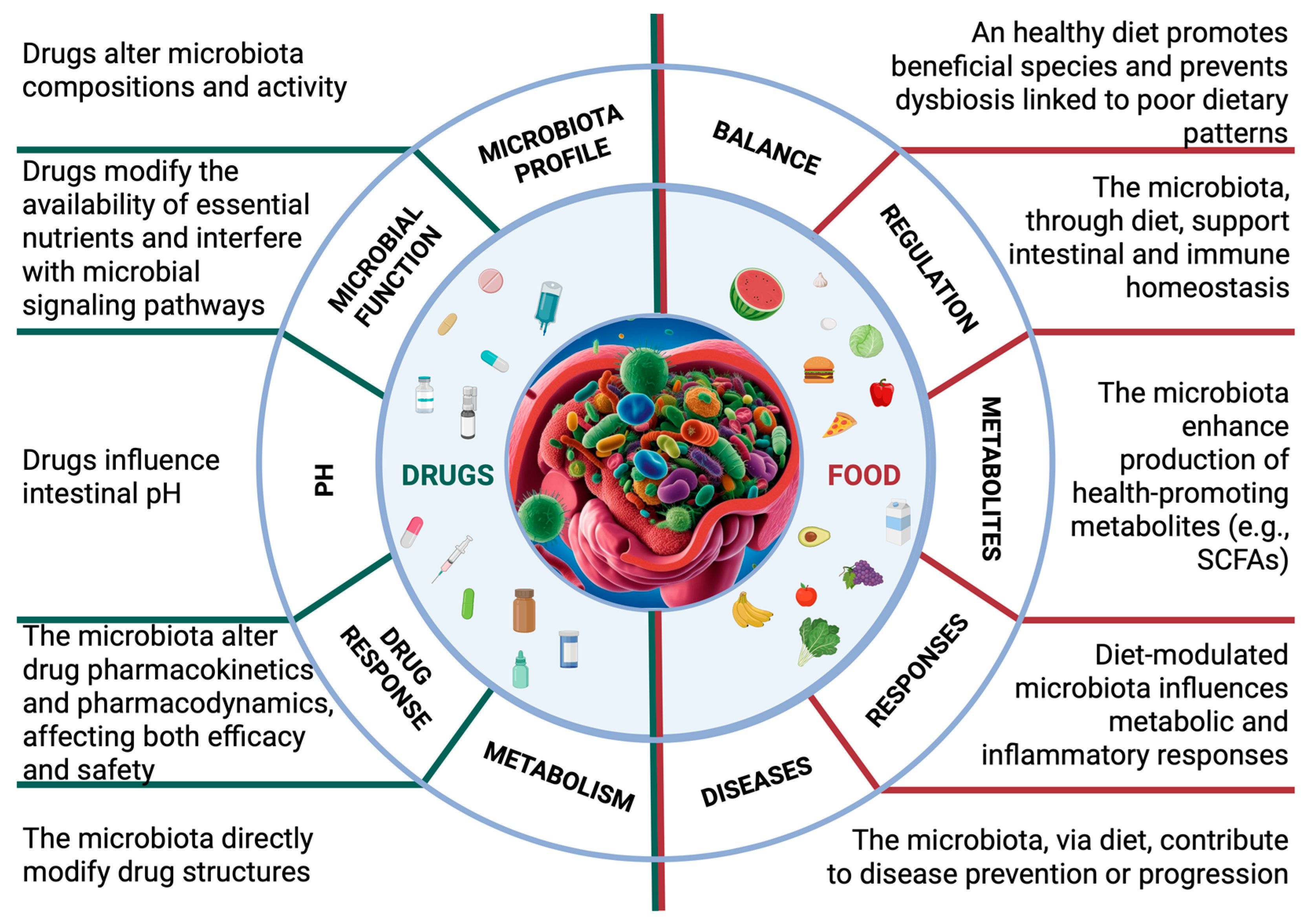
2.2. The Effects of Drugs on the Microbiota
2.3. The Impact of Diet on the Microbiota
3. The Microbiota–Gut–Brain Axis: Foundations and Physiological Implications
4. Involvement of the Microbiota–Gut–Brain Axis in Parkinson’s Disease
4.1. The Small Intestine in Parkinson’s Disease: A Crucial Site for Pathogenesis
4.2. Gut Microbial Products Influence α-Synuclein Aggregation and Neurotoxicity
5. The Role of Gut Microbiota in the Pharmacological Management of Parkinson’s Disease
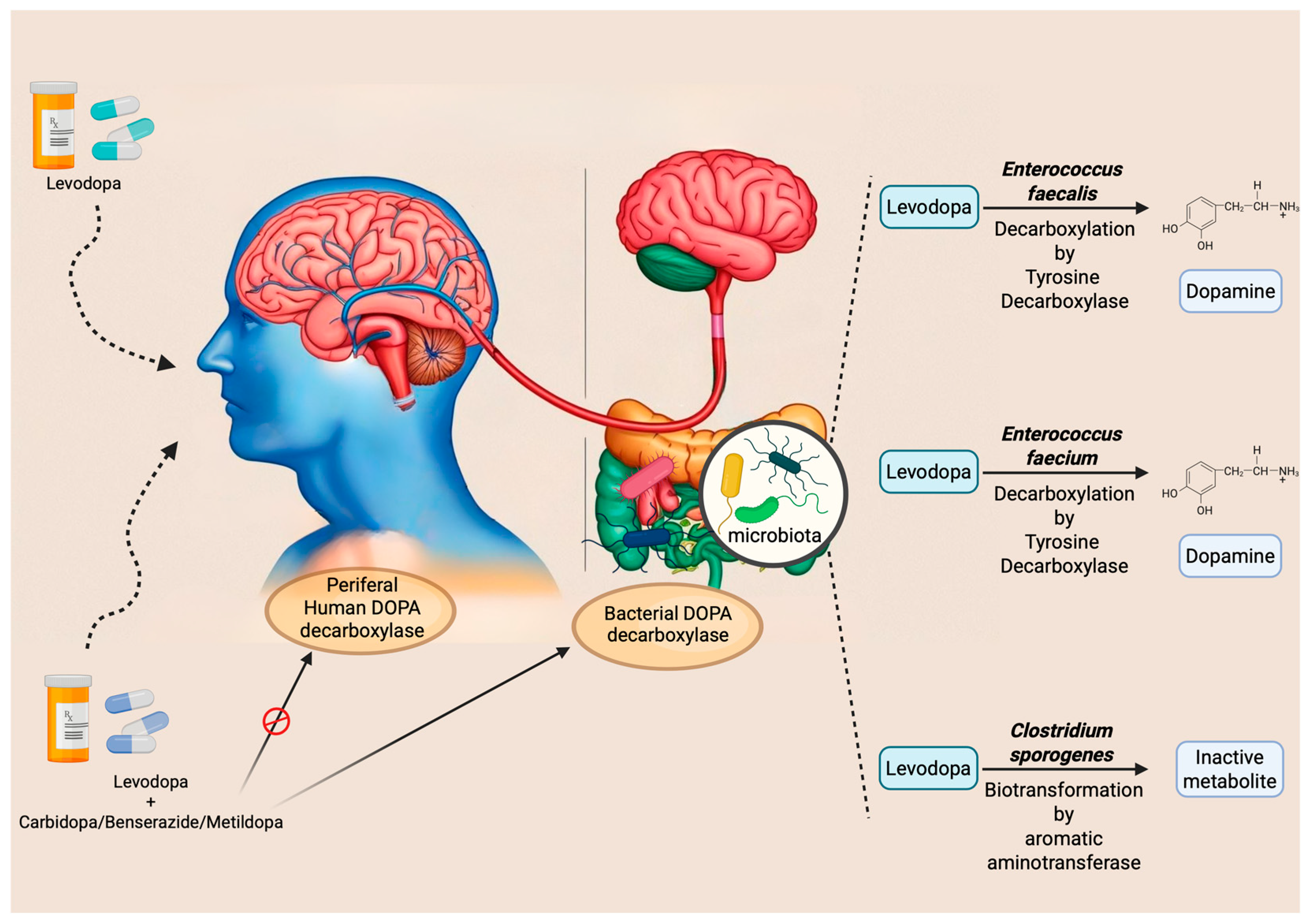
5.1. Levodopa
5.2. Dopamine Agonists
5.3. COMT Inhibitors
6. Potential Strategies of Microbial Intervention in Parkinson’s Disease
6.1. Food (Diet, Prebiotics)
6.2. Probiotics
6.3. Synbiotics
6.4. Antibiotics
6.5. Amyloid Inhibitors
7. Potential Developments
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2023, 64, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-Aging. An Evolutionary Perspective on Immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Witkowski, J.M.; Olivieri, F.; Larbi, A. The Integration of Inflammaging in Age-Related Diseases. Semin. Immunol. 2018, 40, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Cresci, G.A.; Bawden, E. Gut Microbiome: What We Do and Don’t Know. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2015, 30, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Bodogai, M.; O’Connell, J.; Kim, K.; Kim, Y.; Moritoh, K.; Chen, C.; Gusev, F.; Vaughan, K.; Shulzhenko, N.; Mattison, J.A.; et al. Commensal Bacteria Contribute to Insulin Resistance in Aging by Activating Innate B1a Cells. Sci. Transl. Med. 2018, 10, eaat4271. [Google Scholar] [CrossRef] [PubMed]
- Schepici, G.; Silvestro, S.; Bramanti, P.; Mazzon, E. The Gut Microbiota in Multiple Sclerosis: An Overview of Clinical Trials. Cell Transplant. 2019, 28, 1507–1527. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Embong, H.; Othman, F.; Ghazi, H.F.; Maruthey, N.; Bahari, H. Probiotics for Alzheimer’s Disease: A Systematic Review. Nutrients 2021, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gao, G.; Kwok, L.-Y.; Sun, Z. Gut Microbiome-Targeted Therapies for Alzheimer’s Disease. Gut Microbes 2023, 15, 2271613. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the Diagnosis of Parkinson’s Disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Krzyzanowski, B.; Mullan, A.F.; Turcano, P.; Camerucci, E.; Bower, J.H.; Savica, R. Air Pollution and Parkinson Disease in a Population-Based Study. JAMA Netw. Open 2024, 7, e2433602. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.C.; Chuang, Y.-H.; Shih, I.-F.; Keener, A.; Bordelon, Y.; Bronstein, J.M.; Ritz, B. The Association Between Lifestyle Factors and Parkinson’s Disease Progression and Mortality. Mov. Disord. Off. J. Mov. Disord. Soc. 2019, 34, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Proano, A.C.; Viteri, J.A.; Orozco, E.N.; Calle, M.A.; Costa, S.C.; Reyes, D.V.; German-Montenegro, M.; Moncayo, D.F.; Tobar, A.C.; Moncayo, J.A. Gut Microbiota and Its Repercussion in Parkinson’s Disease: A Systematic Review in Occidental Patients. Neurol. Int. 2023, 15, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Heravi, F.S.; Naseri, K.; Hu, H. Gut Microbiota Composition in Patients with Neurodegenerative Disorders (Parkinson’s and Alzheimer’s) and Healthy Controls: A Systematic Review. Nutrients 2023, 15, 4365. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. (Encinitas). 2014, 13, 17–22. [Google Scholar] [PubMed]
- Shanahan, F.; Ghosh, T.S.; O’Toole, P.W. The Healthy Microbiome-What Is the Definition of a Healthy Gut Microbiome? Gastroenterology 2021, 160, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the Human Microbiome. Nutr. Rev. 2012, 70 (Suppl. S1), S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Vascellari, S.; Palmas, V.; Melis, M.; Pisanu, S.; Cusano, R.; Uva, P.; Perra, D.; Madau, V.; Sarchioto, M.; Oppo, V.; et al. Gut Microbiota and Metabolome Alterations Associated with Parkinson’s Disease. mSystems 2020, 5, e00561-20. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.H.; Chong, C.W.; Lim, S.-Y.; Yap, I.K.S.; Teh, C.S.J.; Loke, M.F.; Song, S.-L.; Tan, J.Y.; Ang, B.H.; Tan, Y.Q.; et al. Gut Microbial Ecosystem in Parkinson Disease: New Clinicobiological Insights from Multi-Omics. Ann. Neurol. 2021, 89, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, H.; Ito, M.; Ishida, T.; Hamaguchi, T.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Ueyama, J.; Shimamura, T.; Mori, H.; et al. Meta-Analysis of Gut Dysbiosis in Parkinson’s Disease. Mov. Disord. 2020, 35, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; You, L.; Lei, H.; Li, X. Association between Increased and Decreased Gut Microbiota Abundance and Parkinson’s Disease: A Systematic Review and Subgroup Meta-Analysis. Exp. Gerontol. 2024, 191, 112444. [Google Scholar] [CrossRef] [PubMed]
- Toh, T.S.; Chong, C.W.; Lim, S.-Y.; Bowman, J.; Cirstea, M.; Lin, C.-H.; Chen, C.-C.; Appel-Cresswell, S.; Finlay, B.B.; Tan, A.H. Gut Microbiome in Parkinson’s Disease: New Insights from Meta-Analysis. Park. Relat. Disord. 2022, 94, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-Analysis of the Parkinson’s Disease Gut Microbiome Suggests Alterations Linked to Intestinal Inflammation. NPJ Park. Dis. 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Bove, F.; Gabrielli, M.; Petracca, M.; Zocco, M.A.; Ragazzoni, E.; Barbaro, F.; Piano, C.; Fortuna, S.; Tortora, A.; et al. The Role of Small Intestinal Bacterial Overgrowth in Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2013, 28, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Pinto, M.F.; Candeias, E.; Melo-Marques, I.; Esteves, A.R.; Maranha, A.; Magalhães, J.D.; Carneiro, D.R.; Sant’Anna, M.; Pereira-Santos, A.R.; Abreu, A.E.; et al. Gut-First Parkinson’s Disease Is Encoded by Gut Dysbiome. Mol. Neurodegener. 2024, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- de Waal, T.; Brouwers, J.; Berben, P.; Flanagan, T.; Tack, J.; Vandenberghe, W.; Vanuytsel, T.; Augustijns, P. Characterization of Aspirated Duodenal Fluids from Parkinson’s Disease Patients. Pharmaceutics 2023, 15, 1243. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Y.; Chen, D.; Xu, X.; Li, W.; Li, K.; He, J.; Su, W.; Luo, Q. The Alteration of Intestinal Mucosal α-Synuclein Expression and Mucosal Microbiota in Parkinson’s Disease. Appl. Microbiol. Biotechnol. 2023, 107, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Freedberg, D.E.; Toussaint, N.C.; Chen, S.P.; Ratner, A.J.; Whittier, S.; Wang, T.C.; Wang, H.H.; Abrams, J.A. Proton Pump Inhibitors Alter Specific Taxa in the Human Gastrointestinal Microbiome: A Crossover Trial. Gastroenterology 2015, 149, 883–885.e9. [Google Scholar] [CrossRef] [PubMed]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive Impact of Non-Antibiotic Drugs on Human Gut Bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Saad, R.; Rizkallah, M.R.; Aziz, R.K. Gut Pharmacomicrobiomics: The Tip of an Iceberg of Complex Interactions between Drugs and Gut-Associated Microbes. Gut Pathog. 2012, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Buschmann, M.M.; Gilbert, J.A. Pharmacomicrobiomics: The Holy Grail to Variability in Drug Response? Clin. Pharmacol. Ther. 2019, 106, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Javdan, B.; Lopez, J.G.; Chankhamjon, P.; Lee, Y.-C.J.; Hull, R.; Wu, Q.; Wang, X.; Chatterjee, S.; Donia, M.S. Personalized Mapping of Drug Metabolism by the Human Gut Microbiome. Cell 2020, 181, 1661–1679.e22. [Google Scholar] [CrossRef] [PubMed]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between Drugs and the Gut Microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Koppel, N.; Maini Rekdal, V.; Balskus, E.P. Chemical Transformation of Xenobiotics by the Human Gut Microbiota. Science 2017, 356, eaag2770. [Google Scholar] [CrossRef] [PubMed]
- Klünemann, M.; Andrejev, S.; Blasche, S.; Mateus, A.; Phapale, P.; Devendran, S.; Vappiani, J.; Simon, B.; Scott, T.; Kafkia, E.; et al. Bioaccumulation of Therapeutic Drugs by Human Gut Bacteria. Nature 2021, 597, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.J. Antibiotic Use and Its Consequences for the Normal Microbiome. Science 2016, 352, 544–545. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Tilg, H.; Gasbarrini, A. Antibiotics as Deep Modulators of Gut Microbiota: Between Good and Evil. Gut 2016, 65, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Morgun, A.; Dzutsev, A.; Dong, X.; Greer, R.L.; Sexton, D.J.; Ravel, J.; Schuster, M.; Hsiao, W.; Matzinger, P.; Shulzhenko, N. Uncovering Effects of Antibiotics on the Host and Microbiota Using Transkingdom Gene Networks. Gut 2015, 64, 1732–1743. [Google Scholar] [CrossRef] [PubMed]
- Bonder, M.J.; Tigchelaar, E.F.; Cai, X.; Trynka, G.; Cenit, M.C.; Hrdlickova, B.; Zhong, H.; Vatanen, T.; Gevers, D.; Wijmenga, C.; et al. The Influence of a Short-Term Gluten-Free Diet on the Human Gut Microbiome. Genome Med. 2016, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Verdi, S.; Maxan, M.-E.; Shin, C.M.; Zierer, J.; Bowyer, R.C.E.; Martin, T.; Williams, F.M.K.; Menni, C.; Bell, J.T.; et al. Gut Microbiota Associations with Common Diseases and Prescription Medications in a Population-Based Cohort. Nat. Commun. 2018, 9, 2655. [Google Scholar] [CrossRef] [PubMed]
- Vich Vila, A.; Collij, V.; Sanna, S.; Sinha, T.; Imhann, F.; Bourgonje, A.R.; Mujagic, Z.; Jonkers, D.M.A.E.; Masclee, A.A.M.; Fu, J.; et al. Impact of Commonly Used Drugs on the Composition and Metabolic Function of the Gut Microbiota. Nat. Commun. 2020, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The Interplay between Diet and the Gut Microbiome: Implications for Health and Disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, Stability and Resilience of the Human Gut Microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.K.; Boudry, G.; Lemay, D.G.; Raybould, H.E. Changes in Intestinal Barrier Function and Gut Microbiota in High-Fat Diet-Fed Rats Are Dynamic and Region Dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G840–G851. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, E.K.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.C.; Yannakoulia, M.; Panagiotakos, D.B.; Kyriacou, A. Adherence to the Mediterranean Diet Is Associated with the Gut Microbiota Pattern and Gastrointestinal Characteristics in an Adult Population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Linehan, K.; Dempsey, E.M.; Ryan, C.A.; Ross, R.P.; Stanton, C. First Encounters of the Microbial Kind: Perinatal Factors Direct Infant Gut Microbiome Establishment. Microbiome Res. Rep. 2022, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Vatanen, T.; Jabbar, K.S.; Ruohtula, T.; Honkanen, J.; Avila-Pacheco, J.; Siljander, H.; Stražar, M.; Oikarinen, S.; Hyöty, H.; Ilonen, J.; et al. Mobile Genetic Elements from the Maternal Microbiome Shape Infant Gut Microbial Assembly and Metabolism. Cell 2022, 185, 4921–4936.e15. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, C.L.J.; Onnerfält, J.; Xu, J.; Molin, G.; Ahrné, S.; Thorngren-Jerneck, K. The Microbiota of the Gut in Preschool Children with Normal and Excessive Body Weight. Obes. Silver Spring Md 2012, 20, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- D’Aversa, F.; Tortora, A.; Ianiro, G.; Ponziani, F.R.; Annicchiarico, B.E.; Gasbarrini, A. Gut Microbiota and Metabolic Syndrome. Intern. Emerg. Med. 2013, 8 (Suppl. S1), S11–S15. [Google Scholar] [CrossRef] [PubMed]
- Stanislawski, M.A.; Dabelea, D.; Lange, L.A.; Wagner, B.D.; Lozupone, C.A. Gut Microbiota Phenotypes of Obesity. NPJ Biofilms Microbiomes 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, W. Nutrition Classics. Experiments and Observations on the Gastric Juice and the Physiology of Digestion. By William Beaumont. Plattsburgh. Printed by F. P. Allen. 1833. Nutr. Rev. 1977, 35, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Almy, T.P. Experimental Studies on the Irritable Colon. Am. J. Med. 1951, 10, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016, 150, 1262–1279.e2. [Google Scholar] [CrossRef] [PubMed]
- Mönnikes, H.; Tebbe, J.J.; Hildebrandt, M.; Arck, P.; Osmanoglou, E.; Rose, M.; Klapp, B.; Wiedenmann, B.; Heymann-Mönnikes, I. Role of Stress in Functional Gastrointestinal Disorders. Evidence for Stress-Induced Alterations in Gastrointestinal Motility and Sensitivity. Dig. Dis. 2001, 19, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Dupont, P.; Aziz, Q.; Fukudo, S. Understanding Neurogastroenterology from Neuroimaging Perspective: A Comprehensive Review of Functional and Structural Brain Imaging in Functional Gastrointestinal Disorders. J. Neurogastroenterol. Motil. 2018, 24, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Lee, J.Y.; Jung, S.W.; Shin, S.Y.; Ryu, H.S.; Jang, S.-H.; Kwon, J.G.; Kim, Y.S. Brain-Gut-Microbiota Axis. Korean J. Gastroenterol. 2023, 81, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A.; Tack, J.; Ford, A.C.; Szigethy, E.; Törnblom, H.; Van Oudenhove, L. Neuromodulators for Functional Gastrointestinal Disorders (Disorders of Gut-Brain Interaction): A Rome Foundation Working Team Report. Gastroenterology 2018, 154, 1140–1171.e1. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chen, H.; Cheng, Y.; Xu, F.; Ruan, G.; Ying, S.; Tang, W.; Chen, L.; Chen, M.; Lv, L.; et al. Fecal Microbiota Transplantation Relieves Gastrointestinal and Autism Symptoms by Improving the Gut Microbiota in an Open-Label Study. Front. Cell. Infect. Microbiol. 2021, 11, 759435. [Google Scholar] [CrossRef] [PubMed]
- Hartsough, L.A.; Park, M.; Kotlajich, M.V.; Lazar, J.T.; Han, B.; Lin, C.-C.J.; Musteata, E.; Gambill, L.; Wang, M.C.; Tabor, J.J. Optogenetic Control of Gut Bacterial Metabolism to Promote Longevity. eLife 2020, 9, e56849. [Google Scholar] [CrossRef] [PubMed]
- Opara, J.; Małecki, A.; Małecka, E.; Socha, T. Motor Assessment in Parkinson’s Disease. Ann. Agric. Environ. Med. AAEM 2017, 24, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Gibb, W.R.; Lees, A.J. The Relevance of the Lewy Body to the Pathogenesis of Idiopathic Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Stocchi, F.; Bravi, D.; Emmi, A.; Antonini, A. Parkinson Disease Therapy: Current Strategies and Future Research Priorities. Nat. Rev. Neurol. 2024, 20, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Cloud, L.J.; Greene, J.G. Gastrointestinal Features of Parkinson’s Disease. Curr. Neurol. Neurosci. Rep. 2011, 11, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Han, D.; Cheng, Q.; Zhang, P.; Zhao, C.; Min, J.; Wang, F. Association of Levels of Physical Activity with Risk of Parkinson Disease: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2018, 1, e182421. [Google Scholar] [CrossRef] [PubMed]
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal Gut Microbiota Modulates Brain Development and Behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed]
- Caradonna, E.; Nemni, R.; Bifone, A.; Gandolfo, P.; Costantino, L.; Giordano, L.; Mormone, E.; Macula, A.; Cuomo, M.; Difruscolo, R.; et al. The Brain-Gut Axis, an Important Player in Alzheimer and Parkinson Disease: A Narrative Review. J. Clin. Med. 2024, 13, 4130. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Rüb, U.; Gai, W.P.; Del Tredici, K. Idiopathic Parkinson’s Disease: Possible Routes by Which Vulnerable Neuronal Types May Be Subject to Neuroinvasion by an Unknown Pathogen. J. Neural Transm. 2003, 110, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The Role of Microbiota-Gut-Brain Axis in Neuropsychiatric and Neurological Disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. Parkinson’s Disease: A Dual-Hit Hypothesis. Neuropathol. Appl. Neurobiol. 2007, 33, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Chau, S.W.H.; Liu, Y.; Chan, J.W.Y.; Wang, J.; Ma, S.L.; Zhang, J.; Chan, P.K.S.; Yeoh, Y.K.; Chen, Z.; et al. Gut Microbiome Dysbiosis across Early Parkinson’s Disease, REM Sleep Behavior Disorder and Their First-Degree Relatives. Nat. Commun. 2023, 14, 2501. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.; Zhang, K.; Paul, K.C.; Folle, A.D.; Del Rosario, I.; Jacobs, J.P.; Keener, A.M.; Bronstein, J.M.; Ritz, B. Diet and the Gut Microbiome in Patients with Parkinson’s Disease. NPJ Park. Dis. 2024, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.; Kim, S.-I.; Park, S.-H.; Kim, J.-M.; Lee, J.-Y.; Chung, S.J.; Kim, J.W.; Ahn, T.-B.; Park, K.W.; Shin, J.H.; et al. Diagnostic Accuracy and Predictors of Alpha-Synuclein Accumulation in the Gastrointestinal Tract of Parkinson’s Disease. Npj Park. Dis. 2024, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Emmi, A.; Sandre, M.; Russo, F.P.; Tombesi, G.; Garrì, F.; Campagnolo, M.; Carecchio, M.; Biundo, R.; Spolverato, G.; Macchi, V.; et al. Duodenal Alpha-Synuclein Pathology and Enteric Gliosis in Advanced Parkinson’s Disease. Mov. Disord. 2023, 38, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Campagnolo, M.; Weis, L.; Sandre, M.; Tushevski, A.; Russo, F.P.; Savarino, E.; Carecchio, M.; Stocco, E.; Macchi, V.; De Caro, R.; et al. Immune Landscape of the Enteric Nervous System Differentiates Parkinson’s Disease Patients from Controls: The PADUA-CESNE Cohort. Neurobiol. Dis. 2024, 200, 106609. [Google Scholar] [CrossRef] [PubMed]
- Vascellari, S.; Orrù, C.D.; Groveman, B.R.; Parveen, S.; Fenu, G.; Pisano, G.; Piga, G.; Serra, G.; Oppo, V.; Murgia, D.; et al. α-Synuclein Seeding Activity in Duodenum Biopsies from Parkinson’s Disease Patients. PLoS Pathog. 2023, 19, e1011456. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.J.; Kulkarni, S.; Pasricha, T.S. Upper Gastrointestinal Mucosal Damage and Subsequent Risk of Parkinson Disease. JAMA Netw. Open 2024, 7, e2431949. [Google Scholar] [CrossRef] [PubMed]
- Vidović, M.; Rikalovic, M.G. Alpha-Synuclein Aggregation Pathway in Parkinson’s Disease: Current Status and Novel Therapeutic Approaches. Cells 2022, 11, 1732. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Challis, C.; Jain, N.; Moiseyenko, A.; Ladinsky, M.S.; Shastri, G.G.; Thron, T.; Needham, B.D.; Horvath, I.; Debelius, J.W.; et al. A Gut Bacterial Amyloid Promotes α-Synuclein Aggregation and Motor Impairment in Mice. eLife 2020, 9, e53111. [Google Scholar] [CrossRef] [PubMed]
- Wittung-Stafshede, P. Gut Power: Modulation of Human Amyloid Formation by Amyloidogenic Proteins in the Gastrointestinal Tract. Curr. Opin. Struct. Biol. 2022, 72, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.; Horvath, I.; Wittung-Stafshede, P. Crosstalk Between Alpha-Synuclein and Other Human and Non-Human Amyloidogenic Proteins: Consequences for Amyloid Formation in Parkinson’s Disease. J. Park. Dis. 2020, 10, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Foltynie, T.; Bruno, V.; Fox, S.; Kühn, A.A.; Lindop, F.; Lees, A.J. Medical, Surgical, and Physical Treatments for Parkinson’s Disease. Lancet 2024, 403, 305–324. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.P.; Bianchine, J.R.; Spiegel, H.E.; Rivera-Calimlim, L.; Hersey, R.M. Metabolism of Levodopa in Patients with Parkinson’s Disease. Radioactive and Fluorometric Assays. Arch. Neurol. 1971, 25, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Nyholm, D.; Lennernäs, H. Irregular Gastrointestinal Drug Absorption in Parkinson’s Disease. Expert Opin. Drug Metab. Toxicol. 2008, 4, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Maini Rekdal, V.; Bess, E.N.; Bisanz, J.E.; Turnbaugh, P.J.; Balskus, E.P. Discovery and Inhibition of an Interspecies Gut Bacterial Pathway for Levodopa Metabolism. Science 2019, 364, eaau6323. [Google Scholar] [CrossRef] [PubMed]
- van Kessel, S.P.; Frye, A.K.; El-Gendy, A.O.; Castejon, M.; Keshavarzian, A.; van Dijk, G.; El Aidy, S. Gut Bacterial Tyrosine Decarboxylases Restrict Levels of Levodopa in the Treatment of Parkinson’s Disease. Nat. Commun. 2019, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, X.; Mo, C.; Liu, X.; Li, J.; Yan, Z.; Qian, Y.; Lai, Y.; Xu, S.; Yang, X.; et al. Association Between Microbial Tyrosine Decarboxylase Gene and Levodopa Responsiveness in Patients with Parkinson Disease. Neurology 2022, 99, e2443–e2453. [Google Scholar] [CrossRef] [PubMed]
- Camargo, S.M.R.; Vuille-dit-Bille, R.N.; Mariotta, L.; Ramadan, T.; Huggel, K.; Singer, D.; Götze, O.; Verrey, F. The Molecular Mechanism of Intestinal Levodopa Absorption and Its Possible Implications for the Treatment of Parkinson’s Disease. J. Pharmacol. Exp. Ther. 2014, 351, 114–123. [Google Scholar] [CrossRef] [PubMed]
- van Kessel, S.P.; de Jong, H.R.; Winkel, S.L.; van Leeuwen, S.S.; Nelemans, S.A.; Permentier, H.; Keshavarzian, A.; El Aidy, S. Gut Bacterial Deamination of Residual Levodopa Medication for Parkinson’s Disease. BMC Biol. 2020, 18, 137. [Google Scholar] [CrossRef] [PubMed]
- Rosebraugh, M.; Voight, E.A.; Moussa, E.M.; Jameel, F.; Lou, X.; Zhang, G.G.Z.; Mayer, P.T.; Stolarik, D.; Carr, R.A.; Enright, B.P.; et al. Foslevodopa/Foscarbidopa: A New Subcutaneous Treatment for Parkinson’s Disease. Ann. Neurol. 2021, 90, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.; Tolosa, E.; Stocchi, F.; Ferreira, J.J.; Rascol, O.; Antonini, A.; Poewe, W. Optimizing Levodopa Therapy, When and How? Perspectives on the Importance of Delivery and the Potential for an Early Combination Approach. Expert Rev. Neurother. 2023, 23, 15–24. [Google Scholar] [CrossRef] [PubMed]
- LeWitt, P.A.; Hauser, R.A.; Pahwa, R.; Isaacson, S.H.; Fernandez, H.H.; Lew, M.; Saint-Hilaire, M.; Pourcher, E.; Lopez-Manzanares, L.; Waters, C.; et al. Safety and Efficacy of CVT-301 (Levodopa Inhalation Powder) on Motor Function during off Periods in Patients with Parkinson’s Disease: A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet Neurol. 2019, 18, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Zibetti, M.; Merola, A.; Ricchi, V.; Marchisio, A.; Artusi, C.A.; Rizzi, L.; Montanaro, E.; Reggio, D.; De Angelis, C.; Rizzone, M.; et al. Long-Term Duodenal Levodopa Infusion in Parkinson’s Disease: A 3-Year Motor and Cognitive Follow-up Study. J. Neurol. 2013, 260, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.A.; Dutta, S. Population Pharmacokinetics of Levodopa in Subjects with Advanced Parkinson’s Disease: Levodopa-Carbidopa Intestinal Gel Infusion vs. Oral Tablets. Br. J. Clin. Pharmacol. 2014, 78, 94–105. [Google Scholar] [CrossRef] [PubMed]
- van Kessel, S.P.; Bullock, A.; van Dijk, G.; El Aidy, S. Parkinson’s Disease Medication Alters Small Intestinal Motility and Microbiota Composition in Healthy Rats. mSystems 2022, 7, e0119121. [Google Scholar] [CrossRef] [PubMed]
- Scheperjans, F.; Aho, V.; Pereira, P.A.B.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut Microbiota Are Related to Parkinson’s Disease and Clinical Phenotype. Mov. Disord. Off. J. Mov. Disord. Soc. 2015, 30, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Barichella, M.; Severgnini, M.; Cilia, R.; Cassani, E.; Bolliri, C.; Caronni, S.; Ferri, V.; Cancello, R.; Ceccarani, C.; Faierman, S.; et al. Unraveling Gut Microbiota in Parkinson’s Disease and Atypical Parkinsonism. Mov. Disord. Off. J. Mov. Disord. Soc. 2019, 34, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Hill-Burns, E.M.; Debelius, J.W.; Morton, J.T.; Wissemann, W.T.; Lewis, M.R.; Wallen, Z.D.; Peddada, S.D.; Factor, S.A.; Molho, E.; Zabetian, C.P.; et al. Parkinson’s Disease and Parkinson’s Disease Medications Have Distinct Signatures of the Gut Microbiome. Mov. Disord. Off. J. Mov. Disord. Soc. 2017, 32, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Grün, D.; Zimmer, V.C.; Kauffmann, J.; Spiegel, J.; Dillmann, U.; Schwiertz, A.; Faßbender, K.; Fousse, M.; Unger, M.M. Impact of Oral COMT-Inhibitors on Gut Microbiota and Short Chain Fatty Acids in Parkinson’s Disease. Parkinsonism Relat. Disord. 2020, 70, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Bourdeau-Julien, I.; Castonguay-Paradis, S.; Rochefort, G.; Perron, J.; Lamarche, B.; Flamand, N.; Di Marzo, V.; Veilleux, A.; Raymond, F. The Diet Rapidly and Differentially Affects the Gut Microbiota and Host Lipid Mediators in a Healthy Population. Microbiome 2023, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Pietrucci, D.; Cerroni, R.; Unida, V.; Farcomeni, A.; Pierantozzi, M.; Mercuri, N.B.; Biocca, S.; Stefani, A.; Desideri, A. Dysbiosis of Gut Microbiota in a Selected Population of Parkinson’s Patients. Parkinsonism Relat. Disord. 2019, 65, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Cantu-Jungles, T.M.; Rasmussen, H.E.; Hamaker, B.R. Potential of Prebiotic Butyrogenic Fibers in Parkinson’s Disease. Front. Neurol. 2019, 10, 663. [Google Scholar] [CrossRef] [PubMed]
- Bedarf, J.R.; Romano, S.; Heinzmann, S.S.; Duncan, A.; Traka, M.H.; Ng, D.; Segovia-Lizano, D.; Simon, M.-C.; Narbad, A.; Wüllner, U.; et al. A Prebiotic Dietary Pilot Intervention Restores Faecal Metabolites and May Be Neuroprotective in Parkinson’s Disease. NPJ Park. Dis. 2025, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Riegelman, E.; Xue, K.S.; Wang, J.-S.; Tang, L. Gut–Brain Axis in Focus: Polyphenols, Microbiota, and Their Influence on α-Synuclein in Parkinson’s Disease. Nutrients 2024, 16, 2041. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.; Forsyth, C.B.; Shaikh, M.; Voigt, R.M.; Engen, P.A.; Ramirez, V.; Keshavarzian, A. Diet in Parkinson’s Disease: Critical Role for the Microbiome. Front. Neurol. 2019, 10, 1245. [Google Scholar] [CrossRef] [PubMed]
- Avallone, R.; Vitale, G.; Bertolotti, M. Omega-3 Fatty Acids and Neurodegenerative Diseases: New Evidence in Clinical Trials. Int. J. Mol. Sci. 2019, 20, 4256. [Google Scholar] [CrossRef] [PubMed]
- Hernando, S.; Requejo, C.; Herran, E.; Ruiz-Ortega, J.A.; Morera-Herreras, T.; Lafuente, J.V.; Ugedo, L.; Gainza, E.; Pedraz, J.L.; Igartua, M.; et al. Beneficial Effects of N-3 Polyunsaturated Fatty Acids Administration in a Partial Lesion Model of Parkinson’s Disease: The Role of Glia and NRf2 Regulation. Neurobiol. Dis. 2019, 121, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pardo, P.; Dodiya, H.B.; Broersen, L.M.; Douna, H.; van Wijk, N.; Lopes da Silva, S.; Garssen, J.; Keshavarzian, A.; Kraneveld, A.D. Gut–Brain and Brain–Gut Axis in Parkinson’s Disease Models: Effects of a Uridine and Fish Oil Diet. Nutr. Neurosci. 2018, 21, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Dong, W.; Chang, K.; Yan, Y.; Liu, X. Efficacy of Probiotic Supplements on Parkinson’s Disease: A Systematic Review and Meta-Analysis. Complement. Ther. Med. 2024, 82, 103045. [Google Scholar] [CrossRef] [PubMed]
- Fang, X. Microbial Treatment: The Potential Application for Parkinson’s Disease. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2019, 40, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, D.; Ancusa, O.E.; Georgescu, L.A.; Ionita, I.; Reisz, D. Nonmotor Gastrointestinal Disorders in Older Patients with Parkinson’s Disease: Is There Hope? Clin. Interv. Aging 2016, 11, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Lee, S.C.; Ham, C.; Kim, Y.W. Effect of Probiotic Supplementation on Gastrointestinal Motility, Inflammation, Motor, Non-Motor Symptoms and Mental Health in Parkinson’s Disease: A Meta-Analysis of Randomized Controlled Trials. Gut Pathog. 2023, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, Z.; Zhao, L.; Zhao, Y.; Yang, G.; Wang, C.; Gao, L.; Niu, C.; Li, S. Lactobacillus Plantarum DP189 Reduces α-SYN Aggravation in MPTP-Induced Parkinson’s Disease Mice via Regulating Oxidative Damage, Inflammation, and Gut Microbiota Disorder. J. Agric. Food Chem. 2022, 70, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Aktas, B.; Aslim, B.; Ozdemir, D.A. A Neurotherapeutic Approach with Lacticaseibacillus Rhamnosus E9 on Gut Microbiota and Intestinal Barrier in MPTP-Induced Mouse Model of Parkinson’s Disease. Sci. Rep. 2024, 14, 15460. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chu, C.; Yu, L.; Zhai, Q.; Wang, S.; Zhao, J.; Zhang, H.; Chen, W.; Tian, F. Neuroprotective Effects of Bifidobacterium Breve CCFM1067 in MPTP-Induced Mouse Models of Parkinson’s Disease. Nutrients 2022, 14, 4678. [Google Scholar] [CrossRef] [PubMed]
- Valvaikar, S.; Vaidya, B.; Sharma, S.; Bishnoi, M.; Kondepudi, K.K.; Sharma, S.S. Supplementation of Probiotic Bifidobacterium Breve Bif11 Reverses Neurobehavioural Deficits, Inflammatory Changes and Oxidative Stress in Parkinson’s Disease Model. Neurochem. Int. 2024, 174, 105691. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhao, F.; Liu, Y.; Ma, T.; Jin, H.; Quan, K.; Leng, B.; Zhao, J.; Yuan, X.; Li, Z.; et al. Probiotics Synergized with Conventional Regimen in Managing Parkinson’s Disease. NPJ Park. Dis. 2022, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Tamtaji, O.R.; Taghizadeh, M.; Daneshvar Kakhaki, R.; Kouchaki, E.; Bahmani, F.; Borzabadi, S.; Oryan, S.; Mafi, A.; Asemi, Z. Clinical and Metabolic Response to Probiotic Administration in People with Parkinson’s Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. Edinb. Scotl. 2019, 38, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P. Probiotics for Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 4121. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Lim, Y.L.; Yaow, C.Y.L.; Ng, W.K.; Thumboo, J.; Liew, T.M. Effect of Probiotic Supplementation on Gut Microbiota in Patients with Major Depressive Disorders: A Systematic Review. Nutrients 2023, 15, 1351. [Google Scholar] [CrossRef] [PubMed]
- Barathikannan, K.; Chelliah, R.; Rubab, M.; Daliri, E.B.-M.; Elahi, F.; Kim, D.-H.; Agastian, P.; Oh, S.-Y.; Oh, D.H. Gut Microbiome Modulation Based on Probiotic Application for Anti-Obesity: A Review on Efficacy and Validation. Microorganisms 2019, 7, 456. [Google Scholar] [CrossRef] [PubMed]
- Barichella, M.; Pacchetti, C.; Bolliri, C.; Cassani, E.; Iorio, L.; Pusani, C.; Pinelli, G.; Privitera, G.; Cesari, I.; Faierman, S.A.; et al. Probiotics and Prebiotic Fiber for Constipation Associated with Parkinson Disease: An RCT. Neurology 2016, 87, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Sanctuary, M.R.; Kain, J.N.; Chen, S.Y.; Kalanetra, K.; Lemay, D.G.; Rose, D.R.; Yang, H.T.; Tancredi, D.J.; German, J.B.; Slupsky, C.M.; et al. Pilot Study of Probiotic/Colostrum Supplementation on Gut Function in Children with Autism and Gastrointestinal Symptoms. PLoS ONE 2019, 14, e0210064. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Du, Z.R.; Wang, X.; Sun, X.R.; Zhao, Q.; Zhao, F.; Wong, W.T.; Wong, K.H.; Dong, X.-L. Polymannuronic Acid Prebiotic plus Lacticaseibacillus Rhamnosus GG Probiotic as a Novel Synbiotic Promoted Their Separate Neuroprotection against Parkinson’s Disease. Food Res. Int. Ott. Ont 2022, 155, 111067. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.; Bonazzi, P.; Scarpellini, E.; Bendia, E.; Lauritano, E.C.; Fasano, A.; Ceravolo, M.G.; Capecci, M.; Rita Bentivoglio, A.; Provinciali, L.; et al. Prevalence of Small Intestinal Bacterial Overgrowth in Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2011, 26, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Malservisi, S.; Veneto, G.; Ferrieri, A.; Corazza, G.R. Rifaximin versus Chlortetracycline in the Short-Term Treatment of Small Intestinal Bacterial Overgrowth. Aliment. Pharmacol. Ther. 2000, 14, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M. Review of Rifaximin as Treatment for SIBO and IBS. Expert Opin. Investig. Drugs 2009, 18, 349–358. [Google Scholar] [CrossRef] [PubMed]
- van Kessel, S.P.; El Aidy, S. Contributions of Gut Bacteria and Diet to Drug Pharmacokinetics in the Treatment of Parkinson’s Disease. Front. Neurol. 2019, 10, 1087. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lu, J.; Wei, K.; Wei, J.; Tian, P.; Yue, M.; Wang, Y.; Hong, D.; Li, F.; Wang, B.; et al. Neuroprotective Effect of Ceftriaxone on MPTP-Induced Parkinson’s Disease Mouse Model by Regulating Inflammation and Intestinal Microbiota. Oxid. Med. Cell. Longev. 2021, 2021, 9424582. [Google Scholar] [CrossRef] [PubMed]
- Baizabal-Carvallo, J.F.; Alonso-Juarez, M.; Fekete, R. Intestinal Decontamination Therapy for Dyskinesia and Motor Fluctuations in Parkinson’s Disease. Front. Neurol. 2021, 12, 729961. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.L.; Chorell, E.; Taylor, J.D.; Åden, J.; Götheson, A.; Li, F.; Koch, M.; Sefer, L.; Matthews, S.J.; Wittung-Stafshede, P.; et al. The Bacterial Curli System Possesses a Potent and Selective Inhibitor of Amyloid Formation. Mol. Cell 2015, 57, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Kobayashi, H.; Chuta, H.; Matsuyoshi, N.; Kato, Y.; Ogasawara, H. CsgI (YccT) Is a Novel Inhibitor of Curli Fimbriae Formation in Escherichia Coli Preventing CsgA Polymerization and Curli Gene Expression. Int. J. Mol. Sci. 2023, 24, 4357. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.Ø.; Kumar, A.; Shin, B.; Stylianou, F.; Sewell, L.; Xu, Y.; Otzen, D.E.; Pedersen, J.S.; Matthews, S.J. FapA Is an Intrinsically Disordered Chaperone for Pseudomonas Functional Amyloid FapC. J. Mol. Biol. 2023, 435, 167878. [Google Scholar] [CrossRef] [PubMed]
- Katsipis, G.; Avgoulas, D.I.; Geromichalos, G.D.; Petala, M.; Pantazaki, A.A. In Vitro and In Silico Evaluation of the Serrapeptase Effect on Biofilm and Amyloids of Pseudomonas Aeruginosa. Appl. Microbiol. Biotechnol. 2023, 107, 7269–7285. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.W.; Abbott, R.D.; Petrovitch, H.; Tanner, C.M.; White, L.R. Pre-Motor Features of Parkinson’s Disease: The Honolulu-Asia Aging Study Experience. Parkinsonism Relat. Disord. 2012, 18 (Suppl. S1), S199–S202. [Google Scholar] [CrossRef] [PubMed]
| Study Groups | Sample Size | Microbiota Sampling Method | Main Results | ||
|---|---|---|---|---|---|
| Preclinical study | Munoz-Pinto et al. [27] | Untreated mice vs. HC 1 mice vs. PD 2 mice | 115 mice (46 untreated; 23 HC 1 mice; 46 PD 2 mice) | Human fecal material, terminal ileum mucosa biopsies, mouse fecal pellets, and terminal ileum mucosa-associated material | PD dysbiosis may activate a toxic gut-to-brain pathway. Fecal transplants from PD patients into mice can induce immune, functional, inflammatory, and pathological alterations. |
| Clinical studies | Fasano et al. [26] | PD 2 patients vs. HCs 1 | 33 PD 2 patients and 30 HCs 1 | The LBT 3 and GBT 4 were used to assess the presence of SIBO | SIBO is associated with more severe motor fluctuations |
| de Waal et al. [28] | PD 2 patients vs. HC 1 | 9 PD patients (6 males, 3 females) and 9 (4 males, 5 females) | Duodenal fluid collected via nasoduodenal tube | Duodenal fluid analysis in PD patients revealed dysbiosis, altered microbial metabolites, and increased α-synuclein accumulation, supporting the role of the small intestine in disease progression. | |
| Shi et al. [29] | PD group vs. control group | 19 patients and 22 controls | Duodenal mucosal biopsies | This study revealed differences in OSyn distribution within the sigmoid mucosa between PD patients and healthy controls; significant changes in the microbiome composition in the gut mucosa of PD patients suggested the potential diagnostic relevance of OSyn/αSyn levels in the sigmoid mucosa for PD. | |
| trial NCT06003608 | PD2 patients vs. HC1 | Total Participants: 100 | SIMBA capsule | This clinical trial is ongoing. If this sampling method is effective, it will enable minimally invasive sampling of the microbiome and metabolome from regions of the small intestine that are difficult to reach using conventional approaches. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrossa, G.; Misenti, V.; Faggin, S.; Giron, M.C.; Antonini, A. The Small Intestinal Microbiota and the Gut–Brain Axis in Parkinson’s Disease: A Narrative Review. Biomedicines 2025, 13, 1769. https://doi.org/10.3390/biomedicines13071769
Carrossa G, Misenti V, Faggin S, Giron MC, Antonini A. The Small Intestinal Microbiota and the Gut–Brain Axis in Parkinson’s Disease: A Narrative Review. Biomedicines. 2025; 13(7):1769. https://doi.org/10.3390/biomedicines13071769
Chicago/Turabian StyleCarrossa, Gloria, Valentina Misenti, Sofia Faggin, Maria Cecilia Giron, and Angelo Antonini. 2025. "The Small Intestinal Microbiota and the Gut–Brain Axis in Parkinson’s Disease: A Narrative Review" Biomedicines 13, no. 7: 1769. https://doi.org/10.3390/biomedicines13071769
APA StyleCarrossa, G., Misenti, V., Faggin, S., Giron, M. C., & Antonini, A. (2025). The Small Intestinal Microbiota and the Gut–Brain Axis in Parkinson’s Disease: A Narrative Review. Biomedicines, 13(7), 1769. https://doi.org/10.3390/biomedicines13071769







