Perioperative Oral Immunonutrient Regulation of Intestinal Barrier and Gut Microbiota in Patients with Gastric Cancer, a Randomized Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Intervention Protocol
2.4. Data Collection
2.4.1. Immune and Nutritional Indicators
2.4.2. Intestinal Barrier Indicators
2.4.3. Fecal Metagenomic Sequencing
2.4.4. Bioinformatics Analysis
2.5. Statistical Analysis
2.6. Sample Size and Ethics
3. Results
3.1. Descriptive Analysis
3.2. Perioperative Immune and Nutrition Parameter Changes
3.3. Perioperative Intestinal Barrier Marker Changes
3.4. Gene Differential Analysis
3.4.1. Phylum-Level Differences
3.4.2. Genus-Level Differences
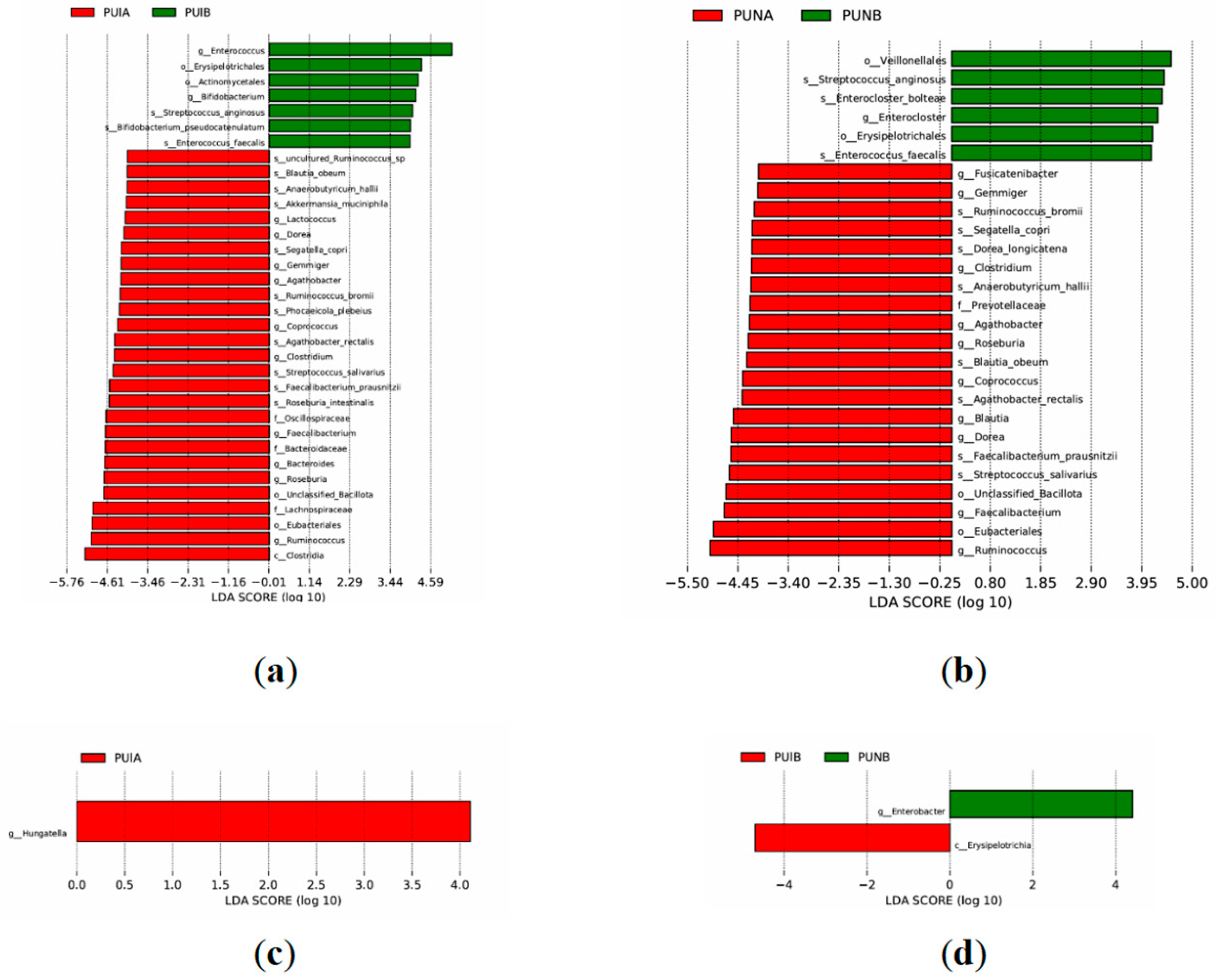
3.4.3. Species-Level Differences
3.5. Differential Gene Function Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, R.; Yin, Z.; Chen, X.; Mao, R.; Zheng, X.; Yuan, M.; Li, H.; Lu, Y.; Liu, S.; et al. Gut microbiota-driven metabolic alterations reveal the distinct pathogenicity of chemotherapy-induced cachexia in gastric cancer. Pharmacol. Res. 2024, 209, 107476. [Google Scholar] [CrossRef]
- Tan, S.; Zhou, F.; Zhang, Z.; Wang, J.; Xu, J.; Zhuang, Q.; Meng, Q.; Xi, Q.; Jiang, Y.; Wu, G. Beta-1 blocker reduces inflammation and preserves intestinal barrier function after open abdominal surgery. Surgery 2021, 169, 885–893. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Camara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Wang, S.L.; Zhang, F.M.; Chen, C.B.; Dong, Q.T.; Liu, S.; Yu, Z.; Shen, X.; Zhuang, C.L. Comparison between AWGC-cachexia and GLIM-malnutrition in patients with gastric cancer. Eur. J. Surg. Oncol. 2024, 50, 108580. [Google Scholar]
- Heneghan, H.M.; Zaborowski, A.; Fanning, M.; McHugh, A.; Doyle, S.; Moore, J.; Ravi, N.; Reynolds, J.V. Prospective Study of Malabsorption and Malnutrition After Esophageal and Gastric Cancer Surgery. Ann. Surg. 2015, 262, 803–807; discussion 807–808. [Google Scholar] [CrossRef]
- Freitas, R.D.S.; Campos, M.M. Protective Effects of Omega-3 Fatty Acids in Cancer-Related Complications. Nutrients 2019, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.J.; Douglas, C.; McCullough, F.S.; Stanworth, S.J.; Calder, P.C. Impact of enteral immunonutrition on infectious complications and immune and inflammatory markers in cancer patients undergoing chemotherapy: A systematic review of randomised controlled trials. Clin. Nutr. 2022, 41, 2135–2146. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S.; Chen, C.; Xie, B.; Fang, Z.; Hu, W.; Chen, J.; Fu, H.; He, H. Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-kappaB pathway following experimental traumatic brain injury. J. Neuroinflamm. 2017, 14, 143. [Google Scholar] [CrossRef] [PubMed]
- Maulydia, M.; Rehatta, N.M.; Soedarmo, S.M. Effects of glutamine and arginine combination on pro- and anti-inflammatory cytokines. Open Vet. J. 2023, 13, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Zhao, R.Y.; Li, H.H.; Xu, X.M.; Cui, H.; Deng, H.; Chen, L.; Wei, B. Oral administration of asparagine and 3-indolepropionic acid prolongs survival time of rats with traumatic colon injury. Mil. Med. Res. 2022, 9, 37. [Google Scholar] [CrossRef]
- Ma, Y.; Shan, K.; Huang, Z.; Zhao, D.; Zhang, M.; Ke, W.; Li, C. Bile Acid Derivatives Effectively Prevented High-Fat Diet-Induced Colonic Barrier Dysfunction. Mol. Nutr. Food Res. 2023, 67, e2200649. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Fak, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Backhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergstrom, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Backhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef] [PubMed]
- Zeller, G.; Tap, J.; Voigt, A.Y.; Sunagawa, S.; Kultima, J.R.; Costea, P.I.; Amiot, A.; Bohm, J.; Brunetti, F.; Habermann, N.; et al. Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol. Syst. Biol. 2014, 10, 766. [Google Scholar] [CrossRef]
- Huson, D.H.; Mitra, S.; Ruscheweyh, H.J.; Weber, N.; Schuster, S.C. Integrative analysis of environmental sequences using MEGAN4. Genome Res. 2011, 21, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, H.; Cai, X.; Zhong, H.; Feng, Q.; Sunagawa, S.; Arumugam, M.; Kultima, J.R.; Prifti, E.; Nielsen, T.; et al. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 2014, 32, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liang, S.; Jia, H.; Stadlmayr, A.; Tang, L.; Lan, Z.; Zhang, D.; Xia, H.; Xu, X.; Jie, Z.; et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat. Commun. 2015, 6, 6528. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Lee, Y. What repeated measures analysis of variances really tells us. Korean J. Anesthesiol. 2015, 68, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Efremova, I.; Maslennikov, R.; Medvedev, O.; Kudryavtseva, A.; Avdeeva, A.; Krasnov, G.; Romanikhin, F.; Diatroptov, M.; Fedorova, M.; Poluektova, E.; et al. Gut Microbiota and Biomarkers of Intestinal Barrier Damage in Cirrhosis. Microorganisms 2024, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, I.; Calder, P.C. Pros and Cons of Long-Chain Omega-3 Polyunsaturated Fatty Acids in Cardiovascular Health. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 383–406. [Google Scholar] [CrossRef]
- Elisia, I.; Yeung, M.; Kowalski, S.; Wong, J.; Rafiei, H.; Dyer, R.A.; Atkar-Khattra, S.; Lam, S.; Krystal, G. Omega 3 supplementation reduces C-reactive protein, prostaglandin E(2) and the granulocyte/lymphocyte ratio in heavy smokers: An open-label randomized crossover trial. Front. Nutr. 2022, 9, 1051418. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, C.; Liang, W.; Li, L.; Du, J.; Pan, C.; Chen, B.; Chen, Y.; Wang, Y. omega-3 and omega-6 Polyunsaturated Fatty Acids Regulate the Proliferation, Invasion and Angiogenesis of Gastric Cancer Through COX/PGE Signaling Pathway. Front. Oncol. 2022, 12, 802009. [Google Scholar]
- Mayer, K.; Fegbeutel, C.; Hattar, K.; Sibelius, U.; Kramer, H.J.; Heuer, K.U.; Temmesfeld-Wollbruck, B.; Gokorsch, S.; Grimminger, F.; Seeger, W. Omega-3 vs. omega-6 lipid emulsions exert differential influence on neutrophils in septic shock patients: Impact on plasma fatty acids and lipid mediator generation. Intensive Care Med. 2003, 29, 1472–1481. [Google Scholar] [CrossRef]
- Almeida, E.B.; Silva, K.P.H.; Paixao, V.; Amaral, J.B.D.; Rossi, M.; Xavier-Navarro, R.A.; Barros, K.V.; Silveira, V.L.F.; Vieira, R.P.; Oliveira, L.V.F.; et al. A Mixture of Polyunsaturated Fatty Acids omega-3 and omega-6 Reduces Melanoma Growth by Inhibiting Inflammatory Mediators in the Murine Tumor Microenvironment. Int. J. Mol. Sci. 2019, 20, 3765. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; Khedr, N.F.; El-Bahrawy, H.A.; Helal, S.A. Upregulation of PPAR-gamma mediates the renoprotective effect of omega-3 PUFA and ferulic acid in gentamicin-intoxicated rats. Biomed. Pharmacother. 2018, 99, 504–510. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.; Fan, S.; Wu, S.; Yang, F.; Fang, Z.; Fu, H.; Li, Y. Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-kappaB pathway following experimental traumatic brain injury. J. Neuroinflamm. 2018, 15, 116. [Google Scholar] [CrossRef]
- Anez-Bustillos, L.; Dao, D.T.; Finkelstein, A.; Pan, A.; Cho, B.S.; Mitchell, P.D.; Gura, K.M.; Bistrian, B.R.; Puder, M. Metabolic and Inflammatory Effects of an omega-3 Fatty Acid-Based Eucaloric Ketogenic Diet in Mice With Endotoxemia. JPEN J. Parenter. Enteral Nutr. 2019, 43, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Fell, G.L.; Cho, B.S.; Pan, A.; Nose, V.; Anez-Bustillos, L.; Dao, D.T.; Baker, M.A.; Nandivada, P.; Gura, K.M.; Puder, M. A Comparison of Fish Oil Sources for Parenteral Lipid Emulsions in a Murine Model. JPEN J. Parenter. Enteral Nutr. 2017, 41, 181–187. [Google Scholar] [CrossRef]
- Tejero, J.; Hunt, A.P.; Santolini, J.; Lehnert, N.; Stuehr, D.J. Mechanism and regulation of ferrous heme-nitric oxide (NO) oxidation in NO synthases. J. Biol. Chem. 2019, 294, 7904–7916. [Google Scholar] [CrossRef] [PubMed]
- Stadler, J.; Harbrecht, B.G.; Di Silvio, M.; Curran, R.D.; Jordan, M.L.; Simmons, R.L.; Billiar, T.R. Endogenous nitric oxide inhibits the synthesis of cyclooxygenase products and interleukin-6 by rat Kupffer cells. J. Leukoc. Biol. 1993, 53, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Liang, Y.; Meng, X.; Zhao, Y.; Fan, H.; Hou, S. The Role of Long Noncoding RNAs in Intestinal Health and Diseases: A Focus on the Intestinal Barrier. Biomolecules 2023, 13, 1674. [Google Scholar] [CrossRef]
- Diez-Sainz, E.; Lorente-Cebrian, S.; Aranaz, P.; Riezu-Boj, J.I.; Martinez, J.A.; Milagro, F.I. Potential Mechanisms Linking Food-Derived MicroRNAs, Gut Microbiota and Intestinal Barrier Functions in the Context of Nutrition and Human Health. Front. Nutr. 2021, 8, 586564. [Google Scholar] [CrossRef]
- Shen, J.; Wang, S.; Xia, H.; Han, S.; Wang, Q.; Wu, Z.; Zhuge, A.; Li, S.; Chen, H.; Lv, L.; et al. Akkermansia muciniphila attenuated lipopolysaccharide-induced acute lung injury by modulating the gut microbiota and SCFAs in mice. Food Funct. 2023, 14, 10401–10417. [Google Scholar] [CrossRef]
- Qiao, C.M.; Huang, W.Y.; Zhou, Y.; Quan, W.; Niu, G.Y.; Li, T.; Zhang, M.X.; Wu, J.; Zhao, L.P.; Zhao, W.J.; et al. Akkermansia muciniphila Is Beneficial to a Mouse Model of Parkinson’s Disease, via Alleviated Neuroinflammation and Promoted Neurogenesis, with Involvement of SCFAs. Brain Sci. 2024, 14, 238. [Google Scholar] [CrossRef]
- Gonzalez, C.G.; Mills, R.H.; Kordahi, M.C.; Carrillo-Terrazas, M.; Secaira-Morocho, H.; Widjaja, C.E.; Tsai, M.S.; Mittal, Y.; Yee, B.A.; Vargas, F.; et al. The Host-Microbiome Response to Hyperbaric Oxygen Therapy in Ulcerative Colitis Patients. Cell Mol. Gastroenterol. Hepatol. 2022, 14, 35–53. [Google Scholar] [CrossRef]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef]
- Haghikia, A.; Jorg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.H.; May, C.; Wilck, N.; et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2015, 43, 817–829. [Google Scholar] [CrossRef]
- de Oliveira, M.T.; de Oliveira, F.L.; Salgaço, M.K.; Mesa, V.; Sartoratto, A.; Duailibi, K.; Raimundo, B.V.B.; Ramos, W.S.; Sivieri, K. Restoring Balance: Probiotic Modulation of Microbiota, Metabolism, and Inflammation in SSRI-Induced Dysbiosis Using the SHIME® Model. Pharmaceuticals 2025, 18, 1132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Gong, J.; Huang, W.; Liu, W.; Ma, C.; Liang, R.; Chen, Y.; Xie, Z.; Li, P.; Liao, Q. Structural Analysis and Antioxidant and Immunoregulatory Activities of an Exopolysaccharide Isolated from Bifidobacterium longum subsp. longum XZ01. Molecules 2023, 28, 7448. [Google Scholar] [CrossRef]
- D’Ambrosio, S.; Dabous, A.; Sadiq, S.; Casillo, A.; Schiraldi, C.; Cassese, E.; Bedini, E.; Corsaro, M.M.; Cimini, D. Bifidobacterium animalis subsp. lactis HN019 live probiotics and postbiotics: Production strategies and bioactivity evaluation for potential therapeutic properties. Front. Bioeng. Biotechnol. 2024, 12, 1379574. [Google Scholar]
- Schroeder, B.O.; Birchenough, G.M.H.; Stahlman, M.; Arike, L.; Johansson, M.E.V.; Hansson, G.C.; Backhed, F. Bifidobacteria or Fiber Protects against Diet-Induced Microbiota-Mediated Colonic Mucus Deterioration. Cell Host Microbe 2018, 23, 27–40 e27. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, L.; Yang, S.; Tan, P.; Lei, W.; Yang, H.; Gao, Z. Lactobacillus acidophilus 6074 Fermented Jujube Juice Ameliorated DSS-induced Colitis via Repairing Intestinal Barrier, Modulating Inflammatory Factors, and Gut Microbiota. Mol. Nutr. Food Res. 2025, 69, e202400568. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Huang, C.; Ning, Z.; Zhang, Y.; Zhuang, M.; Yang, W.; Wang, X.; Wang, J.; Zhang, L.; Xiao, H.; et al. Ruminococcus gnavus plays a pathogenic role in diarrhea-predominant irritable bowel syndrome by increasing serotonin biosynthesis. Cell Host Microbe 2023, 31, 33–44 e35. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, A.; Lkhagvajav, T.; Mishima, R.; Therdtatha, P.; Jamiyan, D.; Purevdorj, C.; Sonomtseren, S.; Chimeddorj, B.; Namdag, B.; Lee, Y.K.; et al. Gut microbiome signatures associated with type 2 diabetes in obesity in Mongolia. Front. Microbiol. 2024, 15, 1355396. [Google Scholar] [CrossRef]
- Wong, C.C.; Fong, W.; Yu, J. Gut microbes promote chemoradiotherapy resistance via metabolic cross-feeding. Cancer Cell 2023, 41, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Hegerle, N.; Nkeze, J.; Sen, S.; Jamindar, S.; Nasrin, S.; Sen, S.; Permala-Booth, J.; Sinclair, J.; Tapia, M.D.; et al. The Diversity of Lipopolysaccharide (O) and Capsular Polysaccharide (K) Antigens of Invasive Klebsiella pneumoniae in a Multi-Country Collection. Front. Microbiol. 2020, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yuan, R.; Cui, X.; Cui, Y.; Han, S.; Wang, Q.Q.; Chen, Y.; Huang, L.; Yang, S.; Xu, Q.; et al. Anemoside B4 protects against Klebsiella pneumoniae- and influenza virus FM1-induced pneumonia via the TLR4/Myd88 signaling pathway in mice. Chin. Med. 2020, 15, 68. [Google Scholar] [CrossRef]
- Walev, I.; Hombach, M.; Bobkiewicz, W.; Fenske, D.; Bhakdi, S.; Husmann, M. Resealing of large transmembrane pores produced by streptolysin O in nucleated cells is accompanied by NF-kappaB activation and downstream events. FASEB J. 2002, 16, 237–239. [Google Scholar] [CrossRef]
- Khalil, M.A.; Alorabi, J.A.; Al-Otaibi, L.M.; Ali, S.S.; Elsilk, S.E. Antibiotic Resistance and Biofilm Formation in Enterococcus spp. Isolated from Urinary Tract Infections. Pathogens 2022, 12, 34. [Google Scholar] [CrossRef]
- Lipski, A.; Herve, M.; Lombard, V.; Nurizzo, D.; Mengin-Lecreulx, D.; Bourne, Y.; Vincent, F. Structural and biochemical characterization of the beta-N-acetylglucosaminidase from Thermotoga maritima: Toward rationalization of mechanistic knowledge in the GH73 family. Glycobiology 2015, 25, 319–330. [Google Scholar] [CrossRef][Green Version]
- Ketudat Cairns, J.R.; Esen, A. beta-Glucosidases. Cell Mol. Life Sci. 2010, 67, 3389–3405. [Google Scholar] [CrossRef]
- Wu, X.R.; He, X.H.; Xie, Y.F. Characteristics of gut microbiota dysbiosis in patients with colorectal polyps. World J. Gastrointest. Oncol. 2025, 17, 98872. [Google Scholar] [CrossRef]
- Yin, L.L.; Qi, P.Q.; Hu, Y.F.; Fu, X.J.; He, R.S.; Wang, M.M.; Deng, Y.J.; Xiong, S.Y.; Yu, Q.W.; Hu, J.P.; et al. Dysbiosis promotes recurrence of adenomatous polyps in the distal colorectum. World J. Gastrointest. Oncol. 2024, 16, 3600–3623. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Yin, S.; Yu, J.; Wang, J.; Jin, X.; Wang, Y.; Liu, H.; Sun, G. Improvement in Glycolipid Metabolism Parameters After Sup-plementing Fish Oil-Derived Omega-3 Fatty Acids Is Associated with Gut Microbiota and Lipid Metabolites in Type 2 Diabetes Mellitus. Nutrients 2024, 16, 3755. [Google Scholar] [CrossRef]
- Khan, M.N.; Xie, Z.; Bukhari, S.M.B.; Nielsen, D.S.; Imran, M. Dairy-based multi-strain probiotic community successfully miti-gated obesity-related gut microbiota dysbiosis in vitro (CoMiniGut). J. Med. Microbiol. 2024, 73. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, G.; Xu, Y.L.; Fang, Q.; Ye, L.; Wang, C.H.; Liu, X.S. Preoperative Status of Gut Microbiota Predicts Postoperative Delirium in Patients With Gastric Cancer. Front. Psychiatry 2022, 13, 852269. [Google Scholar] [CrossRef]
- Xiao, H.; Xiao, Y.; Quan, H.; Liu, W.; Pan, S.; Ouyang, Y. Intra-abdominal infection after radical gastrectomy for gastric cancer: Incidence, pathogens, risk factors and outcomes. Int. J. Surg. 2017, 48, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Jardon, K.M.; Goossens, G.H.; Most, J.; Galazzo, G.; Venema, K.; Penders, J.; Blaak, E.E. Examination of sex-specific interactions between gut microbiota and host metabolism after 12-week combined polyphenol supplementation in individuals with overweight or obesity. Gut Microbes 2024, 16, 2392875. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Del-Campo, A.; Gozalbo-Rovira, R.; Moya-Gonzalvez, E.M.; Alberola, J.; Rodriguez-Diaz, J.; Yebra, M.J. Infant gut microbiota modulation by human milk disaccharides in humanized microbiome mice. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]






| Variables | Groups | p | |
|---|---|---|---|
| Control Group (n = 29) | Experimental Group (n = 29) | ||
| Age (years) | 59.207 ± 10.311 | 57.828 ± 12.795 | 0.653 |
| Gender, n (%) Male | 21 (72.41) | 19 (65.52) | 0.570 |
| Female | 8 (27.59) | 10 (34.48) | |
| BMI (kg/m2) | 23.254 ± 3.207 | 23.345 ± 3.434 | 0.918 |
| Hospitalization time (days) Surgical procedure, n (%) | 16.414 ± 10.995 | 13.448 ± 2.585 | 0.167 |
| Total gastrectomy | 12 (41.38) | 9 (31.03) | 0.644 |
| Distal gastrectomy | 1 (3.45) | 2 (6.90) | |
| Proximal gastrectomy | 16 (55.17) | 18 (62.07) | |
| Neoadjuvant chemotherapy, n (%) Yes No | 10 (34.48) 19 (65.52) | 11 (37.93) 18 (62.07) | 0.785 |
| Operation time (hours) | 3.224 ± 0.867 | 3.103 ± 0.763 | 0.576 |
| AJCC stage I | 13 (44.83) | 12 (41.38) | 0.157 |
| II | 10 (34.48) | 5 (17.24) | |
| III | 6 (20.69) | 12 (41.38) | |
| Postoperative complication, n (%) Yes No | 23(79.31) 6(20.69) | 24 (82.76) 5 (17.24) | 0.738 |
| Variables | Statistical Indicators | F | p |
|---|---|---|---|
| White blood cell (109/L) | Time | 2.462 | 0.064 |
| Group | 0.254 | 0.859 | |
| Absolute value of lymphocytes (109/L) | Time | 0.501 | 0.682 |
| Group | 0.196 | 0.899 | |
| CD3+ T cell (cells/μL) | Time | 0.691 | 0.558 |
| Group | 0.069 | 0.976 | |
| CD4+ T cell (cells/μL) | Time | 0.968 | 0.409 |
| Group | 0.171 | 0.916 | |
| CD8+ T cell (cells/μL) | Time | 0.692 | 0.558 |
| Group | 0.061 | 0.980 | |
| Hypersensitive C-reactive protein (mg/L) | Time | 0.653 | 0.582 |
| Group | 0.264 | 0.852 | |
| Interleukin-6 (pg/mL) | Time | 1.033 | 0.355 |
| Group | 0.212 | 0.792 | |
| Immunoglobulin G (g/L) | Time | 1.241 | 0.297 |
| Group | 0.891 | 0.447 | |
| Total protein (g/L) | Time | 0.832 | 0.478 |
| Group | 0.918 | 0.434 | |
| Albumin (g/L) | Time | 0.164 | 0.921 |
| Group | 0.858 | 0.464 | |
| Prealbumin (mg/L) | Time | 0.448 | 0.719 |
| Group | 0.448 | 0.719 | |
| Hemoglobin (g/L) | Time | 0.775 | 0.510 |
| Group | 0.956 | 0.415 |
| Variables | Statistical Indicators | F | p |
|---|---|---|---|
| Diamine oxidase (U/mL) | Time | 11.364 | 0.000 *** |
| Group | 2.770 | 0.043 * | |
| D-lactate (mg/L) | Time | 20.263 | 0.000 *** |
| Group | 0.462 | 0.541 | |
| Endotoxin (EU/mL) | Time | 2.844 | 0.039 * |
| Group | 2.772 | 0.043 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Liu, G.; Wang, Y.; Li, J.; Zhang, C.; Zhang, Y.; Ye, X.; Kang, W. Perioperative Oral Immunonutrient Regulation of Intestinal Barrier and Gut Microbiota in Patients with Gastric Cancer, a Randomized Controlled Clinical Trial. Biomedicines 2025, 13, 2163. https://doi.org/10.3390/biomedicines13092163
Zheng Z, Liu G, Wang Y, Li J, Zhang C, Zhang Y, Ye X, Kang W. Perioperative Oral Immunonutrient Regulation of Intestinal Barrier and Gut Microbiota in Patients with Gastric Cancer, a Randomized Controlled Clinical Trial. Biomedicines. 2025; 13(9):2163. https://doi.org/10.3390/biomedicines13092163
Chicago/Turabian StyleZheng, Zicheng, Guanmo Liu, Yihua Wang, Jie Li, Chenggang Zhang, Yajun Zhang, Xin Ye, and Weiming Kang. 2025. "Perioperative Oral Immunonutrient Regulation of Intestinal Barrier and Gut Microbiota in Patients with Gastric Cancer, a Randomized Controlled Clinical Trial" Biomedicines 13, no. 9: 2163. https://doi.org/10.3390/biomedicines13092163
APA StyleZheng, Z., Liu, G., Wang, Y., Li, J., Zhang, C., Zhang, Y., Ye, X., & Kang, W. (2025). Perioperative Oral Immunonutrient Regulation of Intestinal Barrier and Gut Microbiota in Patients with Gastric Cancer, a Randomized Controlled Clinical Trial. Biomedicines, 13(9), 2163. https://doi.org/10.3390/biomedicines13092163







