Beyond Latency: Chronic Toxoplasma Infection and Its Unveiled Behavioral and Clinical Manifestations—A 30-Year Research Perspective
Abstract
1. Introduction: Toxoplasma gondii
2. Toxoplasmosis, Behavior and Personality
2.1. Toxoplasma and Behavior
2.1.1. Toxoplasma and Behavior Change in Rodents and Mice
2.1.2. Toxoplasma and Behavior Change in Non-Human Primates
2.1.3. Toxoplasma and Behavior Change in Humans
2.2. Toxoplasma and Personality
2.2.1. Toxoplasma and Cattell’s 16PF
2.2.2. Toxoplasma and NEO PI–R and TCI
2.2.3. What Is Behind the Opposite Effects of Toxoplasma on the Personality of Men and Women?
3. Toxoplasma and Sexual Behavior: Key Research Directions
3.1. Sexual Transmission of Toxoplasma
3.2. The Sexual Behavior of Toxoplasma-Infected Individuals
3.3. The Sexual Behavior of Uninfected Individuals Towards Toxoplasma-Infected Individuals
4. Toxoplasma and Cognition
4.1. Toxoplasma, Reaction Time, and Processing Speed
4.2. Toxoplasma Infection and Cognitive Outcomes
4.3. Toxoplasma and Intelligence
5. Toxoplasma Infection and Mental Health
5.1. Schizophrenia
5.2. Autism
5.3. Attention Deficit–Hyperactivity Disorder
5.4. Bipolar Disorder
5.5. Anxiety
5.6. Depression
5.7. Obsessive–Compulsive Disorder
5.8. Antisocial Personality Disorder
5.9. Learning Disabilities
5.10. Substance Use Disorder
5.11. Neurotropic Zoonoses: Synergistic and Antagonistic Effects of Toxoplasma, Bartonella, and Borrelia Infections on Mental Health
6. Toxoplasma and Physical Health
6.1. Health Burden of Toxoplasma Infection
6.2. Toxoplasma Infection and Reproductive Outcomes
6.2.1. Effects of Toxoplasma Infection on Pregnancy
6.2.2. Effect of Toxoplasma Infection of a Mother on Postnatal Development of Children
6.2.3. Effects of Toxoplasma Infection on Male-to-Female Birth Ratios
6.2.4. Toxoplasma Infection and Human Fertility
Male Fertility
Female Fertility
7. Biology of the Mechanisms Underlying the Effects of Toxoplasma Infection
7.1. Neurotransmitters
7.1.1. Dopamine
7.1.2. Glutamate and GABA
7.1.3. Serotonin
7.1.4. Norepinephrine
7.2. Hormones
7.2.1. Testosterone
7.2.2. Vasopressin
7.2.3. Oxytocin
7.2.4. Cortisol
7.3. The Immune System and Toxoplasmosis
7.3.1. Immune-Mediated Physical Health Effects
7.3.2. Immune-Mediated Mental Health Effects
7.3.3. Immune-Mediated Reproductive Effects
8. Host and Parasite Factors Modulating the Effects of Toxoplasma Infection
8.1. Modifying Effect of Host Sex on Toxoplasma-Associated Behavioral and Health Changes
8.1.1. Animal Studies
8.1.2. Human Studies
8.2. Rhesus Factor
8.3. Strain of the Parasite
8.3.1. Strain-Dependent Effects in Animal Models
8.3.2. Strain-Dependent Effects in Humans
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Johnson, S.K.; Johnson, P.T.J. Toxoplasmosis: Recent advances in understanding the link between infection and host behavior. Annu. Rev. Anim. Biosci. 2021, 9, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Wesołowski, R.; Pawłowska, M.; Smoguła, M.; Szewczyk-Golec, K. Advances and challenges in diagnostics of toxoplasmosis in HIV-infected patients. Pathogens 2023, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.H.; Pavey, C.; O’Handley, R.; Vyas, A. Behavioral biology of Toxoplasma gondii infection. Parasit. Vectors 2021, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Jones, J.L. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasit. 2008, 38, 1257–1278. [Google Scholar] [CrossRef] [PubMed]
- Bogitsh, J.B.; Carter, C.E.; Oeltmann, T.N. Med Parasitol; Elsevier, Inc.: Amsterdam, The Netherlands, 2013. [Google Scholar]
- McAllister, M.M. A decade of discoveries in veterinary protozoology changes our concept of “subclinical” toxoplasmosis. Vet. Parasitol. 2005, 132, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.E.; Chirukandoth, S.; Dubey, J.P. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim. Health Res. Rev. 2005, 6, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Barnard, C.J.; Behnke, J.M. Parasitism and Host Behaviour; Taylor and Francis: New York, NY, USA, 1990; Volume 1. [Google Scholar]
- Flegr, J. Xenoadaptations. In Encyclopedia of Sexual Psychology and Behavior; Shackelford, T.K., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–5. [Google Scholar]
- Webster, J.P. The effect of Toxoplasma gondii and other parasites on activity levels in wild and hybrid Rattus norvegicus. Parasitology 1994, 109, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.P.; Brunton, C.F.A.; Macdonald, D.W. Effect of Toxoplasma gondii upon neophobic behaviour in wild brown rats, Rattus norvegicus. Parasitology 1994, 109, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.; Hutchison, W.M.; Aitken, P.P.; Graham, D.I. The effect of congenital and adult-acquired Toxoplasma infections on activity and responsiveness to novel stimulation in mice. Ann. Trop. Med. Parasitol. 1983, 77, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.; Aitken, P.P.; Graham, D.I. Toxoplasma infection and response to novelty in mice. Z. Parasitenkd. 1984, 70, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, W.M.; Bradley, M.; Cheyne, W.M.; Wells, B.W.P.; Hay, J. Behavioural abnormalities in Toxoplasma-infected mice. Ann. Trop. Med. Parasit. 1980, 74, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, W.M.; Aitken, P.P.; Wells, W.P. Chronic Toxoplasma infections and familiarity-novelty discrimination in the mouse. Ann. Trop. Med. Parasit. 1980, 74, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Piekarski, G.; Zippelius, H.M.; Witting, P.A. Auswirkungen einer latenten Toxoplasma-Infektion auf das Lernvermogen von weissen Laboratoriumsratten and Mausen. Z. Parasitenkd. 1978, 57, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Witting, P.A. Learning capacity and memory of normal and Toxoplasma-infected laboratory rats and mice. Z. Parasitenkd. 1979, 61, 29–51. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J. Influence of latent toxoplasmosis on the phenotype of intermediate hosts. Folia Parasitol. 2010, 57, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Hrdý, I. Influence of chronic toxoplasmosis on some human personality factors. Folia Parasitol. 1994, 41, 122–126. [Google Scholar]
- Flegr, J.; Zitkova, S.; Kodym, P.; Frynta, D. Induction of changes in human behaviour by the parasitic protozoan Toxoplasma gondii. Parasitology 1996, 113, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Hari Dass, S.A.; Vyas, A. Toxoplasma gondii infection reduces predator aversion in rats through epigenetic modulation in the host medial amygdala. Mol. Ecol. 2014, 23, 6114–6122. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, J.W.; McConkey, G.A.; Westhead, D.R. The transferome of metabolic genes explored: Analysis of the horizontal transfer of enzyme encoding genes in unicellular eukaryotes. Genome Biol. 2009, 10, R36. [Google Scholar] [CrossRef] [PubMed]
- Castello, A.; Bruschetta, G.; Giunta, R.P.; Marino, A.M.F.; Ferlazzo, A.M. The effect of Toxoplasma gondii on plasma serotonin concentration in sheep. Vet. World 2018, 11, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Xu, C.; Zhao, G.H.; Xie, H.H. Epigenetic manipulation of psychiatric behavioral disorders induced by Toxoplasma gondii. Front. Cell. Infect. Microbiol. 2022, 12, 803502. [Google Scholar] [CrossRef] [PubMed]
- David, C.N.; Frias, E.S.; Szu, J.I.; Vieira, P.A.; Hubbard, J.A.; Lovelace, J.; Michael, M.; Worth, D.; McGovern, K.E.; Ethell, I.M.; et al. GLT-1-dependent disruption of CNS glutamate homeostasis and neuronal function by the protozoan parasite Toxoplasma gondii. PLoS Pathog. 2016, 12, e1005643. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Latifi, A.; Kaňková, Š. Toxoplasma infection and its sequential impact on physical health, stress, and anxiety: A large cross-sectional study testing the stress-coping hypothesis. medRxiv 2024. [Google Scholar] [CrossRef]
- Flegr, J.; Prandota, J.; Sovickova, M.; Israili, Z.H. Toxoplasmosis—A global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLoS ONE 2014, 9, e90203. [Google Scholar] [CrossRef] [PubMed]
- Torrey, E.F.; Bartko, J.J.; Lun, Z.R.; Yolken, R.H. Antibodies to Toxoplasma gondii in patients with schizophrenia: A meta-analysis. Schizophr. Bull. 2007, 33, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Afifi, M.A.; Jiman-Fatani, A.A.; Al-Rabia, M.W.; Al-Hussainy, N.H.; El Saadany, S.; Mayah, W. More than an association: Latent toxoplasmosis might provoke a local oxidative stress that triggers the development of bipolar disorder. J. Microsc. Ultrastruct. 2018, 6, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.L.; Wang, B.Y.; Wu, S.Z.; Yang, Z.H.; Xin, Z.X.; Zheng, S.Y.; Zou, W.H.; Zhang, C.; Chen, J.T.; Peng, H.J. Population-based cohort study of Toxoplasma gondii P22 antibody positivity correlation with anxiety. J. Affect. Disord. 2024, 359, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Akaltun, I.; Kara, T.; Ayaydin, H.; Alyanak, B.; Beka, H.; Agacfidan, A. The relation between serum Toxoplasma gondii IgG antibody in children and ADHD and its severity. Psychiatry Clin. Psychopharmacol. 2019, 29, 326–331. [Google Scholar] [CrossRef]
- Gajewski, P.D.; Falkenstein, M.; Hengstler, J.G.; Golka, K. Toxoplasma gondii impairs memory in infected seniors. Brain Behav. Immun. 2014, 36, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Egorov, A.I.; Converse, R.R.; Griffin, S.M.; Styles, J.N.; Sams, E.; Hudgens, E.; Wade, T.J. Latent Toxoplasma gondii infections are associated with elevated biomarkers of inflammation and vascular injury. BMC Infect. Dis. 2021, 21, 188. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, F. The interplay of dopamine metabolism abnormalities and mitochondrial defects in the pathogenesis of schizophrenia. Transl. Psychiatr. 2022, 12, 464. [Google Scholar] [CrossRef] [PubMed]
- Pålsson, E.; Sellgren, C.; Pelanis, A.; Zetterberg, H.; Blennow, K.; Landén, M. Altered brain dopamine metabolism is a trait marker for bipolar disorder. Biomark. Neuropsychiatry 2023, 9, 100078. [Google Scholar] [CrossRef]
- Hlaváčová, J.; Flegr, J.; Řežábek, K.; Calda, P.; Kaňková, Š. Male-to-female presumed transmission of toxoplasmosis between sexual partners. Am. J. Epidemiol. 2021, 190, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A. Nuts and bolts of the behavioural manipulation by Toxoplasma gondii. Folia Parasitol. 2024, 71, 6. [Google Scholar] [CrossRef] [PubMed]
- Abdoli, A.; Ghaffarifar, F.; Sharifi, Z.; Taghipour, A. Toxoplasma gondii infection and testosterone alteration: A systematic review and meta-analyses. PLoS ONE 2024, 19, e0297362. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, E.A.; Smith, J.E.; Pinney, J.W.; Westhead, D.R.; McConkey, G.A. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PLoS ONE 2009, 4, e4801. [Google Scholar] [CrossRef] [PubMed]
- Hodková, H.; Kodym, P.; Flegr, J. Poorer results of mice with latent toxoplasmosis in learning tests: Impaired learning processes or the novelty discrimination mechanism? Parasitology 2007, 134, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Skallová, A.; Kodym, P.; Frynta, D.; Flegr, J. The role of dopamine in Toxoplasma-induced behavioural alterations in mice: An ethological and ethopharmacological study. Parasitology 2006, 133, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.P. Who makes the decisions? Uncovering the evolutionary implications and clinical applications of Toxoplasma gondii’s Fatal Feline Attraction. Folia Parasitol. 2025, 72, 016. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.; Aitken, P.P.; Hair, D.M.; Hutchison, W.M.; Graham, D.I. The effect of congenital Toxoplasma infection on mouse activity and relative preference for exposed areas over a series of trials. Ann. Trop. Med. Parasit. 1984, 78, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.P.; Hegglin, D.; Briner, T.; Ruetten, M.; Muller, N.; More, G.; Frey, C.F.; Deplazes, P.; Basso, W. High prevalence rates of Toxoplasma gondii in cat-hunted small mammals-Evidence for parasite induced behavioural manipulation in the natural environment? Int. J. Parasitol. Parasites Wildl. 2023, 20, 108–116. [Google Scholar] [CrossRef]
- Berdoy, M.; Webster, J.P.; Macdonald, D.W. Fatal attraction in rats infected with Toxoplasma gondii. Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 1591–1594. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; Kim, S.K.; Giacomini, N.; Boothroyd, J.C.; Sapolsky, R.M. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc. Natl. Acad. Sci. USA 2007, 104, 6442–6447. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Kumar, V.; Dass, S.A.H.; Vyas, A. Toxoplasma gondii infection enhances testicular steroidogenesis in rats. Mol. Ecol. 2013, 22, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Markos, A. Masterpiece of epigenetic engineering—How Toxoplasma gondii reprogrammes host brains to change fear to sexual attraction. Mol. Ecol. 2014, 23, 5934–5936. [Google Scholar] [CrossRef] [PubMed]
- Cano-Terriza, D.; Puig-Ribas, M.; Jimenez-Ruiz, S.; Cabezon, O.; Almeria, S.; Galan-Relano, A.; Dubey, J.P.; Garcia-Bocanegra, I. Risk factors of Toxoplasma gondii infection in hunting, pet and watchdogs from southern Spain and northern Africa. Parasitol. Int. 2016, 65, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H.; Yang, Y.; Su, C. Recent epidemiologic, clinical, and genetic diversity of Toxoplasma gondii infections in non-human primates. Res. Vet. Sci. 2021, 136, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Boesch, C. The effects of leopard predation on grouping patterns in forest chimpanzees. Behaviour 1991, 117, 220–241. [Google Scholar] [CrossRef]
- Nakazawa, N.; Hanamura, S.; Inoue, E.; Nakatsukasa, M.; Nakamura, M. A leopard ate a chimpanzee: First evidence from East Africa. J. Hum. Evol. 2013, 65, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Poirotte, C.; Kappeler, P.M.; Ngoubangoye, B.; Bourgeois, S.; Moussodji, M.; Charpentier, M.J.E. Morbid attraction to leopard urine in Toxoplasma-infected chimpanzees. Curr. Biol. 2016, 26, R98–R99. [Google Scholar] [CrossRef] [PubMed]
- Lindová, J.; Novotná, M.; Havlíček, J.; Jozífková, E.; Skallová, A.; Kolbeková, P.; Hodný, Z.; Kodym, P.; Flegr, J. Gender differences in behavioural changes induced by latent toxoplasmosis. Int. J. Parasit. 2006, 36, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.B.; Brenner, L.A.; Cloninger, C.R.; Langenberg, P.; Igbide, A.; Giegling, I.; Hartmann, A.M.; Konte, B.; Friedl, M.; Brundin, L.; et al. “Latent” infection with Toxoplasma gondii: Association with trait aggression and impulsivity in healthy adults. J. Psychiatr. Res. 2015, 60, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Coccaro, E.F.; Lee, R.; Groer, M.W.; Can, A.; Coussons-Read, M.; Postolache, T.T. Toxoplasma gondii infection: Relationship with aggression in psychiatric subjects. J. Clin. Psychiatry 2016, 77, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Hamid, N.; Azizy, B.; Hamidinejad, H. Toxoplasma gondii infection and aggression in autistic children. Pediatr. Infect. Dis. J. 2022, 41, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Havlíček, J.; Kodym, P.; Malý, M.; Šmahel, Z. Increased risk of traffic accidents in subjects with latent toxoplasmosis: A retrospective case-control study. BMC Infect. Dis. 2002, 2, art-11. [Google Scholar] [CrossRef] [PubMed]
- Yereli, K.; Balcioglu, I.C.; Ozbilgin, A. Is Toxoplasma gondii a potential risk for traffic accidents in Turkey? Forensic Sci. Int. 2006, 163, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, E.V.; Kondrashin, A.V.; Sergiev, V.P.; Morozova, L.F.; Turbabina, N.A.; Maksimova, M.S.; Brazhnikov, A.I.; Shevchenko, S.B.; Morozov, E.N. Significance of chronic toxoplasmosis in epidemiology of road traffic accidents in Russian Federation. PLoS ONE 2017, 12, e0184930. [Google Scholar] [CrossRef] [PubMed]
- Ghasemirad, H.; Aalazade, M.S.; Shariatpanahi, M.; Owliaey, H.; Kargar, M.; Ghasemirad, M.; Zare, M. Higher risk of car accidents in older patients with Toxoplasma gondii in Yazd province, center of Iran: A Cohort Study. Preprint 2022. [Google Scholar] [CrossRef]
- Rayatdoost, E.; Chegin, M.; Taghipour, A.; Shadmand, E.; Rezaei, F.; Falahi, S.; Kenarkoohi, A.; Badri, M.; Solhjoo, K.; Abdoli, A. Latent toxoplasmosis, Cytomegalovirus, and Herpes Simplex Virus infections and risk of motorcycle accidents: A case-control study in a county with a high rate of motorcycle injuries in Iran. PLoS ONE 2024, 19, e0307950. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Dama, M. Does prevalence of latent toxoplasmosis correlate with nation-wide rate of traffic accidents? Folia Parasitol. 2014, 6, 485–494. [Google Scholar] [CrossRef]
- Gohardehi, S.; Sharif, M.; Sarvi, S.; Moosazadeh, M.; Alizadeh-Navaei, R.; Hosseini, S.A.; Amouei, A.; Pagheh, A.; Sadeghi, M.; Daryani, A. The potential risk of toxoplasmosis for traffic accidents: A systematic review and meta-analysis. Exp. Parasitol. 2018, 191, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Sutterland, A.L.; Kuin, A.; Kuiper, B.; van Gool, T.; Leboyer, M.; Fond, G.; de Haan, L. Driving us mad: The association of Toxoplasma gondii with suicide attempts and traffic accidents—A systematic review and meta-analysis. Psychol. Med. 2019, 49, 1608–1623. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Esquivel, C.; Torres-Castorena, A.; Liesenfeld, O.; Estrada-Martinez, S.; Urbina-Alvarez, J.D. High seroprevalence of Toxoplasma gondii infection in a subset of Mexican patients with work accidents and low socioeconomic status. Parasit Vectors 2012, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.K.; Fitza, M.A.; Lerner, D.A.; Calhoun, D.M.; Beldon, M.A.; Chan, E.T.; Johnson, P.T.J. Risky business: Linking Toxoplasma gondii infection and entrepreneurship behaviours across individuals and countries. Proc. R. Soc. B-Biol. Sci. 2018, 285, 20180822. [Google Scholar] [CrossRef]
- Flegr, J.; Kodym, P.; Tolarová, V. Correlation of duration of latent Toxoplasma gondii infection with personality changes in women. Biol. Psychol. 2000, 53, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Havlíček, J. Changes in the personality profile of young women with latent toxoplasmosis. Folia Parasitol. 1999, 46, 22–28. [Google Scholar]
- Flegr, J.; Novotná, M.; Fialová, A.; Kolbeková, P.; Gašová, Z. The influence of RhD phenotype on toxoplasmosis- and age-associated changes in personality profile of blood donors. Folia Parasitol. 2010, 57, 143–150. [Google Scholar] [CrossRef]
- Lindová, J.; Příplatová, L.; Flegr, J. Higher extraversion and lower conscientiousness in humans infected with Toxoplasma. Eur. J. Personal. 2012, 26, 285–291. [Google Scholar] [CrossRef]
- Flegr, J.; Preiss, M.; Klose, J. Toxoplasmosis-associated difference in intelligence and personality in men depends on their Rhesus blood group but not ABO blood group. PLoS ONE 2013, 8, e61272. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, K.D. Can the common brain parasite, Toxoplasma gondii, influence human culture? Proc. R. Soc. B-Biol. Sci. 2006, 273, 2749–2755. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Preiss, M.; Klose, J.; Havlíček, J.; Vitáková, M.; Kodym, P. Decreased level of psychobiological factor novelty seeking and lower intelligence in men latently infected with the protozoan parasite Toxoplasma gondii. Dopamine, a missing link between schizophrenia and toxoplasmosis? Biol. Psychol. 2003, 63, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Lindová, J.; Kuběna, A.A.; Šturcová, A.; Křivohlavá, R.; Novotná, M.; Rubešová, A.; Havlíček, J.; Kodym, P.; Flegr, J. Pattern of money allocation in experimental games supports the stress hypothesis of gender differences in Toxoplasma gondii-induced behavioural changes. Folia Parasitol. 2010, 57, 136–142. [Google Scholar] [CrossRef]
- Alvarado-Esquivel, C.; Estrada-Martinez, S.; Ramos-Nevarez, A.; Perez-Alamos, A.R.; Beristain-Garcia, I.; Alvarado-Felix, A.O.; Cerrillo-Soto, S.M.; Alvarado-Felix, G.A.; Guido-Arreola, C.A.; Saenz-Soto, L.; et al. Is Toxoplasma gondii infection associated with sexual promiscuity? A cross-sectional study. Pathogens 2021, 10, 1393. [Google Scholar] [CrossRef] [PubMed]
- Sýkorová, K.; Flegr, J. Faster life history strategy manifests itself by lower age at menarche, higher sexual desire, and earlier reproduction in people with worse health. Sci. Rep. 2021, 11, 11254. [Google Scholar] [CrossRef] [PubMed]
- Thrall, P.H.; Antonovics, J.; Dobson, A.P. Sexually transmitted diseases in polygynous mating systems: Prevalence and impact on reproductive success. Proc. Biol. Sci. 2000, 267, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Dass, S.A.H.; Vasudevan, A.; Dutta, D.; Soh, L.J.T.; Sapolsky, R.M.; Vyas, A. Protozoan parasite Toxoplasma gondii manipulates mate choice in rats by enhancing attractiveness of males. PLoS ONE 2011, 6, e27229. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.B.; Costa, A.J.; Jordao, S.; Paim, B.B.; Pinto, F.R.; Di Mauro, D.C. Toxoplasma gondii in semen of experimentally infected swine. Pesqui. Vet. Bras. 2007, 27, 430–434. [Google Scholar] [CrossRef]
- Tong, W.H.; Hlavacova, J.; Abdulai-Saiku, S.; Kankova, S.; Flegr, J.; Vyas, A. Presence of Toxoplasma gondii tissue cysts in human semen: Toxoplasmosis as a potential sexually transmissible infection. J. Infect. 2023, 86, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Boyer, K.M.; Holfels, M.; Roizen, N.; Swisher, C.; Mack, D.; Remington, J.; Withers, S.; Meier, P.; McLeod, R. Risk factors for Toxoplasma gondii infection in mothers of infants with congenital toxoplasmosis: Implications for prenatal management and screening. Am. J. Obstet. Gynecol. 2005, 192, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Vesco, G.; Villari, S.; Buffolano, W. What do we know about risk factors for infection in humans with Toxoplasma gondii and how can we prevent infections? Zoonoses Public Health 2010, 57, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Klapilová, K.; Kaňková, Š. Toxoplasmosis can be a sexually transmitted infection with serious clinical consequences. Not all routes of infection are created equal. Med. Hypotheses 2014, 83, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Kodym, P.; Malý, M.; Švandová, E.; Lekatková, H.; Badoutová, M.; Vlková, J.; Beneš, C.; Zástěra, M. Toxoplasma in the Czech Republic 1923–1999: First case to widespread outbreak: In Recent trends in research on congenital toxoplasmosis. Int. J. Parasit. 2000, 30, 11–18. [Google Scholar] [CrossRef]
- Torrey, E.F.; Bartko, J.J.; Yolken, R.H. Toxoplasma gondii and other risk factors for schizophrenia: An update. Schizophr. Bull. 2012, 38, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Holub, D.; Flegr, J.; Dragomirecka, E.; Rodriguez, M.; Preiss, M.; Novak, T.; Cermak, J.; Horacek, J.; Kodym, P.; Libiger, J.; et al. Differences in onset of disease and severity of psychopathology between toxoplasmosis-related and toxoplasmosis-unrelated schizophrenia. Acta Psychiat Scand. 2013, 127, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Esquivel, C.; Sanchez-Anguiano, L.F.; Hernandez-Tinoco, J.; Arreola-Chaidez, E.; Lopez, J.; Salcido-Meraz, K.I.; Estrada-Martinez, S.; Navarrete-Flores, J.A.; Perez-Alamos, A.R.; Hernandez-Ochoa, M.; et al. High seroprevalence of Toxoplasma gondii infection in female sex workers: A case-control study. Eur. J. Microbiol. Immunol. 2015, 5, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Kaňková, Š.; Hlaváčová, J.; Flegr, J. Oral sex: A new, and possibly the most dangerous, route of toxoplasmosis transmission. Med. Hypotheses 2020, 141, 109725. [Google Scholar] [CrossRef] [PubMed]
- Disko, R.; Braveny, I.; Vogel, P. Studies on the occurrence of Toxoplasma gondii in the human ejaculate. Z. Tropenmed. Parasitol. 1971, 22, 391–396. [Google Scholar] [PubMed]
- Ullmann, J.; Kodym, P.; Flegr, J.; Berenova, D.; Jirsová, S.; Kanková, S. Oral sex as a potential route for Toxoplasma gondii transmission: Experiment with human semen and laboratory mice model. Acta Parasitol. 2024, 69, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Sýkorová, K.; Fiala, V.; Hlaváčová, J.; Kaňková, Š.; Flegr, J. Redheaded women are more sexually active than other women, but it is probably due to their suitors. Front. Psychol. 2022, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Trigunaite, A.; Dimo, J.; Jørgensen, T.N. Suppressive effects of androgens on the immune system. Cell. Immunol. 2015, 294, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, S.A.; Brett, R.; Alexander, J.; Pratt, J.; Roberts, C.W. Neuropsychiatric disease and Toxoplasma gondii infection. Neuroimmunomodulation 2009, 16, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Kuba, R. The relation of Toxoplasma infection and sexual attraction to fear, danger, pain, and submissiveness. Evol. Psychol. 2016, 14, 1474704916659746. [Google Scholar] [CrossRef]
- Flegr, J. Does Toxoplasma infection increase sexual masochism and submissiveness? Yes and no. Commun. Integr. Biol. 2017, 10, e1303590. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Escudero, D.Q. Impaired health status and increased incidence of diseases in Toxoplasma-seropositive subjects—An explorative cross-sectional study. Parasitology 2016, 143, 1974–1989. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A. Parasite-augmented mate choice and reduction in innate fear in rats infected by Toxoplasma gondii. J. Exp. Biol. 2013, 216, 120–126. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baum, M.J.; Kelliher, K.R. Complementary roles of the main and accessory olfactory systems in mammalian mate recognition. Annu. Rev. Physiol. 2009, 71, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Penn, D.; Potts, W.K. Chemical signals and parasite-mediated sexual selection. Trends Ecol. Evol. 1998, 13, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P.; Christe, P.; Lux, E. Parasitism, host immune function, and sexual selection. Q. Rev. Biol. 1999, 74, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Hodková, H. Behavioral and Neurophysiological Manifestations of Latent Toxoplasmosis in Mice. Diploma Thesis, Charles University, Prague, Czech Republic, 2006; p. 104.
- Flegr, J.; Hrušková, M.; Hodný, Z.; Novotná, M.; Hanušová, J. Body height, body mass index, waist-hip ratio, fluctuating asymmetry and second to fourth digit ratio in subjects with latent toxoplasmosis. Parasitology 2005, 130, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Hodková, H.; Kolbeková, P.; Skallová, A.; Lindová, J.; Flegr, J. Higher perceived dominance in Toxoplasma infected men—A new evidence for role of increased level of testosterone in toxoplasmosis-associated changes in human behavior. Neuroendocrinol. Lett. 2007, 28, 110–114. [Google Scholar] [PubMed]
- Borraz-Leon, J.I.; Rantala, M.J.; Krams, I.A.; Cerda-Molina, A.L.; Contreras-Garduno, J. Are Toxoplasma-infected subjects more attractive, symmetrical, or healthier than non-infected ones? Evidence from subjective and objective measurements. PeerJ 2022, 10, e13122. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Lindová, J.; Kodym, P. Sex-dependent toxoplasmosis-associated differences in testosterone concentration in humans. Parasitology 2008, 135, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Roved, J.; Westerdahl, H.; Hasselquist, D. Sex differences in immune responses: Hormonal effects, antagonistic selection, and evolutionary consequences. Horm. Behav. 2017, 88, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Guerrero, G.; Gonzalez-Reyes, R.E.; de-la-Torre, A.; Medina-Rincon, G.; Nava-Mesa, M.O. Pathophysiological mechanisms of cognitive impairment and neurodegeneration by Toxoplasma gondii infection. Brain Sci. 2020, 10, 369. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Chvátalová, V.; Příplatová, L.; Tureček, P.; Kodym, P.; Šebánková, B.; Kaňková, Š. Cognitive effects of Toxoplasma and CMV infections: A cross-sectional study of 557 young adults considering modulation by sex and Rh factor. Pathogens 2024, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Havlíček, J.; Gašová, Z.; Smith, A.P.; Zvára, K.; Flegr, J. Decrease of psychomotor performance in subjects with latent ‘asymptomatic’ toxoplasmosis. Parasitology 2001, 122, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Příplatová, L.; Šebanková, B.; Flegr, J. Contrasting effect of prepulse signals on performance of Toxoplasma-infected and Toxoplasma-free subjects in an acoustic reaction times test. PLoS ONE 2014, 9, e112771. [Google Scholar] [CrossRef] [PubMed]
- de Haan, L.; Sutterland, A.L.; Schotborgh, J.V.; Schirmbeck, F.; de Haan, L. Association of Toxoplasma gondii seropositivity with cognitive function in healthy people: A systematic review and meta-analysis. JAMA Psychiatry 2021, 78, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Klose, J.; Novotná, M.; Berenreitterová, M.; Havlíček, J. Increased incidence of traffic accidents in Toxoplasma-infected military drivers and protective effect RhD molecule revealed by a large-scale prospective cohort study. BMC Infect. Dis. 2009, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Novotná, M.; Havlíček, J.; Smith, A.P.; Kolbeková, P.; Skallová, A.; Klose, J.; Gašová, Z.; Písačka, M.; Sechovská, M.; Flegr, J. Toxoplasma and reaction time: Role of toxoplasmosis in the origin, preservation and geographical distribution of Rh blood group polymorphism. Parasitology 2008, 135, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Novotná, M.; Lindová, J.; Havlíček, J. Neurophysiological effect of the Rh factor. Protective role of the RhD molecule against Toxoplasma-induced impairment of reaction times in women. Neuroendocrinol. Lett. 2008, 29, 475–481. [Google Scholar] [PubMed]
- Mahmoudvand, H.; Sheibani, V.; Shojaee, S.; Mirbadie, S.R.; Keshavarz, H.; Esmaeelpour, K.; Keyhani, A.R.; Ziaali, N. Toxoplasma gondii infection potentiates cognitive impairments of Alzheimer’s disease in the Balb/c mice. J. Parasitol. 2016, 102, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.; Robinson, S.A.; Kim, D.G.; Yan, A.; Cleland, T.A.; Bynoe, M.S. Toxoplasma gondii alters NMDAR signaling and induces signs of Alzheimer’s disease in wild-type, C57BL/6 mice. J. Neuroinflamm. 2018, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Möhle, L.; Israel, N.; Paarmann, K.; Krohn, M.; Pietkiewicz, S.; Müller, A.; Dunay, I.R. Chronic Toxoplasma gondii infection enhances β-amyloid phagocytosis and clearance by recruited monocytes. Acta Neuropathol. Commun. 2016, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Kusbeci, O.Y.; Miman, O.; Yaman, M.; Aktepe, O.C.; Yazar, S. Could Toxoplasma gondii have any role in Alzheimer disease? Alzheimer Dis. Assoc. Disord. 2011, 25, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.E.; Gale, S.D.; Erickson, L.; Wilson, E.; Nielsen, B.; Kauwe, J.; Hedges, D.W. Seroprevalence and serointensity of latent Toxoplasma gondii in a sample of elderly adults with and without alzheimer disease. Alzheimer Dis. Assoc. Disord. 2016, 30, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Galeh, T.M.; Ghazvini, H.; Mohammadi, M.; Sarvi, S.; Azizi, S.; Asgarian-Omran, H.; Hajizadeh, F.; Daryani, A. Effects of diverse types of Toxoplasma gondii on the outcome of Alzheimer’s disease in the rat model. Microb. Pathog. 2023, 174, 8. [Google Scholar] [CrossRef] [PubMed]
- Gale, S.D.; Erickson, L.D.; Thacker, E.L.; Mitchell, E.L.; Brown, B.L.; Hedges, D.W. Toxoplasma gondii seropositivity and serointensity and cognitive function in adults. PLoS Neglect. Trop. Dis. 2020, 14, e0008733. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Zhao, Q.; Chen, H.; Li, M.; Zhang, Z.; Qu, Z.; Yang, C.; Lin, X.; Ma, W.; Standlee, C.R. Toxoplasma gondii seropositivity and cognitive functioning in older adults: An analysis of cross-sectional data of the National Health and Nutrition Examination Survey 2011–2014. BMJ Open 2024, 14, e071513. [Google Scholar] [CrossRef] [PubMed]
- Mendy, A.; Vieira, E.R.; Albatineh, A.N.; Gasana, J. Toxoplasma gondii seropositivity and cognitive functions in school-aged children. Parasitology 2015, 142, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Dincel, G.C.; Atmaca, H.T. Role of oxidative stress in the pathophysiology of Toxoplasma gondii infection. Int. J. Immunopathol. Pharmacol. 2016, 29, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Colzato, L.; Zhang, W.X.; Beste, C.; Stock, A.K. Dissociating direct and indirect effects: A theoretical framework of how latent toxoplasmosis affects cognitive profile across the lifespan. Neurobiol. Aging 2021, 102, 119–128. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xu, D.X.; Yan, Z.Y.; Wu, Y.S.; Zhang, Y.S.; Tian, X.K.; Zhu, J.H.; Liu, Z.Z.; Cheng, W.P.; Zheng, K.Y.; et al. A metabolite attenuates neuroinflammation, synaptic loss and cognitive deficits induced by chronic infection of Toxoplasma gondii. Front. Immunol. 2022, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Jones-Brando, L.; Torrey, E.F.; Yolken, R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr. Res. 2003, 62, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, N.; Daban-Huard, C.; Lajnef, M.; Gadel, R.; Le Corvoisier, P.; Delavest, M.; Carde, S.; Lepine, J.P.; Jamain, S.; Houenou, J.; et al. Cognitive deterioration among bipolar disorder patients infected by Toxoplasma gondii is correlated to interleukin 6 levels. J. Affect. Disord. 2015, 179, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, F.; Stallings, C.; Origoni, A.; Katsafanas, E.; Schweinfurth, L.; Savage, C.; Khushalani, S.; Yolken, R. Antibodies to Toxoplasma gondii and cognitive functioning in schizophrenia, bipolar disorder, and nonpsychiatric controls. J. Nerv. Ment. Dis. 2014, 202, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, A.L.; Coelho, D.; Scoriels, L.; Mambrini, J.; Antonelli, L.; Henriques, P.; Teixeira-Carvalho, A.; Martins, O.; Mineo, J.; Bahia-Oliveira, L.; et al. Effects of Toxoplasma gondii infection on cognition, symptoms, and response to digital cognitive training in schizophrenia. Schizophrenia 2022, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Veleva, I.; Stoychev, K.; Stoimenova-Popova, M.; Stoyanov, L.; Mineva-Dimitrova, E.; Angelov, I. Toxoplasma gondii seropositivity and cognitive function in adults with schizophrenia. Schizophr. Res. Cogn. 2022, 30, 6. [Google Scholar] [CrossRef] [PubMed]
- Millard, S.J.; Bearden, C.E.; Karlsgodt, K.H.; Sharpe, M.J. The prediction-error hypothesis of schizophrenia: New data point to circuit-specific changes in dopamine activity. Neuropsychopharmacology 2022, 47, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Kerr, J.R.; Lafuente, M.J.; Maclean, A.; Chibalina, M.V.; Liu, B.; Burke, B.; Bevan, S.; Nasir, J. Altered expression and coregulation of dopamine signalling genes in schizophrenia and bipolar disorder. Neuropathol. Appl. Neurobiol. 2011, 37, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Previc, F.H. Dopamine and the origins of human intelligence. Brain Cogn. 1999, 41, 299–350. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, Y.; Tan, S.; Tian, X.; Meng, X.; Li, Y.; Zhou, B.; Zhao, G.; Ge, X.; He, C.; et al. Alterations in gut microbiota contribute to cognitive deficits induced by chronic infection of Toxoplasma gondii. Brain Behav. Immun. 2024, 119, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Ullmann, J.; Toman, J. Parasitic manipulation or side effects? The effects of past Toxoplasma gondii and Borrelia spp. infections on human personality and cognitive performance are not mediated by impaired health. Folia Parasitol. 2023, 70, 13. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Horáček, J. Toxoplasmosis, but not borreliosis, is associated with psychiatric disorders and symptoms. Schizophr. Res. 2018, 197, 603–604. [Google Scholar] [CrossRef] [PubMed]
- Minto, A.; Roberts, F.J. The psychiatric complications of toxoplasmosis. Lancet 1959, 1, 1180–1182. [Google Scholar] [CrossRef] [PubMed]
- Torrey, E.F. The linking of toxoplasmosis and schizophrenia. Folia Parasitol. 2024, 71, 7. [Google Scholar] [CrossRef] [PubMed]
- Sutterland, A.L.; Fond, G.; Kuin, A.; Koeter, M.W.; Lutter, R.; van Gool, T.; Yolken, R.; Szoke, A.; Leboyer, M.; de Haan, L. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: Systematic review and meta-analysis. Acta Psychiat. Scand. 2015, 132, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Příplatová, L.; Hampl, R.; Bičíková, M.; Řípová, D.; Mohr, P. Difference of neuro- and immunomodulatory steroids and selected hormone and lipid concentrations between Toxoplasma-free and Toxoplasma-infected but not CMV-free and CMV-infected schizophrenia patients. Neuroendocrinol. Lett. 2014, 35, 20–27. [Google Scholar] [PubMed]
- Wang, H.L.; Wang, G.H.; Li, Q.Y.; Shu, C.; Jiang, M.S.; Guo, Y. Prevalence of Toxoplasma infection in first-episode schizophrenia and comparison between Toxoplasma-seropositive and Toxoplasma-seronegative schizophrenia. Acta Psychiat Scand. 2006, 114, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Celik, T.; Kartalci, S.; Aytas, O.; Akarsu, G.A.; Gozukara, H.; Unal, S. Association between latent toxoplasmosis and clinical course of schizophrenia—Continuous course of the disease is characteristic for Toxoplasma gondii-infected patients. Folia Parasitol. 2015, 62, 015. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J. Predictors of Toxoplasma gondii infection in Czech and Slovak populations: The possible role of cat-related injuries and risky sexual behavior in the parasite transmission. Epidemiol. Infect. 2017, 145, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Fond, G.; Macgregor, A.; Tamouza, R.; Hamdani, N.; Meary, A.; Leboyer, M.; Dubremetz, J.F. Comparative analysis of anti-toxoplasmic activity of antipsychotic drugs and valproate. Eur. Arch. Psych. Clin. Neurosci. 2014, 264, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Prandovszky, E.; Gaskell, E.; Martin, H.; Dubey, J.P.; Webster, J.P.; McConkey, G.A. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS ONE 2011, 6, e23866. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Horáček, J. Negative effects of latent toxoplasmosis on mental health. Front. Psychiatry 2020, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.H.; Al-Shewy, K.A.H.; Abdin, E.M.; Hasan, H.M. Seroprevalence of toxoplasmosis among children with autism. Egypt. J. Neurol. Psychiatr. Neurosurg. 2024, 60, 7. [Google Scholar] [CrossRef]
- Nayeri, T.; Sarvi, S.; Moosazadeh, M.; Hosseininejad, Z.; Sharif, M.; Amouei, A.; Daryani, A. Relationship between toxoplasmosis and autism: A systematic review and meta-analysis. Microb. Pathog. 2020, 147, 104434. [Google Scholar] [CrossRef] [PubMed]
- Pavăl, D.; Micluția, I.V. The dopamine hypothesis of autism spectrum disorder revisited: Current status and future prospects. Dev. Neurosci. 2021, 43, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Sourander, A.; Hinkka-Yli-Salomäki, S.; McKeague, I.W.; Sundvall, J.; Surcel, H.M. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol. Psychiatry 2014, 19, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Prandota, J. Autism spectrum disorders may be due to cerebral toxoplasmosis associated with chronic neuroinflammation causing persistent hypercytokinemia that resulted in an increased lipid peroxidation, oxidative stress, and depressed metabolism of endogenous and exogenous substances. Res. Autism Spectr. Disord. 2010, 4, 119–155. [Google Scholar] [CrossRef]
- Maisarah, A.; Mohamad, S.; Husain, M.; Abdullah, S.; Noordin, R. Association between infection with Toxoplasma gondii and psychiatric disorders. Folia Parasitol. 2022, 69, 008. [Google Scholar] [CrossRef] [PubMed]
- Noori, M.A.; Al-Hasnawi, S.M.J.; Al-Haidari, A.F.; Hamza, D.M. Toxoplasma gondii seropositivity and attention deficit hyperactivity disorder. J. Glob. Sci. Res. 2020, 10, 871–879. [Google Scholar]
- Khademvatan, S.; Riahi, F.; Izadi-Mazidi, M.; Khajeddin, N.; Yousefi, E. Toxoplasma gondii exposure and the risk of attention deficit hyperactivity disorder in children and adolescents. Pediatr. Infect. Dis. J. 2018, 37, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, T.; Sarvi, S.; Moosazadeh, M.; Hosseininejad, Z.; Amouei, A.; Daryani, A. Toxoplasma gondii infection and risk of attention-deficit hyperactivity disorder: A systematic review and meta-analysis. Pathog. Glob. Health 2020, 114, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, N.; Daban-Huard, C.; Lajnef, M.; Richard, J.R.; Delavest, M.; Godin, O.; Le Guen, E.; Vederine, F.E.; Lepine, J.P.; Jamain, S.; et al. Relationship between Toxoplasma gondii infection and bipolar disorder in a French sample. J. Affect. Disord. 2013, 148, 444–448. [Google Scholar] [CrossRef] [PubMed]
- de Barros, J.; Barbosa, I.G.; Salem, H.; Rocha, N.P.; Kummer, A.; Okusaga, O.O.; Soares, J.C.; Teixeira, A.L. Is there any association between Toxoplasma gondii infection and bipolar disorder? A systematic review and meta-analysis. J. Affect. Disord. 2017, 209, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Cossu, G.; Preti, A.; Gyppaz, D.; Gureje, O.; Carta, M.G. Association between toxoplasmosis and bipolar disorder: A systematic review and meta-analysis. J. Psychiatr. Res. 2022, 153, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Chmielowska, M.; Koola, M.M.; Srihari, V.H. Gender differences within the psychosis spectrum. J. Neuropsychiatry Clin. Neurosci. 2019, 31, 64–71. [Google Scholar] [CrossRef]
- Seeman, M.V. Women who suffer from schizophrenia: Critical issues. World J. Psychiatry 2018, 8, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Petkari, E.; Mayoral, F.; Moreno-Küstner, B. Gender matters in schizophrenia-spectrum disorders: Results from a healthcare users epidemiological study in Malaga, Spain. Compr. Psychiatry 2017, 72, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Hazell, C.M.; Berry, C.; Bogen-Johnston, L.; Banerjee, M. Creating a hierarchy of mental health stigma: Testing the effect of psychiatric diagnosis on stigma. BJPsych Open 2022, 8, e174. [Google Scholar] [CrossRef] [PubMed]
- Groër, M.W.; Yolken, R.H.; Xiao, J.C.; Beckstead, J.W.; Fuchs, D.; Mohapatra, S.S.; Seyfang, A.; Postolache, T.T. Prenatal depression and anxiety in Toxoplasma gondii-positive women. Am. J. Obstet. Gynecol. 2011, 204, 433.e1–433.e7. [Google Scholar] [CrossRef] [PubMed]
- Markovitz, A.A.; Simanek, A.M.; Yolken, R.H.; Galea, S.; Koenen, K.C.; Chen, S.; Aiello, A.E. Toxoplasma gondii and anxiety disorders in a community-based sample. Brain Behav. Immun. 2015, 43, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Traskman-Bendz, L.; Janelidze, S.; Langenberg, P.; Saleh, A.; Constantine, N.; Okusaga, O.; Bay-Richter, C.; Brundin, L.; Postolache, T.T. Toxoplasma gondii immunoglobulin G antibodies and nonfatal suicidal self-directed violence. J. Clin. Psychiatry 2012, 73, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Gale, S.D.; Brown, B.L.; Berrett, A.; Erickson, L.D.; Hedges, D.W. Association between latent toxoplasmosis and major depression, generalised anxiety disorder and panic disorder in human adults. Folia Parasitol. 2014, 61, 285–292. [Google Scholar] [CrossRef]
- de Bles, N.J.; van der Does, J.E.H.; Kortbeek, L.M.; Hofhuis, A.; van Grootheest, G.; Vollaard, A.M.; Schoevers, R.A.; van Hemert, A.M.; Penninx, B.; Rius-Ottenheim, N.; et al. Toxoplasma gondii seropositivity in patients with depressive and anxiety disorders. Brain Behav. Immun. Health 2021, 11, 100197. [Google Scholar] [CrossRef] [PubMed]
- Dionisie, V.; Filip, G.A.; Manea, M.C.; Manea, M.; Riga, S. The anti-inflammatory role of SSRI and SNRI in the treatment of depression: A review of human and rodent research studies. Inflammopharmacology 2021, 29, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Kar, N.; Misra, B. Toxoplasma seropositivity and depression: A case report. BMC Psychiatry 2004, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Nasirpour, S.; Kheirandish, F.; Fallahi, S. Depression and Toxoplasma gondii infection: Assess the possible relationship through a seromolecular case-control study. Arch. Microbiol. 2020, 203, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Rahiminezhad, A.; Latifi, A.; Rostami, R.; Farahani, H. Psychopathology of latent toxoplasmosis in infected individuals: Roles of age, duration of infection, and sex. Med. J. Tabriz Univ. Med. Sci. Health Serv. 2024, 46, 61–73. [Google Scholar] [CrossRef]
- Miman, O.; Mutlu, E.A.; Ozcan, O.; Atambay, M.; Karlidag, R.; Unal, S. Is there any role of Toxoplasma gondii in the etiology of obsessive-compulsive disorder? Psychiatry Res. 2010, 177, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Horáček, J. Toxoplasma-infected subjects report an obsessive-compulsive disorder diagnosis more often and score higher in obsessive-compulsive inventory. Eur. Psychiatry 2017, 40, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, F.; Sayyah, M.; Tavalla, M.; Arjmand, R. Toxoplasmosis in treatment-resistant obsessive-compulsive disorder patients. Acta Parasitol. 2022, 67, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Nayeri Chegeni, T.; Sarvi, S.; Amouei, A.; Moosazadeh, M.; Hosseininejad, Z.; Aghayan, S.A.; Daryani, A. Relationship between toxoplasmosis and obsessive compulsive disorder: A systematic review and meta-analysis. PLoS Neglect. Trop. Dis. 2019, 13, e0007306. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric, A. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; DSM-5-TR; American Psychiatric Publishing: Arlington, VA, USA, 2022. [Google Scholar]
- Akgul, O. The effects of latent Toxoplasma gondii infection on the behavior and personality characteristics of university students. Anadolu Psikiyatri Dergisi 2020, 21, 70–76. [Google Scholar] [CrossRef]
- Flegr, J. Effects of Toxoplasma on human behavior. Schizophr. Bull. 2007, 33, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Salais, A.; Munoz-Larreta, F.Y.; Garcia-Perez, S.I.; Serrato-Enriquez, A.I.; Rivas-Gonzalez, M.A.; Sifuentes-Alvarez, A.; Rabago-Sanchez, E.; Beristain-Garcia, I.; Alvarado-Esquivel, C. Survey on the association between Toxoplasma gondii infection and violent behavior in inmates. PLoS ONE 2023, 18, e0284202. [Google Scholar] [CrossRef] [PubMed]
- Berrett, A.N.; Gale, S.D.; Erickson, L.D.; Thacker, E.L.; Brown, B.L.; Hedges, D.W. Toxoplasma gondii seropositivity and substance use in US adults. Folia Parasitol. 2018, 65, 11. [Google Scholar] [CrossRef] [PubMed]
- Elmorsy, E.; Mahmoud, E.H.M.; Rakha, S.A.; Shoaib, M. An association between latent toxoplasmosis and substance abuse: An Egyptian Center Study. J. Addict. Dis. 2019, 37, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Bahreini, M.S.; Jahromi, S.S.; Radfar, A.H.; Salemi, A.M.; Dastan, N.; Asgari, Q. The Relationship of Latent Toxoplasmosis and Cigarette Smoking: Seroprevalence, Risk Factor, and Case-Control Study in Fars Province, Southern Iran. Pathogens 2022, 11, 1274. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J. Neurological and neuropsychiatric consequences of chronic Toxoplasma infection. Clin. Microbiol. Rep. 2015, 2, 163–172. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B.; Maggi, R.G.; Nicholson, W.L.; Cherry, N.A.; Woods, C.W. Bartonella sp bacteremia in patients with neurological and neurocognitive dysfunction. J. Clin. Microbiol. 2008, 46, 2856–2861. [Google Scholar] [CrossRef] [PubMed]
- Stewart, Z.; Korsapathy, S.; Frohlich, F. Crowd-sourced investigation of a potential relationship between Bartonella-associated cutaneous lesions and neuropsychiatric symptoms. Front. Psychiatry 2023, 14, 1244121. [Google Scholar] [CrossRef] [PubMed]
- Delaney, S.; Robveille, C.; Maggi, R.G.; Lashnits, E.; Kingston, E.; Liedig, C.; Murray, L.; Fallon, B.A.; Breitschwerdt, E.B. Bartonella species bacteremia in association with adult psychosis. Front. Psychiatry 2024, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Guirguis, V.; Pupillo, F.; Rodrigues, S.; Walker, N.; Roth, H.; Liedig, C.E.; Maggi, R.G.; Breitschwerdt, E.B.; Frohlich, F. Bartonella spp. infection in people with Mild Cognitive Impairment: A pilot study. PLoS ONE 2024, 19, e0307060. [Google Scholar] [CrossRef] [PubMed]
- Lashnits, E.; Maggi, R.; Jarskog, F.; Bradley, J.; Breitschwerdt, E.; Frohlich, F. Schizophrenia and Bartonella spp. infection: A pilot case-control study. Vector-Borne Zoonotic Dis. 2021, 21, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Preiss, M.; Balatova, P. Depressiveness and neuroticism in Bartonella seropositive and seronegative subjects-Preregistered case-controls study. Front. Psychiatry 2018, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Hodny, Z. Cat scratches, not bites, are associated with unipolar depression—Cross-sectional study. Parasit. Vectors 2016, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Hanauer, D.A.; Ramakrishnan, N.; Seyfried, L.S. Describing the relationship between cat bites and human depression using data from an electronic health record. PLoS ONE 2013, 8, e70585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wong, W.K.; Upton, A.; Thomas, M.G. Neuropsychiatric symptoms are common in immunocompetent adult patients with Toxoplasma gondii acute lymphadenitis. Scand. J. Infect. Dis. 2013, 45, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Šebánková, B.; Flegr, J. Physical and mental health status in Toxoplasma-infected women before and three years after they learn about their infection: Manipulation or side-effects of impaired health? Front. Ecol. Evol. 2017, 5, 144. [Google Scholar] [CrossRef]
- Flegr, J. Thirty years of studying latent toxoplasmosis: Behavioural, physiological, and health insights. Folia Parasitol. 2025, 72, 005. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Hrdá, Š.; Kodym, P. Influence of latent ‘asymptomatic’ toxoplasmosis on body weight of pregnant women. Folia Parasitol. 2005, 52, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Kaňková, Š.; Flegr, J. Longer pregnancy and slower fetal development in women with latent “asymptomatic” toxoplasmosis BMC Infect. Dis. 2007, 7, 114. [Google Scholar] [CrossRef]
- Kaňková, Š.; Šulc, J.; Flegr, J. Increased pregnancy weight gain in women with latent toxoplasmosis and RhD-positivity protection against this effect. Parasitology 2010, 137, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Kaňková, Š.; Flegr, J.; Calda, P. An elevated blood glucose level and increased incidence of gestational diabetes mellitus in pregnant women with latent toxoplasmosis. Folia Parasitol. 2015, 62, 056. [Google Scholar] [CrossRef] [PubMed]
- Kaňková, Š.; Procházková, L.; Flegr, J.; Calda, P.; Springer, D.; Potluková, E. Effects of latent toxoplasmosis on autoimmune thyroid diseases in pregnancy. PLoS ONE 2014, 9, e110878. [Google Scholar] [CrossRef] [PubMed]
- Kaňkova, S.; Šulc, J.; Křivohlavá, R.; Kuběna, A.; Flegr, J. Slower postnatal motor development in infants of mothers with latent toxoplasmosis during the first 18 months of life. Early Hum. Dev. 2012, 88, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Kaňková, Š.; Šulc, J.; Nouzová, K.; Fajfrlik, K.; Frynta, D.; Flegr, J. Women infected with parasite Toxoplasma have more sons. Naturwissenschaften 2007, 94, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Kankova, S. The effects of toxoplasmosis on sex ratio at birth. Early Hum. Dev. 2020, 141, 104874. [Google Scholar] [CrossRef] [PubMed]
- James, W.H.; Grech, V. Can offspring sex ratios help to explain the endocrine effects of toxoplasmosis infection on human behaviour? Early Hum. Dev. 2018, 122, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Abdulai-Saiku, S.; Vyas, A. Loss of predator aversion in female rats after Toxoplasma gondii infection is not dependent on ovarian steroids. Brain Behav. Immun. 2017, 65, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Kaňková, Š.; Kodym, P.; Frynta, D.; Vavřinová, R.; Kuběna, A.; Flegr, J. Influence of latent toxoplasmosis on the secondary sex ratio in mice. Parasitology 2007, 134, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Dama, M.S.; Novakova, L.M.; Flegr, J. Do differences in Toxoplasma prevalence influence global variation in secondary sex ratio? Preliminary ecological regression study. Parasitology 2016, 143, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Trivers, R.L.; Willard, D.E. Natural selection of parental ability to vary the sex ratio of offspring. Science 1973, 179, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Tyebji, S.; Hannan, A.J.; Tonkin, C.J. Pathogenic infection in male mice changes sperm small rna profiles and transgenerationally alters offspring behavior. Cell Rep. 2020, 31, 107573. [Google Scholar] [CrossRef] [PubMed]
- Lopes, W.D.; da Costa, A.J.; Santana, L.F.; Dos Santos, R.S.; Rossanese, W.M.; Lopes, W.C.; Costa, G.H.; Sakamoto, C.A.; Dos Santos, T.R. Aspects of Toxoplasma infection on the reproductive system of experimentally infected rams (Ovis aries). J. Parasitol. Res. 2009, 2009, 602803. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, F.; Regadera, J.; Mayer, R.; Sanchez, S.; Nistal, M. Protozoan infections in the male genital tract. J. Urol. 1996, 156, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Crider, S.R.; Horstman, W.G.; Massey, G.S. Toxoplasma orchitis—Report of a case and a review of the literature. Am. J. Med. 1988, 85, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Tabares Tejada, P.; Cardona Maya, W.D. Toxoplasma gondii infection in the male reproductive system: A systematic review. Acta Parasitol. 2025, 70, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.H.; Lu, Y.J.; Wang, R.B.; Song, L.M.; Shi, F.; Gao, Q.F.; Luo, Y.F.; Gu, X.F.; Wang, P. Survey of infection of Toxoplasma gondii in infertile couples in Suzhou countryside. Zhonghua Nan Ke Xue 2002, 8, 350–352. [Google Scholar] [PubMed]
- Flegr, J.; Preiss, M. Friends with malefit. The effects of keeping dogs and cats, sustaining animal-related injuries and Toxoplasma infection on health and quality of life. PLoS ONE 2019, 14, e0221988. [Google Scholar] [CrossRef] [PubMed]
- Kaňková, S.; Flegr, J.; Calda, P. The influence of latent toxoplasmosis on women’s reproductive function: Four cross-sectional studies. Folia Parasitol. 2015, 62, 041. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pavlinová, J.; Kinčeková, J.; Ostró, A.; Saksun, L.; Vasilková, Z.; Königová, A. Parasitic infections and pregnancy complications. Helminthologia 2011, 48, 8–12. [Google Scholar] [CrossRef]
- Abdoli, A.; Dalimi, A.; Soltanghoraee, H.; Ghaffarifar, F. Molecular detection and genotypic characterization of Toxoplasma gondii in paraffin-embedded fetoplacental tissues of women with recurrent spontaneous abortion. Int. J. Fertil. Steril. 2017, 10, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, S.; Khademvatan, S.; Jafari, R.; Vazifekhah, S.; Yousefi, E.; Behroozi-Lak, T. Prevalence and risk factors of Toxoplasma gondii infection among women with miscarriage and their aborted fetuses in the northwest of Iran. PLoS ONE 2023, 18, e0283493. [Google Scholar] [CrossRef] [PubMed]
- Giorgino, F.L.; Mega, M. Toxoplasmosis and habitual abortion. Our experience. Clin. Exp. Obstet. Gynecol. 1981, 8, 132–134. [Google Scholar] [PubMed]
- Qublan, H.S.; Jumaian, N.; Abu-Salem, A.; Hamadelil, F.Y.; Mashagbeh, M.; Abdel-Ghani, F. Toxoplasmosis and habitual abortion. J. Obstet. Gynaecol. 2002, 22, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, N.; Gorgani-Firouzjaee, T.; Moulana, Z.; Chehrazi, M.; Ghaffari, S. Toxoplasma gondii infection and spontaneous abortion: A systematic review and meta-analysis. Microb. Pathog. 2021, 158, 105070. [Google Scholar] [CrossRef] [PubMed]
- Stock, A.K.; Dajkic, D.; Kohling, H.L.; von Heinegg, E.H.; Fiedler, M.; Beste, C. Humans with latent toxoplasmosis display altered reward modulation of cognitive control. Sci. Rep. 2017, 7, 10170. [Google Scholar] [CrossRef] [PubMed]
- Skallová, A.; Novotná, M.; Kolbeková, P.; Gašová, Z.; Veselý, V.; Flegr, J. Decreased level of novelty seeking in blood donors infected with Toxoplasma. Neuroendocrinol. Lett. 2005, 26, 480–486. [Google Scholar] [PubMed]
- Rossini, J.C.; Lopes, C.S.; Dirscherl, F.P.; Silva, D.A.O.; Mineo, J.R. Altered visual attention behavior of Toxoplasma gondii-infected individuals. Psychol. Neurosci. 2019, 12, 485–494. [Google Scholar] [CrossRef]
- Chudasama, Y.; Robbins, T.W. Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacology 2004, 29, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Atsumi, T.; Poirot, R.; Lee, Y.A.; Kato, A.; Goto, Y. Dopamine-dependent visual attention preference to social stimuli in nonhuman primates. Psychopharmacology 2017, 234, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Matzel, L.D.; Sauce, B. A multi-faceted role of dual-state dopamine signaling in working memory, attentional control, and intelligence. Front. Behav. Neurosci. 2023, 17, 1060786. [Google Scholar] [CrossRef] [PubMed]
- Rihet, P.; Possamai, C.A.; Micallef-Roll, J.; Blin, O.; Hasbroucq, T. Dopamine and human information processing: A reaction-time analysis of the effect of levodopa in healthy subjects. Psychopharmacology 2002, 163, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, S.F.; Gomes, F.V.; Grace, A.A. Dysregulation of midbrain dopamine system and the pathophysiology of schizophrenia. Front. Psychiatry 2020, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.S.; Lin, C.Y.; Chiu, N.T.; Yang, Y.K.; Chen, P.S.; Chang, H.H. Changes in striatal dopamine transporters in bipolar disorder and valproate treatment. Eur. Psychiatry 2021, 64, e9. [Google Scholar] [CrossRef] [PubMed]
- Denys, D.; de Vries, F.; Cath, D.; Figee, M.; Vulink, N.; Veltman, D.J.; van der Doef, T.F.; Boellaard, R.; Westenberg, H.; van Balkom, A.; et al. Dopaminergic activity in Tourette syndrome and obsessive-compulsive disorder. Eur. Neuropsychopharmacol. 2013, 23, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Yolken, R.H.; Dickerson, F.B.; Torrey, E.F. Toxoplasma and schizophrenia. Parasite Immunol. 2009, 31, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Ayano, G. Dopamine: Receptors, functions, synthesis, pathways, locations and mental disorders: Review of literatures. J. Ment. Disord. Treat. 2016, 2, 2. [Google Scholar] [CrossRef]
- Chaudhury, A.; Ramana, B.V. Schizophrenia and bipolar disorders: The Toxoplasma connection. Trop. Parasitol. 2019, 9, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Hassel, B.; Dingledine, R. Glutamate and glutamate receptors. In Basic Neurochemistry; Academic Press: Cambridge, MA, USA, 2012; pp. 342–366. [Google Scholar]
- Yuen, E.Y.; Wei, J.; Liu, W.; Zhong, P.; Li, X.; Yan, Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 2012, 73, 962–977. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, E.A.; Anand, C.; Khatib, D.; Diwadkar, V.A.; Stanley, J.A. Working memory modulates glutamate levels in the dorsolateral prefrontal cortex during (1)H fMRS. Front. Psychiatry 2018, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.M.; Forster, L.; Pawellek, M.; Malloni, W.M.; Ahn, S.; Tse, P.U.; Greenlee, M.W. Visual attention modulates glutamate-glutamine levels in vestibular cortex: Evidence from magnetic resonance spectroscopy. J. Neurosci. 2021, 41, 1970–1981. [Google Scholar] [CrossRef] [PubMed]
- Gallinat, J.; Kunz, D.; Senkowski, D.; Kienast, T.; Seifert, F.; Schubert, F.; Heinz, A. Hippocampal glutamate concentration predicts cerebral theta oscillations during cognitive processing. Psychopharmacology 2006, 187, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Sawa, A.; Iyo, M. Increased levels of glutamate in brains from patients with mood disorders. Biol. Psychiatry 2007, 62, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, K.; Bhattacharyya, S.; Christopher, R.; Khanna, S. Glutamatergic dysfunction in OCD. Neuropsychopharmacology 2005, 30, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T. Glutamate and schizophrenia: Beyond the dopamine hypothesis. Cell. Mol. Neurobiol. 2006, 26, 365–384. [Google Scholar] [CrossRef] [PubMed]
- Rojas, D.C. The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. J. Neural Transm. (Vienna) 2014, 121, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.J.; Bures, M.; Johnson, M.P.; Linden, A.M.; Monn, J.A.; Schoepp, D.D. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat. Rev. Drug Discov. 2005, 4, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.M.; Carrillo, G.L.; Su, J.M.; Lindsay, D.S.; Fox, M.A.; Blader, I.J. Toxoplasma gondii infections alter GABAergic synapses and signaling in the central nervous system. MBio 2015, 6, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Bando, H.; Fukuda, Y.; Watanabe, N.; Olawale, J.T.; Kato, K. Depletion of intracellular glutamine pools triggers Toxoplasma gondii stage conversion in human glutamatergic neurons. Front. Cell. Infect. Microbiol. 2021, 11, 788303. [Google Scholar] [CrossRef] [PubMed]
- Latifi, A.; Flegr, J. Toxoplasma infection. In Encyclopedia of Sexual Psychology and Behavior; Shackelford, T.K., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–14. [Google Scholar]
- Lucchese, G. From toxoplasmosis to schizophrenia via nMda dysfunction: Peptide overlap between Toxoplasma gondii and N-Methyl-D-aspartate receptors as a potential Mechanistic Link. Front. Psychiatry 2017, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Acquarone, M.; Poleto, A.; Perozzo, A.F.; Gonçalves, P.F.R.; Panizzutti, R.; Menezes, J.R.L.; Neves, G.A.; Barbosa, H.S. Social preference is maintained in mice with impaired startle reflex and glutamate/D-serine imbalance induced by chronic cerebral toxoplasmosis. Sci. Rep. 2021, 11, 14029. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.L.; Azmitia, E.C. Structure and function of the brain serotonin system. Physiol. Rev. 1992, 72, 165–229. [Google Scholar] [CrossRef] [PubMed]
- Lucki, I. The spectrum of behaviors influenced by serotonin. Biol. Psychiatry 1998, 44, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Jonnakuty, C.; Gragnoli, C. What do we know about serotonin? J. Cell. Physiol. 2008, 217, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Leathwood, P.D. Tryptophan availability and serotonin synthesis. Proc. Nutr. Soc. 1987, 46, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Pfefferkorn, E.R. Interferon-gamma blocks the growth of Toxoplasma gondii in human-fibroblasts by inducing the host-cells to degrade tryptophan. Proc. Natl. Acad. Sci. USA 1984, 81, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.W.; Feng, G.S. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991, 5, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Rahdar, M.; Farbod, Y.; Seydinejad, S.; Zarrin, M. The effect of chronic experimental toxoplasmosis on some brain neurotransmitters level and behavior changes. Exp. Parasitol. 2023, 251, 7. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Lee, L.T.; Yang, Y.K. Serotonin and mental disorders: A concise review on molecular neuroimaging evidence. Clin. Psychopharmacol. Neurosci. 2014, 12, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.; Rashidi, N.; Nurgali, K.; Apostolopoulos, V. The Role of tryptophan metabolites in neuropsychiatric disorders. Int. J. Mol. Sci. 2022, 23, 9968. [Google Scholar] [CrossRef] [PubMed]
- Anguelova, M.; Benkelfat, C.; Turecki, G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: II. Suicidal behavior. Mol. Psychiatry 2003, 8, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Bremshey, S.; Groß, J.; Renken, K.; Masseck, O.A. The role of serotonin in depression-A historical roundup and future directions. J. Neurochem. 2024, 168, 1751–1779. [Google Scholar] [CrossRef] [PubMed]
- Coccaro, E.F.; Fanning, J.R.; Phan, K.L.; Lee, R. Serotonin and impulsive aggression. CNS Spectr. 2015, 20, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Sinopoli, V.M.; Burton, C.L.; Kronenberg, S.; Arnold, P.D. A review of the role of serotonin system genes in obsessive-compulsive disorder. Neurosci. Biobehav. Rev. 2017, 80, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.M.; Abd El-Fatah, A.S.; Rizk, M.M.; Hassan, E.E. Latent toxoplasmosis is associated with depression and suicidal behavior. Arch. Suicide Res. 2020, 26, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.O.; Lima, F.W.D.; Rocha, R.B.A.; Bah, H.A.F.; Carvalho, C.F.; Menezes, J.A. Interaction of Toxoplasma gondii infection and elevated blood lead levels on children’s neurobehavior. Neurotoxicology 2020, 78, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Alsaady, I.; Tedford, E.; Alsaad, M.; Bristow, G.; Kohli, S.; Murray, M.; Reeves, M.; Vijayabaskar, M.S.; Clapcote, S.J.; Wastling, J.; et al. Downregulation of the central noradrenergic system by Toxoplasma gondii infection. Infect. Immun. 2019, 87, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Sugama, S.; Kakinuma, Y. Noradrenaline as a key neurotransmitter in modulating microglial activation in stress response. Neurochem. Int. 2021, 143, 104943. [Google Scholar] [CrossRef] [PubMed]
- Laing, C.; Blanchard, N.; McConkey, G.A. Noradrenergic signaling and neuroinflammation crosstalk regulate Toxoplasma gondi-induced behavioral changes. Trends Immunol. 2020, 41, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Xin, Y.; Peng, B.; Liu, S. Norepinephrine system at the interface of attention and reward. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 125, 110751. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.; Johansson, M.; Agren, H.; Friberg, P. Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness: Evidence in support of the catecholamine hypothesis of mood disorders. Arch. Gen. Psychiatry 2000, 57, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Ressler, K.J.; Nemeroff, C.B. Role of norepinephrine in the pathophysiology of neuropsychiatric disorders. CNS Spectr. 2001, 6, 663–666, 670. [Google Scholar] [CrossRef] [PubMed]
- Nanjappa, M.S.; Voyiaziakis, E.; Pradhan, B.; Mannekote Thippaiah, S. Use of selective serotonin and norepinephrine reuptake inhibitors (SNRIs) in the treatment of autism spectrum disorder (ASD), comorbid psychiatric disorders and ASD-associated symptoms: A clinical review. CNS Spectr. 2022, 27, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Morilak, D.A.; Barrera, G.; Echevarria, D.J.; Garcia, A.S.; Hernandez, A.; Ma, S.; Petre, C.O. Role of brain norepinephrine in the behavioral response to stress. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Maletic, V.; Eramo, A.; Gwin, K.; Offord, S.J.; Duffy, R.A. The role of norepinephrine and its α-adrenergic receptors in the pathophysiology and treatment of major depressive disorder and schizophrenia: A systematic review. Front. Psychiatry 2017, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Cairney, P.; McConkey, G. Pathophysiological mechanisms of Toxoplasma gondii infection in the central nervous system (CNS). In Neurobiology of Infectious Diseases; Academic Press: Cambridge, MA, USA, 2025; pp. 337–346. [Google Scholar]
- Singh, D.K.; Dass, S.A.H.; Abdulai-Saiku, S.; Vyas, A. Testosterone acts within the medial amygdala of rats to reduce innate fear to predator odor akin to the effects of Toxoplasma gondii infection. Front. Psychiatry 2020, 11, 630. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J. Hormones and human sexual behavior. J. Sex. Marital. Ther. 1984, 10, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Bain, J. The many faces of testosterone. Clin. Interv. Aging 2007, 2, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, G.; Limoncin, E.; Carosa, E.; Di Sante, S.; Gravina, G.L.; Mollaioli, D.; Gianfrilli, D.; Lenzi, A.; Jannini, E.A. Is testosterone a food for the brain? Sex Med. Rev. 2016, 4, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Zghair, K.H.; Al-Qadhi, B.N.; Mahmood, S.H. The effect of toxoplasmosis on the level of some sex hormones in males blood donors in Baghdad. J. Parasit. Dis. 2015, 39, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, R.A.; Al-awadi, H.M. Changes in testosterone, progesterone and prolactin levels in pregnant women with chronic toxoplasmosis. Med. J. Babylon. 2013, 10, 699–708. [Google Scholar]
- Zouei, N.; Shojaee, S.; Mohebali, M.; Keshavarz, H. The association of latent toxoplasmosis and level of serum testosterone in humans. BMC Res. Notes 2018, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Carrier, N.; Saland, S.K.; Duclot, F.; He, H.; Mercer, R.; Kabbaj, M. The anxiolytic and antidepressant-like effects of testosterone and estrogen in gonadectomized male rats. Biol. Psychiatry 2015, 78, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Roncati, L.; Manenti, A.; Pusiol, T.; Piscioli, F.; Barbolini, G.; Maiorana, A. Testosterone aromatization to estradiol in course of ovarian functioning brenner tumor associated with endometrial carcinoma and endometriosis (Roncati-Manenti triad). Int. J. Gynecol. Cancer 2016, 26, 1461–1464. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.V.; Jordan, C.L.; Breedlove, S.M. Testosterone works through androgen receptors to modulate neuronal response to anxiogenic stimuli. Neurosci. Lett. 2021, 753, 135852. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, H.K.; Lee, H.J.; Macbeth, A.H.; Young, W.S., 3rd. Vasopressin: Behavioral roles of an “original” neuropeptide. Prog. Neurobiol. 2008, 84, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Frank, E.; Landgraf, R. The vasopressin system--from antidiuresis to psychopathology. Eur. J. Pharmacol. 2008, 583, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann-Peruzatto, J.M.; Lazzari, V.M.; de Moura, A.C.; Almeida, S.; Giovenardi, M. Examining the role of vasopressin in the modulation of parental and sexual behaviors. Front. Psychiatry 2015, 6, 130. [Google Scholar] [CrossRef] [PubMed]
- Morales-Medina, J.C.; Witchey, S.K.; Caldwell, H.K. The role of vasopressin in anxiety and depression. In Melatonin, Neuroprotective Agents and Antidepressant Therapy; López-Muñoz, F., Srinivasan, V., de Berardis, D., Álamo, C., Kato, T., Eds.; Springer: New Delhi, India, 2016. [Google Scholar]
- Campbell, A. Oxytocin and human social behavior. Pers. Soc. Psychol. Rev. 2010, 14, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Lischke, A.; Gamer, M.; Berger, C.; Grossmann, A.; Hauenstein, K.; Heinrichs, M.; Herpertz, S.C.; Domes, G. Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology 2012, 37, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Radke, S.; Volman, I.; Kokal, I.; Roelofs, K.; de Bruijn, E.R.A.; Toni, I. Oxytocin reduces amygdala responses during threat approach. Psychoneuroendocrinology 2017, 79, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Ayers, L.W.; Missig, G.; Schulkin, J.; Rosen, J.B. Oxytocin reduces background anxiety in a fear-potentiated startle paradigm: Peripheral vs central administration. Neuropsychopharmacology 2011, 36, 2488–2497. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.; Becker, B.; Scheele, D.; Scholz, C.; Preckel, K.; Schlaepfer, T.E.; Grinevich, V.; Kendrick, K.M.; Maier, W.; Hurlemann, R. Oxytocin facilitates the extinction of conditioned fear in humans. Biol. Psychiatry 2015, 78, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Abdulai-Saiku, S.; Vyas, A. Toxoplasma gondii infection causes an atypical abundance of oxytocin and its receptor in the female rat brain. Pathogens 2021, 10, 1495. [Google Scholar] [CrossRef] [PubMed]
- Mazael, N.A.; Shakir, O.M. Impact of Toxoplasma gondii on prostaglandin, progesterone, oxytocin and anti-müllerian in abortion women. Int. J. Biol. Sci. 2024, 6, 45–49. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Osório, F.L. Peripheral oxytocin concentrations in psychiatric disorders—A systematic review and methanalysis: Further evidence. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 117, 110561. [Google Scholar] [CrossRef] [PubMed]
- Chaulagain, R.P.; Shrestha, Y.; Shrestha, H.; Bhandari, R.; Gurung, P. The neurobiological impact of oxytocin in mental health disorders: A comprehensive review. Ann. Med. Surg. 2025, 87, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Katsu, Y.; Baker, M.E. Subchapter 123D—Cortisol. In Handbook of Hormones, 2nd ed.; Ando, H., Ukena, K., Nagata, S., Eds.; Academic Press: San Diego, CA, USA, 2021; pp. 947–949. [Google Scholar]
- Fallah, M.; Ehsan, T.S.; Salehi, I.; Maghsood, A.H.; Matini, M.; Pooyandehravan, A. Effect of acute toxoplasmosis on anxiety and cortisol and interleukin-17 levels in male rats: An experimental study. Zahedan J. Res. Med. Sci. 2021, 23, e95733. [Google Scholar] [CrossRef]
- Laubach, Z.M.; Gering, E.; Yang, E.; Montgomery, T.M.; Getty, T.; Holekamp, K.E. Associations between Toxoplasma gondii infection and steroid hormone levels in spotted hyenas. Int. J. Parasitol. Parasites Wildl. 2022, 17, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Shirbazou, S.; Abasian, L.; Talebi, M.F. Effects of Toxoplasma gondii infection on plasma testosterone and cortisol level and stress index on patients referred to Sina hospital, Tehran. Jundishapur J. Microbiol. 2011, 4, 167–173. [Google Scholar]
- El-Gebaly, N.; Abd-Eltawab, M.; Hamed, A.; Mahfouz, N.; Adel, S.; Mahfoz, A.; Rehan, M.; Elsebaei, E. Insights into the interplay of latent toxoplasmosis, testosterone, cortisol and oxidative stress in screened schizophrenic patients in Egypt. Parasitol. United J. 2019, 12, 102–109. [Google Scholar] [CrossRef]
- Heck, A.L.; Handa, R.J. Sex differences in the hypothalamic-pituitary-adrenal axis’ response to stress: An important role for gonadal hormones. Neuropsychopharmacology 2019, 44, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Jellyman, J.K.; Valenzuela, O.A.; Fowden, A.L. HORSE SPECIES SYMPOSIUM: Glucocorticoid programming of hypothalamic-pituitary-adrenal axis and metabolic function: Animal studies from mouse to horse. J. Anim. Sci. 2015, 93, 3245–3260. [Google Scholar] [CrossRef] [PubMed]
- Rohleder, N.; Wolf, J.M.; Kirschbaum, C. Glucocorticoid sensitivity in humans-interindividual differences and acute stress effects. Stress 2003, 6, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Mcleod, R.; Estes, R.G.; Mack, D.G.; Cohen, H. Immune-response of mice to ingested Toxoplasma gondii—A model of Toxoplasma infection acquired by ingestion. J. Infect. Dis. 1984, 149, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.G.; Nitzgen, B.; Reichmann, G.; Hadding, U. Cytokine responses induced by Toxoplasma gondii in astrocytes and microglial cells. Eur. J. Immunol. 1997, 27, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Matowicka-Karna, J.; Dymicka-Piekarska, V.; Kemona, H. Does Toxoplasma gondii infection affect the levels of IgE and cytokines (IL-5, IL-6, IL-10, IL-12, and TNF-alpha)? Clin. Dev. Immunol. 2009, 2009, 374696. [Google Scholar] [CrossRef]
- Mohyuddin, H.; Laffon, B.; Teixeira, J.P.; Costa, S.; Teixeira-Gomes, A.; Pásaro, E.; Constantine, N.; Dagdag, A.; Ortmeyer, H.K.; Tizenberg, B.; et al. Toxoplasma gondii IgG serointensity is positively associated with frailty. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2024, 79, glad228. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, J.; Flegr, J.; Nouzová, K.; Včelák, J.; Kaňková, Š. Chronic inflammation in pregnant women with latent toxoplasmosis and explanation of discordant results of serological tests for toxoplasmosis. Folia Parasitol. 2025, 72, 21. [Google Scholar] [CrossRef]
- Lopes, C.S.; Carvalho, R.J.V.; da Silva, T.L.; Barros, H.L.S.; Costa, L.V.S.; Mota, D.; Barbosa, B.F.; Vieira, L.S.; de Araujo, T.M.; Costa, A.R.; et al. Pregnant women chronically infected by Toxoplasma gondii with depressive disorder: Differential modulation of pro-inflammatory and anti-inflammatory cytokines. Pathogens 2025, 14, 15. [Google Scholar] [CrossRef]
- Flegr, J.; Stříž, I. Potential immunomodulatory effects of latent toxoplasmosis in humans. BMC Infect. Dis. 2011, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Sana, M.; Rashid, M.; Rashid, I.; Akbar, H.; Gomez-Marin, J.E.; Dimier-Poisson, I. Immune response against toxoplasmosis-some recent updates RH: Toxoplasma gondii immune response. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221078436. [Google Scholar] [CrossRef] [PubMed]
- Bliss, S.K.; Marshall, A.J.; Zhang, Y.; Denkers, E.Y. Human polymorphonuclear leukocytes produce IL-12, TNF-α, and the chemokines macrophage-inflammatory protein-1α and -1β in response to Toxoplasma gondii antigens. J. Immunol. 1999, 162, 7369–7375. [Google Scholar] [CrossRef] [PubMed]
- Ihara, F.; Yamamoto, M. The role of IFN-γ-mediated host immune responses in monitoring and the elimination of Toxoplasma gondii infection. Int. Immunol. 2024, 36, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Bisetegn, H.; Debash, H.; Ebrahim, H.; Mahmood, N.; Gedefie, A.; Tilahun, M.; Alemayehu, E.; Mohammed, O.; Feleke, D.G. Global seroprevalence of Toxoplasma gondii infection among patients with mental and neurological disorders: A systematic review and meta-analysis. Health Sci. Rep. 2023, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Babekir, A.; Mostafa, S.; Obeng-Gyasi, E. The association of Toxoplasma gondii IgG and cardiovascular biomarkers. Int. J. Environ. Res. Public Health 2021, 18, 4908. [Google Scholar] [CrossRef] [PubMed]
- Babekir, A.; Mostafa, S.; Obeng-Gyasi, E. The association of Toxoplasma gondii IgG antibody and chronic kidney disease biomarkers. Microorganisms 2022, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Babekir, A.; Mostafa, S.; Obeng-Gyasi, E. The association of Toxoplasma gondii with the combination of cardiovascular disease, chronic kidney disease, or chronic liver disease: A preliminary study. Med. Sci. 2023, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Saraav, I.; Cervantes-Barragan, L.; Olias, P.; Fu, Y.; Wang, Q.; Wang, L.; Mack, M.; Baldridge, M.T.; Stappenbeck, T.; Colonna, M.; et al. Chronic Toxoplasma gondii infection enhances susceptibility to colitis. Proc. Natl. Acad. Sci. USA 2021, 118, e2106730118. [Google Scholar] [CrossRef] [PubMed]
- Shapira, Y.; Agmon-Levin, N.; Selmi, C.; Petrikova, J.; Barzilai, O.; Ram, M.; Bizzaro, N.; Valentini, G.; Matucci-Cerinic, M.; Anaya, J.M.; et al. Prevalence of anti-Toxoplasma antibodies in patients with autoimmune diseases. J. Autoimmun. 2012, 39, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, R.G.; Eassa, S.H.; Mohammad, F.K.; Eassa, S. Plasma cholinesterase activity in patients with rheumatoid arthritis and toxoplasmosis. Cureus 2023, 15, e50979. [Google Scholar] [CrossRef] [PubMed]
- Reeves, G.; Mazaheri, S.; Snitker, S.; Langenberg, P.; Giegling, I.; Hartmann, A.; Konte, B.; Friedl, M.; Okusaga, O.; Groer, M.; et al. A positive association between T. gondii seropositivity and obesity. Front. Public Health 2013, 1, 73. [Google Scholar] [CrossRef] [PubMed]
- Salem, D.A.; Salem, N.A.; Hendawy, S.R. Association between Toxoplasma gondii infection and metabolic syndrome in obese adolescents: A possible immune-metabolic link. Parasitol. Int. 2021, 83, 102343. [Google Scholar] [CrossRef] [PubMed]
- Majidiani, H.; Datuand, S.; Daryani, A.; Galuan-Ramirez, M.D.; Foroutan-Rad, M. Is chronic toxoplasmosis a risk factor for diabetes mellitus? A systematic review and meta-analysis of case-control studies. Braz. J. Infect. Dis. 2016, 20, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Kim, B.S.; Im, H.I. Pathophysiological role of neuroinflammation in neurodegenerative diseases and psychiatric disorders. Int. Neurourol. J. 2016, 20, S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.G.C.; Nani, J.V.; Oses, J.P.; Brietzke, E.; Hayashi, M.A.F. Neuroinflammation and glial cell activation in mental disorders. Brain Behav. Immun. Health 2020, 2, 100034. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, G.M.; Cousins, L.; Deakin, J.; Lennox, B.R.; Yolken, R.; Jones, P.B. Inflammation and immunity in schizophrenia: Implications for pathophysiology and treatment. Lancet Psychiatry 2015, 2, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Fries, G.R.; Walss-Bass, C.; Bauer, M.E.; Teixeira, A.L. Revisiting inflammation in bipolar disorder. Pharmacol. Biochem. Behav. 2019, 177, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Coelho, D.R.A.; Salvi, J.D.; Vieira, W.F.; Cassano, P. Inflammation in obsessive-compulsive disorder: A literature review and hypothesis-based potential of transcranial photobiomodulation. J. Neurosci. Res. 2024, 102, e25317. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The bidirectional relationship of depression and inflammation: Double trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Kappelmann, N.; Moser, S.; Davey Smith, G.; Burgess, S.; Jones, P.B.; Khandaker, G.M. Role of inflammation in depression and anxiety: Tests for disorder specificity, linearity and potential causality of association in the UK Biobank. EClinicalMedicine 2021, 38, 100992. [Google Scholar] [CrossRef] [PubMed]
- Fard, M.T.; Cribb, L.; Nolidin, K.; Savage, K.; Wesnes, K.; Stough, C. Is there a relationship between low-grade systemic inflammation and cognition in healthy people aged 60–75 years? Behav. Brain Res. 2020, 383, 112502. [Google Scholar] [CrossRef] [PubMed]
- Hostomská, L.; J¡rovec, O.; Horáčková, M.; Hrubcová, M. Mongolismus und latente Toxoplasmosis der Mutter. Endokrinologie 1957, 34, 295–304. [Google Scholar]
- Kaňková, Š.; Holáň, V.; Zajícová, A.; Kodym, P.; Flegr, J. Modulation of immunity in mice with latent toxoplasmosis—The experimental support for the immunosupression hypothesis of Toxoplasma-induced changes in reproduction of mice and humans. Parasitol. Res. 2010, 107, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.M.; Elmehankar, M.S.; Azab, M.S.; El-Tantawy, N.L.; Abdel-Aziz, A. Sex dichotomy in the course of experimental latent toxoplasmosis. Exp. Parasitol. 2019, 202, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Mendy, A.; Vieira, E.R.; Albatineh, A.N.; Gasana, J. Immediate rather than delayed memory impairment in older adults with latent toxoplasmosis. Brain Behav. Immun. 2015, 45, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.N.B.; Menezes, I.R.R.; Palmer, J.L.; Brys, I. Chronic Toxoplasma gondii infection is associated with decreased working memory performance in women. Estud. Psicol. 2024, 41, e210112. [Google Scholar] [CrossRef]
- Alvarado-Esquivel, C.; Estrada-Martinez, S.; Perez-Alamos, A.R.; Beristain-Garcia, I.; Alvarado-Felix, A.O.; Alvarado-Felix, G.A.; Sifuentes-Alvarez, A. Toxoplasma gondii infection and suicidal behavior in people with alcohol consumption. Pathogens 2021, 10, 734. [Google Scholar] [CrossRef] [PubMed]
- Hlaváčová, J.; Flegr, J.; Fiurášková, K.; Kaňková, Š. Relationship between latent toxoplasmosis and depression in clients of a Center for Assisted Reproduction. Pathogens 2021, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Borráz-León, J.I.; Rantala, M.J.; Luoto, S.; Krams, I.; Contreras-Garduño, J.; Cerda-Molina, A.L.; Krama, T. Toxoplasma gondii and psychopathology: Latent infection Is associated with interpersonal sensitivity, psychoticism, and higher testosterone levels in men, but not in women. Adapt. Hum. Behav. Physiol. 2021, 7, 28–42. [Google Scholar] [CrossRef]
- Huang, L.; You, X.; Lu, Z.; Zhou, X.; He, L.; Zou, C.; Wang, Q. Toxoplasma gondii infection and cardiovascular mortality: Sex-specific differences in a United States population-based cohort study. BMC Infect. Dis. 2024, 24, 1029. [Google Scholar] [CrossRef] [PubMed]
- Carritt, B.; Kemp, T.J.; Poulter, M. Evolution of the human RH (rhesus) blood group genes: A 50 year old prediction (partially) fulfilled. Hum. Mol. Genet. 1997, 6, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Flegel, W.A. Molecular genetics of RH and its clinical application. Transfus. Clin. Biol. 2006, 13, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Prandota, J. Rhesus-associated glycoprotein (RhAG) phenotype of the red blood cells modulates T. gondii infection-associated psychomotor performance reaction times and changes in the human personality profile. Impaired function of the CO2, AQP1, and AQP4 gas channels may cause hypoxia and thus enhance neuroinflammation in autistic individuals. In Neuroinflammation: Pathogenesis, Mechanisms and Management; Gemma, C., Ed.; Nova Publishers: New York, NY, USA, 2012; pp. 423–439. [Google Scholar]
- Flegr, J.; Šebánková, B.; Příplatová, L.; Chvátalová, V.; Kaňková, Š. Lower performance of Toxoplasma-infected, Rh-negative subjects in the weight holding and hand-grip tests. PLoS ONE 2018, 13, e0200346. [Google Scholar] [CrossRef] [PubMed]
- Daniels, G. Human Blood Groups; Blackwell Publishers: Oxford, UK, 2002; pp. 198–200. [Google Scholar]
- Flegr, J.; Toman, J.; Hula, M.; Kankova, S. The role of balancing selection in maintaining human RhD blood group polymorphism: A preregistered cross-sectional study. J. Evol. Biol. 2021, 34, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Příplatová, L.; Hlaváčová, J.; Šebánková, B.; Žďárský, E.; Kaňková, Š. The importance of being heterozygote: Effects of RHD-genotype-sex interaction on the physical and mental health of a non-clinical population. Sci. Rep. 2021, 11, 21960. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J. Heterozygote advantage probably maintains Rhesus factor blood group polymorphism: Ecological regression study. PLoS ONE 2016, 11, e0147955. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Hoffmann, R.; Dammann, M. Worse health status and higher incidence of health disorders in Rhesus negative subjects. PLoS ONE 2015, 10, e0141362. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Kuba, R.; Kopecký, R. Rhesus-minus phenotype as a predictor of sexual desire and behavior, wellbeing, mental health, and fecundity. PLoS ONE 2020, 15, e0236134. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Geryk, J.; Volny, J.; Klose, J.; Cernochova, D. Rhesus factor modulation of effects of smoking and age on psychomotor performance, intelligence, personality profile, and health in Czech soldiers. PLoS ONE 2012, 7, e49478. [Google Scholar] [CrossRef] [PubMed]
- Halmin, M.; Rostgaard, K.; Lee, B.K.; Wikman, A.; Norda, R.; Nielsen, K.R.; Pedersen, O.B.; Holmqvist, J.; Hjalgrim, H.; Edgren, G. Length of storage of red blood cells and patient survival after blood transfusion a binational cohort study. Ann. Intern. Med. 2017, 166, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Saeij, J.P.J.; Boyle, J.P.; Boothroyd, J.C. Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol. 2005, 21, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Dardé, M.L. Toxoplasma gondii, “new” genotypes and virulence. Parasite 2008, 15, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Kannan, G.; Moldovan, K.; Xiao, J.C.; Yolken, R.H.; Jones-Brando, L.; Pletnikov, M.V. Toxoplasma gondii strain-dependent effects on mouse behaviour. Folia Parasitol. 2010, 57, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Ingram, W.M.; Goodrich, L.M.; Robey, E.A.; Eisen, M.B. Mice infected with low-virulence strains of Toxoplasma gondii lose their innate aversion to cat urine, even after extensive parasite clearance. PLoS ONE 2013, 8, e75246. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Yang, D.; Qin, K.; Liu, L.; Jin, M.; Zhang, F.; Zhu, J.; Wang, J.; Luo, Q.; Du, J.; et al. Studies on the mechanism of Toxoplasma gondii Chinese 1 genotype Wh6 strain causing mice abnormal cognitive behavior. Parasit. Vectors 2023, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.K.; Pyo, K.H.; Shin, K.Y.; Hwang, Y.S.; Lim, H.; Lee, S.J.; Moon, J.H.; Lee, S.H.; Suh, Y.H.; Chai, J.Y.; et al. Toxoplasma gondii infection in the brain inhibits neuronal degeneration and learning and memory impairments in a Murine Model of Alzheimer’s disease. PLoS ONE 2012, 7, e33312. [Google Scholar] [CrossRef] [PubMed]
- Cabral, C.M.; McGovern, K.E.; MacDonald, W.R.; Franco, J.; Koshy, A.A. Dissecting amyloid beta deposition using distinct strains of the neurotropic parasite Toxoplasma gondii as a novel tool. ASN Neuro 2017, 9, 1759091417724915. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.C.; Prandovszky, E.; Kannan, G.; Pletnikov, M.V.; Dickerson, F.; Severance, E.G.; Yolken, R.H. Toxoplasma gondii: Biological parameters of the connection to schizophrenia. Schizophr. Bull. 2018, 44, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.J.; Koshy, A.A. Latent toxoplasmosis effects on rodents and humans: How much is real and how much is media hype? MBio 2020, 11, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Castaño Barrios, L.; Da Silva Pinheiro, A.P.; Gibaldi, D.; Silva, A.A.; Machado Rodrigues, E.S.P.; Roffê, E.; da Costa Santiago, H.; Tostes Gazzinelli, R.; Mineo, J.R.; Silva, N.M.; et al. Behavioral alterations in long-term Toxoplasma gondii infection of C57BL/6 mice are associated with neuroinflammation and disruption of the blood brain barrier. PLoS ONE 2021, 16, e0258199. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.K.; Strassmann, P.S.; Lee, I.P.; Sapolsky, R.M. Patterns of Toxoplasma gondii cyst distribution in the forebrain associate with individual variation in predator odor avoidance and anxiety-related behavior in male Long-Evans rats. Brain Behav. Immun. 2014, 37, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Miyagaki, M.; Zong, Y.; Yang, M.; Zhang, J.; Zou, Y.; Ohno-Matsui, K.; Kamoi, K. Ocular toxoplasmosis: Advances in Toxoplasma gondii biology, clinical manifestations, diagnostics, and therapy. Pathogens 2024, 13, 898. [Google Scholar] [CrossRef] [PubMed]
- de-la-Torre, A.; Sauer, A.; Pfaff, A.W.; Bourcier, T.; Brunet, J.; Speeg-Schatz, C.; Ballonzoli, L.; Villard, O.; Ajzenberg, D.; Sundar, N.; et al. Severe South American ocular toxoplasmosis is associated with decreased Ifn-γ/Il-17a and increased Il-6/Il-13 intraocular levels. PLoS Negl. Trop. Dis. 2013, 7, e2541. [Google Scholar] [CrossRef] [PubMed]
- Genot, S.; Franck, J.; Forel, J.M.; Rebaudet, S.; Ajzenberg, D.; de Paula, A.M.; Dardé, M.L.; Stein, A.; Ranque, S. Severe Toxoplasma gondii I/III recombinant-genotype encephalitis in a human immunodeficiency virus patient. J. Clin. Microbiol. 2007, 45, 3138–3140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ghosn, J.; Paris, L.; Ajzenberg, D.; Carcelain, G.; Dardé, M.L.; Tubiana, R.; Bossi, P.; Bricaire, F.; Katlama, C. Atypical toxoplasmic manifestation after discontinuation of maintenance therapy in a human immunodeficiency virus type 1-infected patient with immune recovery. Clin. Infect. Dis. 2003, 37, e112–e114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Delhaes, L.; Ajzenberg, D.; Sicot, B.; Bourgeot, P.; Dardé, M.L.; Dei-Cas, E.; Houfflin-Debarge, V. Severe congenital toxoplasmosis due to a Toxoplasma gondii strain with an atypical genotype: Case report and review. Prenat. Diagn. 2010, 30, 902–905. [Google Scholar] [CrossRef] [PubMed]
- Stajner, T.; Vasiljević, Z.; Vujić, D.; Marković, M.; Ristić, G.; Mićić, D.; Pasić, S.; Ivović, V.; Ajzenberg, D.; Djurković-Djaković, O. Atypical strain of Toxoplasma gondii causing fatal reactivation after hematopoietic stem cell transplantion in a patient with an underlying immunological deficiency. J. Clin. Microbiol. 2013, 51, 2686–2690. [Google Scholar] [CrossRef] [PubMed]
- Patrat-Delon, S.; Gangneux, J.P.; Lavoué, S.; Lelong, B.; Guiguen, C.; le Tulzo, Y.; Robert-Gangneux, F. Correlation of parasite load determined by quantitative PCR to clinical outcome in a heart transplant patient with disseminated toxoplasmosis. J. Clin. Microbiol. 2010, 48, 2541–2545. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.C.; Buka, S.L.; Cannon, T.D.; Suzuki, Y.; Viscidi, R.P.; Torrey, E.F.; Yolken, R.H. Serological pattern consistent with infection with type I Toxoplasma gondii in mothers and risk of psychosis among adult offspring. Microbes Infect. 2009, 11, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
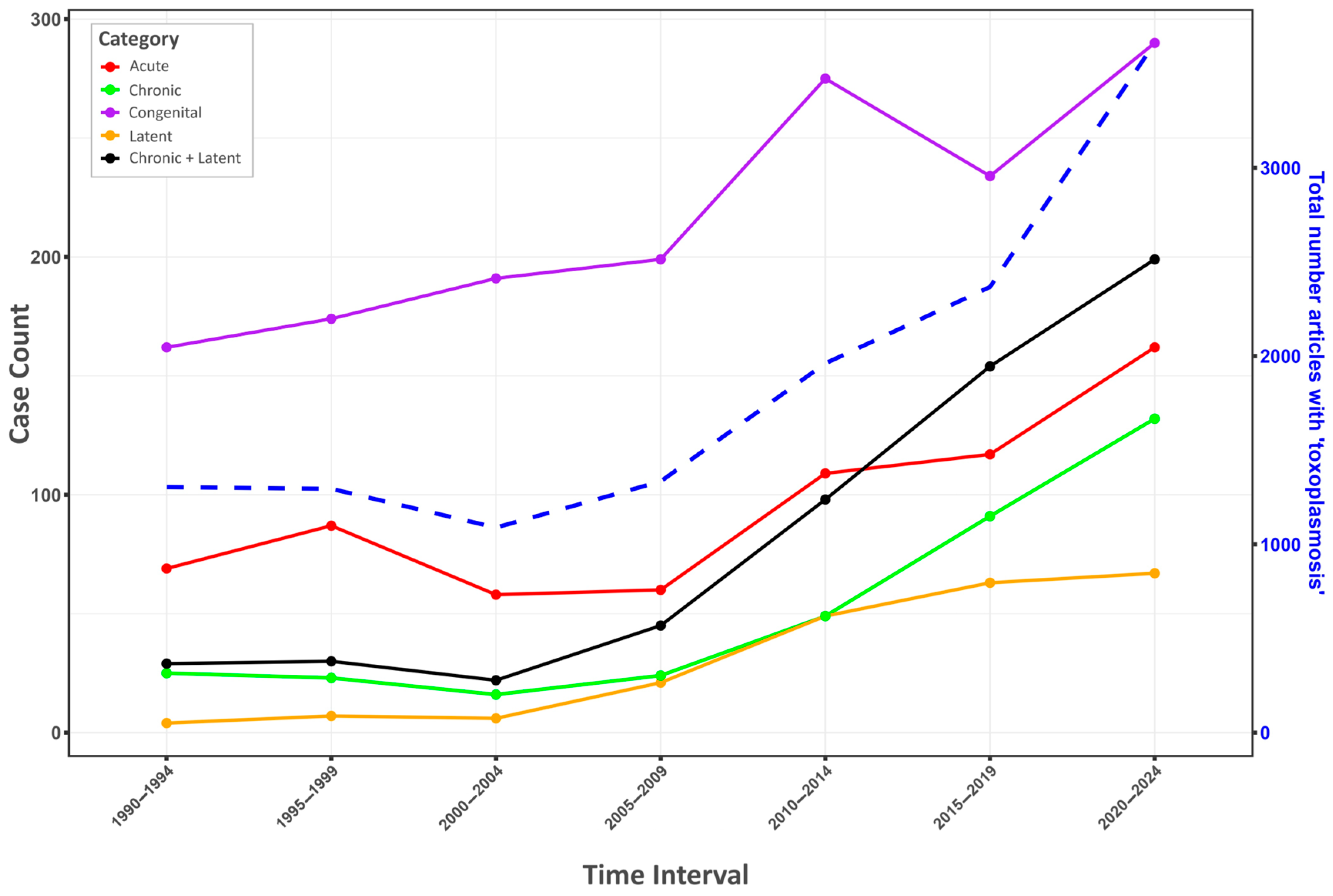
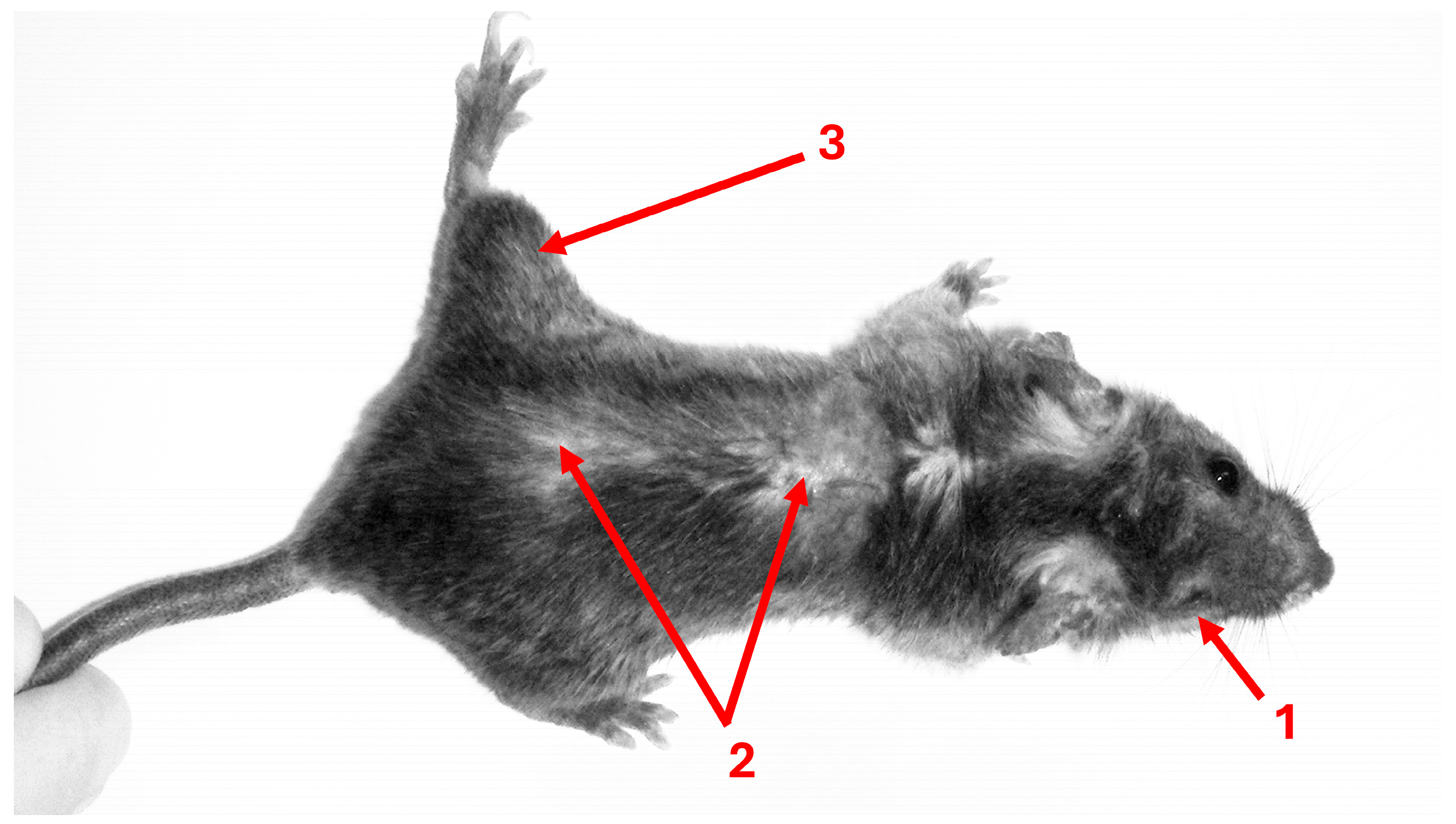
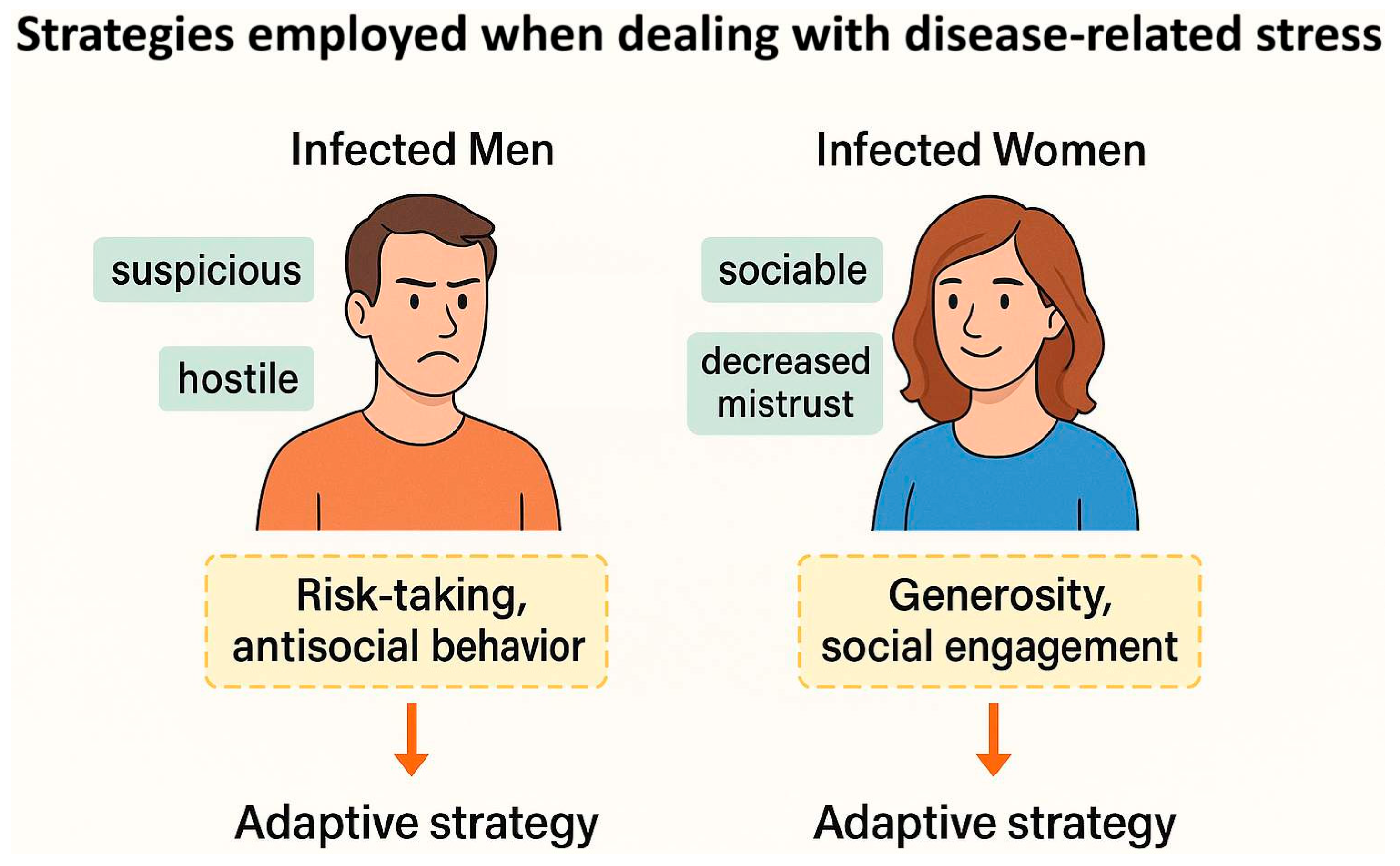
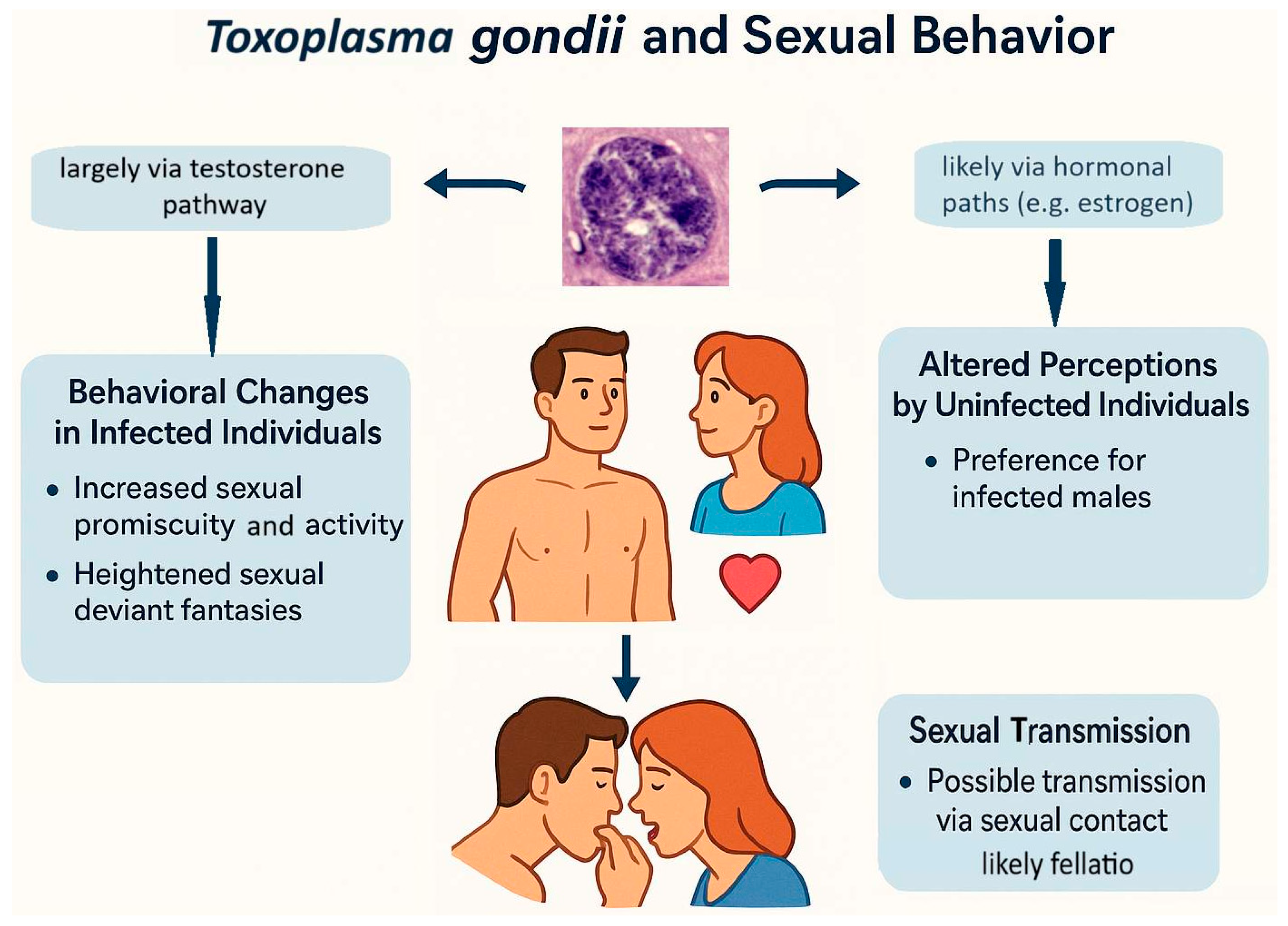
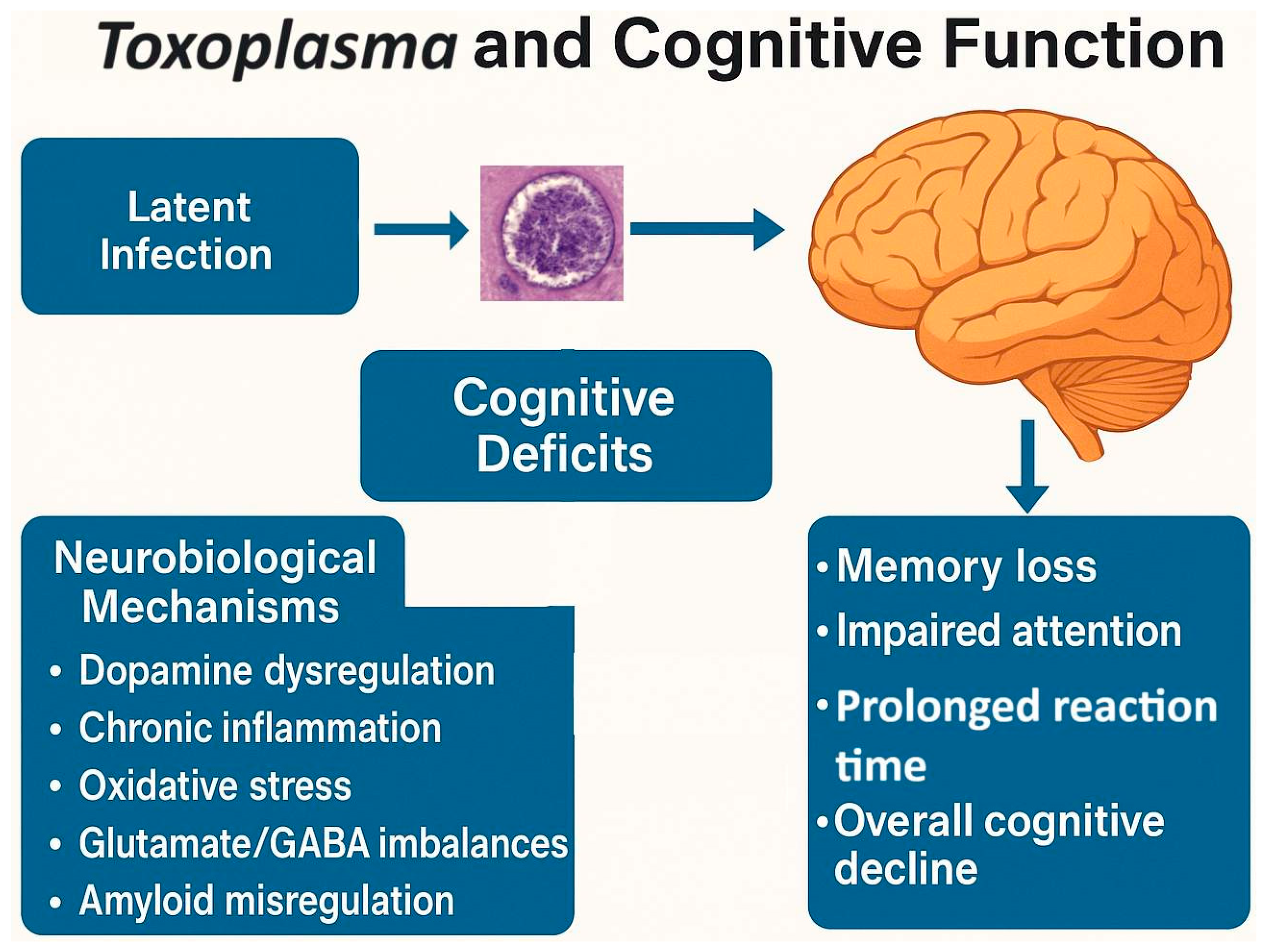
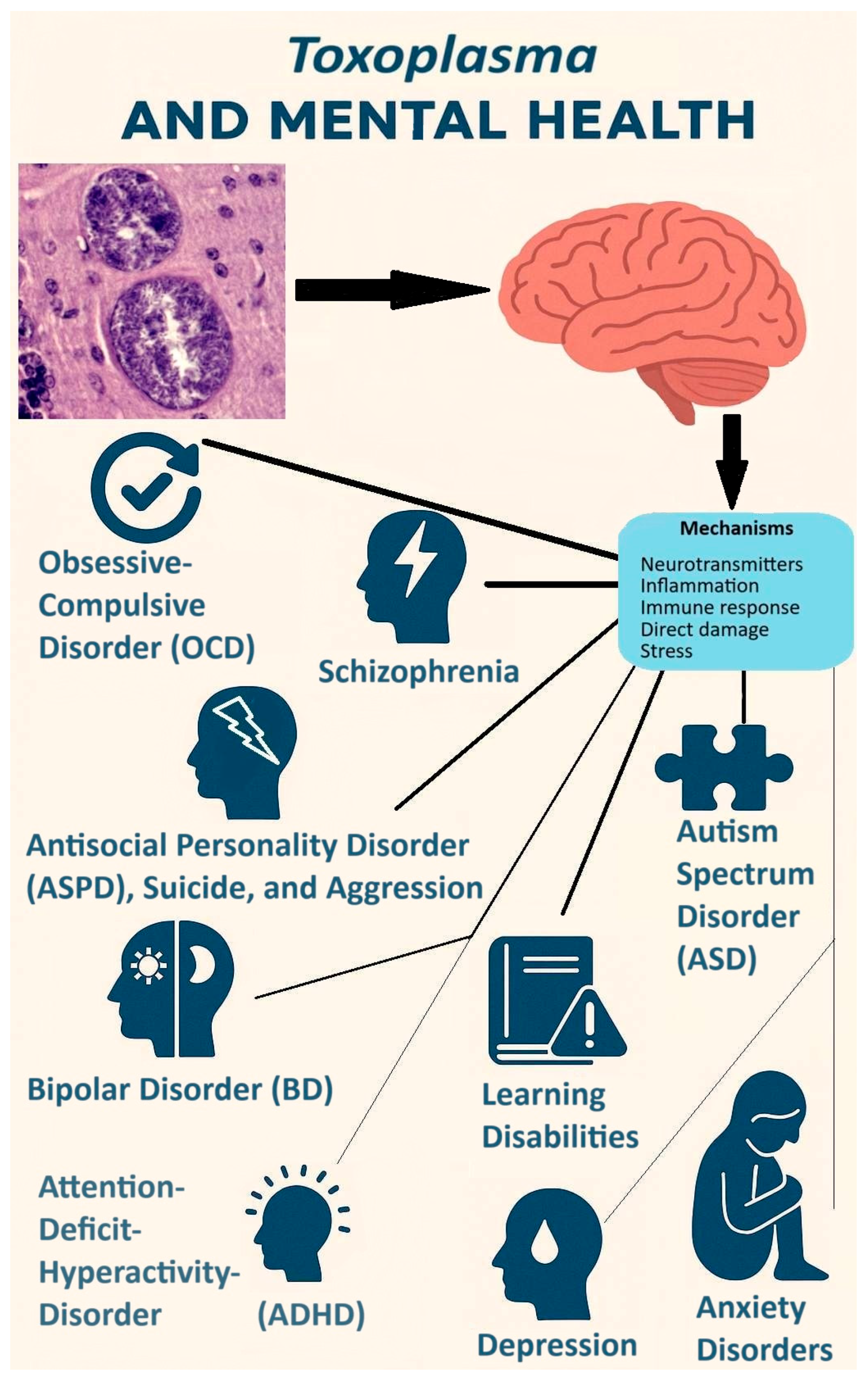
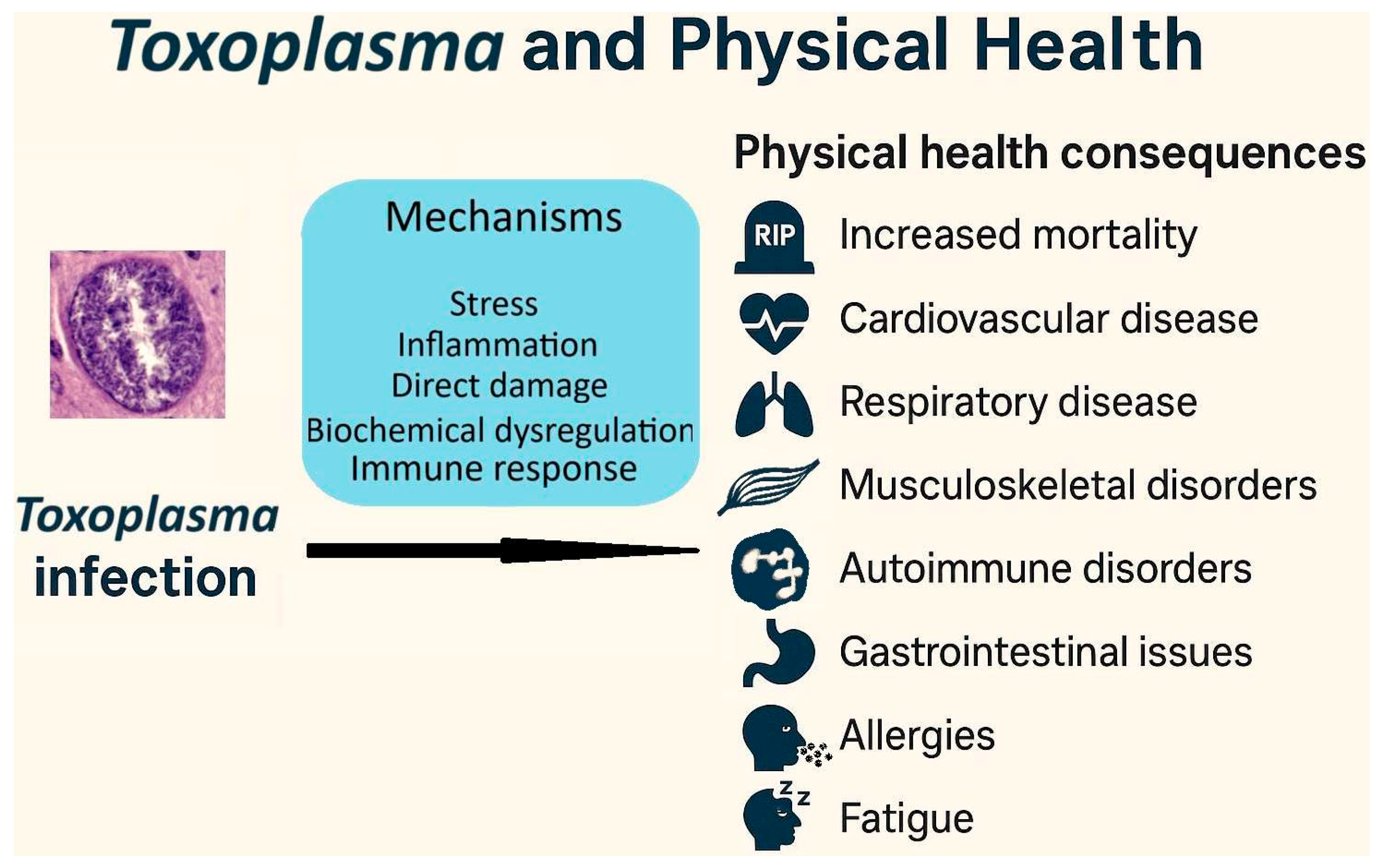
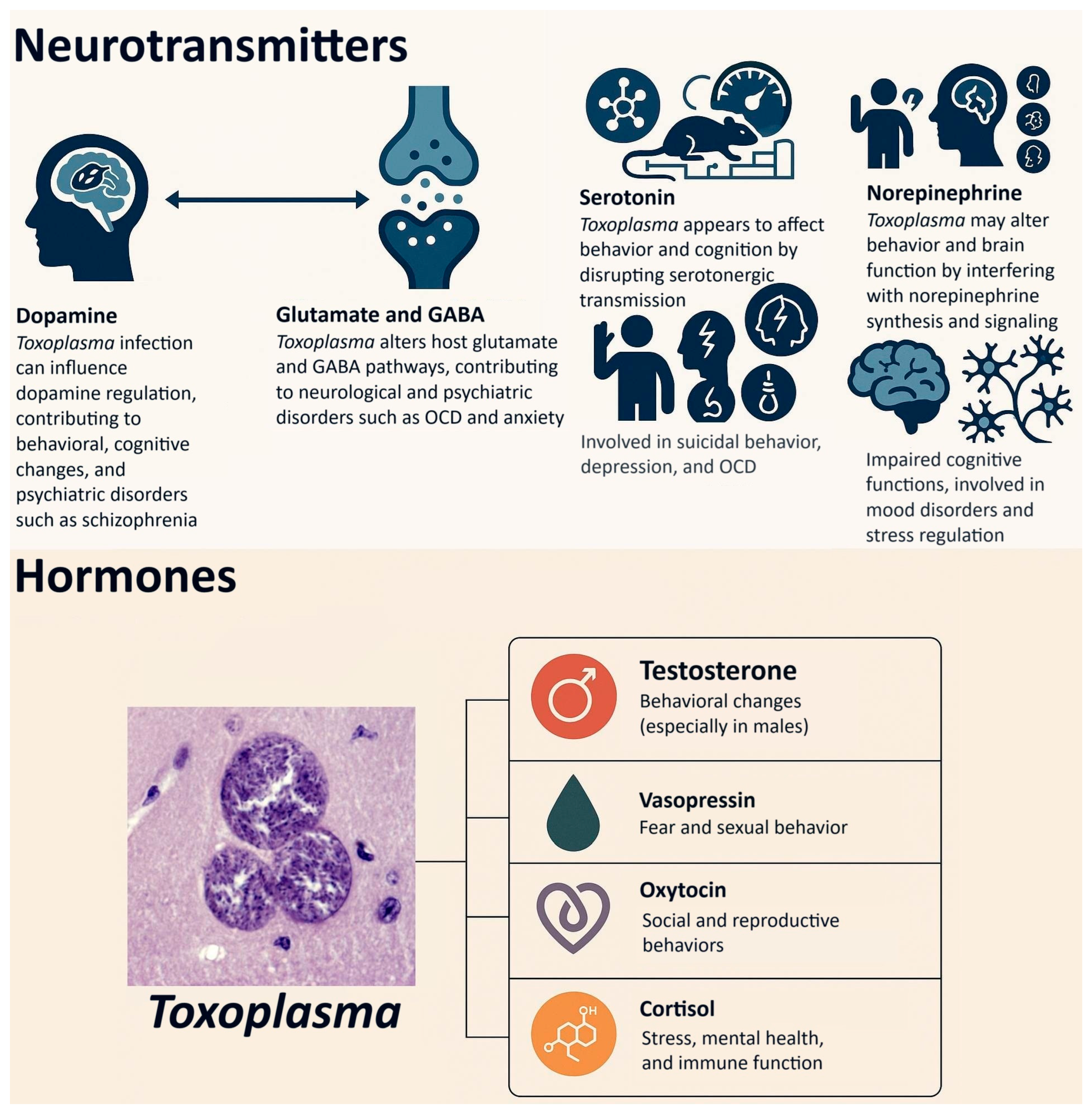
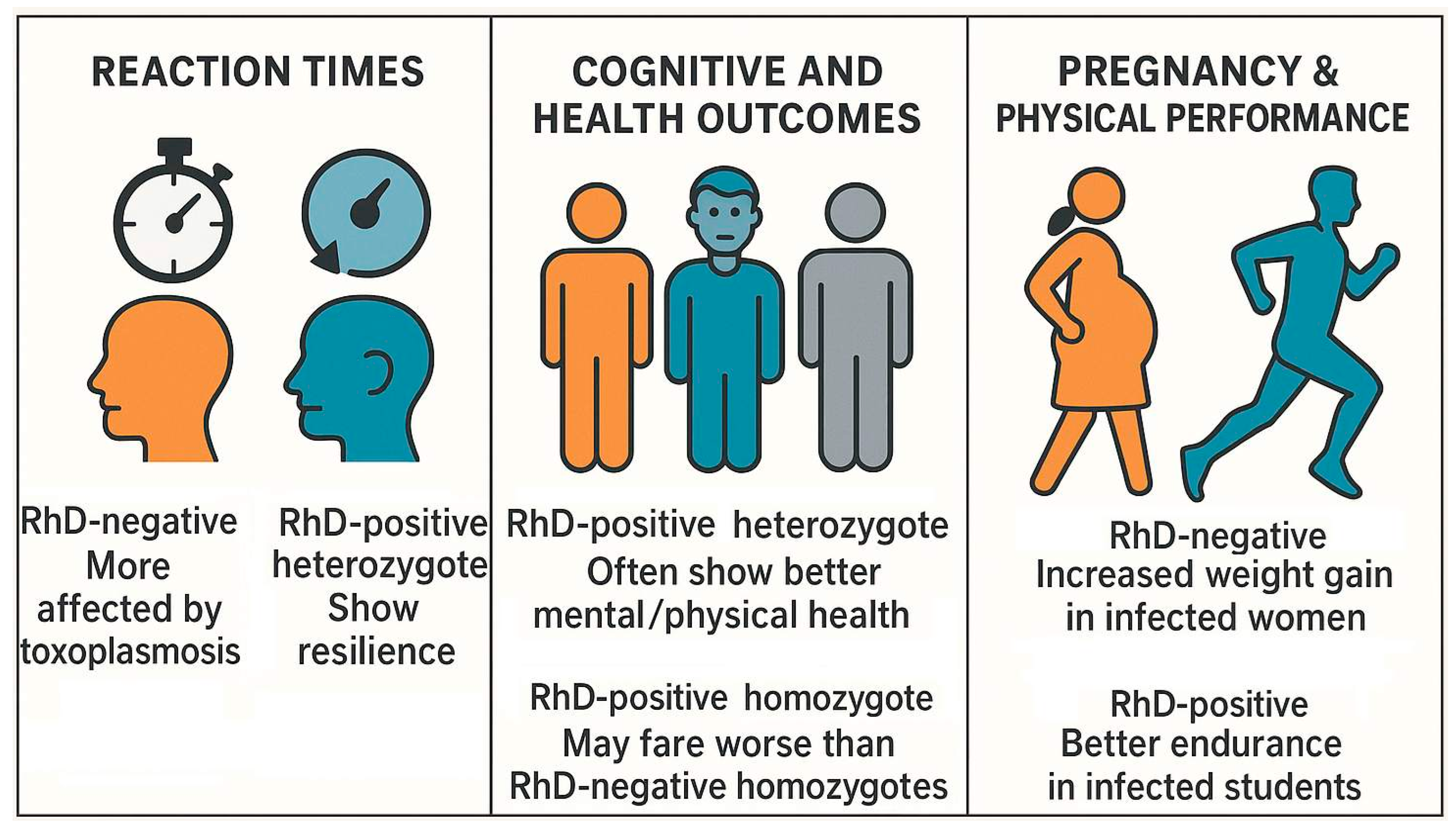
| Type of Toxoplasmosis | Description | Duration | Symptoms | Clinical Relevance |
|---|---|---|---|---|
| Acute toxoplasmosis | Recently infected individuals showing pronounced but transient symptoms of an active infection. | Days to weeks | Fever, lymphadenopathy, fatigue | Usually self-limiting in immunocompetent individuals |
| Subclinical (latent) toxoplasmosis | Long-term (potentially lifelong) infection with subtle, often non-specific symptoms or behavioral changes, some of which may worsen over time. | Lifelong | Mild neurological, immunological, or behavioral symptoms; elevated risk of diverse somatic and psychiatric disorders with often more severe course | Often undiagnosed; possible impact on host behavior |
| Chronic toxoplasmosis | Long-term (potentially lifelong) infection with persistent or recurrent clinical symptoms that may require medical treatment. | Lifelong | Chronic headaches, muscle pain, neuropsychiatric issues | May require long-term management |
| Congenital toxoplasmosis | Infection acquired in utero via vertical transmission from an infected mother, especially during primary maternal infection in pregnancy. | Lifelong | Visual and hearing impairments, cognitive deficits; in severe cases, hydrocephalus, chorioretinitis, and cerebral calcifications, miscarriage | High clinical importance; requires early intervention |
| Cerebral toxoplasmosis (toxoplasmic encephalitis) | Reactivation of latent infection in immunocompromised individuals, primarily affecting the brain. | Variable, often progressive without treatment | Headaches, seizures, focal neurological deficits, altered mental status, fever | Severe, life-threatening opportunistic infection; requires urgent medical treatment |
| Topic | Key Findings | References |
|---|---|---|
| Toxoplasma and behavior in rodents | Increased activity, reduced fear of cat odors, behavioral manipulation to increase predation by cats. | [10,44] |
| Toxoplasma and behavior in non-human primates | Loss of aversion to predator urine (e.g., leopard urine in chimpanzees); strain-specific behavior changes. | [53] |
| Toxoplasma and behavior in humans | Sex-specific behavior differences, increased aggression/impulsivity, elevated risk of traffic/workplace accidents, altered decision-making. | [19,66,67] |
| Toxoplasma and personality (Cattell’s 16PF) | Sex-dependent personality shifts (e.g., lower conscientiousness in men, higher in women); interaction with RhD phenotype. | [19,70] |
| Personality (NEO PI-R, TCI) | Lower conscientiousness, higher extraversion, reduced novelty seeking; infection duration effects vary by test used. | [71,72,74] |
| Explaining opposite effects by sex | Evolutionary, physiological, and psychological explanations (e.g., stress-coping strategies, hormone modulation). | [54] |
| Topic | Key Findings | References |
|---|---|---|
| Altered sexual behavior in Infected Individuals | Infected individuals, especially men, show increased sexual promiscuity and activity; linked to elevated testosterone and dopamine levels, possibly due to parasitic manipulation. | [21,76] |
| Attractiveness to uninfected individuals | Infected males (rats, possibly humans) are found more attractive by uninfected females, potentially due to elevated testosterone and associated traits like dominance and symmetry. | [79] |
| Behavioral manipulation and predation | Infected rodents lose fear of cat odors, possibly via amygdala and limbic system changes, facilitating parasite transmission to feline hosts. | [21] |
| Possibility of sexual transmission | Evidence exists for sexual transmission in animals and potential in humans via semen; observed in sex workers and correlated with STD prevalence. | [81,88,89] |
| Qualitative changes in sexual preferences | Infected individuals report more violent or atypical sexual fantasies but engage less due to poorer health. | [95,97] |
| Sexual behavior of uninfected individuals toward infected | Despite typical aversion to infected mates, Toxoplasma-infected males may be preferred due to heightened masculine traits linked to testosterone. | [104,106] |
| Topic | Key Findings | References |
|---|---|---|
| Cognitive outcomes (general) | Cognitive impairments include poorer working memory, reasoning, attention, and verbal fluency. | [112] |
| Reaction time and processing speed | Toxoplasma-infected individuals show slower reaction times, especially as tests progress; effects moderated by RhD phenotype. | [110,114,115] |
| Executive function and memory | Older adults with infection show impaired memory; middle-aged adults show impaired executive functioning; mixed findings on memory. | [32,122,123] |
| Youth cognition | Infected children (12–16) have lower reading/memory and effects moderated by vitamin E levels. | [124] |
| Psychiatric patients | In bipolar and schizophrenia patients, Toxoplasma associated with worse cognitive scores; antipsychotics may confound effects. | [130,131] |
| Alzheimer’s disease (AD) | Animal models suggest mixed effects, some studies show worsened symptoms, others show protection. Human data are inconsistent. | [116,117,119,120] |
| Intelligence | Mixed findings: some report lower intelligence in men, higher in RhD-negative women; recent studies suggest reduced fluid intelligence. | [20,69,72] |
| Topic | Key Findings | References |
|---|---|---|
| Schizophrenia | Strong association, higher symptom severity and continuous illness in infected; dopamine synthesis linked to parasite | [74,86,141] |
| Autism spectrum disorder | Moderate to strong association, especially in men; dopamine dysfunction and prenatal immune response, infection may influence neurodevelopment via dopamine pathways | [147,148,150] |
| ADHD | Weak to moderate association, recent large study shows significant association, stronger in men | [31,148] |
| Bipolar disorder | Mixed, earlier studies suggest association, but a recent large study shows non-significant effects | [29,148,159] |
| Anxiety | Controversially, some studies have found association with general anxiety, whilst others have not, which is possibly stronger in infected women than infected men | [148,165,166,168] |
| Depression | Weak association with toxoplasmosis, no significant association with major depression but possible effect on mood | [141,148] |
| Obsessive-compulsive disorder (OCD) | Strong association, consistent across studies; infection linked to treatment resistance | [175,176] |
| Antisocial personality disorder (ASPD) | Moderate association, limited direct evidence, but personality traits and aggression linked to infection, Toxoplasma-seropositive men show more aggression, less conscientiousness; | [137,148] |
| Learning disabilities (LDs) | Moderate association, two studies show significant association with higher odds of LD | [148,175] |
| Substance use disorder | Mixed evidence on the link between Toxoplasma infection and addiction; findings vary by study and drug | [182,183,184] |
| Neurotropic zoonoses (Bartonella/Borrelia interaction) | Complex, co-infections with Bartonella or Borrelia may modify Toxoplasma’s impact on mental health (to alter risks of depression and PTSD) | [138,191] |
| Topic | Key Findings | References |
|---|---|---|
| Ecological findings | 88 countries, WHO data on 129 diseases; Toxoplasma seroprevalence correlated with disease burden (23/128 diseases) and mortality (12 diseases) | [27] |
| Cross-sectional and case-control findings | Toxoplasma-infected individuals even before knowledge of infection, especially in RhD-negative individuals score worse on health variables | [195] |
| Health → Anxiety pathway | Health deterioration mediates stress and anxiety | [26] |
| Pregnancy | Lower weight and longer pregnancy in infected women | [197] |
| Pregnancy and RhD | Higher weight gain in RhD-negative, infected women | [197] |
| Gestational diabetes | Higher glucose levels and gestational diabetes mellitus in infected women | [200] |
| Thyroid function | Mild hormonal differences, potential for autoimmune insight in pregnant women | [201] |
| Human infant development | Greater weight gain, delayed motor milestones | [202] |
| Sex ratio in both humans and animal studies | Acute infection associated with higher male ratio; chronic infection associated with lower male births | [204,205] |
| Men’s fertility | Lower sperm quality, higher subfertility risk | [214,215] |
| Women’s fertility | Older age, longer to conceive, higher assisted reproductive technology use | [217,218] |
| Miscarriage risk in pregnant women | Chronic infection nearly doubled miscarriage risk | [97,218] |
| Category | Mechanism | Key Findings | References |
|---|---|---|---|
| Neurotransmitters | Dopamine | Increased dopamine production; linked to behavioral changes, reduced reward sensitivity, altered cognition, and mental disorders like schizophrenia. | [39,224,229,231] |
| Glutamate and GABA | Altered glutamate and GABA levels; associated with cognitive impairments, psychiatric disorders (e.g., OCD, schizophrenia), and seizures. | [25,27,97] | |
| Serotonin | Decreased brain serotonin due to tryptophan depletion; linked to mood disorders, impulsivity, and suicidal behavior. | [148,258,263,272] | |
| Norepinephrine | Suppressed norepinephrine synthesis; impacts behavior, cognition, and immune regulation. | [265,266,268] | |
| Hormones | Testosterone | Elevated testosterone in males; alters fear and sexual behavior, possibly linked to psychiatric outcomes. | [21,278,281] |
| Vasopressin | Increased expression in male rodents; mediates loss of predator fear through interaction with testosterone. | [21,278] | |
| Oxytocin | Increased in infected females; modulates socioemotional behavior and may influence risk-taking and emotional regulation. | [292,297,298] | |
| Cortisol | Altered cortisol levels; may modulate anxiety and stress response with variable results across species and individuals. | [302,303,304] | |
| Immune system | General immune effects | Pro- and anti-inflammatory cytokine shifts; impacts immune balance and may support parasite persistence. | [311,316] |
| Physical health | Linked to cardiovascular, liver, kidney diseases, and autoimmune disorders via chronic inflammation. | [320,321,322,324] | |
| Cognitive and mental health | Inflammatory responses associated with cognitive impairments and psychiatric disorders. | [112,148,249] | |
| Reproductive effects | Possible immune suppression linked to altered sex ratios, Down syndrome prevalence, and prolonged pregnancies. | [198,207,337] |
| Topic | Key Findings | References |
|---|---|---|
| Host sex—animal studies | Sex-specific behavioral effects; different immune responses and hormone influences; males and females may show opposite behaviors or mechanisms. | [41,206,278] |
| Host sex—human studies | Opposite personality and behavioral traits between sexes; infected men show lower sociality, women show higher conscientiousness. Sex-specific cognitive and mental health effects. | [19,71,109,122] |
| RhD status—reaction times | RhD-negative individuals more affected by toxoplasmosis; RhD-positive heterozygotes show resilience. | [114,115] |
| RhD status—cognitive/health outcomes | RhD-positive heterozygotes often show better mental/physical health. Some studies suggest RhD-positive homozygotes may fare worse than RhD-negative homozygotes. | [351,352,353] |
| RhD status—pregnancy and physical performance | Increased weight gain in RhD-negative, Toxoplasma-infected women. Better endurance in RhD-positive infected students. | [199,349] |
| Parasite strain—animal models | Some behavioral changes appear strain-general; for example, the loss of innate fear of cat odors, whilst other outcomes are strain-specific; for instance, Type II not Pru strain, induced memory deficits in mice | [360,361] |
| Parasite strain—human studies | Type I/atypical strains linked to more severe ocular and psychiatric outcomes; Type II less severe. Strain typing remains a challenge. | [369,370,376] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latifi, A.; Flegr, J. Beyond Latency: Chronic Toxoplasma Infection and Its Unveiled Behavioral and Clinical Manifestations—A 30-Year Research Perspective. Biomedicines 2025, 13, 1731. https://doi.org/10.3390/biomedicines13071731
Latifi A, Flegr J. Beyond Latency: Chronic Toxoplasma Infection and Its Unveiled Behavioral and Clinical Manifestations—A 30-Year Research Perspective. Biomedicines. 2025; 13(7):1731. https://doi.org/10.3390/biomedicines13071731
Chicago/Turabian StyleLatifi, Ashkan, and Jaroslav Flegr. 2025. "Beyond Latency: Chronic Toxoplasma Infection and Its Unveiled Behavioral and Clinical Manifestations—A 30-Year Research Perspective" Biomedicines 13, no. 7: 1731. https://doi.org/10.3390/biomedicines13071731
APA StyleLatifi, A., & Flegr, J. (2025). Beyond Latency: Chronic Toxoplasma Infection and Its Unveiled Behavioral and Clinical Manifestations—A 30-Year Research Perspective. Biomedicines, 13(7), 1731. https://doi.org/10.3390/biomedicines13071731







