Novel Benzenesulfonamide Derivatives of 5′-Aminospirotriazolotriazine Exhibit Anti-Inflammatory Activity by Suppressing Pro-Inflammatory Mediators: In Vitro and In Vivo Evaluation Using a Rat Model of Carrageenan-Induced Paw Edema
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
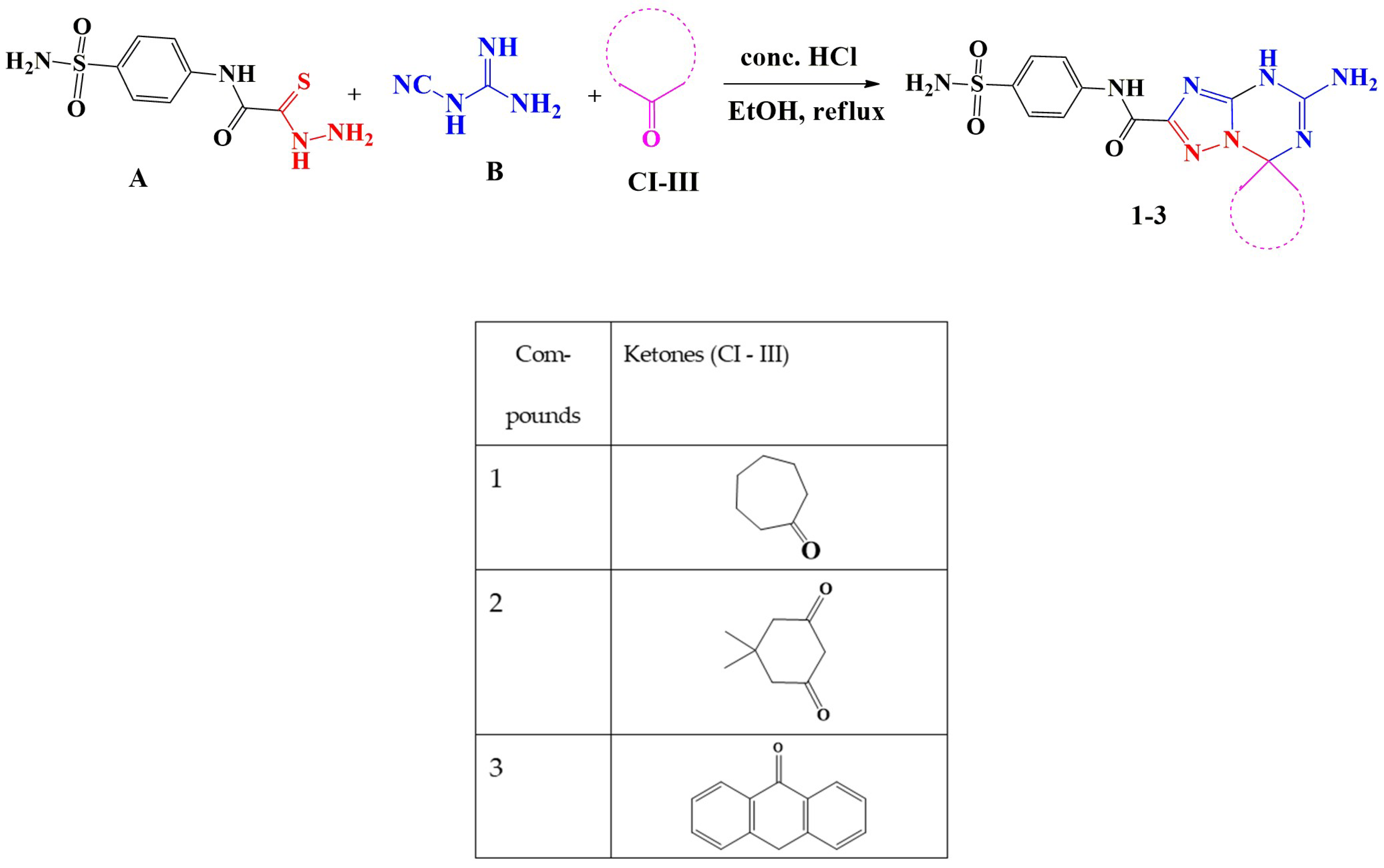
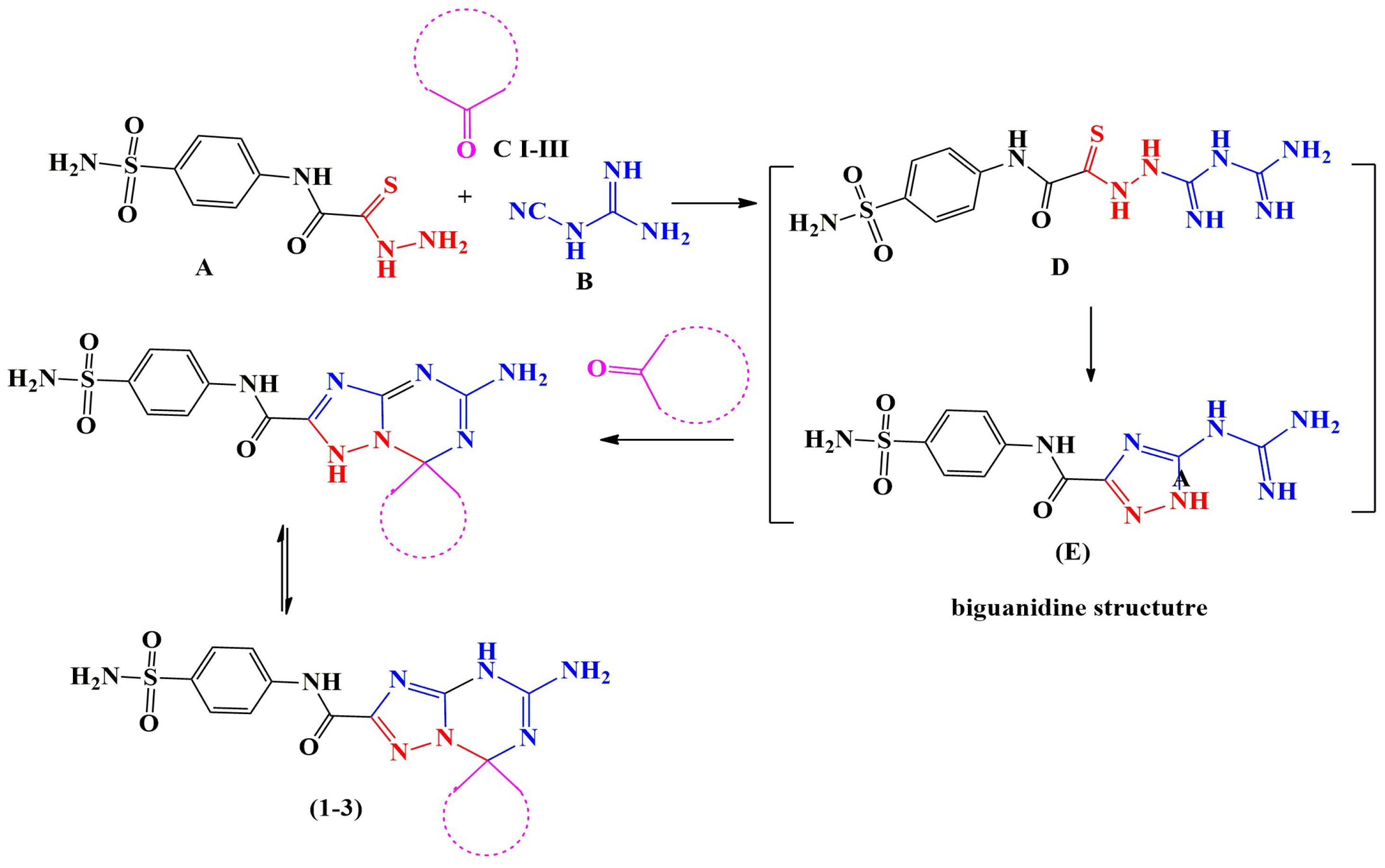
General Procedures for the Synthesis of 5′-Amino-N-(4-sulfamoylphenyl)-spiro[1,7′-[1,2,4]triazolo[1,5-a][1,3,5]triazine]-2′-carboxamide Derivatives (1–3)
2.2. Reagents and Reference Drugs
2.3. Ethical Considerations
2.4. Biological Activity
2.4.1. In Vitro Anti-Inflammatory Activity Assays
Bovine Serum Albumin Denaturation Inhibition Assay
Egg Albumin Protein Denaturation Inhibition Assay
Red Blood Cell Membrane Stabilization Assay
- Principle
- Preparing erythrocyte suspension
- Hypotonicity induced hemolysis
In Vitro COX Inhibition Assay
Cell Viability and Cytotoxicity Evaluation (MTT Assay)
2.4.2. In Vivo Anti-Inflammatory Activity
Pilot Study to Assess Acute Toxicity
Experimental Animals and Carrageenan-Induced Paw Edema
Biochemical and Histopathological Examination
2.5. Statistical Analysis
3. Results
3.1. Chemistry
3.2. Evaluation of Biological Activity
3.2.1. In Vitro Anti-Inflammatory Activities
Effect on Protein Denaturation
Effect on Erythrocyte Membrane Stability
In Vitro COX Inhibitory Assay
Cell Cytotoxicity
3.2.2. In Vivo Anti-Inflammatory Activities
Acute Toxicity Study
Effect on Carrageenan-Induced Edema
Inflammatory Cytokine Changes
Oxidative Stress Biomarker Changes
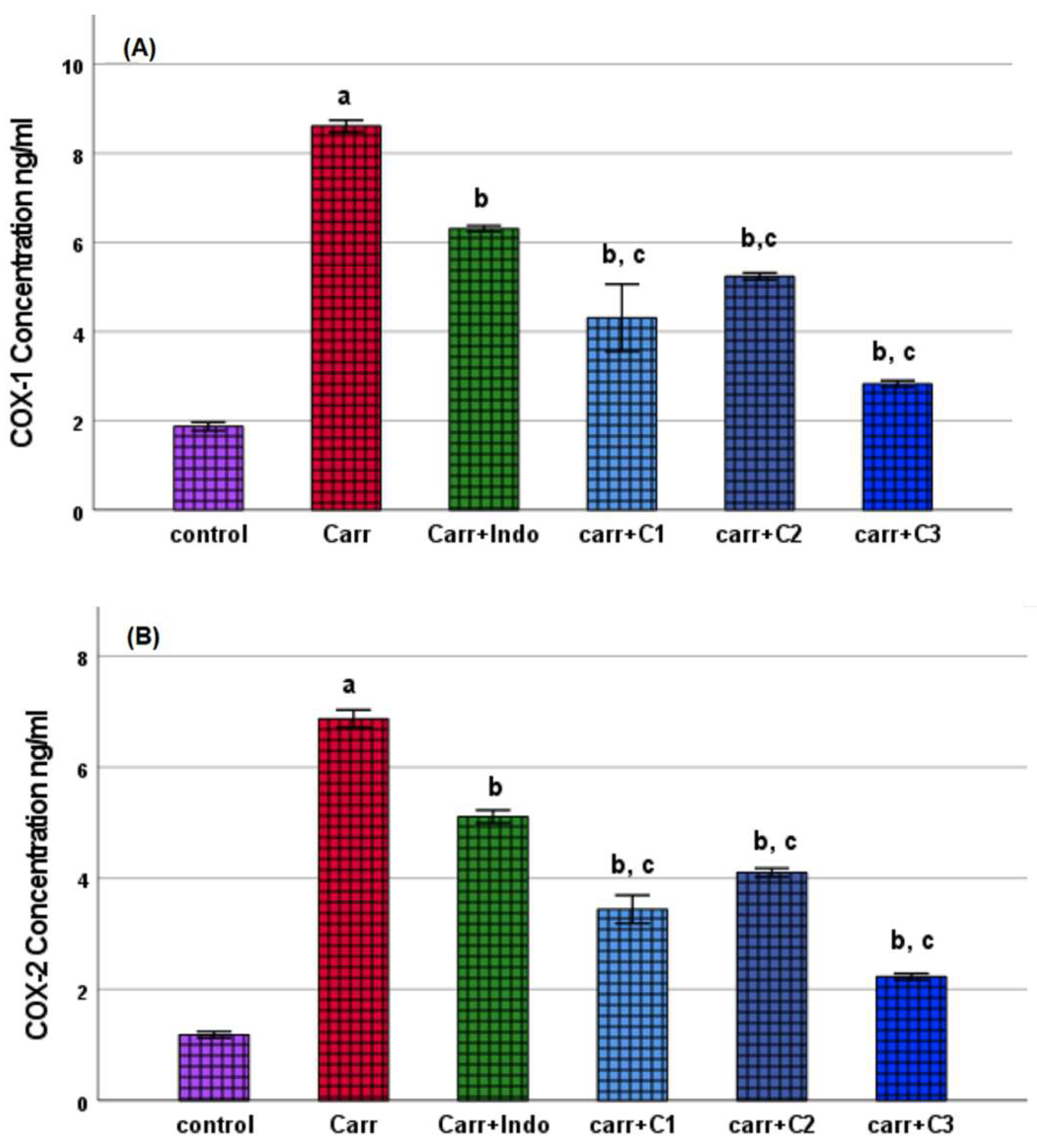
COX Inhibition Assay
Assessment of Therapeutic Index and COX Inhibitory Profiles
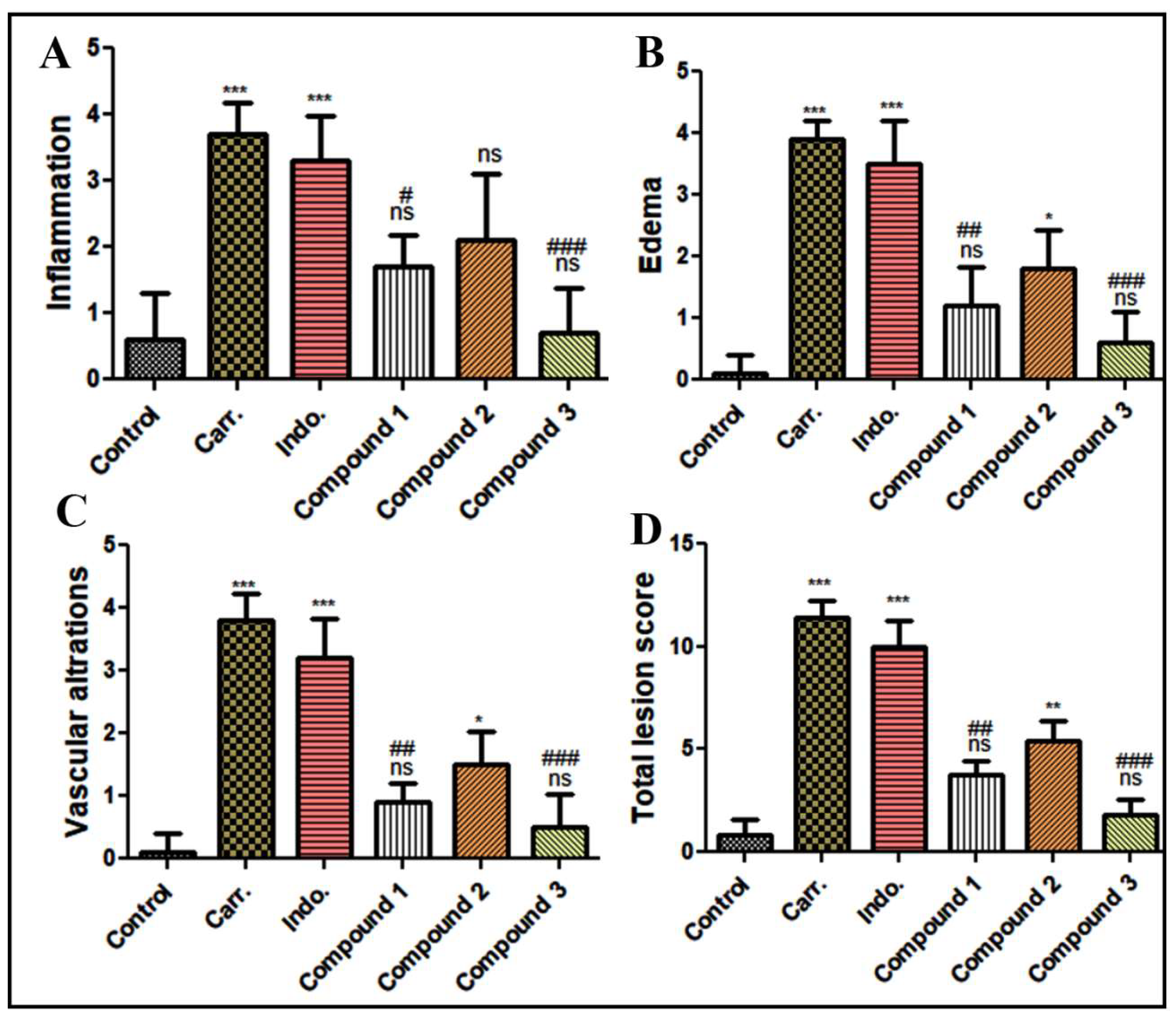
Histopathological Assessment of Paw Tissue
Histomorphometry Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSAIDs | non-steroidal anti-inflammatory drugs |
| PBS | phosphate-buffered saline |
| OECD | Organization for Economic Co-operation and Development |
| ED 50 | median effective dose |
| LD 50 | median lethal dose |
| INF- γ | Interferon γ |
| NF-κB | nuclear factor kappa B |
| MAPKs | mitogen-activated protein kinases |
| COX | cyclooxygenase |
| RBC | red blood cell |
| BSA | bovine serum albumin |
| Indo | indomethacin |
| Carr | carrageenan |
| C1, C2, C3 | synthesized compounds 1–3 |
| C.F. | chemical formula |
| Mp. | melting point |
| M.W | molecular weight |
References
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Busquets-Cortés, C.; Capó, X.; Tejada, S.; Tur, J.A.; Pons, A.; Sureda, A. Cyclooxygenase-2 inhibitors as a therapeutic target in inflammatory diseases. Curr. Med. Chem. 2019, 26, 3225–3241. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Derry, S.; Phillips, C.J.; McQuay, H.J. Nonsteroidal anti-inflammatory drugs (NSAIDs), cyxlooxygenase-2 selective inhibitors (coxibs) and gastrointestinal harm: Review of clinical trials and clinical practice. BMC Musculoskelet. Disord. 2006, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zarghi, A.; Arfaei, S. Selective COX-2 inhibitors: A review of their structure-activity relationships. Iran. J. Pharm. Res. IJPR 2011, 10, 655. [Google Scholar] [PubMed]
- Dogné, J.-M.; Supuran, C.T.; Pratico, D. Adverse cardiovascular effects of the coxibs. J. Med. Chem. 2005, 48, 2251–2257. [Google Scholar] [CrossRef]
- Ankali, K.N.; Rangaswamy, J.; Shalavadi, M.; Naik, N.; naik Krishnamurthy, G. Synthesis and molecular docking of novel 1, 3-thiazole derived 1, 2, 3-triazoles and in vivo biological evaluation for their anti anxiety and anti inflammatory activity. J. Mol. Struct. 2021, 1236, 130357. [Google Scholar] [CrossRef]
- Banerjee, A.; Hung, C.-H.D.; Lo, K.L. An anatomy of credit risk transfer between sovereign and financials in the Eurozone crisis. J. Int. Financ. Mark. Inst. Money 2016, 41, 102–120. [Google Scholar] [CrossRef][Green Version]
- Mohamed, L.W.; El-Badry, O.M.; El-Ansary, A.K.; Ismael, A. Design & synthesis of novel oxazolone & triazinone derivatives and their biological evaluation as COX-2 inhibitors. Bioorganic Chem. 2017, 72, 308–314. [Google Scholar] [CrossRef]
- Asadi, P.; Alvani, M.; Hajhashemi, V.; Rostami, M.; Khodarahmi, G. Design, synthesis, biological evaluation, and molecular docking study on triazine based derivatives as anti-inflammatory agents. J. Mol. Struct. 2021, 1243, 130760. [Google Scholar] [CrossRef]
- Yang, X.; Michiels, T.J.; de Jong, C.; Soethoudt, M.; Dekker, N.; Gordon, E.; van der Stelt, M.; Heitman, L.H.; van der Es, D.; IJzerman, A.P. An affinity-based probe for the human adenosine A2A receptor. J. Med. Chem. 2018, 61, 7892–7901. [Google Scholar] [CrossRef]
- Jörg, M.; May, L.T.; Mak, F.S.; Lee, K.C.K.; Miller, N.D.; Scammells, P.J.; Capuano, B. Synthesis and pharmacological evaluation of dual acting ligands targeting the adenosine A2A and dopamine D2 receptors for the potential treatment of parkinson’s disease. J. Med. Chem. 2015, 58, 718–738. [Google Scholar] [CrossRef] [PubMed]
- Marín-Ocampo, L.; Veloza, L.A.; Abonia, R.; Sepúlveda-Arias, J.C. Anti-inflammatory activity of triazine derivatives: A systematic review. Eur. J. Med. Chem. 2019, 162, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Zacharie, B.; Abbott, S.D.; Duceppe, J.S.; Gagnon, L.; Grouix, B.; Geerts, L.; Gervais, L.; Sarra-Bournet, F.; Perron, V.; Wilb, N.; et al. Design and Synthesis of New 1, 3, 5-Trisubstituted Triazines for the Treatment of Cancer and Inflammation. ChemistryOpen 2018, 7, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Dinari, M.; Mokhtari, N.; Taymouri, S.; Arshadi, M.; Abbaspourrad, A. Covalent polybenzimidazole-based triazine frameworks: A robust carrier for non-steroidal anti-inflammatory drugs. Mater. Sci. Eng. C 2020, 108, 110482. [Google Scholar] [CrossRef]
- Vadivu, R.; Lakshmi, K. In vitro and in vivo anti-inflammatory activity of leaves of Symplocos cochinchinensis (Lour) Moore ssp Laurina. Bangladesh J. Pharmacol. 2008, 3, 121–124. [Google Scholar] [CrossRef]
- Yasmine, Y.; JEBA, C. In vitro cytotoxicity activity of hydroalcoholic extract of polyherbal churnam (COFRI) in normal liver and kidney cell lines. Asian J. Pharm. Clin. Res. 2019, 12, 74–77. [Google Scholar] [CrossRef]
- El-Dershaby, N.H.; El-Hawash, S.A.; Kassab, S.E.; Daabees, H.G.; Abdel Moneim, A.E.; El-Miligy, M.M. Rational design and synthesis of new selective cox-2 inhibitors with in vivo pge2-lowering activity by tethering benzenesulfonamide and 1, 2, 3-triazole pharmacophores to some NSAIDs. Pharmaceuticals 2022, 15, 1165. [Google Scholar] [CrossRef]
- Elkanzi, N.A.; Kadry, A.M.; Ryad, R.M.; Bakr, R.B.; Ali El-Remaily, M.A.E.A.A.; Ali, A.M. Efficient and recoverable bio-organic catalyst cysteine for synthesis, docking study, and antifungal activity of new bio-active 3, 4-dihydropyrimidin-2 (1 H)-ones/thiones under microwave irradiation. ACS Omega 2022, 7, 22839–22849. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Kadry, A.M.; Bekhit, S.A.; Abourehab, M.A.; Amagase, K.; Ibrahim, T.M.; El-Saghier, A.M.; Bekhit, A.A. Spiro heterocycles bearing piperidine moiety as potential scaffold for antileishmanial activity: Synthesis, biological evaluation, and in silico studies. J. Enzym. Inhib. Med. Chem. 2023, 38, 330–342. [Google Scholar] [CrossRef]
- Abd Allah, O.A.; El-Saghier, A.M.; Kadry, A.M. Synthesis, structural stability calculation, and antibacterial evaluation of novel 3, 5-diphenylcyclohex-2-en-1-one derivatives. Synth. Commun. 2015, 45, 944–957. [Google Scholar] [CrossRef]
- Toma, A.; Mogoşan, C.; Vlase, L.; Leonte, D.; Zaharia, V. Heterocycles 39. Synthesis, characterization and evaluation of the anti-inflammatory activity of thiazolo [3, 2-b][1, 2, 4] triazole derivatives bearing pyridin-3/4-yl moiety. Med. Chem. Res. 2017, 26, 2602–2613. [Google Scholar] [CrossRef]
- El-Saghier, A.M.; Mohamed, M.A.; Abd-Allah, O.A.; Kadry, A.M.; Ibrahim, T.M.; Bekhit, A.A. Green synthesis, antileishmanial activity evaluation, and in silico studies of new amino acid-coupled 1, 2, 4-triazoles. Med. Chem. Res. 2019, 28, 169–181. [Google Scholar] [CrossRef]
- El-Saghier, A.M.; Enaili, S.S.; Abdou, A.; Hamed, A.M.; Kadry, A.M. An operationally simple, one-pot, convenient synthesis, and in vitro anti-inflammatory activity of some new spirotriazolotriazine derivatives. J. Heterocycl. Chem. 2024, 61, 146–162. [Google Scholar] [CrossRef]
- Saleem, A.; Saleem, M.; Akhtar, M.F. Antioxidant, anti-inflammatory and antiarthritic potential of Moringa oleifera Lam: An ethnomedicinal plant of Moringaceae family. S. Afr. J. Bot. 2020, 128, 246–256. [Google Scholar] [CrossRef]
- Patel, S.; Zaveri, M. Trypsin and protein denaturation inhibitory activity of different fractionation and isolated compound of leaf and root of Justicia Gendarussa. Int. J. Pharm. Sci. Res. 2014, 5, 5564–5571. [Google Scholar]
- Pavithra, T.; Smitha, K.; Kulashekar, K.; Kumar, B.A. Evaluation of in vitro anti-arthritic activity of Vitex negundo against the denaturation of protein. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 87–90. [Google Scholar]
- Jo, W.S.; Yang, K.M.; Choi, Y.J.; Jeong, C.H.; Ahn, K.J.; Nam, B.H.; Lee, S.W.; Seo, S.Y.; Jeong, M.H. In vitro and in vivo anti-inflammatory effects of pegmatite. Mol. Cell. Toxicol. 2010, 6, 195–202. [Google Scholar] [CrossRef]
- Adnan, A.Z.; Armin, F.; Sudji, I.R.; Novida, M.; Roesma, D.I.; Ali, H.A.; Fauzana, A. In vitro anti-inflammatory activity test of tinocrisposide and freeze-dried aqueous extract of Tinospora crispa stems on human red blood cell by increasing membrane stability experiment. In Vitro 2019, 12, 125–129. [Google Scholar]
- Aidoo, D.B.; Konja, D.; Henneh, I.T.; Ekor, M. Protective effect of bergapten against human erythrocyte hemolysis and protein denaturation in vitro. Int. J. Inflamm. 2021, 2021, 1279359. [Google Scholar] [CrossRef]
- Shinde, U.; Phadke, A.; Nair, A.; Mungantiwar, A.; Dikshit, V.; Saraf, M. Membrane stabilizing activity—A possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia 1999, 70, 251–257. [Google Scholar] [CrossRef]
- Bahuguna, A.; Khan, I.; Bajpai, V.K.; Kang, S.C. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J. Pharmacol. 2017, 12, 8. [Google Scholar] [CrossRef]
- Kim, K.H.; Im, H.-W.; Karmacharya, M.B.; Kim, S.; Min, B.-H.; Park, S.R.; Choi, B.H. Low-intensity ultrasound attenuates paw edema formation and decreases vascular permeability induced by carrageenan injection in rats. J. Inflamm. 2020, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Mogilski, S.; Kubacka, M.; Łażewska, D.; Więcek, M.; Głuch-Lutwin, M.; Tyszka-Czochara, M.; Bukowska-Strakova, K.; Filipek, B.; Kieć-Kononowicz, K. Aryl-1, 3, 5-triazine ligands of histamine H 4 receptor attenuate inflammatory and nociceptive response to carrageen, zymosan and lipopolysaccharide. Inflamm. Res. 2017, 66, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Bachar, S.C.; Rahmatullah, M. Analgesic and antiinflammatory activity of methanolic extract of Acalypha indica Linn. Pak. J. Pharm. Sci. 2010, 23, 256–258. [Google Scholar] [PubMed]
- Elkhateeb, O.; Badawy, M.E.; Tohamy, H.G.; Abou-Ahmed, H.; El-Kammar, M.; Elkhenany, H. Curcumin-infused nanostructured lipid carriers: A promising strategy for enhancing skin regeneration and combating microbial infection. BMC Vet. Res. 2023, 19, 206. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Andersen, K.-J.; Schjønsby, H.; Skagen, D. Enzyme activities in human and rat jejunal mucosa. Scand. J. Gastroenterol. 1983, 18, 241–249. [Google Scholar] [CrossRef]
- Al-Farhan, B.S.; Basha, M.T.; Abdel Rahman, L.H.; El-Saghier, A.M.; Abou El-Ezz, D.; Marzouk, A.A.; Shehata, M.R.; Abdalla, E.M. Synthesis, dft calculations, antiproliferative, bactericidal activity and molecular docking of novel mixed-ligand salen/8-hydroxyquinoline metal complexes. Molecules 2021, 26, 4725. [Google Scholar] [CrossRef]
- Hussein, A.H.M.; El-Adasy, A.-B.A.; El-Saghier, A.M.; Olish, M.; Abdelmonsef, A.H. Synthesis, characterization, in silico molecular docking, and antibacterial activities of some new nitrogen-heterocyclic analogues based on ap-phenolic unit. RSC Adv. 2022, 12, 12607–12621. [Google Scholar] [CrossRef]
- Abdelhamid, A.A.; Aref, S.A.; Ahmed, N.A.; Elsaghier, A.M.; Abd El Latif, F.M.; Al-Ghamdi, S.N.; Gad, M.A. Design, synthesis, and toxicological activities of novel insect growth regulators as insecticidal agents against Spodoptera littoralis (Boisd.). ACS Omega 2022, 8, 709–717. [Google Scholar] [CrossRef]
- El-Saghier, A.; Abd El-Halim, H.; Abdel-Rahman, L.H.; Kadry, A. Green synthesis of new trizole based heterocyclic amino acids ligands and their transition metal complexes. Characterization, kinetics, antimicrobial and docking studies. Appl. Organomet. Chem. 2019, 33, e4641. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Jahanban-Esfahlan, R.; Roufegarinejad, L.; Tabibiazar, M.; Amarowicz, R. Recent developments in the detection of bovine serum albumin. Int. J. Biol. Macromol. 2019, 138, 602–617. [Google Scholar] [CrossRef]
- Kiranmayi, G.; Anusha, V.; Chandrika, Y.; Satya Priya, I.V.; Swetha, S.; Krishn, Y.V. Preliminary phytochemical screening and in vitro evaluation of anti-inflammatory, antiarthritic, and thrombolytic activities of ethanolic leaf extract of Bauhinia purpurea. Int. J. Green Pharm. 2018, 12, 241–247. [Google Scholar]
- Arida, A.; Protogerou, A.D.; Kitas, G.D.; Sfikakis, P.P. Systemic inflammatory response and atherosclerosis: The paradigm of chronic inflammatory rheumatic diseases. Int. J. Mol. Sci. 2018, 19, 1890. [Google Scholar] [CrossRef] [PubMed]
- Shilpa, K.; Chacko, N.; Shetty, P. Investigation of anti-arthritic activity (in-vitro models) of Hibiscus hispidissimus Griffith. J. Phytopharm. 2018, 7, 60–65. [Google Scholar] [CrossRef]
- Deshpande, V.D.; Jadhav, V.; Kadam, V. In-vitro anti-arthritic activity of Abutilon indicum (Linn.) sweet. J. Pharm. Res. 2009, 2, 644–645. [Google Scholar]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Falzon, M.; Nielsch, A.; Burke, M.D. Denaturation of cytochrome P-450 by indomethacin and other non-steroidal antiinflammatory drugs: Evidence for a surfactant mechanism and a selective effect of a p-chlorophenyl moiety. Biochem. Pharmacol. 1986, 35, 4019–4024. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Raza, S.A.; Saleem, A.; Hamid, I.; Ashraf Baig, M.M.F.; Sharif, A.; Sohail, K.; Javaid, Z.; Saleem, U.; Rasul, A. Appraisal of anti-arthritic and anti-inflammatory potential of folkloric medicinal plant Peganum harmala. Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2022, 22, 49–63. [Google Scholar] [CrossRef]
- Esho, B.A.; Samuel, B.; Akinwunmi, K.F.; Oluyemi, W.M. Membrane stabilization and inhibition of protein denaturation as mechanisms of the anti-inflammatory activity of some plant species. Trends Pharm. Sci. 2021, 7, 269–278. [Google Scholar]
- Sabalingam, S. In-vitro approaches to evaluate the anti-inflammatory potential of phytochemicals: A Review. J. Drug Deliv. Ther. 2025, 15, 187–192. [Google Scholar] [CrossRef]
- Chahardoli, A.; Qalekhani, F.; Hajmomeni, P.; Shokoohinia, Y.; Fattahi, A. Enhanced hemocompatibility, antimicrobial and anti-inflammatory properties of biomolecules stabilized AgNPs with cytotoxic effects on cancer cells. Sci. Rep. 2025, 15, 1186. [Google Scholar] [CrossRef]
- Kulesza, A.; Paczek, L.; Burdzinska, A. The role of COX-2 and PGE2 in the regulation of immunomodulation and other functions of mesenchymal stromal cells. Biomedicines 2023, 11, 445. [Google Scholar] [CrossRef]
- Ballerini, P.; Contursi, A.; Bruno, A.; Mucci, M.; Tacconelli, S.; Patrignani, P. Inflammation and cancer: From the development of personalized indicators to novel therapeutic strategies. Front. Pharmacol. 2022, 13, 838079. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, D.; Kumar, M.; Proestos, C.; Oz, F.; Gupta, P.; Kumar, A.; Kundu, P.; Kaur, J.; Maurya, V.K.; Anjali, A.; et al. A narrative review on the anti-inflammatory efficacy of Bougainvillea spectabilis Willd. and its various applications. J. Agric. Food Res. 2023, 12, 100570. [Google Scholar] [CrossRef]
- Muller, P.Y.; Milton, M.N. The determination and interpretation of the therapeutic index in drug development. Nat. Rev. Drug Discov. 2012, 11, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, J.; Liu, X.; Chen, X.; Wang, J. Decoding nature: Multi-target anti- inflammatory mechanisms of natural products in the TLR4/NF-κB pathway. Front. Pharmacol. 2025, 15, 1467193. [Google Scholar] [CrossRef]
- El-Dershaby, N.H.; El-Hawash, S.A.; Kassab, S.E.; Dabees, H.G.; Abdel Moneim, A.E.; Abdel Wahab, I.A.; Abd-Alhaseeb, M.M.; El-Miligy, M.M. Rational design of biodegradable sulphonamide candidates treating septicaemia by synergistic dual inhibition of COX-2/PGE2 axis and DHPS enzyme. J. Enzym. Inhib. Med. Chem. 2022, 37, 1737–1751. [Google Scholar] [CrossRef]
- Carroll, P.; Kellow, A. The OECD: A Decade of Transformation: 2011–2021; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2021. [Google Scholar]
- Martínez-Rizo, A.B.; Fosado-Rodríguez, R.; Torres-Romero, J.C.; Lara-Riegos, J.C.; Ramírez-Camacho, M.A.; Herrera, A.L.A.; de la Torre, F.E.V.; Góngora, E.C.; Arana-Argáez, V.E. Models in vivo and in vitro for the study of acute and chronic inflammatory activity: A comprehensive review. Int. Immunopharmacol. 2024, 135, 112292. [Google Scholar] [CrossRef]
- Calimag, K.P.D.; Arbis, C.C.H.; Collantes, T.M.A.; Bariuan, J.V.; Ang, M.J.C.; Cervancia, C.A.; Desamero, M.J.M.; Estacio, M.A.C. Attenuation of carrageenan-induced hind paw edema and plasma TNF-α level by Philippine stingless bee (Tetragonula biroi Friese) propolis. Exp. Anim. 2021, 70, 185–193. [Google Scholar] [CrossRef]
- Haftcheshmeh, S.M.; Abedi, M.; Mashayekhi, K.; Mousavi, M.J.; Navashenaq, J.G.; Mohammadi, A.; Momtazi-Borojeni, A.A. Berberine as a natural modulator of inflammatory signaling pathways in the immune system: Focus on NF-κB, JAK/STAT, and MAPK signaling pathways. Phytother. Res. 2022, 36, 1216–1230. [Google Scholar] [CrossRef]
- Yang, L.; Liu, R.; Fang, Y.; He, J. Anti-inflammatory effect of phenylpropanoids from Dendropanax dentiger in TNF-α-induced MH7A cells via inhibition of NF-κB, Akt and JNK signaling pathways. Int. Immunopharmacol. 2021, 94, 107463. [Google Scholar] [CrossRef]
- Es-Sai, B.; Wahnou, H.; Benayad, S.; Rabbaa, S.; Laaziouez, Y.; El Kebbaj, R.; Limami, Y.; Duval, R.E. Gamma-Tocopherol: A Comprehensive Review of Its Antioxidant, Anti-Inflammatory, and Anticancer Properties. Molecules 2025, 30, 653. [Google Scholar] [CrossRef]
- Fatima, H.; Shahid, M.; Jamil, A.; Naveed, M. Therapeutic potential of selected medicinal plants against carrageenan induced inflammation in rats. Dose-Response 2021, 19, 15593258211058028. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Heilmann, R.M.; Paital, B.; Patel, A.; Yadav, V.K.; Wong, D.; Jergens, A.E. Oxidative stress, hormones, and effects of natural antioxidants on intestinal inflammation in inflammatory bowel disease. Front. Endocrinol. 2023, 14, 1217165. [Google Scholar] [CrossRef]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The interplay between cytokines, inflammation, and antioxidants: Mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef]








| Observation | Control | Compound 1 | Compound 2 | Compound 3 |
|---|---|---|---|---|
| Change in skin color | Normal | Normal | Normal | Normal |
| Diarrhea | Absent | Present | Present | Present |
| Seizures | Absent | Absent | Absent | Absent |
| Micturition | Normal | Normal | Normal | Normal |
| Drowsiness | Absent | Absent | Absent | Absent |
| Dyspnea | Absent | Absent | Absent | Absent |
| Weight loss | No | No | No | No |
| Ability to feed | Normal | Normal | Normal | Normal |
| Death | No | No | No | No |
| Groups/ Parameters | CRP (ng/mL) | IL-1α (pg/mL) | IL-1β (pg/mL) | IL-6 (pg/mL) | TNF-α (pg/mL) |
|---|---|---|---|---|---|
| Control | 1.70 ± 0.05 | 39.41 ± 0.88 | 53.25 ± 0.58 | 10.54 ± 0.24 | 6.49 ± 0.31 |
| Carr | 10.04 ± 0.70 a | 105.72 ± 0.73 a | 150.16 ± 0.60 a | 21.08 ± 0.51 a | 13.90 ± 0.34 a |
| Carr + Indo | 6.23 ± 0.22 b | 79.28 ± 0.55 b | 92.86 ± 0.47 b | 18.49 ± 0.27 b | 11.30 ± 0.30 b |
| Carr + C1 | 4.19 ± 0.28 b | 56.97 ± 0.79 b | 64.98 ± 0.30 b | 14.44 ± 0.26 b | 8.27 ± 0.19 b |
| Carr + C2 | 3.02 ± 0.28 b | 62.82 ± 0.94 b | 77.98 ± 0.45 b | 16.14 ± 0.16 b | 9.38 ± 0.20 b |
| Carr + C3 | 2.20 ± 0.36 b | 40.66 ± 0.43 b | 60.38 ± 0.73 b | 11.39 ± 0.23 b | 7.16 ± 0.13 b |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Compound | IC50 (µg/mL) | COX-2 Inhibition % | COX-1 Inhibition % | COX-2 Inhibition Effective Dose | TI (IC50/COX-2 Effective Dose) | Interpretation |
|---|---|---|---|---|---|---|
| 1 | 362.78 | 98% | 70% | 49 | ~7.4 | Safest profile. Strong efficacy with minimal cytotoxic risk. Suitable for further development. |
| 2 | 327.88 | 98% | 69% | 49 | ~6.6 | Very good safety margin. Slightly lower than C1 but still acceptable. Promising candidate. |
| 3 | 148.13 | 99% | 70% | 49.5 | ~2.9 | Narrow safety margin. Most efficacious but also most cytotoxic. Further optimization or dose control is needed. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamed, A.M.; Enaili, S.S.; I. Mohammed, W.; Abouelella, A.M.A.; Mohana, Z.E.E.; Monir, D.M.; Soliman, S.S.; Hamouda, E.E.M.; Elatif, H.M.A.; El-Saghier, A.M. Novel Benzenesulfonamide Derivatives of 5′-Aminospirotriazolotriazine Exhibit Anti-Inflammatory Activity by Suppressing Pro-Inflammatory Mediators: In Vitro and In Vivo Evaluation Using a Rat Model of Carrageenan-Induced Paw Edema. Biomedicines 2025, 13, 1732. https://doi.org/10.3390/biomedicines13071732
Hamed AM, Enaili SS, I. Mohammed W, Abouelella AMA, Mohana ZEE, Monir DM, Soliman SS, Hamouda EEM, Elatif HMA, El-Saghier AM. Novel Benzenesulfonamide Derivatives of 5′-Aminospirotriazolotriazine Exhibit Anti-Inflammatory Activity by Suppressing Pro-Inflammatory Mediators: In Vitro and In Vivo Evaluation Using a Rat Model of Carrageenan-Induced Paw Edema. Biomedicines. 2025; 13(7):1732. https://doi.org/10.3390/biomedicines13071732
Chicago/Turabian StyleHamed, Amany M., Souhaila S. Enaili, Walaa I. Mohammed, Azza M. A. Abouelella, Zeyad Elsayed Eldeeb Mohana, Dina M. Monir, Safaa S. Soliman, Elsayed Eldeeb Mehana Hamouda, Hytham Mahmoud Abd Elatif, and Ahmed M. El-Saghier. 2025. "Novel Benzenesulfonamide Derivatives of 5′-Aminospirotriazolotriazine Exhibit Anti-Inflammatory Activity by Suppressing Pro-Inflammatory Mediators: In Vitro and In Vivo Evaluation Using a Rat Model of Carrageenan-Induced Paw Edema" Biomedicines 13, no. 7: 1732. https://doi.org/10.3390/biomedicines13071732
APA StyleHamed, A. M., Enaili, S. S., I. Mohammed, W., Abouelella, A. M. A., Mohana, Z. E. E., Monir, D. M., Soliman, S. S., Hamouda, E. E. M., Elatif, H. M. A., & El-Saghier, A. M. (2025). Novel Benzenesulfonamide Derivatives of 5′-Aminospirotriazolotriazine Exhibit Anti-Inflammatory Activity by Suppressing Pro-Inflammatory Mediators: In Vitro and In Vivo Evaluation Using a Rat Model of Carrageenan-Induced Paw Edema. Biomedicines, 13(7), 1732. https://doi.org/10.3390/biomedicines13071732







