Independent Effects of Hypothyroidism and Obesity on Endometrial Cancer Risk Revealed by Mendelian Randomisation
Abstract
1. Introduction
2. Materials and Methods
2.1. Genome-Wide Association Study (GWAS) Summary Statistics
2.2. IV Selection
2.3. Mendelian Randomisation Analysis
2.4. Mendelian Randomisation Sensitivity Analyses
2.5. Multivariable Mendelian Randomisation Analysis
3. Results
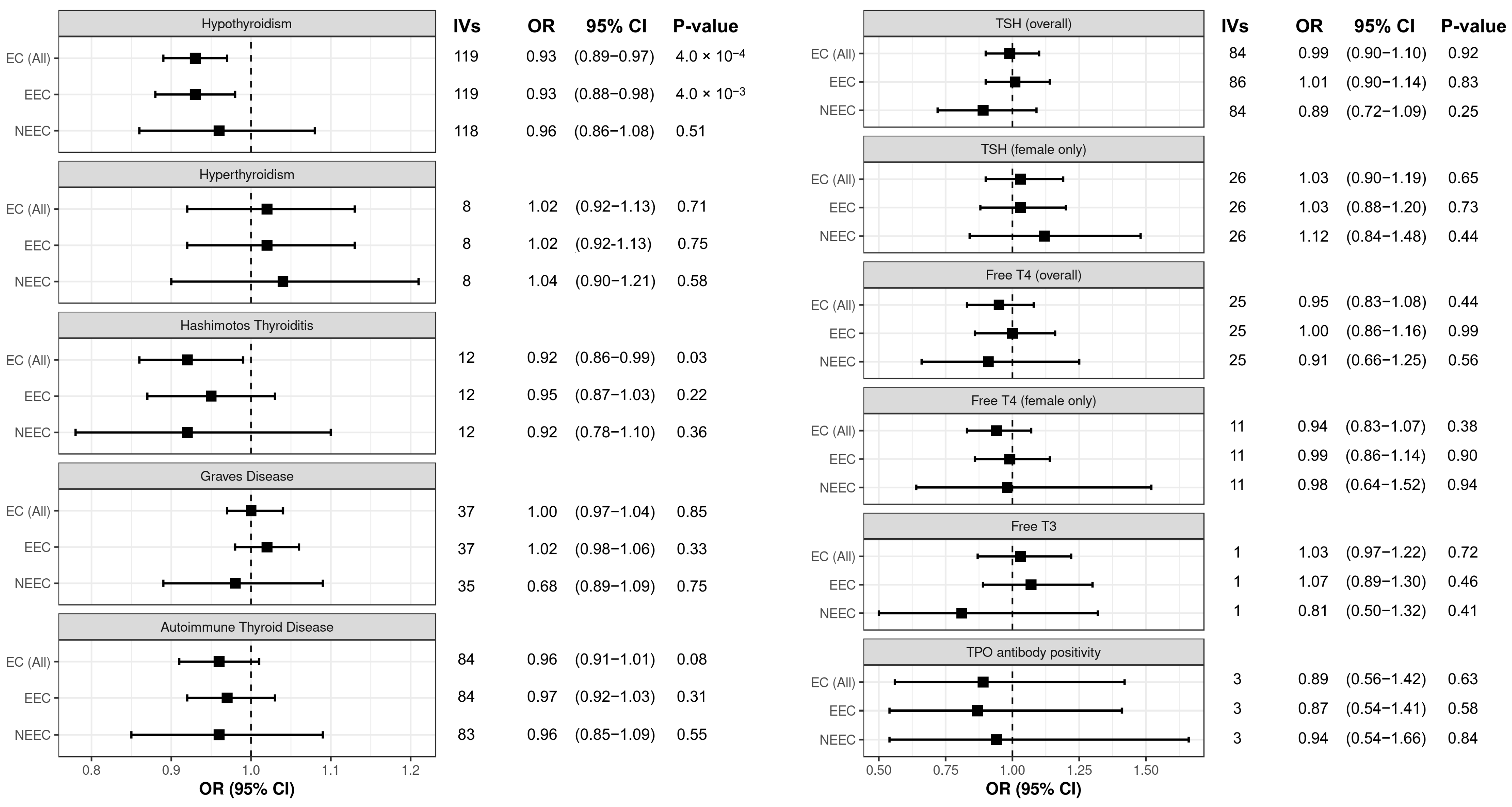
3.1. Hypothyroidism Is Causally Associated with Decreased Endometrial Cancer Risk
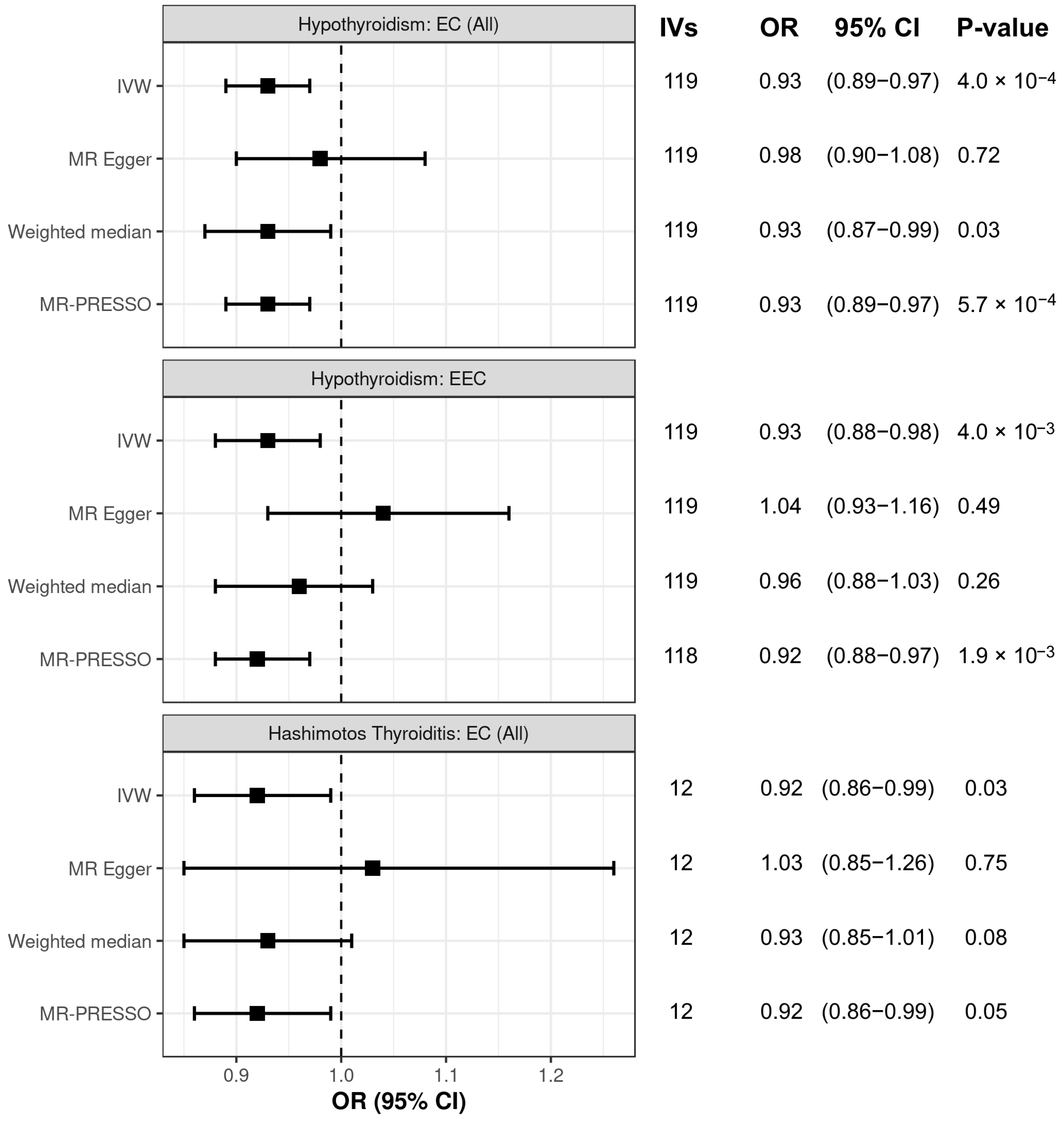
3.2. Sensitivity Analysis of Associations Between Thyroid Dysfunction and Endometrial Cancer Risk
3.3. BMI and Hypothyroidism Are Independently Associated with Endometrial Cancer Risk
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CI | Confidence interval |
| EEC | Endometrioid endometrial cancer |
| EC | Endometrial cancer |
| GWAS | Genome-wide association study |
| IV | Instrumental variable |
| IVW | Inverse-variance weighted |
| NEEC | Non-endometrioid endometrial cancer |
| OR | Odds ration |
| SLE | Systemic lupus erythematosus |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| TPO | Thyroid peroxidase |
| TSH | Thyroid stimulating hormone |
References
- Koskas, M.; Amant, F.; Mirza, M.R.; Creutzberg, C.L. Cancer of the corpus uteri: 2021 update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. S1), 45–60. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Glubb, D.M.; O’Mara, T.A. 10 Years of GWAS discovery in endometrial cancer: Aetiology, function and translation. EBioMedicine 2022, 77, 103895. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Swanson, S.A.; Labrecque, J.A. Are Mendelian randomization investigations immune from bias due to reverse causation? Eur. J. Epidemiol. 2021, 36, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Walczak, K.; Sieminska, L. Obesity and Thyroid Axis. Int. J. Environ. Res. Public Health 2021, 18, 9434. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.F.; Ocarino, N.M.; Serakides, R. Thyroid hormones and female reproduction. Biol. Reprod. 2018, 99, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Brinton, L.A.; Sakoda, L.C.; Frederiksen, K.; Sherman, M.E.; Kjaer, S.K.; Graubard, B.I.; Olsen, J.H.; Mellemkjaer, L. Relationships of uterine and ovarian tumors to pre-existing chronic conditions. Gynecol. Oncol. 2007, 107, 487–494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prinzi, N.; Sorrenti, S.; Baldini, E.; De Vito, C.; Tuccilli, C.; Catania, A.; Coccaro, C.; Bianchini, M.; Nesca, A.; Grani, G.; et al. Association of Thyroid Diseases with Primary Extra-Thyroidal Malignancies in Women: Results of a Cross-Sectional Study of 6,386 Patients. PLoS ONE 2015, 10, e0122958. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, R.; Wang, J. Relationship between Hypothyroidism and Endometrial Cancer. Aging Dis. 2019, 10, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Yurkovetsky, Z.; Ta’asan, S.; Skates, S.; Rand, A.; Lomakin, A.; Linkov, F.; Marrangoni, A.; Velikokhatnaya, L.; Winans, M.; Gorelik, E.; et al. Development of multimarker panel for early detection of endometrial cancer. High diagnostic power of prolactin. Gynecol. Oncol. 2007, 107, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Laurberg, P.; Knudsen, N.; Andersen, S.; Carlé, A.; Pedersen, I.B.; Karmisholt, J. Thyroid Function and Obesity. Eur. Thyroid. J. 2012, 1, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, X.; Han, Y.; Zhang, F.; Lin, Z.; Wang, H.; Teng, W.; Shan, Z. Causal Association Between Serum Thyrotropin and Obesity: A Bidirectional, Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2021, 106, e4251–e4259. [Google Scholar] [CrossRef] [PubMed]
- Song, R.H.; Wang, B.; Yao, Q.M.; Li, Q.; Jia, X.; Zhang, J.A. The Impact of Obesity on Thyroid Autoimmunity and Dysfunction: A Systematic Review and Meta-Analysis. Front. Immunol. 2019, 10, 2349. [Google Scholar] [CrossRef] [PubMed]
- Hyppönen, E.; Mulugeta, A.; Zhou, A.; Santhanakrishnan, V.K. A data-driven approach for studying the role of body mass in multiple diseases: A phenome-wide registry-based case-control study in the UK Biobank. Lancet Digit. Health 2019, 1, e116–e126. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Kar, S.; Vithayathil, M.; Carter, P.; Mason, A.M.; Burgess, S.; Larsson, S.C. Causal associations of thyroid function and dysfunction with overall, breast and thyroid cancer: A two-sample Mendelian randomization study. Int. J. Cancer 2020, 147, 1895–1903. [Google Scholar] [CrossRef] [PubMed]
- O’Mara, T.A.; Glubb, D.M.; Amant, F.; Annibali, D.; Ashton, K.; Attia, J.; Auer, P.L.; Beckmann, M.W.; Black, A.; Bolla, M.K.; et al. Identification of nine new susceptibility loci for endometrial cancer. Nat. Commun. 2018, 9, 3166. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.; Chavez, J.; Canalli, M.H.B.; Zanetti, C.R. Determination of IgG Subclasses and Avidity of Antithyroid Peroxidase Antibodies in Patients with Subclinical Hypothyroidism—A Comparison with Patients with Overt Hypothyroidism. Horm. Res. Paediatr. 2003, 59, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Kho, P.F.; Mortlock, S.; Endometrial Cancer Association Consortium; International Endometriosis Genetics Consortium; Rogers, P.A.W.; Nyholt, D.R.; Montgomery, G.W.; Spurdle, A.B.; Glubb, D.M.; O’Mara, T.A. Genetic analyses of gynecological disease identify genetic relationships between uterine fibroids and endometrial cancer, and a novel endometrial cancer genetic risk region at the WNT4 1p36.12 locus. Hum. Genet. 2021, 140, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.H.; Thompson, D.J.; O’Mara, T.A.; Painter, J.N.; Glubb, D.M.; Flach, S.; Lewis, A.; French, J.D.; Freeman-Mills, L.; Church, D.; et al. Five endometrial cancer risk loci identified through genome-wide association analysis. Nat. Genet. 2016, 48, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Saevarsdottir, S.; Olafsdottir, T.A.; Ivarsdottir, E.V.; Halldorsson, G.H.; Gunnarsdottir, K.; Sigurdsson, A.; Johannesson, A.; Sigurdsson, J.K.; Juliusdottir, T.; Lund, S.H.; et al. FLT3 stop mutation increases FLT3 ligand level and risk of autoimmune thyroid disease. Nature 2020, 584, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, S.; Briend, M.; Abner, E.; Couture, C.; Li, Z.; Bosse, Y.; Theriault, S.; Esko, T.; Arsenault, B.J.; Mathieu, P. Genetic association and Mendelian randomization for hypothyroidism highlight immune molecular mechanisms. iScience 2022, 25, 104992. [Google Scholar] [CrossRef] [PubMed]
- Teumer, A.; Chaker, L.; Groeneweg, S.; Li, Y.; Di Munno, C.; Barbieri, C.; Schultheiss, U.T.; Traglia, M.; Ahluwalia, T.S.; Akiyama, M.; et al. Genome-wide analyses identify a role for SLC17A4 and AADAT in thyroid hormone regulation. Nat. Commun. 2018, 9, 4455. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.D.; Simmonds, M.J.; Walker, N.M.; Burren, O.; Brand, O.J.; Guo, H.; Wallace, C.; Stevens, H.; Coleman, G.; Wellcome Trust Case Control, C.; et al. Seven newly identified loci for autoimmune thyroid disease. Hum. Mol. Genet. 2012, 21, 5202–5208. [Google Scholar] [CrossRef] [PubMed]
- Sakaue, S.; Kanai, M.; Tanigawa, Y.; Karjalainen, J.; Kurki, M.; Koshiba, S.; Narita, A.; Konuma, T.; Yamamoto, K.; Akiyama, M.; et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 2021, 53, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Popović, M.; Matana, A.; Torlak, V.; Boutin, T.; Brdar, D.; Gunjaca, I.; Kalicanin, D.; Kolcic, I.; Boraska Perica, V.; Punda, A.; et al. Genome-wide meta-analysis identifies novel loci associated with free triiodothyronine and thyroid-stimulating hormone. J. Endocrinol. Investig. 2019, 42, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Medici, M.; Porcu, E.; Pistis, G.; Teumer, A.; Brown, S.J.; Jensen, R.A.; Rawal, R.; Roef, G.L.; Plantinga, T.S.; Vermeulen, S.H.; et al. Identification of novel genetic Loci associated with thyroid peroxidase antibodies and clinical thyroid disease. PLoS Genet. 2014, 10, e1004123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Brumpton, B.; Kabil, O.; Gudmundsson, J.; Thorleifsson, G.; Weinstock, J.; Zawistowski, M.; Nielsen, J.B.; Chaker, L.; Medici, M.; et al. GWAS of thyroid stimulating hormone highlights pleiotropic effects and inverse association with thyroid cancer. Nat. Commun. 2020, 11, 3981. [Google Scholar] [CrossRef] [PubMed]
- Pulit, S.L.; Stoneman, C.; Morris, A.P.; Wood, A.R.; Glastonbury, C.A.; Tyrrell, J.; Yengo, L.; Ferreira, T.; Marouli, E.; Ji, Y.; et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 2019, 28, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, N.; Tung, J.Y.; Kiefer, A.K.; Hinds, D.A.; Francke, U.; Mountain, J.L.; Do, C.B. Novel associations for hypothyroidism include known autoimmune risk loci. PLoS ONE 2012, 7, e34442. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.A.; Thompson, J.R. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: The role of the I2 statistic. Int. J. Epidemiol. 2016, 45, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinsky, J.; Bonilla, C.; Haycock, P.C.; Langdon, R.J.Q.; Lotta, L.A.; Langenberg, C.; Relton, C.L.; Lewis, S.J.; Evans, D.M.; Consortium, P.; et al. Circulating Selenium and Prostate Cancer Risk: A Mendelian Randomization Analysis. J. Natl. Cancer Inst. 2018, 110, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Davey Smith, G.; Hemani, G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014, 23, R89–R98. [Google Scholar] [CrossRef] [PubMed]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Timpson, N.J.; Higgins, J.P.T.; Dimou, N.; Langenberg, C.; et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): Explanation and elaboration. BMJ 2021, 375, n2233. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.F.D.; Minelli, C.; Sheehan, N.A.; Thompson, J.R. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat. Med. 2015, 34, 2926–2940. [Google Scholar] [CrossRef] [PubMed]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef] [PubMed]
- Brion, M.J.; Shakhbazov, K.; Visscher, P.M. Calculating statistical power in Mendelian randomization studies. Int. J. Epidemiol. 2013, 42, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Yavorska, O.O.; Burgess, S. MendelianRandomization: An R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 2017, 46, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Mincer, D.L.; Jialal, I. Hashimoto Thyroiditis. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Makker, V.; Rasco, D.; Vogelzang, N.J.; Brose, M.S.; Cohn, A.L.; Mier, J.; Di Simone, C.; Hyman, D.M.; Stepan, D.E.; Dutcus, C.E.; et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: An interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.H.; Lee, C.H.; Makker, V.; Rasco, D.; Dutcus, C.E.; Wu, J.; Stepan, D.E.; Shumaker, R.C.; Motzer, R.J. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients with Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J. Clin. Oncol. 2020, 38, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.; Zhao, W.D.; Tao, J.H. Causal effects of systemic lupus erythematosus on endometrial cancer: A univariable and multivariable Mendelian randomization study. Front. Oncol. 2022, 12, 930243. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yuan, J.; Zhou, H.; Wang, Y.; Tian, G.; Liu, X.; Wang, X.; Tang, M.; Meng, X.; Kou, C.; et al. Association Between Systemic Lupus Erythematosus and Primary Hypothyroidism: Evidence from Complementary Genetic Methods. J. Clin. Endocrinol. Metab. 2023, 108, 941–949. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glubb, D.M.; Wang, X.; O’Mara, T.A. Independent Effects of Hypothyroidism and Obesity on Endometrial Cancer Risk Revealed by Mendelian Randomisation. Biomedicines 2025, 13, 1729. https://doi.org/10.3390/biomedicines13071729
Glubb DM, Wang X, O’Mara TA. Independent Effects of Hypothyroidism and Obesity on Endometrial Cancer Risk Revealed by Mendelian Randomisation. Biomedicines. 2025; 13(7):1729. https://doi.org/10.3390/biomedicines13071729
Chicago/Turabian StyleGlubb, Dylan M., Xuemin Wang, and Tracy A. O’Mara. 2025. "Independent Effects of Hypothyroidism and Obesity on Endometrial Cancer Risk Revealed by Mendelian Randomisation" Biomedicines 13, no. 7: 1729. https://doi.org/10.3390/biomedicines13071729
APA StyleGlubb, D. M., Wang, X., & O’Mara, T. A. (2025). Independent Effects of Hypothyroidism and Obesity on Endometrial Cancer Risk Revealed by Mendelian Randomisation. Biomedicines, 13(7), 1729. https://doi.org/10.3390/biomedicines13071729





