High-Fat-Diet-Induced Metabolic Disorders: An Original Cause for Neurovascular Uncoupling Through the Imbalance of Glutamatergic Pathways
Abstract
1. Introduction
2. Materials and Methods
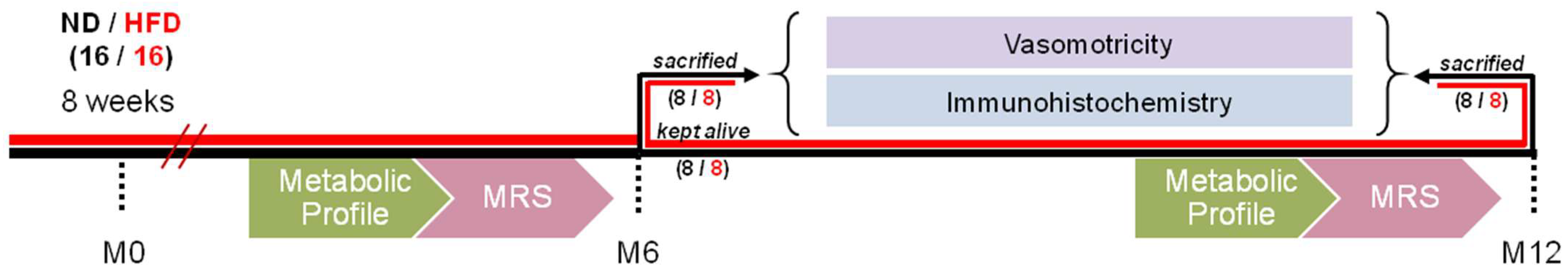
2.1. Animal Feed and Experimental Design
2.2. Metabolic Profile
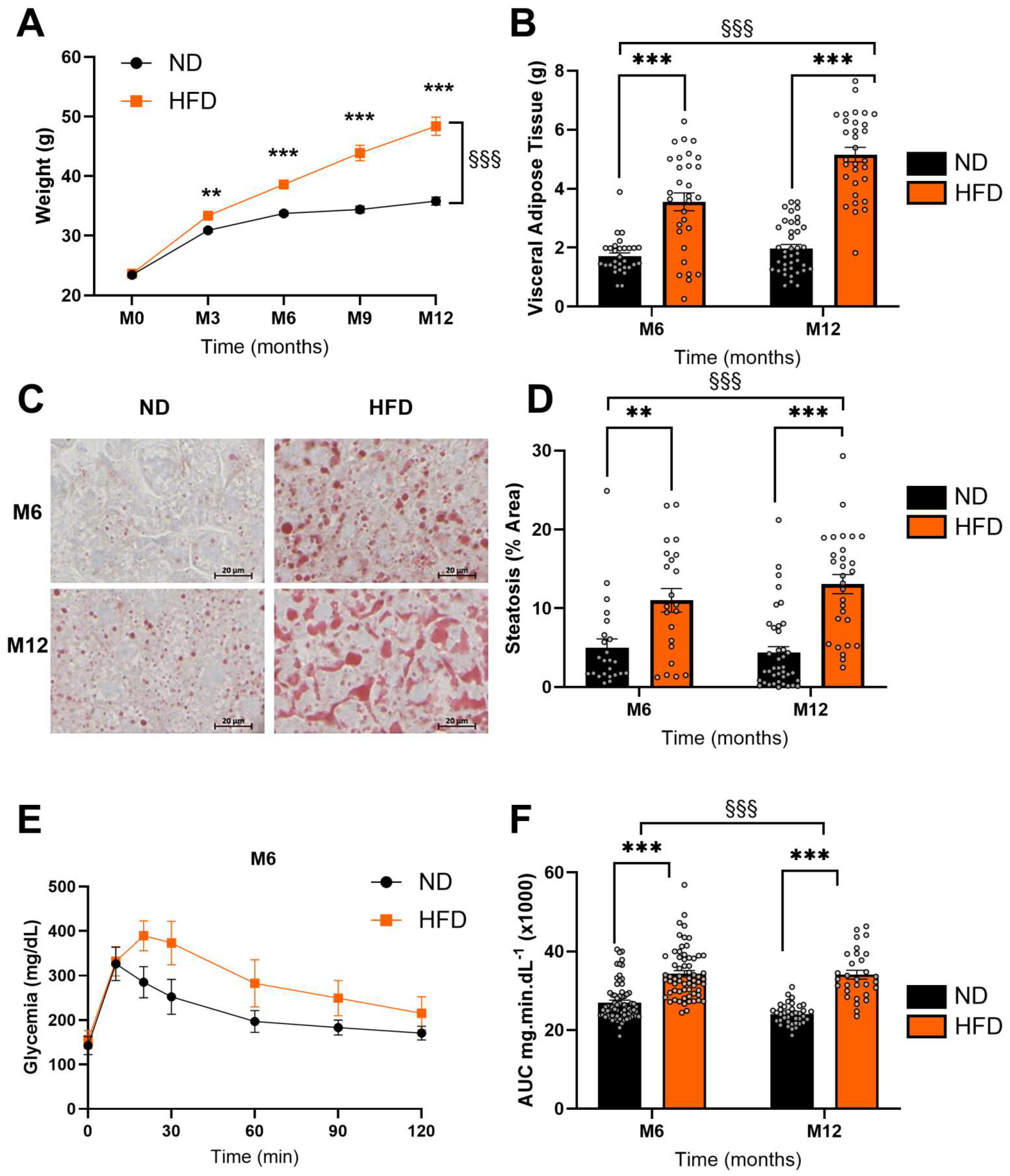
2.2.1. Body Weight
2.2.2. Fasting Blood Glucose and Oral Glucose Tolerance Test (OGTT)
2.2.3. Plasma Metabolic Assays
2.2.4. Hepatic Steatosis
2.2.5. Visceral Adiposity
2.2.6. Blood Pressure
2.3. Vasomotricity Studies
2.3.1. Ex Vivo Analysis of Basilar Artery Reactivity
Preparation
Myogenic Tone
2.3.2. Analysis of the Reactivity of Intraparenchymal Arterioles Ex Vivo
Preparation
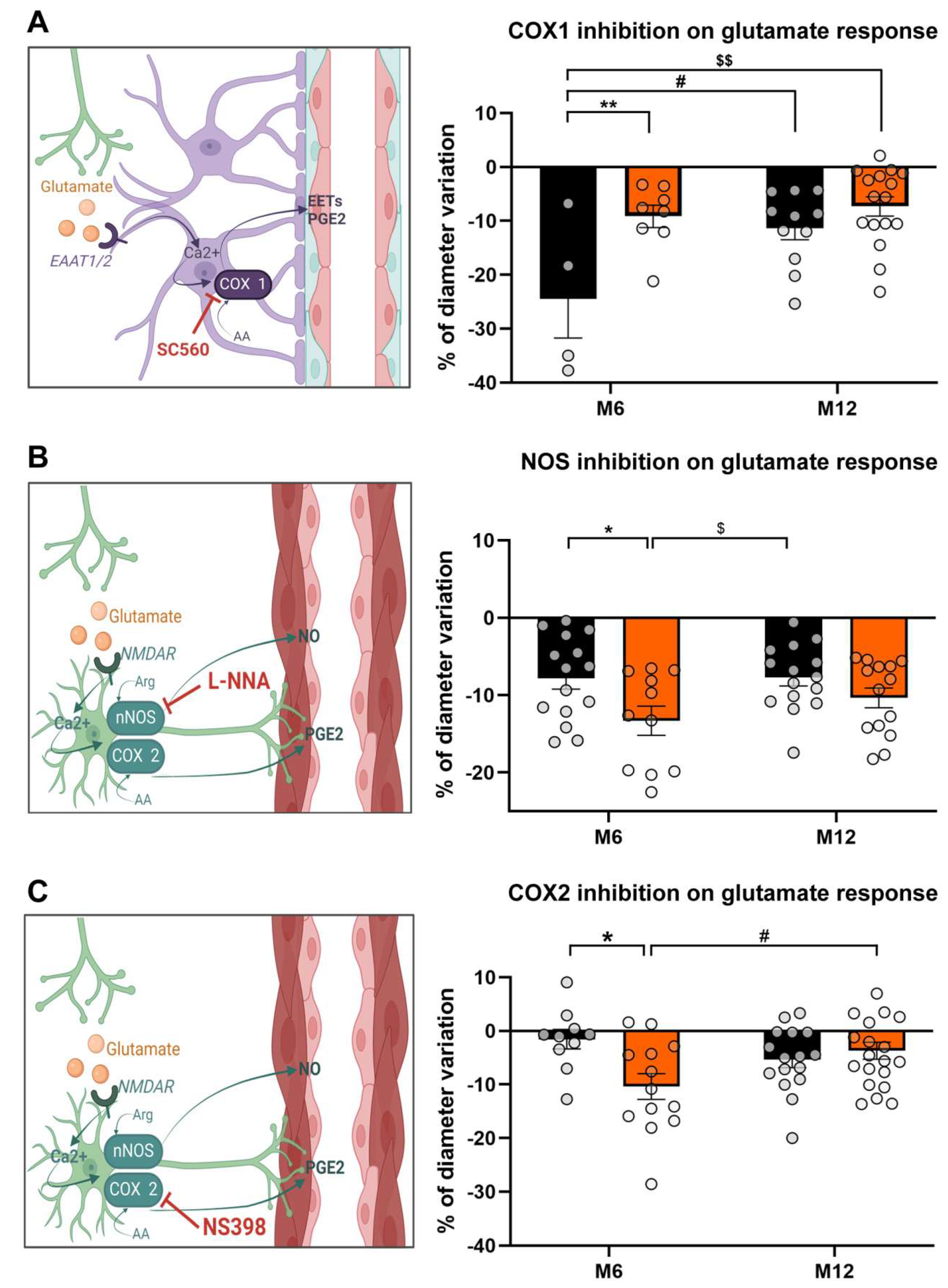
Pharmacological Modulations
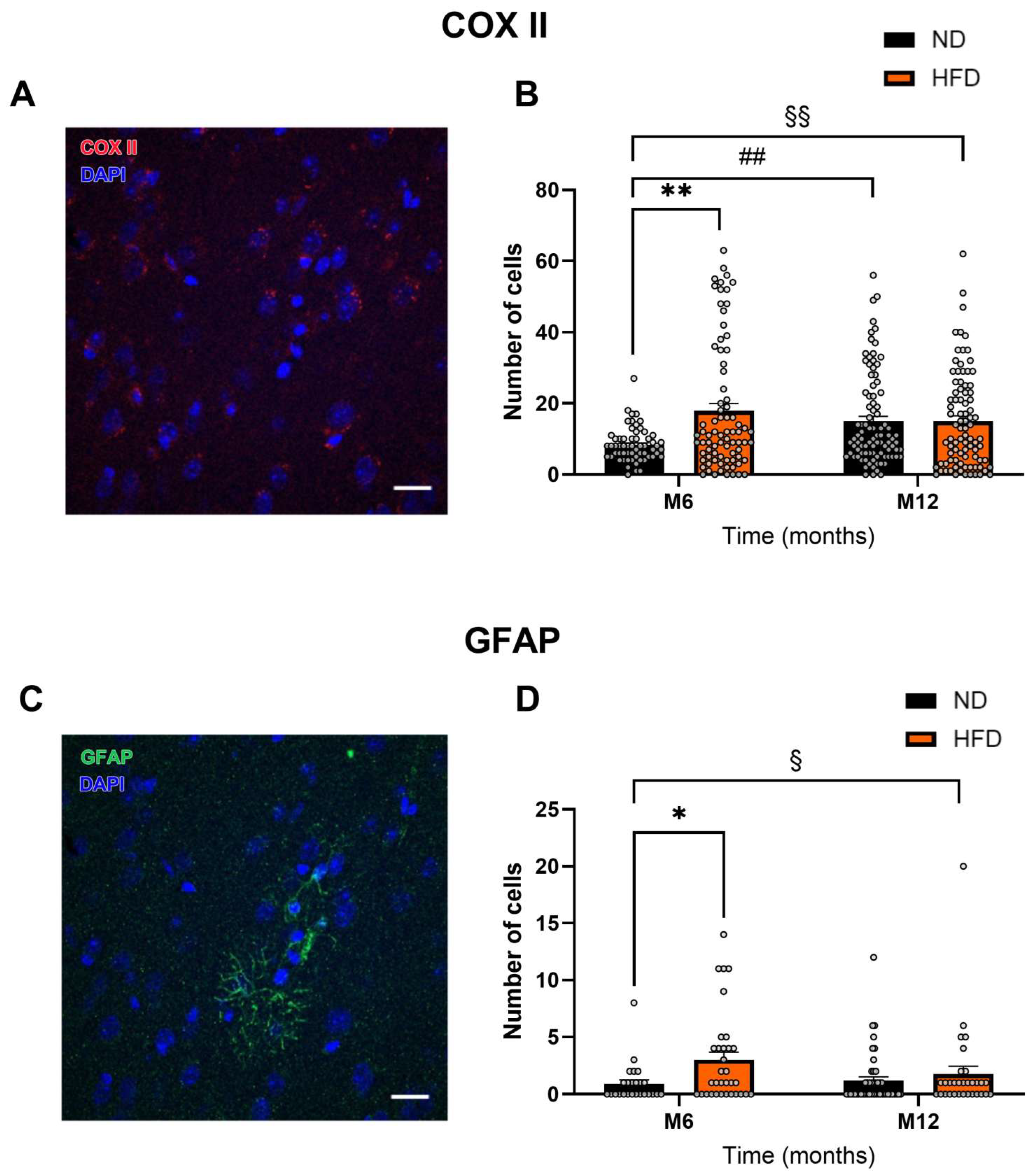
2.4. Immunohistochemistry
2.5. Magnetic Resonance Spectrometry (MRS) Procedure
2.6. Statistical Analysis
2.7. Figures
3. Results
3.1. HFD Induced Significant Metabolic Disturbances
3.2. HFD Alters the Myogenic Tone in the Basilar Artery and Glutamate-Mediated Vasodilation in Parenchymal Arterioles
3.3. HFD Unbalances Glutamate Vasodilator Pathways
3.4. HFD Induces Astrocytic Activation
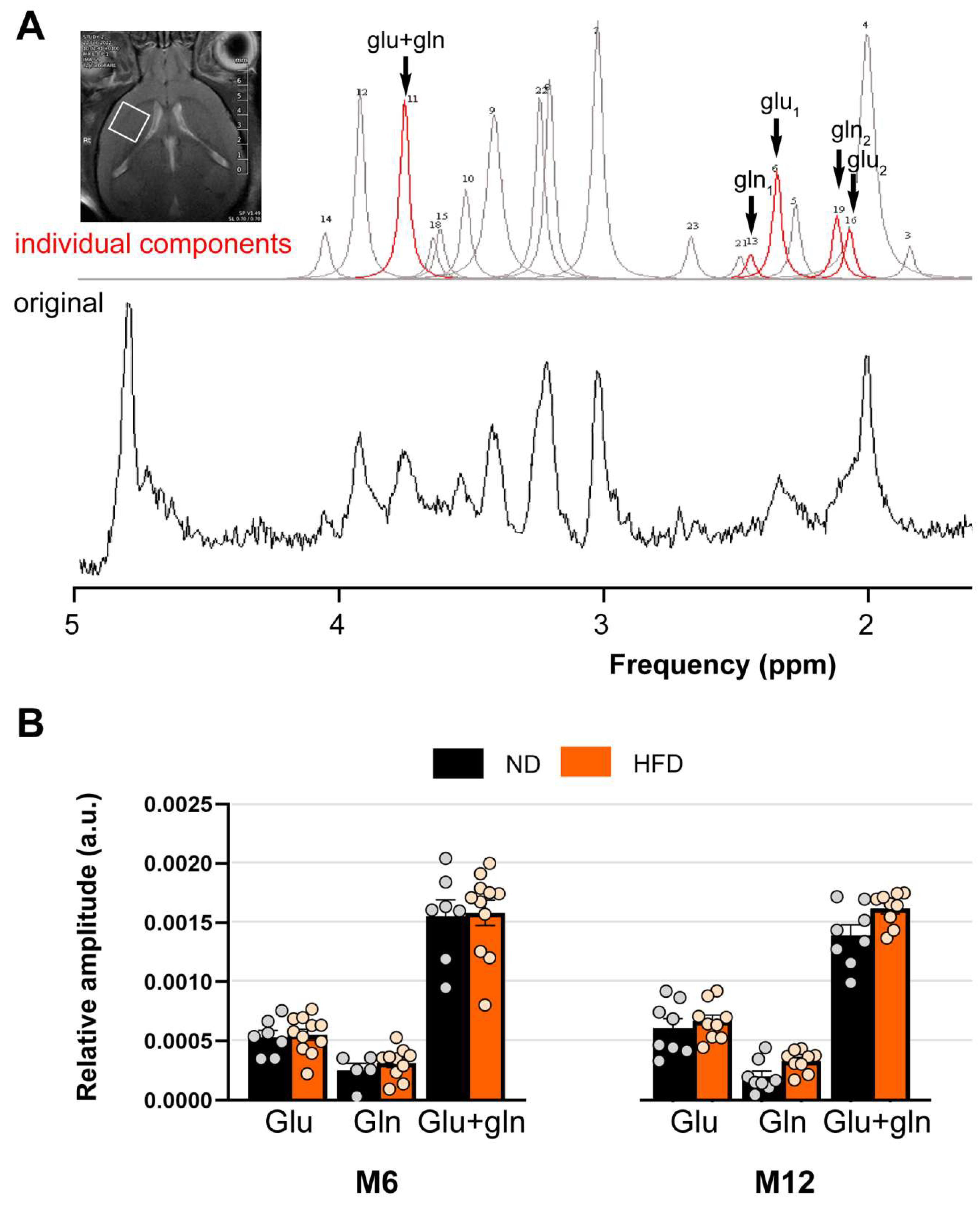
3.5. HFD Does Not Alter the Availability of Glutamate and Glutamine in the Brain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Arachidonic Acid |
| aCSF | Artificial CerebroSpinal Fluid |
| AUC | Area Under the Curve |
| BBB | Blood–Brain Barrier |
| COX I | Cyclooxygenase I |
| COX II | Cyclooxygenase II |
| DIC | Differential Interference Contrast |
| EAAT 1/2 | Excitatory Animo Acid Transporter 1/2 |
| EETs | Epoxyeicosatrienoic Acids |
| HFD | High-Fat Diet |
| IOD | Integrated Optical Density |
| L-NNA | Nω-Nitro-L-Arginine |
| ND | Normal Diet |
| NMDAR | N-methyl-D-aspartate Receptor |
| nNOS | Neuronal NO Synthase |
| NO | Nitric Oxide |
| OGTT | Oral Glucose Tolerance Test |
| Pap | Papaverine |
| PGE2 | Prostaglandin E2 |
| SEM | Standard Error of the Mean |
| VAT | Visceral Adipose Tissue |
References
- National Institute on Aging. Cognitive Health and Older Adults. 2020. Available online: https://www.nia.nih.gov/health/brain-health/cognitive-health-and-older-adults (accessed on 30 May 2024).
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 30 May 2024).
- Farooqui, A.A.; Farooqui, T.; Panza, F.; Frisardi, V. Metabolic syndrome as a risk factor for neurological disorders. Cell. Mol. Life Sci. 2012, 69, 741–762. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef] [PubMed]
- Morys, F.; Dadar, M.; Dagher, A. Association Between Midlife Obesity and Its Metabolic Consequences, Cerebrovascular Disease, and Cognitive Decline. J. Clin. Endocrinol. Metab. 2021, 106, e4260–e4274. [Google Scholar] [CrossRef]
- Whitmer, R.A.; Sidney, S.; Selby, J.; Johnston, S.C.; Yaffe, K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005, 64, 277–281. [Google Scholar] [CrossRef]
- Pétrault, O.; Pétrault, M.; Ouk, T.; Bordet, R.; Bérézowski, V.; Bastide, M. Visceral adiposity links cerebrovascular dysfunction to cognitive impairment in middle-aged mice. Neurobiol. Dis. 2019, 130, 104536. [Google Scholar] [CrossRef]
- Stapleton, P.A.; James, M.E.; Goodwill, A.G.; Frisbee, J.C. Obesity and vascular dysfunction. Pathophysiology 2008, 15, 79–89. [Google Scholar] [CrossRef]
- Steinberg, H.O.; Chaker, H.; Leaming, R.; Johnson, A.; Brechtel, G.; Baron, A.D. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J. Clin. Investig. 1996, 97, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.; Ogrodnik, M.; Wenzel, J.; Stölting, I.; Huber, L.; Will, O.; Peschke, E.; Matschl, U.; Hövener, J.-B.; Schwaninger, M.; et al. Telmisartan prevents high-fat diet-induced neurovascular impairments and reduces anxiety-like behavior. J. Cereb. Blood Flow. Metab. 2021, 41, 2356–2369. [Google Scholar] [CrossRef]
- Obadia, N.; Andrade, G.; Leardini-Tristão, M.; Albuquerque, L.; Garcia, C.; Lima, F.; Daleprane, J.; Castro-Faria-Neto, H.C.; Tibiriçá, E.; Estato, V. TLR4 mutation protects neurovascular function and cognitive decline in high-fat diet-fed mice. J. Neuroinflamm. 2022, 19, 104. [Google Scholar] [CrossRef]
- Petroff, O.A.C. GABA and glutamate in the human brain. Neuroscientist 2002, 8, 562–573. [Google Scholar] [CrossRef]
- Fergus, A.; Lee, K.S. Regulation of cerebral microvessels by glutamatergic mechanisms. Brain Res. 1997, 754, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Zonta, M.; Sebelin, A.; Gobbo, S.; Fellin, T.; Pozzan, T.; Carmignoto, G. Glutamate-mediated cytosolic calcium oscillations regulate a pulsatile prostaglandin release from cultured rat astrocytes. J. Physiol. 2003, 553, 407–414. [Google Scholar] [CrossRef]
- Busija, D.W.; Bari, F.; Domoki, F.; Louis, T. Mechanisms involved in the cerebrovascular dilator effects of N-methyl-d-aspartate in cerebral cortex. Brain Res. Rev. 2007, 56, 89–100. [Google Scholar] [CrossRef]
- Lacroix, A.; Toussay, X.; Anenberg, E.; Lecrux, C.; Ferreirós, N.; Karagiannis, A.; Plaisier, F.; Chausson, P.; Jarlier, F.; Burgess, S.A.; et al. COX-2-Derived Prostaglandin E2 Produced by Pyramidal Neurons Contributes to Neurovascular Coupling in the Rodent Cerebral Cortex. J. Neurosci. 2015, 35, 11791–11810. [Google Scholar] [CrossRef]
- Niwa, K.; Araki, E.; Morham, S.G.; Ross, M.E.; Iadecola, C. Cyclooxygenase-2 Contributes to Functional Hyperemia in Whisker-Barrel Cortex. J. Neurosci. 2000, 20, 763. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, S.; Blair, A.R.; Deluca, N.; Fam, B.C.; Proietto, J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1323–E1332. [Google Scholar] [CrossRef]
- Ouk, T.; Potey, C.; Laprais, M.; Gautier, S.; Hanf, R.; Darteil, R.; Staels, B.; Duriez, P.; Bordet, R. PPARα is involved in the multitargeted effects of a pretreatment with atorvastatin in experimental stroke. Fundam. Clin. Pharmacol. 2014, 28, 294–302. [Google Scholar] [CrossRef]
- Cipolla, M.J.; McCall, A.L.; Lessov, N.; Porter, J.M. Reperfusion decreases myogenic reactivity and alters middle cerebral artery function after focal cerebral ischemia in rats. Stroke 1997, 28, 176–180. [Google Scholar] [CrossRef]
- Naressi, A.; Couturier, C.; Devos, J.M.; Janssen, M.; Mangeat, C.; de Beer, R.; Graveron-Demilly, D. Java-based graphical user interface for the MRUI quantitation package. MAGMA 2001, 12, 141–152. [Google Scholar] [CrossRef]
- Stefan, D.; Cesare, F.D.; Andrasescu, A.; Popa, E.; Lazariev, A.; Vescovo, E.; Strbak, O.; Williams, S.; Starcuk, Z.; Cabanas, M.; et al. Quantitation of magnetic resonance spectroscopy signals: The jMRUI software package. Meas. Sci. Technol. 2009, 20, 104035. [Google Scholar] [CrossRef]
- Vanhamme, L.; van den Boogaart, A.; Van Huffel, S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J. Magn. Reson. 1997, 129, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Krüger, N.; Biwer, L.A.; Good, M.E.; Ruddiman, C.A.; Wolpe, A.G.; DeLalio, L.J.; Murphy, S.; Macal, E.H.; Ragolia, L.; Serbulea, V.; et al. Loss of Endothelial FTO Antagonizes Obesity-Induced Metabolic and Vascular Dysfunction. Circ. Res. 2020, 126, 232–242. [Google Scholar] [CrossRef]
- Cauli, B.; Hamel, E. Revisiting the role of neurons in neurovascular coupling. Front. Neuroenerg. 2010, 2, 9. [Google Scholar] [CrossRef]
- Mishra, A.; Gordon, G.R.; MacVicar, B.A.; Newman, E.A. Astrocyte Regulation of Cerebral Blood Flow in Health and Disease. Cold Spring Harb. Perspect. Biol. 2024, 16, a041354. [Google Scholar] [CrossRef]
- Omori, K.; Kida, T.; Hori, M.; Ozaki, H.; Murata, T. Multiple roles of the PGE2 -EP receptor signal in vascular permeability. Br. J. Pharmacol. 2014, 171, 4879–4889. [Google Scholar] [CrossRef]
- Butler, M.J.; Muscat, S.M.; Caetano-Silva, M.E.; Shrestha, A.; Olmo, B.M.G.; Mackey-Alfonso, S.E.; Massa, N.; Alvarez, B.D.; Blackwell, J.A.; Bettes, M.N.; et al. Obesity-associated memory impairment and neuroinflammation precede widespread peripheral perturbations in aged rats. Immun. Ageing 2025, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Venegas, B.; Muñoz-Arenas, G.; Moran, C.; Vazquez-Roque, R.A.; Flores, G.; Treviño, S.; Diaz, A.; Guevara, J. High-carbohydrate and fat diet consumption causes metabolic deterioration, neuronal damage, and loss of recognition memory in rats. J. Chem. Neuroanat. 2023, 129, 102237. [Google Scholar] [CrossRef]
- Naveed, M.; Smedlund, K.; Zhou, Q.-G.; Cai, W.; Hill, J.W. Astrocyte involvement in metabolic regulation and disease. Trends Endocrinol. Metab. 2025, 36, 219–234. [Google Scholar] [CrossRef]

| Proteins | Lipids | Carbohydrates | Minerals | Cellulose | Humidity | Total | |
|---|---|---|---|---|---|---|---|
| ND | |||||||
| Nutrients (%) | 16.2 | 3.2 | 60.5 | 4.6 | 3.9 | 11.6 | 100 |
| Energy (%) | 19.3 | 8.4 | 72.3 | 100 | |||
| Energy (kcal/kg) | 648 | 288 | 2420 | 3326 | |||
| HFD | |||||||
| Nutrients (%) | 28.7 | 40.1 | 14.2 | 8.2 | 3.1 | 5.6 | 100 |
| Energy (%) | 21.3 | 70.7 | 8 | 100 | |||
| Energy (kcal/kg) | 11.48 | 3620.2 | 567.6 | 5335.8 |
| M12 | M6 | Time vs. Diet | |
|---|---|---|---|
| 73.71 ± 1.89 (23) | 77.11 ± 1.41 (48) | ND | Mean Arterial Pressure (mmHg) |
| 64.95 ± 1.58 (23) | 77.14 ± 1.51 (40) | HFD | |
| 1.98 ± 0.14 (39) | 1.71 ± 0.11 (30) | ND | Visceral Adipose Tissue (g) §§§ |
| 5.18 ± 0.25 (31) *** | 3.56 ± 0.30 (30) *** | HFD | |
| 4.364 ± 0.785 (40) | 5.01 ± 1.114 (24) | ND | Liver lipid accumulation (% Area) §§§ |
| 13.08 ± 1.22 (29) *** | 11.01 ± 1.51 (22) ** | HFD | |
| 65.41 ± 5.84 (39) | 65.11 ± 3.28 (64) | ND | Triglycerides (mg.dL−1) §§ |
| 49.61 ± 2.82 (31) * | 57.09 ± 2.86 (56) | HFD | |
| 148.0 ± 6.17 (39) | 151.0 ± 5.123 (64) | ND | Total cholesterol (mg.dL−1) §§§ |
| 260.8 ± 8.41 (31) *** | 245.9 ± 7.45 (56) *** | HFD | |
| 1.293 ± 0.15 (39) | 1.194 ± 0.10 (64) | ND | Insulin (µg.L−1) §§§ |
| 3.807 ± 0.50 (27) *** | 2.116 ± 0.17 (56) *** | HFD | |
| 140.7 ± 2.51 (39) | 144.1 ± 2.89 (72) | ND | Fasting glycemia (mg.dL−1) §§§ |
| 170.2 ± 4.44 (31) *** | 158.5 ± 3.27 (64) *** | HFD | |
| 24.027 ± 0.41 (39) | 27.03 ± 0.58 (71) | ND | OGTT (AUC. mg.min.dL−1) §§§ |
| 34.092 ± 1.11 (30) ** | 35.33 ± 0.80 (64) *** | HFD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haas, M.; Petrault, M.; Gele, P.; Ouk, T.; Berezowski, V.; Petrault, O.; Bastide, M. High-Fat-Diet-Induced Metabolic Disorders: An Original Cause for Neurovascular Uncoupling Through the Imbalance of Glutamatergic Pathways. Biomedicines 2025, 13, 1712. https://doi.org/10.3390/biomedicines13071712
Haas M, Petrault M, Gele P, Ouk T, Berezowski V, Petrault O, Bastide M. High-Fat-Diet-Induced Metabolic Disorders: An Original Cause for Neurovascular Uncoupling Through the Imbalance of Glutamatergic Pathways. Biomedicines. 2025; 13(7):1712. https://doi.org/10.3390/biomedicines13071712
Chicago/Turabian StyleHaas, Manon, Maud Petrault, Patrick Gele, Thavarak Ouk, Vincent Berezowski, Olivier Petrault, and Michèle Bastide. 2025. "High-Fat-Diet-Induced Metabolic Disorders: An Original Cause for Neurovascular Uncoupling Through the Imbalance of Glutamatergic Pathways" Biomedicines 13, no. 7: 1712. https://doi.org/10.3390/biomedicines13071712
APA StyleHaas, M., Petrault, M., Gele, P., Ouk, T., Berezowski, V., Petrault, O., & Bastide, M. (2025). High-Fat-Diet-Induced Metabolic Disorders: An Original Cause for Neurovascular Uncoupling Through the Imbalance of Glutamatergic Pathways. Biomedicines, 13(7), 1712. https://doi.org/10.3390/biomedicines13071712







