Significant Microvascular Abnormalities Present in Autonomic Nervous System Dysfunction: Results of a Cross-Sectional Study
Abstract
1. Introduction
2. Methods
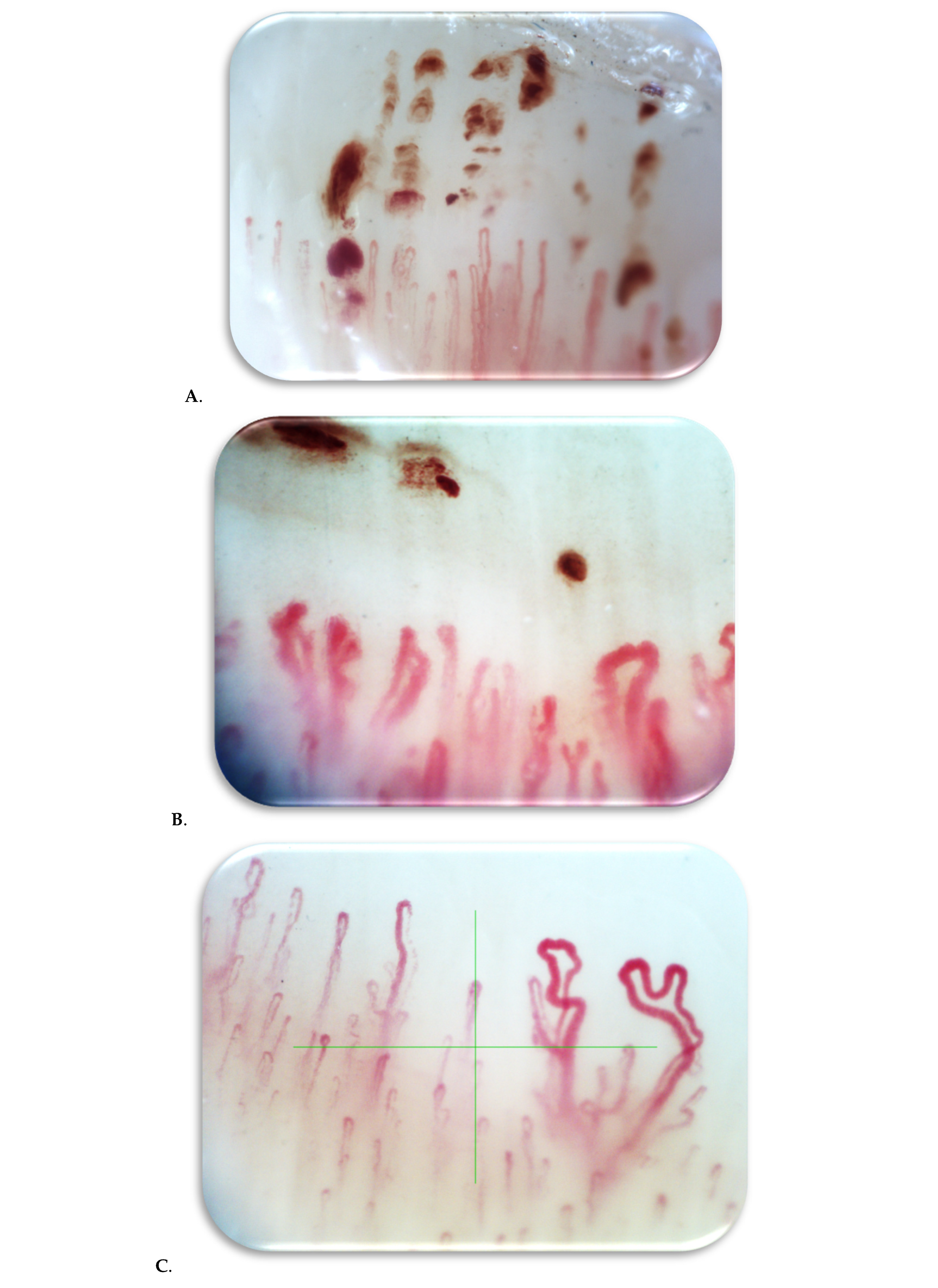
2.1. Nailfold Video Capillaroscopy
2.2. Statistical Methods
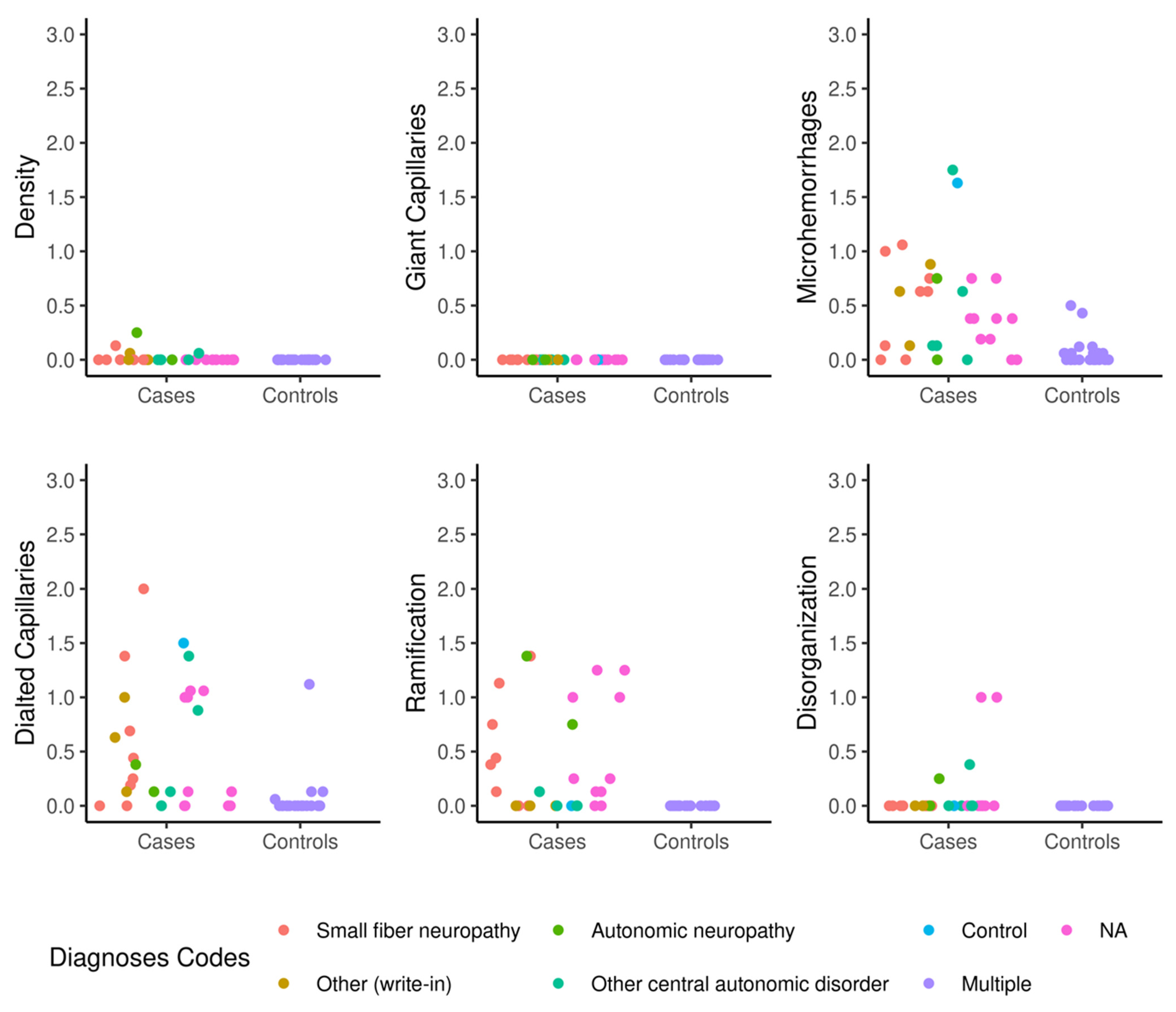
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klimiuk, P.S.; Taylor, L.; Baker, R.D.; Jayson, M.I. Autonomic neuropathy in systemic sclerosis. Ann. Rheum. Dis. 1988, 47, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, L.; Brinth, L.S.; Bergmann, M.L.; Kristensen, B.; Hansen, T.W.; Hasbak, P.; Thomsen, J.F.; Eldrup, E.; Jensen, L.T. Autonomic nervous system activity in primary Raynaud’s phenomenon: Heart rate variability, plasma catecholamines and [123I]MIBG heart scintigraphy. Clin. Physiol. Funct. Imaging 2022, 42, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, N.; Rotondo, C.; Corrado, A.; Cantatore, F.P. Nailfold capillaroscopy: A comprehensive review on common findings and clinical usefulness in non-rheumatic disease. J. Med. Investig. 2021, 68, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Paolino, S.; Smith, V. Nailfold capillaroscopy in rheumatology: Ready for the daily use but with care in terminology. Clin. Rheumatol. 2019, 38, 2293–2297. [Google Scholar] [CrossRef] [PubMed]
- Stacher, G.; Merio, R.; Budka, C.; Schneider, C.; Smolen, J.; Tappeiner, G. Cardiovascular autonomic function, autoantibodies, and esophageal motor activity in patients with systemic sclerosis and mixed connective tissue disease. J. Rheumatol. 2000, 27, 692–697. [Google Scholar] [PubMed]
- Cantatore, F.P.; Corrado, A.; Covelli, M.; Lapadula, G. Morphologic study of the microcirculation in connective tissuediseases. Ann. Ital. Med. Int. 2000, 15, 273–281. [Google Scholar]
- Sulli, A.; Ruaro, B.; Smith, V.; Pizzorni, C.; Zampogna, G.; Gallo, M.; Cutolo, M. Progression of nailfold microvascular damage and antinuclear antibody pattern in systemic sclerosis. J. Rheumatol. 2013, 40, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M. Atlas of Capillaroscopy in Rheumatic Diseases; Elsevier: Milan, Italy, 2015. [Google Scholar]
- Davies, K.; Ng, W.F. Autonomic Nervous System Dysfunction in Primary Sjögren’s Syndrome. Front. Immunol. 2021, 12, 702505. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.Z.; Lester, S.; Lu, T.; Keen, H.; Boundy, K.; Proudman, S.M.; Tonkin, A.; Rischmueller, M. Mild autonomic dysfunction in primary Sjögren’s syndrome: A controlled study. Arthritis Res. Ther. 2008, 10, R31. [Google Scholar] [CrossRef] [PubMed]
- Masini, F.; Galiero, R.; Pafundi, P.C.; Gjeloshi, K.; Pinotti, E.; Ferrara, R.; Romano, C.; Adinolfi, L.E.; Sasso, F.C.; Cuomo, G. Autonomic nervous system dysfunction correlates with microvascular damage in systemic sclerosis patients. J. Scleroderma Relat. Disord. 2021, 6, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, M.; Paradiso, M.; Riccieri, V.; Basili, S.; Mammarella, A.; Valesini, G. Autonomic dysfunction and microvascular damage in systemic sclerosis. Clin. Rheumatol. 2007, 26, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.L.; Russell, J.W.; Hummers, L.K.; McMahan, Z.H. Symptoms of Autonomic Dysfunction in Systemic Sclerosis Assessed by the COMPASS-31 Questionnaire. J. Rheumatol. 2018, 45, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Bellocchi, C.; Carandina, A.; Montinaro, B.; Targetti, E.; Furlan, L.; Rodrigues, G.D.; Tobaldini, E.; Montano, N. The Interplay between Autonomic Nervous System and Inflammation across Systemic Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 2449. [Google Scholar] [CrossRef] [PubMed]
- Terreri, M.T.; Andrade, L.E.; Puccinelli, M.L.; Hilário, M.O.; Goldenberg, J. Nail fold capillaroscopy: Normal findings in children and adolescents. Semin. Arthritis Rheum. 1999, 29, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Nakano, S.; Kikuchi, A.; Matsunaga, Y.T. Nailfold capillary patterns correlate with age, gender, lifestyle habits, and fingertip temperature. PLoS ONE 2022, 17, e0269661. [Google Scholar] [CrossRef] [PubMed]
- Gorasiya, A.R.; Mehta, H.H.; Prakashey, A.; Dave, M. Nailfold Capillaroscopy of Healthy Individuals—An Observational Study. Indian Dermatol. Online J. 2022, 13, 600–605. [Google Scholar] [CrossRef] [PubMed]
| Estimated Difference in Mean NVC Scores (95% Confidence Interval) | |||||||
|---|---|---|---|---|---|---|---|
| Factor | N (%) or Median (IQR) | Capillary Density | Giant Capillaries | Microhemorrhages | Dilated Capillaries | Ramification | Disorganization |
| Age | 59 (53–72) | −0.002 (−0.014, 0.010) | NA | −0.01 (−0.02, 0.002) | −0.01 (−0.03, 0.0001) | −0.02 (−0.03, −0.006) * | NA |
| Female gender | 12 (44%) | 0 (−0.1, 0.1) | NA | 0.1 (−0.3, 0.5) | 0(−0.5, 0.5) | 0.1 (−0.1, 0.3) | |
| BMI | 26.6 (22.6, 30.9) | 0.005 (0.001, 0.009) * | NA | −0.02 (−0.06, 0.02) | −0.05 (−0.09, −0.01) * | 0.02 (−0.01, 0.06) | NA |
| Raynaud’s phenomenon | 2 (7%) | NA | NA | NA (all 0 s in the 2 with disease) | 0 (−1.0, 1.0) | −0.5 (−1.8, 0.8) | NA |
| Orthostatic hypotension | 13 (48%) | 0 (−0.1, 0.1) | NA | −0.2 (−0.6, 0.2) | −0.2 (−0.6, 0.2) | 0.1 (−0.3, 0.5) | −0.1 (−0.3, 0.1) |
| Connective tissue disease diagnosis | 2 (7%) | NA | NA | 0 (−1.0, 1.0) | −0.8 (−1.2, −0.4) | 0 (−0.6, 0.6) | NA |
| Autonomic dysfunction diagnosis (yes) | 3 (11%) | NA | NA | −0.3 (−0.5, 0.1) | 0.3 (−0.1, 0.7) | 0.4 (−0.2, 0.6) | NA |
| Small-fiber neuropathy | 10 (37%) | 0 (−0.1, 0.1) | NA | 0 (−0.3, 0.3) | 0.2 (−0.3, 0.7) | 0.3 (−0.1, 0.7) | NA |
| POTS | 2 (7%) | 0.1 (−0.2, 0.4) | NA | −0.1 (−0.8, 0.6) | −0.2 (−0.6, 0.2) | 0.8 (0.2, 1.4) | 0 (−0.3, 0.3) |
| Neuropathic POTS | 0 | ||||||
| Limited autonomic neuropathy | 2 (7%) | NA | NA | 0.0 (−0.6, 0.6) | −0.4 (−0.7, 0.1) | 0.3 (−1.0, 1.6) | NA |
| Autonomic neuropathy | 8 (30%) | NA | NA | 0.1 (−0.6, 0.4) | 0.1 (−0.6, 0.4) | −0.2 (−0.6, 0.2) | 0 (−0.2, 0.1) |
| Other central autonomic disorders | 3 (11%) | NA | NA | −0.5 (−1.8, 0.8) | −0.8 (−1.4, −0.2) | 0.3 (0, 0.6) | −0.5 (−1.5, 0.5) |
| MSA | 0 | ||||||
| Familial dysautonomia | |||||||
| Pure autonomic failure | 0 | ||||||
| Autoimmune autonomic ganglionopathy | 0 | ||||||
| Qsweat (reduced) | 19 (70%) | 0 (−0.1, 0.1) | NA | 0.2 (−0.1, 0.5) | −0.3 (−0.7, 0.1) | 0.2 (−0.3, 0.7) | 0.2 (−0.1, 0.5) |
| Cardiovagal responses (reduced) | 7 (26%) | NA | NA | −0.1 (−0.6, 0.4) | −1 (−0.6, 0.4) | 0.3 (0, 0.6) | −0.1 (−0.4, 0.2) |
| Cardiovascular adrenergic responses | 10 (38%) | NA | NA | 0 (−0.4, 0.4) | −0.1 (−0.5, 0.3) | −0.2 (−0.5, 0.1) | 0.1 (−0.1, 0.3) |
| Flat-top response | |||||||
| Composite autonomic severity score (CASS) | 3 (2, 5) | 0 (−0.008, 0.006) | NA | −0.01 (−0.12, 0.11) | 0.05 (−0.09, 0.19) | −0.09 (−0.19, 0.01) | NA |
| EMG | 20 (77%) | NA | NA | 0.1 (−0.3, 0.5) | −0.2 (−0.9, 0.5) | 0.1 (−0.3, 0.5) | 0 (−0.2, 0.2) |
| Skin punch biopsy | 0 | ||||||
| ANA positivity | 0 | ||||||
| SSA | 0 | ||||||
| SSA−52+ | |||||||
| SSB | 0 | ||||||
| Autoimmune dysautonomia panel | 0 | ||||||
| Nailfold Videocapillaroscopy Scores | Autonomic Dysfunction (N = 27) | Controls (N = 21) | Total (N = 48) | p Value |
|---|---|---|---|---|
| Capillaroscopy density | 0 (0.1) | 0 (0) | 0 (0) | -- |
| Dilated capillaries | 0.5 (0.6) | 0.1 (0.2) | 0.3 (0.5) | <0.001 |
| Giant capillaries | 0 (0) | 0 (0) | 0 (0) | -- |
| Microhemorrhages | 0.5 (0.5) | 0.1 (0.1) | 0.3 (0.4) | 0.001 |
| Ramification | 0.3 (0.5) | 0 (0) | 0.10 (0.4) | <0.001 |
| Disorganization | 0.1 (0.2) | 0 (0) | 0 (0.2) | -- |
| Mean (standard deviation) are shown for each group | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mumtaz, S.; Arca, K.; Majithia, V.; Cheshire, W.; Hodge, D.; Berianu, F. Significant Microvascular Abnormalities Present in Autonomic Nervous System Dysfunction: Results of a Cross-Sectional Study. Biomedicines 2025, 13, 1242. https://doi.org/10.3390/biomedicines13051242
Mumtaz S, Arca K, Majithia V, Cheshire W, Hodge D, Berianu F. Significant Microvascular Abnormalities Present in Autonomic Nervous System Dysfunction: Results of a Cross-Sectional Study. Biomedicines. 2025; 13(5):1242. https://doi.org/10.3390/biomedicines13051242
Chicago/Turabian StyleMumtaz, Sehreen, Karissa Arca, Vikas Majithia, William Cheshire, David Hodge, and Florentina Berianu. 2025. "Significant Microvascular Abnormalities Present in Autonomic Nervous System Dysfunction: Results of a Cross-Sectional Study" Biomedicines 13, no. 5: 1242. https://doi.org/10.3390/biomedicines13051242
APA StyleMumtaz, S., Arca, K., Majithia, V., Cheshire, W., Hodge, D., & Berianu, F. (2025). Significant Microvascular Abnormalities Present in Autonomic Nervous System Dysfunction: Results of a Cross-Sectional Study. Biomedicines, 13(5), 1242. https://doi.org/10.3390/biomedicines13051242






