Targeting Biomarkers of Proliferation and Inflammation (Ki67, p53, and COX-2) in Actinic Keratoses with Photodynamic Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
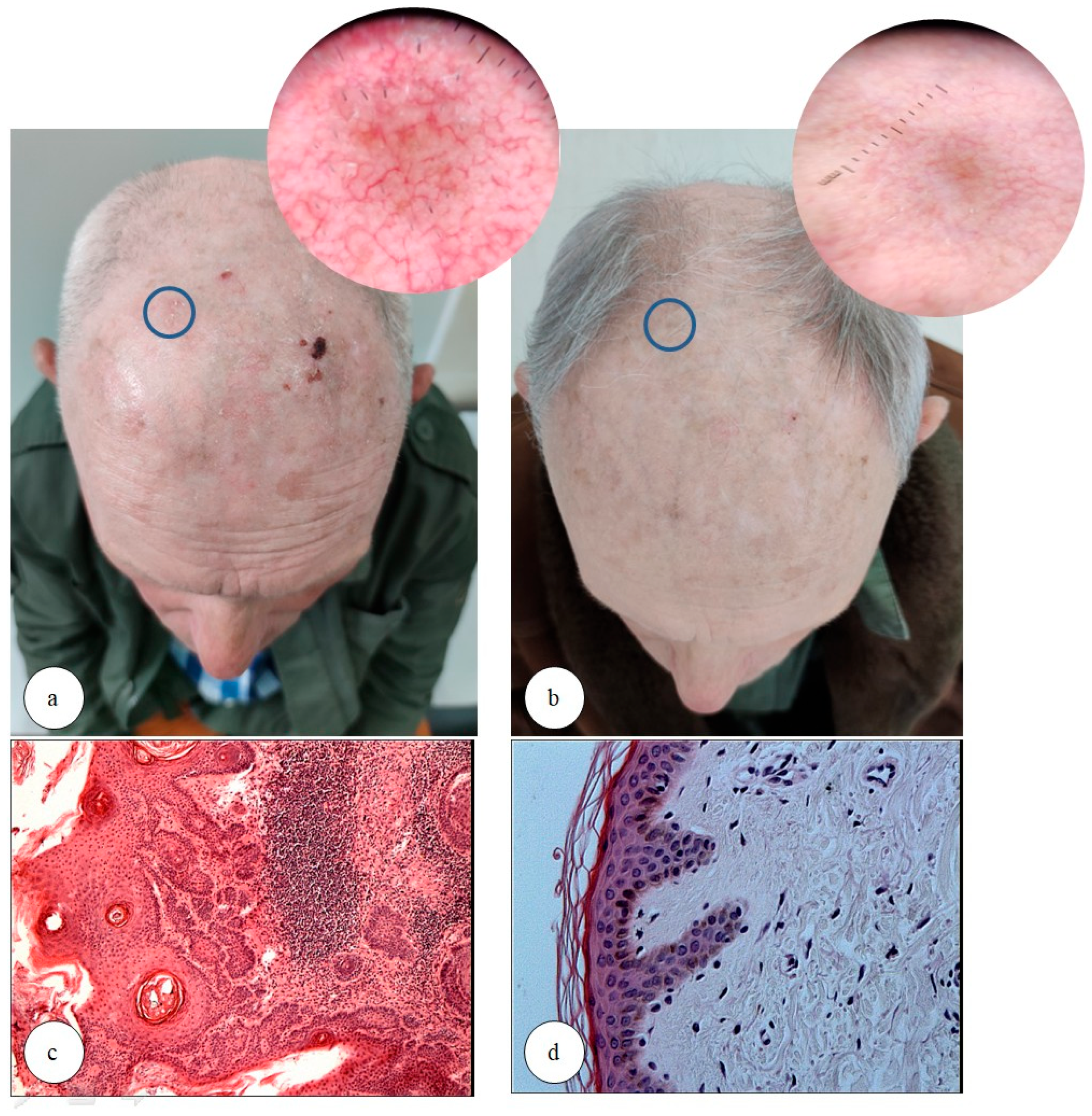
2.2. Study Design and Assessments (Clinical, Dermatoscopic, Histopathological, and Immunohistochemical Evaluation)
2.3. Statistical Analysis
3. Results
3.1. Study Group, Clinical, and Dermatoscopic Evaluation
| Characteristic | n (%) |
|---|---|
| Age (mean = 72.4 ± 8.64; range 54–92) | |
| ≥72.4 | 18 (60) |
| <72.4 | 13 (40) |
| Sex | |
| Male | 24 (77.42) |
| Female | 7 (22.58) |
| Anatomic location | |
| Scalp | 17 (54.84) |
| Face | 9 (29.03) |
| Forearm | 3 (9.68) |
| Trunk | 2 (6.45) |
| Fitzpatrick Skin Type | |
| I | 3 (9.68) |
| II | 26 (83.87) |
| III | 2 (6.45) |
| AK clinical grade (Olsen’s Clasification System) | |
| 1 | |
| 2 | |
| 3 | |
| AK dermatoscopic grade (Dermatoscopic Severity Scale [35] with our modification) | |
| I | 4 (12.9) |
| I/II | 3 (9.68) |
| II | 7 (25.81) |
| II/III | 11 (35.48) |
| III | 6 (19.35) |
| Complete Resolution of Cutaneous Lesions | Olsen 1 | ||||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age | p < 0.001 * | ||||
| ≥72.4 | 8 | 44 | 10 | 56 | |
| <72.4 | 9 | 69 | 4 | 31 | |
| Sex | p = 0.034 | ||||
| Male | 12 | 50 | 12 | 50 | |
| Female | 5 | 71 | 2 | 29 | |
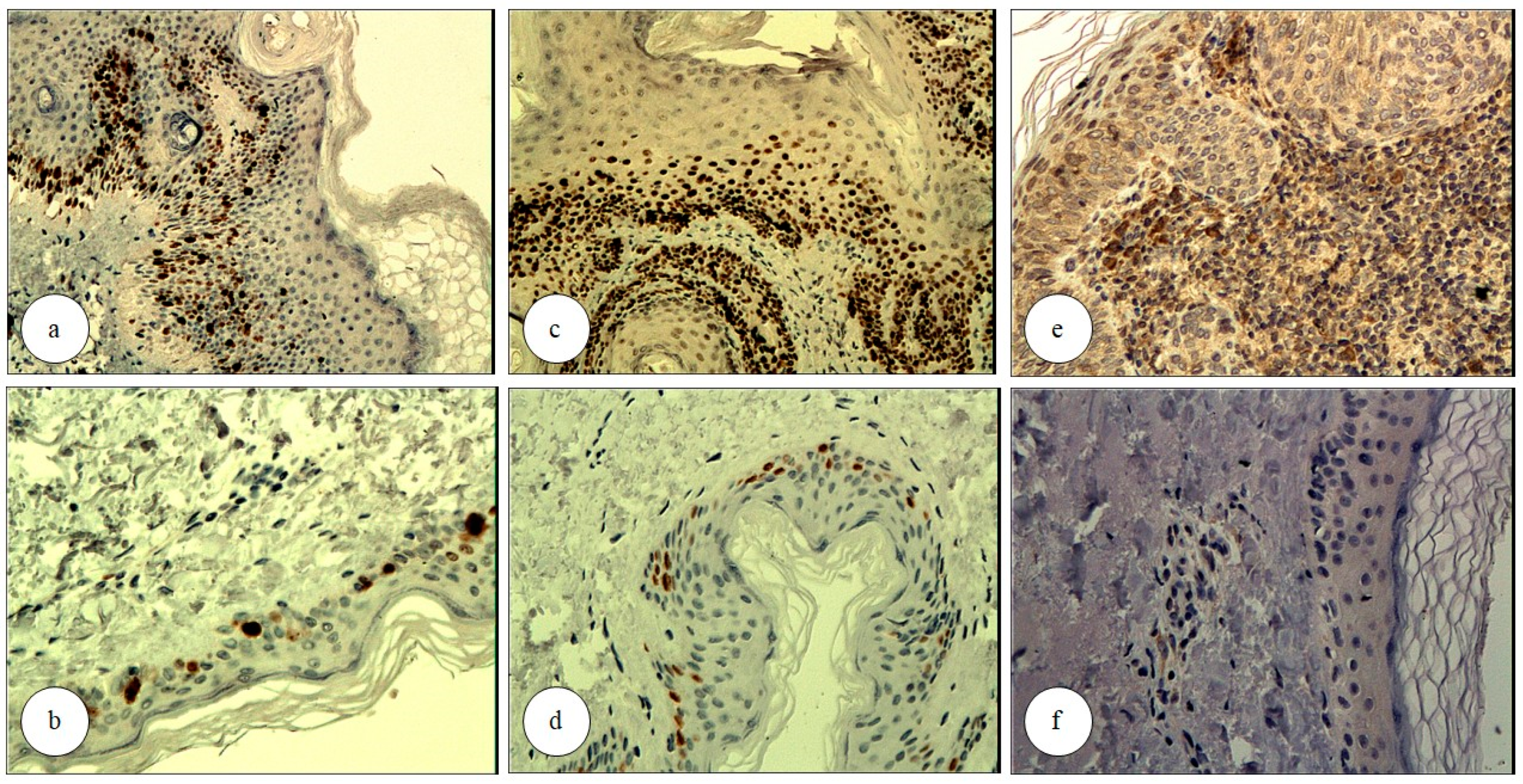
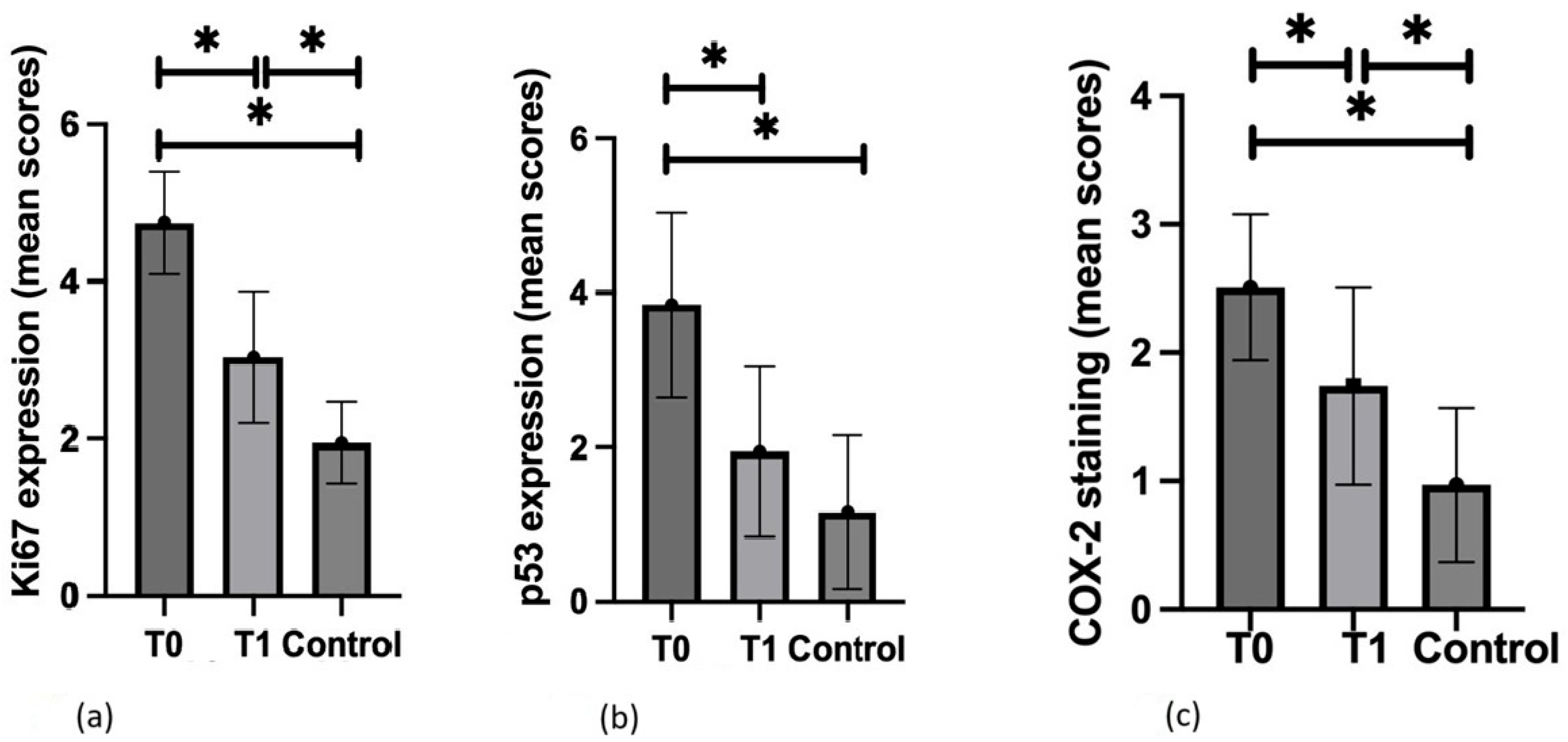
3.2. Ki67
3.3. p53
3.4. COX-2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AK | Actinic keratosis |
| cSCC | Cutaneous squamous cell carcinoma |
| UV | Ultraviolet |
| iSCC | Invasive squamous cell carcinoma |
| BCC | Basal cell carcinoma |
| FC | Field cancerization |
| PDT | Photodynamic therapy |
| c-PDT | Conventional PDT |
| dl-PDT | Daylight PDT |
| ALA | 5-aminolevulinic acid |
| MAL | Methyl aminolevulinate |
| COX-2 | Cyclooxygenase-2 |
| PGE2 | Prostaglandin E2 |
| NMSC | Non-melanoma skin cancer |
| MM | Malignant melanoma |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| HPF | High-power fields |
| AEs | Adverse events |
| c-5-ALAPDT | 5-aminolevulinic acid PDT |
| MAL-cPDT | c-PDT with methyl aminolevulinic acid |
| BD | Bowen disease |
| ROS | Reactive oxygen species |
| KIN | Keratinocytic intraepithelial neoplasia |
References
- Ferrándiz, C.; Plazas, M.J.; Sabaté, M.; Palomino, R.; EPIQA Study Group. Prevalence of actinic keratosis among dermatology outpatients in Spain. Actas Dermo-Sifiliogr. 2016, 107, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Yaldiz, M. Prevalence of actinic keratosis in patients attending the dermatology outpatient clinic. Medicine 2019, 98, e16465. [Google Scholar] [CrossRef]
- Kandolf, L.; Peris, K.; Malvehy, J.; Mosterd, K.; Heppt, M.V.; Fargnoli, M.C.; Berking, C.; Arenberger, P.; Bylaite-Bučinskiene, M.; Del Marmol, V.; et al. European consensus-based interdisciplinary guideline for diagnosis, treatment and prevention of actinic keratoses, epithelial UV-induced dysplasia and field cancerization on behalf of European Association of Dermato-Oncology, European Dermatology Forum, European Academy of Dermatology and Venereology and Union of Medical Specialists (Union Européenne des Médecins Spécialistes). J. Eur. Acad. Dermatol. Venereol. 2024, 38, 1024–1047. [Google Scholar] [CrossRef] [PubMed]
- Heppt, M.V.; Leiter, U.; Steeb, T.; Amaral, T.; Bauer, A.; Becker, J.C.; Breitbart, E.; Breuninger, H.; Diepgen, T.; Dirschka, T.; et al. S3 guideline for actinic keratosis and cutaneous squamous cell carcinoma—Short version, part 1: Diagnosis, interventions for actinic keratoses, care structures and quality-of-care indicators. J. Dtsch. Dermatol. Ges. 2020, 18, 275–294. [Google Scholar] [CrossRef]
- Glogau, R.G. The risk of progression to invasive disease. J. Am. Acad. Dermatol. 2000, 42 Pt. 2, 23–24. [Google Scholar] [CrossRef]
- Balcere, A.; Konrāde-Jilmaza, L.; Pauliņa, L.A.; Čēma, I.; Krūmiņa, A. Clinical Characteristics of Actinic Keratosis Associated with the Risk of Progression to Invasive Squamous Cell Carcinoma: A Systematic Review. J. Clin. Med. 2022, 11, 5899. [Google Scholar] [CrossRef] [PubMed]
- Wehner, M.R.; Linos, E.; Parvataneni, R.; Stuart, S.E.; Boscardin, W.J.; Chren, M.M. Timing of subsequent new tumors in patients who present with basal cell carcinoma or cutaneous squamous cell carcinoma. JAMA Dermatol. 2015, 151, 382–388. [Google Scholar] [CrossRef]
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef]
- Cohen, J.L. Actinic keratosis treatment as a key component of preventive strategies for nonmelanoma skin cancer. J. Clin. Aesthet. Dermatol. 2010, 3, 39–44. [Google Scholar]
- Dirschka, T.; Gupta, G.; Micali, G.; Stockfleth, E.; Basset-Séguin, N.; Del Marmol, V.; Dummer, R.; Jemec, G.B.E.; Malvehy, J.; Peris, K.; et al. Real-world approach to actinic keratosis management: Practical treatment algorithm for office-based dermatology. J. Dermatolog. Treat. 2017, 28, 431–442. [Google Scholar] [CrossRef]
- Willenbrink, T.J.; Ruiz, E.S.; Cornejo, C.M.; Schmults, C.D.; Arron, S.T.; Jambusaria-Pahlajani, A. Field cancerization: Definition, epidemiology, risk factors, and outcomes. J. Am. Acad. Dermatol. 2020, 83, 709–717. [Google Scholar] [CrossRef]
- Eisen, D.B.; Asgari, M.M.; Bennett, D.D.; Connolly, S.M.; Dellavalle, R.P.; Freeman, E.E.; Goldenberg, G.; Leffell, D.J.; Peschin, S.; Sligh, J.E.; et al. Guidelines of care for the management of actinic keratosis. J. Am. Acad. Dermatol. 2021, 85, e209–e233. [Google Scholar] [CrossRef]
- Dirschka, T.; Radny, P.; Dominicus, R.; Mensing, H.; Brüning, H.; Jenne, L.; Karl, L.; Sebastian, M.; Oster-Schmidt, C.; Klövekorn, W.; et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: Results of a multicentre, randomized, observer-blind phase III study in comparison with a registered methyl-5-aminolaevulinate cream and placebo. Br. J. Dermatol. 2012, 166, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Dirschka, T.; Radny, P.; Dominicus, R.; Mensing, H.; Brüning, H.; Jenne, L.; Karl, L.; Sebastian, M.; Oster-Schmidt, C.; Klövekorn, W.; et al. Long-term (6 and 12 months) follow-up of two prospective, randomized, controlled phase III trials of photodynamic therapy with BF-200 ALA and methyl aminolaevulinate for the treatment of actinic keratosis. Br. J. Dermatol. 2013, 168, 825–836. [Google Scholar] [CrossRef]
- Reinhold, U.; Dirschka, T.; Ostendorf, R.; Aschoff, R.; Berking, C.; Philipp-Dormston, W.; Hahn, S.; Lau, K.; Jäger, A.; Schmitz, B.; et al. A randomized, double-blind, phase III, multicentre study to evaluate the safety and efficacy of BF-200 ALA (Ameluz(®)) vs. placebo in the field-directed treatment of mild-to-moderate actinic keratosis with photodynamic therapy (PDT) when using the BF-RhodoLED(®) lamp. Br. J. Dermatol. 2016, 175, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Szeimies, R.-M.; Radny, P.; Sebastian, M.; Borrosch, F.; Dirschka, T.; Krähn-Senftleben, G.; Reich, K.; Pabst, G.; Voss, D.; Foguet, M.; et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: Results of a prospective, randomized, double-blind, placebo-controlled phase III study. Br. J. Dermatol. 2010, 163, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Scalvenzi, M.; Ayala, F.; Fabbrocini, G.; Monfrecola, G. How to treat actinic keratosis? An update. J. Dermatol. Case Rep. 2015, 9, 29–35. [Google Scholar] [CrossRef]
- Miola, A.C.; Castilho, M.A.; Schmitt, J.V.; Marques, M.E.A.; Miot, H.A. Contribution to characterization of skin field cancerization activity: Morphometric, chromatin texture, proliferation, and apoptosis aspects. An. Bras. Dermatol. 2019, 94, 698–703. [Google Scholar] [CrossRef]
- Hollstein, M.; Sidransky, D.; Vogelstein, B.; Harris, C.C. p53 mutations in human cancers. Science 1991, 253, 49–53. [Google Scholar] [CrossRef]
- Carpenter, P.M.; Linden, K.G.; McLaren, C.E.; Li, K.-T.; Arain, S.; Barr, R.J.; Hite, P.; Sun, J.D.; Meyskens, F.L. Nuclear morphometry and molecular biomarkers of actinic keratosis, sun-damaged, and nonexposed skin. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 1996–2002. [Google Scholar] [CrossRef]
- Stratigos, A.; Kapranos, N.; Petrakou, E.; Anastasiadou, A.; Pagouni, A.; Christofidou, E.; Petridis, A.; Papadopoulos, O.; Kokka, E.; Antoniou, C.; et al. Immunophenotypic analysis of the p53 gene in non-melanoma skin cancer and correlation with apoptosis and cell proliferation. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Park, E.J.; Seo, Y.J.; Cho, H.S.; Kim, C.W.; Kim, K.J.; Park, H.R. Immunohistochemical study of cyclooxygenase-2 and p53 expression in skin tumors. J. Dermatol. 2006, 33, 319–325. [Google Scholar] [CrossRef]
- Piipponen, M.; Riihilä, P.; Nissinen, L.; Kähäri, V.M. The Role of p53 in Progression of Cutaneous Squamous Cell Carcinoma. Cancers 2021, 13, 4507. [Google Scholar] [CrossRef]
- Yilmaz, A.S.; Ozer, H.G.; Gillespie, J.L.; Allain, D.C.; Bs, M.N.B.; Furlan, K.C.; Castro, L.T.F.; Peters, S.B.; Nagarajan, P.; Kang, S.Y.; et al. Differential mutation frequencies in metastatic cutaneous squamous cell carcinomas versus primary tumors. Cancer 2017, 123, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- McGregor, J.M.; Yu, C.C.; Dublin, E.A.; Levison, D.A.; MacDonald, D.M. Aberrant expression of p53 tumour-suppressor protein in non-melanoma skin cancer. Br. J. Dermatol. 1992, 127, 463–469. [Google Scholar] [CrossRef]
- Taguchi, M.; Watanabe, S.; Yashima, K.; Murakami, Y.; Sekiya, T.; Ikeda, S. Aberrations of the tumor suppressor p53 gene and p53 protein in solar keratosis in human skin. J. Investig. Dermatol. 1994, 103, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.A.; Einspahr, J.G.; Alberts, D.S.; Balfour, C.A.; Wymer, J.A.; Welch, K.L.; Salasche, S.J.; Bangert, J.L.; Grogan, T.M.; Bozzo, P.O. Analysis of the p53 gene in human precancerous actinic keratosis lesions and squamous cell cancers. Cancer Lett. 1994, 85, 23–29. [Google Scholar] [CrossRef]
- Hedberg, M.L.; Berry, C.T.; Moshiri, A.S.; Xiang, Y.; Yeh, C.J.; Attilasoy, C.; Capell, B.C.; Seykora, J.T. Molecular Mechanisms of Cutaneous Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 3478. [Google Scholar] [CrossRef]
- Prior, S.L.; Griffiths, A.P.; Lewis, P.D. A study of mitochondrial DNA D-loop mutations and p53 status in nonmelanoma skin cancer. Br. J. Dermatol. 2009, 161, 1067–1071. [Google Scholar] [CrossRef]
- Balcere, A.; Sperga, M.; Čēma, I.; Lauskis, G.; Zolovs, M.; Kupfere, M.R.; Krūmiņa, A. Expression of p53, p63, p16, Ki67, Cyclin D, Bcl-2, and CD31 Markers in Actinic Keratosis, In Situ Squamous Cell Carcinoma and Normal Sun-Exposed Skin of Elderly Patients. J. Clin. Med. 2023, 12, 7291. [Google Scholar] [CrossRef]
- Berman, B.; Cockerell, C.J. Pathobiology of actinic keratosis: Ultraviolet-dependent keratinocyte proliferation. J. Am. Acad. Dermatol. 2013, 68 (Suppl. S1), S10–S19. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dubois, R.N. Eicosanoids and cancer. Nat Rev Cancer. 2010, 10, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Elmets, C.A.; Viner, J.L.; Pentland, A.P.; Cantrell, W.; Lin, H.-Y.; Bailey, H.; Kang, S.; Linden, K.G.; Heffernan, M.; Duvic, M.; et al. Chemoprevention of nonmelanoma skin cancer with celecoxib: A randomized, double-blind, placebo-controlled trial. J. Natl. Cancer Inst. 2010, 102, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Zalaudek, I.; Giacomel, J.; Argenziano, G.; Hofmann-Wellenhof, R.; Micantonio, T.; Di Stefani, A.; Oliviero, M.; Rabinovitz, H.; Soyer, H.; Peris, K. Dermoscopy of facial nonpigmented actinic keratosis. Br. J. Dermatol. 2006, 155, 951–956. [Google Scholar] [CrossRef]
- Zalaudek, I.; Piana, S.; Moscarella, E.; Longo, C.; Zendri, E.; Castagnetti, F.; Pellacani, G.; Lallas, A.; Argenziano, G. Morphologic grading and treatment of facial actinic keratosis. Clin. Dermatol. 2014, 32, 80–87. [Google Scholar] [CrossRef]
- Martín, J.M.; Bella-Navarro, R.; Jordá, E. Vascularización en dermatoscopia [Vascular patterns in dermoscopy]. Actas Dermo-Sifiliogr. 2012, 103, 357–375. [Google Scholar] [CrossRef]
- Olsen, E.A.; Abernethy, M.L.; Kulp-Shorten, C.; Callen, J.P.; Glazer, S.D.; Huntley, A.; McCray, M.; Monroe, A.B.; Tschen, E.; Wolf, J.E. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J. Am. Acad. Dermatol. 1991, 24 Pt. 1, 738–743. [Google Scholar] [CrossRef]
- Zalaudek, I.; Giacomel, J.; Schmid, K.; Bondino, S.; Rosendahl, C.; Cavicchini, S.; Tourlaki, A.; Gasparini, S.; Bourne, P.; Keir, J.; et al. Dermatoscopy of facial actinic keratosis, intraepidermal carcinoma, and invasive squamous cell carcinoma: A progression model. J. Am. Acad. Dermatol. 2012, 66, 589–597. [Google Scholar] [CrossRef]
- Zalaudek, I.; Argenziano, G. Dermoscopy of actinic keratosis, intraepidermal carcinoma and squamous cell carcinoma. Curr. Probl. Dermatol. 2015, 46, 70–76. [Google Scholar] [CrossRef]
- Dianzani, C.; Conforti, C.; Giuffrida, R.; Corneli, P.; di Meo, N.; Farinazzo, E.; Moret, A.; Rizzi, G.M.; Zalaudek, I. Current therapies for actinic keratosis. Int. J. Dermatol. 2020, 59, 677–684. [Google Scholar] [CrossRef]
- Park, M.Y.; Sohn, S.; Lee, E.S.; Kim, Y.C. Photorejuvenation induced by 5-aminolevulinic acid photodynamic therapy in patients with actinic keratosis: A histologic analysis. J. Am. Acad. Dermatol. 2010, 62, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Oyama, J.; Ramos-Milaré, Á.C.F.H.; Lera-Nonose, D.S.S.L.; Nesi-Reis, V.; Demarchi, I.G.; Aristides, S.M.A.; Teixeira, J.J.V.; Silveira, T.G.V.; Lonardoni, M.V.C. Photodynamic therapy in wound healing in vivo: A systematic review. Photodiagnosis Photodyn. Ther. 2020, 30, 101682. [Google Scholar] [CrossRef] [PubMed]
- Nesi-Reis, V.; Lera-Nonose, D.S.S.L.; Oyama, J.; Silva-Lalucci, M.P.P.; Demarchi, I.G.; Aristides, S.M.A.; Teixeira, J.J.V.; Silveira, T.G.V.; Lonardoni, M.V.C. Contribution of photodynamic therapy in wound healing: A systematic review. Photodiagnosis Photodyn. Ther. 2018, 21, 294–305. [Google Scholar] [CrossRef]
- Morton, C.A.; Szeimies, R.M.; Basset-Seguin, N.; Calzavara-Pinton, P.; Gilaberte, Y.; Haedersdal, M.; Hofbauer, G.F.L.; Hunger, R.E.; Karrer, S.; Piaserico, S.; et al. European Dermatology Forum guidelines on topical photodynamic therapy 2019 Part 1: Treatment delivery and established indications—Actinic keratoses, Bowen’s disease and basal cell carcinomas. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2225–2238. [Google Scholar] [CrossRef]
- Campione, E.; Di Prete, M.; Di Raimondo, C.; Costanza, G.; Palumbo, V.; Garofalo, V.; Mazzilli, S.; Franceschini, C.; Dika, E.; Bianchi, L.; et al. Topical Treatment of Actinic Keratosis and Metalloproteinase Expression: A Clinico-Pathological Retrospective Study. Int. J. Mol. Sci. 2022, 23, 11351. [Google Scholar] [CrossRef]
- Gellén, E.; Fidrus, E.; Janka, E.; Kollár, S.; Paragh, G.; Emri, G.; Remenyik, É. 5-Aminolevulinic acid photodynamic therapy with and without Er:YAG laser for actinic keratosis: Changes in immune infiltration. Photodiagnosis Photodyn. Ther. 2019, 26, 270–276. [Google Scholar] [CrossRef]
- Abdalla, B.M.Z.; Simas Pedreiro, B.; Garcia Morales, A.; Krutman Zveibil, D.; Paschoal, F.M. Clinical, histopathological and immunohistochemical evaluation of daylight photodynamic therapy in the treatment of field cancerization: A study of 30 cases. J. Dermatolog. Treat. 2022, 33, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Bagazgoitia, L.; Cuevas Santos, J.; Juarranz, A.; Jaén, P. Photodynamic therapy reduces the histological features of actinic damage and the expression of early oncogenic markers. Br. J. Dermatol. 2011, 165, 144–151. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, H.; Li, J. Expression of p63 and cyclooxygenase-2 and their correlation in skin tumors. J. Huazhong Univ. Sci. Technol. Med. Sci. 2007, 27, 206–208. [Google Scholar] [CrossRef]
- Amirnia, M.; Babaie-Ghazani, A.; Fakhrjou, A.; Khodaeiani, E.; Alikhah, H.; Naghavi-Behzad, M.; Zarrintan, A. Immunohistochemical study of cyclooxygenase-2 in skin tumors. J. Dermatolog. Treat. 2014, 25, 380–387. [Google Scholar] [CrossRef]
- Adamska, K.; Pawlaczyk, M.; Bowszyc-Dmochowska, M.; Gornowicz-Piotrowska, J.; Janicka-Jedyńska, M.; Fedorowicz, T.; Żaba, R. Cyclooxygenase-2 expression in actinic keratosis. Postepy Dermatol. Alergol. 2018, 35, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Karampinis, E.; Koumaki, D.; Sgouros, D.; Nechalioti, P.-M.; Toli, O.; Pappa, G.; Papadakis, M.; Georgopoulou, K.-E.; Schulze-Roussaki, A.-V.; Kouretas, D. Non-Melanoma Skin Cancer: Assessing the Systemic Burden of the Disease. Cancers 2025, 17, 703. [Google Scholar] [CrossRef] [PubMed]
- Naharro-Rodriguez, J.; Bacci, S.; Fernandez-Guarino, M. Molecular Biomarkers in Cutaneous Photodynamic Therapy: A Comprehensive Review. Diagnostics 2024, 14, 2724. [Google Scholar] [CrossRef]
- Maltusch, A.; Röwert-Huber, J.; Matthies, C.; Lange-Asschenfeldt, S.; Stockfleth, E. Modes of action of diclofenac 3%/hyaluronic acid 2.5% in the treatment of actinic keratosis. J. Dtsch. Dermatol. Ges. 2011, 9, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Bobyr, I.; Campanati, A.; Consales, V.; Martina, E.; Molinelli, E.; Diotallevi, F.; Brisigotti, V.; Giangiacomi, M.; Ganzetti, G.; Giuliodori, K.; et al. Ingenol mebutate in actinic keratosis: A clinical, videodermoscopic and immunohistochemical study. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 260–266. [Google Scholar] [CrossRef]
- Miola, A.C.; Ferreira, E.R.; Lima, T.R.R.; Schmitt, J.V.; Abbade, L.P.F.; Miot, H.A. Effectiveness and safety of 0·5% colchicine cream vs. photodynamic therapy with methyl aminolaevulinate in the treatment of actinic keratosis and skin field cancerization of the forearms: A randomized controlled trial. Br. J. Dermatol. 2018, 179, 1081–1087. [Google Scholar] [CrossRef]
| Inclusion Criteria | |
| 1 | Signed informed consent for participation in the study. |
| 2 | Age ≥ 18 years. |
| 3 | Presence of a primary AK lesion with characteristic morphology, previously untreated. |
| 4 | Good general health, with no clinically significant conditions as assessed by the physician. |
| 5 | Ability to comprehend study-related information, adhere to protocol requirements, follow medical recommendations, and attend scheduled visits. |
| Exclusion Criteria | |
| 1 | Hypersensitivity to UV or blue light. |
| 2 | Age < 18 years. |
| 3 | Pregnant or breastfeeding women, or those planning pregnancy during the study. |
| 4 | Participation in another clinical trial within 30 days prior to study initiation. |
| 5 | Inability or unwillingness to comply with study requirements. |
| 6 | Presence of acute inflammatory disease at the time of examination, with body temperature > 38 °C. |
| 7 | Active malignancy or history of cancer, including skin cancers (SCC, BCC, MM). |
| 8 | Autoimmune diseases, including Hashimoto’s thyroiditis, Graves’ disease, type 1 diabetes, rheumatoid arthritis, celiac disease, Crohn’s disease, systemic lupus erythematosus, dermatomyositis, polymyositis, ankylosing spondylitis, polymyalgia rheumatica, multiple sclerosis, Sjögren’s syndrome, and vitiligo. |
| 9 | Impaired wound healing. |
| 10 | Blood clotting disorders. |
| 11 | Chronic or intermittent use of NSAIDs during the study period. |
| 12 | Long-term anticoagulant therapy. |
| 13 | Long-term use of medications with known phototoxic effects. |
| 14 | Long-term immunosuppressive, immunomodulatory, or systemic corticosteroid therapy. |
| 15 | Planned hospitalization or surgical procedures during the study period. |
| 16 | Diastolic blood pressure > 95 mmHg or <65 mmHg. |
| 17 | Congenital or acquired immunodeficiency disorders. |
| 18 | Genophotodermatoses associated with increased skin cancer risk, such as xeroderma pigmentosum, Cockayne syndrome, and Bloom syndrome. |
| 19 | Renal dysfunction. |
| 20 | Psychiatric disorders with psychotic symptoms or major depressive disorder. |
| 21 | Substance abuse or dependence (alcohol, prescription medications, or illicit drugs) within the last 12 months. |
| 22 | Use of tanning beds or artificial UV exposure. |
| Grade | Olsen’s Clasification System |
| 1 | lesions are slightly palpable, with the AK being more felt than visible |
| 2 | lesions are moderately thick, easily felt, and visible |
| 3 | lesions are very thick, hyperkeratotic, and/or clearly noticeable as AK |
| Grade | Dermatoscopic Severity Scale [35] with Our Modification |
| I | red pseudo-network pattern with discrete white scales |
| I/II | features of I and II grade |
| II | an erythematous background, the so-called “strawberry pattern” |
| II/III | features of II and III grade |
| III | enlarged follicular openings with keratotic plugs over a scaly, white-to-yellow background |
| The expression of Ki67 and p53 (nuclear staining) was graded based on the mean percentage of immunopositive cells per high-power field (HPF): | |
| Score | Immunopositive Cells (%) |
| 0 | <5% |
| 1 | 6–15% |
| 2 | 16–30% |
| 3 | 31–50% |
| 4 | >50% |
| For Ki67 and p53, the extent of immunopositive cells in the epidermis was graded as follows: | |
| Score | Extent of Immunopositive Cells in the Epidermis |
| 0 | No immunopositive cells |
| 1 | <1/3 of the lower epidermal/lesion level |
| 2 | 1/3–2/3 of the epidermal/lesion level |
| 3 | 2/3 to the entire epidermis |
| The expression of COX-2 (cytoplasmic staining) was graded based on mean immunoexpression intensity: | |
| Score | Immunoexpression Intensity |
| 0 | Not detectable |
| 1 | Mild |
| 2 | Moderate |
| 3 | Strong |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceryn, J.; Lesiak, A.; Ciążyńska, M.; Sobolewska-Sztychny, D.; Noweta, M.; Stasikowska-Kanicka, O.; Ciążyński, K.; Zalaudek, I.; Narbutt, J. Targeting Biomarkers of Proliferation and Inflammation (Ki67, p53, and COX-2) in Actinic Keratoses with Photodynamic Therapy. Biomedicines 2025, 13, 1487. https://doi.org/10.3390/biomedicines13061487
Ceryn J, Lesiak A, Ciążyńska M, Sobolewska-Sztychny D, Noweta M, Stasikowska-Kanicka O, Ciążyński K, Zalaudek I, Narbutt J. Targeting Biomarkers of Proliferation and Inflammation (Ki67, p53, and COX-2) in Actinic Keratoses with Photodynamic Therapy. Biomedicines. 2025; 13(6):1487. https://doi.org/10.3390/biomedicines13061487
Chicago/Turabian StyleCeryn, Justyna, Aleksandra Lesiak, Magdalena Ciążyńska, Dorota Sobolewska-Sztychny, Marcin Noweta, Olga Stasikowska-Kanicka, Karol Ciążyński, Iris Zalaudek, and Joanna Narbutt. 2025. "Targeting Biomarkers of Proliferation and Inflammation (Ki67, p53, and COX-2) in Actinic Keratoses with Photodynamic Therapy" Biomedicines 13, no. 6: 1487. https://doi.org/10.3390/biomedicines13061487
APA StyleCeryn, J., Lesiak, A., Ciążyńska, M., Sobolewska-Sztychny, D., Noweta, M., Stasikowska-Kanicka, O., Ciążyński, K., Zalaudek, I., & Narbutt, J. (2025). Targeting Biomarkers of Proliferation and Inflammation (Ki67, p53, and COX-2) in Actinic Keratoses with Photodynamic Therapy. Biomedicines, 13(6), 1487. https://doi.org/10.3390/biomedicines13061487








