Catheter Ablation of Frequent PVCs in Structural Heart Disease: Impact on Left Ventricular Function and Clinical Outcomes
Abstract
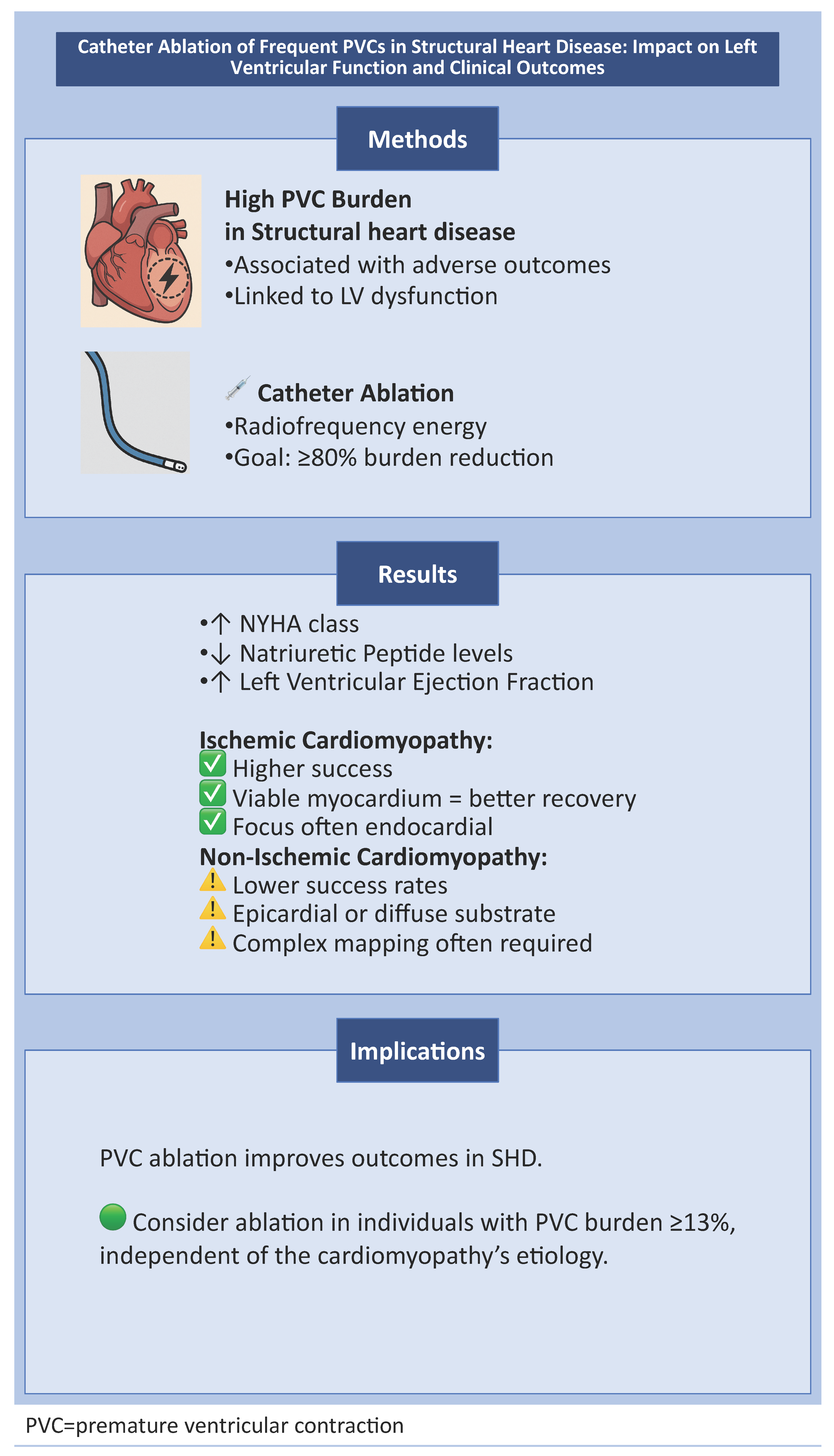
1. PVC Ablation in Structural Heart Disease
2. Methods
- Included adult patients with documented structural heart disease (ischemic or non-ischemic);
- Evaluated the impact of catheter ablation on frequent PVCs (typically defined as a burden >4% on 24 h Holter monitoring);
- Reported at least one post-ablation outcome related to LVEF, heart failure symptoms (e.g., NYHA class), natriuretic peptide levels, or clinical endpoints;
- Published in English in peer-reviewed journals.
3. Results
3.1. Ischemic Cardiomyopathy
3.2. Non-Ischemic Cardiomyopathy
3.3. Mixed Etiologies
| Author | No. of Patients and Patient % on Antiarrhythmic Drugs | Success Rates (at Least 80% Burden Reduction) | PVC Burden Pre/Post-Ablation | LVEF Pre/Post-Ablation | LVEDD Pre/Post-Ablation | BNP Pre/Post-Ablation | NYHA Class Pre/Post-Ablation | Cardiac Mortality Reduction |
|---|---|---|---|---|---|---|---|---|
| Penela 2013 [14] | n = 80 (17 ICMP, 4 non-compaction, 2 valvular heart disease) b-blocker 85%, amiodarone 19% | 85% acutely, 66% in 12 months | 22 ± 13% to not declared | 33.7 ± 8% to 45.8 ± 10.9% | 59.5 ± 5.9 mm to 54.9 ± 6.1 mm in successful procedures | 109 to 60 pg/mL in successful procedures | From 12 patients (23%) with NYHA I at baseline to 42 (79%) | - |
| Penela 2015 [13] | n = 66 (11 ICMP, 3 non-compaction, 1 valvular heart disease, the rest NICMP) | 94% acutely to 76% at 6 months | 21 ± 12 to not declared, in all patients | 28% ± 4% to 42% ± 12% in all patients | 61 ± 6 to 57 ± 6 in all patients | 246 ± 187 to 176 ± 380 pg/mL in all patients | 2 patients with NYHA I (3%) at baseline to 35 (53%) in all patients | - |
| Blaye-Felice 2016 [16] | n = 96 (22 ICMP, 4 valvular heart disease, 1 myocarditis) | 79% in 24 months | 26 ± 12 to 4 ± 7% in successful procedures | 38 ± 10 to 50 ± 13% in all patients | 62 ± 8 to 57 ± 8 in all patients | - | NYHA class I from 49% to 83% in all patients | |
| Wojdyła-Hordyńska 2017 [18] | n = 65 (29 NICMP, 17 ICMP, 7 valvular heart disease) | 83.1% at 6 months | 22,267 ± 12,934 to 2172 ± 3692 in all patients with SHD | 45.2% ± 14.3% to 50.9% ± 13.5% in all patients with SHD | 56.1 ± 8.4 to not declared | - | - | - |
| Abdelhamid 2018 [17] | n = 42 (18 ICMP, 14 NICMP, 7 valvular disease) b-blocker 93%, amiodarone 55% | 90.4% acutely, 78.5% at 6 months in SHD | 30.76 ± 9.91 to 4.8 ± 11.45 in all patients with SHD | 36.8 ± 7.1 to 47.2 ± 11.8 in all patients with SHD | 61.4 ± 6.9 to 57.4 ± 5.9 in all patients with SHD | - | - | - |
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Maggioni, A.P.; Zuanetti, G.; Franzosi, M.G.; Rovelli, F.; Santoro, E.; Staszewsky, L.; Tavazzi, L.; Tognoni, G. Prevalence and prognostic significance of ventricular arrhythmias after acute myocardial infarction in the fibrinolytic era. GISSI-2 results. Circulation 1993, 87, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Echt, D.S.; Liebson, P.R.; Mitchell, L.B.; Peters, R.W.; Obias-Manno, D.; Barker, A.H.; Arensberg, D.; Baker, A.; Friedman, L.; Greene, H.L.; et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N. Engl. J. Med. 1991, 324, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Boas, R.; Thune, J.J.; Pehrson, S.; Køber, L.; Nielsen, J.C.; Videbæk, L.; Haarbo, J.; Korup, E.; Bruun, N.E.; Brandes, A.; et al. Prevalence and prognostic association of ventricular arrhythmia in non-ischaemic heart failure patients: Results from the DANISH trial. Europace 2021, 23, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Milaras, N.; Dourvas, P.; Doundoulakis, I.; Sotiriou, Z.; Nevras, V.; Xintarakou, A.; Laina, A.; Soulaidopoulos, S.; Zachos, P.; Kordalis, A.; et al. Noninvasive electrocardiographic risk factors for sudden cardiac death in dilated ca rdiomyopathy: Is ambulatory electrocardiography still relevant? Heart Fail Rev. 2023, 28, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Simantirakis, E.N.; Koutalas, E.P.; Vardas, P.E. Arrhythmia-induced cardiomyopathies: The riddle of the chicken and the egg still unanswered? Europace 2012, 14, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Fletcher, R.D.; Fisher, S.; Deedwania, P.; Lewis, D.; Massie, B.; Singh, B.; Colling, C.L. Congestive heart failure: Survival trial of antiarrhythmic therapy (CHF STAT). The CHF STAT Investigators. Control Clin. Trials 1992, 13, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.N.; Fletcher, R.D.; Fisher, S.G.; Singh, B.N.; Lewis, H.D.; Deedwania, P.C.; Massie, B.M.; Colling, C.; Lazzeri, D. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N. Engl. J. Med. 1995, 333, 77–82. [Google Scholar] [CrossRef]
- Sarrazin, J.F.; Nault, I. When to Consider Ablation for Premature Ventricular Complexes? Can. J. Cardiol. 2022, 38, 540–543. [Google Scholar] [CrossRef]
- Schleberger, R.; Riess, J.; Brauer, A.; Pinnschmidt, H.O.; Rottner, L.; Moser, F.; Moser, J.; Kany, S.; My, I.; Lemoine, M.D.; et al. Ablation of Outflow Tract Arrhythmias in Patients With and Without Structural Heart Disease-A Comparative Analysis. Front. Cardiovasc. Med. 2022, 9, 910042. [Google Scholar] [CrossRef] [PubMed]
- Marcus, G.M. Evaluation and Management of Premature Ventricular Complexes. Circulation 2020, 141, 1404–1418. [Google Scholar] [CrossRef]
- El Kadri, M.; Yokokawa, M.; Labounty, T.; Mueller, G.; Crawford, T.; Good, E.; Jongnarangsin, K.; Chugh, A.; Ghanbari, H.; Latchamsetty, R.; et al. Effect of ablation of frequent premature ventricular complexes on left ventricular function in patients with nonischemic cardiomyopathy. Heart Rhythm. 2015, 12, 706–713. [Google Scholar] [CrossRef]
- Sarrazin, J.F.; Labounty, T.; Kuhne, M.; Crawford, T.; Armstrong, W.F.; Desjardins, B.; Good, E.; Jongnarangsin, K.; Chugh, A.; Oral, H.; et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm. 2009, 6, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Penela, D.; Acosta, J.; Aguinaga, L.; Tercedor, L.; Ordoñez, A.; Fernández-Armenta, J.; Andreu, D.; Sánchez-Millán, P.J.; Cabanelas, N.; Tolosana, J.M.; et al. Ablation of frequent PVC in patients meeting criteria for primary prevention ICD implant: Safety of withholding the implant. Heart Rhythm. 2015, 12, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Penela, D.; Van Huls Van Taxis, C.; Aguinaga, L.; Fernández-Armenta, J.; Mont, L.; Castel, M.A.; Heras, M.; Tolosana, J.M.; Sitges, M.; Ordóñez, A.; et al. Neurohormonal, structural, and functional recovery pattern after premature ventricular complex ablation is independent of structural heart disease status in patients with depressed left ventricular ejection fraction: A prospective multicenter study. J. Am. Coll. Cardiol. 2013, 62, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Berruezo, A.; Penela, D.; Jáuregui, B.; Soto-Iglesias, D.; Aguinaga, L.; Ordóñez, A.; Fernández-Armenta, J.; Martínez, M.; Tercedor, L.; Bisbal, F.; et al. Mortality and morbidity reduction after frequent premature ventricular complexes ablation in patients with left ventricular systolic dysfunction. Europace 2019, 21, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Sadron Blaye-Felice, M.; Hamon, D.; Sacher, F.; Pascale, P.; Rollin, A.; Duparc, A.; Mondoly, P.; Derval, N.; Denis, A.; Cardin, C.; et al. Premature ventricular contraction-induced cardiomyopathy: Related clinical and electrophysiologic parameters. Heart Rhythm. 2016, 13, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.A.; Samir, R. Reversal of premature ventricular complexes induced cardiomyopathy. Influence of concomitant structural heart disease. Indian Heart J. 2018, 70, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Wojdyła-Hordyńska, A.; Kowalski, O.; Hordyński, G.J.; Dinov, B.; Sommer, P.; Hindricks, G.; Feusette, P.; Arya, A. The effect of radiofrequency catheter ablation of frequent premature ventricular complexes and arrhythmia burden on left ventricular function. Kardiol. Pol. 2017, 75, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Milaras, N.; Kordalis, A.; Tsiachris, D.; Sakalidis, A.; Ntalakouras, I.; Pamporis, K.; Dourvas, P.; Apostolos, A.; Sotiriou, Z.; Arsenos, P.; et al. Ischemia testing and revascularization in patients with monomorphic ventricular tachycardia: A relic of the past? Curr. Probl. Cardiol. 2024, 49, 102358. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Perera, T.; Ganesan, A.; Sullivan, T.; Lau, D.H.; Roberts-Thomson, K.C.; Brooks, A.G.; Sanders, P. Complications of catheter ablation of atrial fibrillation: A systematic review. Circ. Arrhythmia Electrophysiol. 2013, 6, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
| Author | No. of Patients and Patient % on Antiarrhythmic Drugs | Patients | Success Rates (at Least 80% Burden Reduction) | PVC Burden Pre/Post-Ablation | LVEF Pre/Post-Ablation | LVEDD Pre/Post-Ablation | BNP Pre/Post-Ablation | NYHA Class Pre/Post-Ablation | Cardiac Mortality Reduction |
|---|---|---|---|---|---|---|---|---|---|
| Sarrazin 2009 [12] | n = 30 All ICMP (15 ablated, 15 controls) b-blocker 67%, amiodarone 8%, Sotalol 4% | n = 30 All ICMP (15 ablated, 15 controls) | 100% at 3 to 6 months | 21.8 ± 12.5 to 2.6 ± 5.0% all patients | 0.38 ± 0.11 to 0.51 ± 0.09 all patients | 56 ± 11 mm vs. 51 ± 8 mm all patients | - | 1.8 ± 0.8 to 1.3 ± 0.5 all patients | - |
| Berruezo 2019 [15] | n = 101 (22 ICMP) | n = 101 (22 ICMP) | 94% acutely | 21 ± 12% to 3.8 ± 6% in all patients | 32 ± 8% to 39 ± 12% in all patients | 62 ± 7 mm to 59 ± 6 mm in all patients | 36 (78–321) to 68 (32–144) pg/mL in all patients | 2.2 ± 0.6% at baseline to 1.3 ± 0.6% in all patients | HR = 0.18 (95% CI: 0.05–0.66; p = 0.01) in successful procedures |
| Author | No. of patients and PATIENT % on Antiarrhythmic Drugs | Patients | Success Rates (at Least 80% Burden Reduction) | PVC Burden Pre/Post-Ablation | LVEF Pre/Post-Ablation | LVEDD Pre/Post-Ablation | BNP Pre/Post-Ablation | NYHA Class Pre/Post-Ablation | Cardiac Mortality Reduction |
|---|---|---|---|---|---|---|---|---|---|
| El Kadri 2015 [11] | n = 30 All NICMP b-blocker 63% amiodarone 3%, Sotalol 3%, Dofetilide 4% | n = 30 All NICMP | 60% at 48 months | 23.1% ± 8.8% to 1.0% ± 0.9% in successful procedures | 33.9% ± 14.5% to 45.7% ± 17% in successful procedures | 58.8 ± 9.5 to 56.11 ± 8.9 in all patients | - | 2.3 ± 0.6 to 1.1 ± 0.2 in successful procedures | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milaras, N.; Ktenopoulos, N.; Karakasis, P.; Karanikola, A.-E.; Michopoulos, V.; Pamporis, K.; Dourvas, P.; Apostolos, A.; Sotiriou, Z.; Archontakis, S.; et al. Catheter Ablation of Frequent PVCs in Structural Heart Disease: Impact on Left Ventricular Function and Clinical Outcomes. Biomedicines 2025, 13, 1488. https://doi.org/10.3390/biomedicines13061488
Milaras N, Ktenopoulos N, Karakasis P, Karanikola A-E, Michopoulos V, Pamporis K, Dourvas P, Apostolos A, Sotiriou Z, Archontakis S, et al. Catheter Ablation of Frequent PVCs in Structural Heart Disease: Impact on Left Ventricular Function and Clinical Outcomes. Biomedicines. 2025; 13(6):1488. https://doi.org/10.3390/biomedicines13061488
Chicago/Turabian StyleMilaras, Nikias, Nikolaos Ktenopoulos, Paschalis Karakasis, Aikaterini-Eleftheria Karanikola, Vasileios Michopoulos, Konstantinos Pamporis, Panagiotis Dourvas, Anastasios Apostolos, Zoi Sotiriou, Stefanos Archontakis, and et al. 2025. "Catheter Ablation of Frequent PVCs in Structural Heart Disease: Impact on Left Ventricular Function and Clinical Outcomes" Biomedicines 13, no. 6: 1488. https://doi.org/10.3390/biomedicines13061488
APA StyleMilaras, N., Ktenopoulos, N., Karakasis, P., Karanikola, A.-E., Michopoulos, V., Pamporis, K., Dourvas, P., Apostolos, A., Sotiriou, Z., Archontakis, S., Kordalis, A., Gatzoulis, K., & Sideris, S. (2025). Catheter Ablation of Frequent PVCs in Structural Heart Disease: Impact on Left Ventricular Function and Clinical Outcomes. Biomedicines, 13(6), 1488. https://doi.org/10.3390/biomedicines13061488









