Predictive Vascular Changes in OCTA in Diabetic Patients
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. General Characteristics
3.2. OCTA Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DM | Diabetes mellitus |
| DR | Diabetic retinopathy |
| ETDRS | Early Treatment Diabetic Retinopathy Study |
| OCTA | Optical coherence tomography angiography |
| SCP | Superficial capillary plexus |
| DCP | Deep capillary plexus |
| FAZ | Foveal avascular zone |
| FLOW CC | Flow area in the choriocapillaris |
| VD | Vascular density |
| DM + DR | Diabetic patients with diabetic retinopathy |
| DMnoDR | Diabetic patients without diabetic retinopathy |
| NPDR | Non-proliferative diabetic retinopathy |
References
- Avogaro, A.; Fadini, G.P. Microvascular complications in diabetes: A growing concern for cardiologists. Int. J. Cardiol. 2019, 291, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Ishibazawa, A.; Nagaoka, T.; Takahashi, A.; Omae, T.; Tani, T.; Sogawa, K.; Yokota, H.; Yoshida, A. Optical coherence tomography angiography in diabetic retinopathy: A prospective pilot study. Arch. Ophthalmol. 2015, 160, 35–44.e1. [Google Scholar] [CrossRef] [PubMed]
- Sambhav, K.; Abu-Amero, K.K.; Chalam, K.V. Deep capillary macular perfusion indices obtained with OCT angiography correlate with degree of nonproliferative diabetic retinopathy. Eur. J. Ophthalmol. 2017, 27, 716–729. [Google Scholar] [CrossRef]
- Chui, T.Y.P.; Zhong, Z.; Song, H.; Burns, S.A. Foveal avascular zone and its relationship to foveal pit shape. Optom. Vis. Sci. 2012, 89, 602–610. [Google Scholar] [CrossRef]
- Arend, O.; Wolf, S.; Jung, F.; Bertram, B.; Postgens, H.; Toonen, H.; Reim, M. Retinal microcirculation in patients with diabetes mellitus: Dynamic and morphological analysis of perifoveal capillary network. Br. J. Ophthalmol. 1991, 75, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A. Measuring fundus landmarks. Investig. Ophthalmol. Vis. Sci. 1990, 31, 41–42. [Google Scholar]
- Sander, B.; Larsen, M.; Engler, C.; Lund-Andersen, H.; Parving, H. Early changes in diabetic retinopathy: Capillary loss and blood-retina barrier permeability in relation to metabolic control. Acta Ophthalmol. 1994, 72, 553–559. [Google Scholar] [CrossRef]
- Ghassemi, F.; Fadakar, K.; Berijani, S.; Babeli, A.; Gholizadeh, A.; Sabour, S. Quantitative assessment of vascular density in diabetic retinopathy subtypes with optical coherence tomography angiography. BMC Ophthalmol. 2021, 21, 82. [Google Scholar] [CrossRef]
- Durbin, M.K.; An, L.; Shemonski, N.D.; Soares, M.; Santos, T.; Lopes, M.; Neves, C.; Cunha-Vaz, J. Quantification of Retinal Microvascular Density in Optical Coherence Tomographic Angiography Images in Diabetic Retinopathy. JAMA Ophthalmol. 2017, 135, 370–376. [Google Scholar] [CrossRef]
- Agemy, S.A.; Scripsema, N.K.; Shah, C.M.; Chui, T.; Garcia, P.M.; Lee, J.G.; Gentile, R.C.; Hsiao, Y.-S.; Zhou, Q.; Ko, T.; et al. Retinal vascular perfusion density mapping using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina 2015, 35, 2353–2363. [Google Scholar] [CrossRef]
- Al-Sheikh, M.; Akil, H.; Pfau, M.; Sadda, S.R. Swept-Source OCT Angiography Imaging of the Foveal Avascular Zone and Macular Capillary Network Density in Diabetic Retinopathy. Investig. Opthalmol. Vis. Sci. 2016, 57, 3907–3913. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Waheed, N.K.; Moult, E.M.B.; Adhi, M.; Lee, B.; De Carlo, T.; Jayaraman, V.; Baumal, C.R.; Duker, J.S.; Fujimoto, J.G. Ultrahigh speed swept source optical coherence tomography angiography of retinal and choriocapillaris alterations in diabetic patients with and without retinopathy. Retina 2017, 37, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.Y.; Chu, Z.; Shahidzadeh, A.; Wang, R.K.; Puliafito, C.A.; Kashani, A.H. Quantifying Microvascular Density and Morphology in Diabetic Retinopathy Using Spectral-Domain Optical Coherence Tomography Angiography. Investig. Opthalmol. Vis. Sci. 2016, 57, 362–370. [Google Scholar] [CrossRef]
- Samara, W.A.; Shahlaee, A.; Adam, M.K.; Khan, M.A.; Chiang, A.; Maguire, J.I.; Hsu, J.; Ho, A.C. Quantification of Diabetic Macular Ischemia Using Optical Coherence Tomography Angiography and Its Relationship with Visual Acuity. Ophthalmology 2017, 124, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Zahid, S.; Dolz-Marco, R.; Freund, K.B.; Balaratnasingam, C.; Dansingani, K.; Gilani, F.; Mehta, N.; Young, E.; Klifto, M.R.; Chae, B.; et al. Fractal Dimensional Analysis of Optical Coherence Tomography Angiography in Eyes with Diabetic Retinopathy. Investig. Opthalmol. Vis. Sci. 2016, 57, 4940–4947. [Google Scholar] [CrossRef]
- Lupidi, M.; Coscas, G.; Coscas, F.; Fiore, T.; Spaccini, E.; Fruttini, D.; Cagini, C. Retinal microvasculature in nonproliferative diabetic retinopathy: Automated quantitative optical coherence tomography angiography assessment. Ophthalmic Res. 2017, 58, 131–141. [Google Scholar] [CrossRef]
- Carnevali, A.; Sacconi, R.; Corbelli, E.; Tomasso, L.; Querques, L.; Zerbini, G.; Scorcia, V.; Bandello, F.; Querques, G. Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol. 2017, 54, 695–702. [Google Scholar] [CrossRef]
- Bhanushali, D.; Anegondi, N.; Gadde, S.G.; Srinivasan, P.; Chidambara, L.; Yadav, N.K.; Roy, A.S. Linking retinal microvasculature features with severity of diabetic retinopathy using optical coherence tomography angiographyretinal vasculature changes in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 519–525. [Google Scholar] [CrossRef]
- Chu, Z.; Lin, J.; Gao, C.; Xin, C.; Zhang, Q.; Chen, C.-L.; Roisman, L.; Gregori, G.; Rosenfeld, P.J.; Wang, R.K. Quantitative assessment of the retinal microvasculature using optical coherence tomography angiography. J. Biomed. Opt. 2016, 21, 066008. [Google Scholar] [CrossRef]
- Kim, K.; Kim, E.S.; Kim, D.G.; Yu, S.-Y. Progressive retinal neurodegeneration and microvascular change in diabetic retinopathy: Longitudinal study using OCT angiography. Acta Diabetol. 2019, 56, 1275–1282. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Liu, X.; Shi, Y.; Jin, X.; Zhang, Y.; Xu, S.; Zhang, N.; Dong, L.; Zhou, S.; et al. Quantitative analysis of retinal vessel density and thickness changes in diabetes mellitus evaluated using optical coherence tomography angiography: A cross-sectional study. BMC Ophthalmol. 2021, 21, 259. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Yang, D.; Huang, Z.; Zeng, Y.; Wang, J.; Hu, Y.; Zhang, L. Optical coherence tomography angiography discerns preclinical diabetic retinopathy in eyes of patients with type 2 diabetes without clinical diabetic retinopathy. Acta Diabetol. 2018, 55, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, G.; Chihara, E.; Takahashi, H.; Amano, H.; Okazaki, K. Quantitative Retinal Optical Coherence Tomography Angiography in Patients with Diabetes Without Diabetic Retinopathy. Investig. Opthalmol. Vis. Sci. 2017, 58, 190–196. [Google Scholar] [CrossRef]
- Gültekin, B.P.; Hamurcu, M. Evaluation of optical coherence tomography angiography and pattern and flash electroretinography in diabetes mellitus without retinopathy. Ann. Med. 2024, 56, 2397573. [Google Scholar] [CrossRef]
- Simonett, J.M.; Scarinci, F.; Picconi, F.; Giorno, P.; De Geronimo, D.; Di Renzo, A.; Varano, M.; Frontoni, S.; Parravano, M. Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol. 2017, 95, e751–e755. [Google Scholar] [CrossRef]
- Ryu, G.; Kim, I.; Sagong, M. Topographic analysis of retinal and choroidal microvasculature according to diabetic retinopathy severity using optical coherence tomography angiography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.S.; Gao, S.S.; Liu, L.; Lauer, A.K.; Bailey, S.T.; Flaxel, C.J.; Wilson, D.J.; Huang, D.; Jia, Y. Automated Quantification of Capillary Nonperfusion Using Optical Coherence Tomography Angiography in Diabetic Retinopathy. JAMA Ophthalmol. 2016, 134, 367–373. [Google Scholar] [CrossRef]
- Nesper, P.L.; Roberts, P.K.; Onishi, A.C.; Chai, H.; Liu, L.; Jampol, L.M.; Fawzi, A.A. Quantifying Microvascular Abnormalities with Increasing Severity of Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Investig. Opthalmol. Vis. Sci. 2017, 58, 307–315. [Google Scholar] [CrossRef]
- Li, L.; Almansoob, S.; Zhang, P.; Zhou, Y.; Tan, Y.; Gao, L. Quantitative analysis of retinal and choroid capillary ischaemia using optical coherence tomography angiography in type 2 diabetes. Acta Ophthalmol. 2019, 97, 240–246. [Google Scholar] [CrossRef]
- Mastropasqua, R.; Toto, L.; Mastropasqua, A.; Aloia, R.; De Nicola, C.; A Mattei, P.; Di Marzio, G.; Di Nicola, M.; Di Antonio, L. Foveal avascular zone area and parafoveal vessel density measurements in different stages of diabetic retinopathy by optical coherence tomography angiography. Int. J. Ophthalmol. 2017, 10, 1545–1551. [Google Scholar] [CrossRef]
- Conti, F.F.; Qin, V.L.; Rodrigues, E.B.; Sharma, S.; Rachitskaya, A.V.; Ehlers, J.P.; Singh, R.P. Choriocapillaris and retinal vascular plexus density of diabetic eyes using split-spectrum amplitude decorrelation spectral-domain optical coherence tomography angiography. Br. J. Ophthalmol. 2018, 103, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhou, H.; Zhang, Q.; Chu, Z.; de Koo, L.C.O.; Chao, J.R.; Rezaei, K.A.; Saraf, S.S.; Wang, R.K.; Tsai, D.-C. Quantitative assessment of choriocapillaris flow deficits in diabetic retinopathy: A swept-source optical coherence tomography angiography study. PLoS ONE 2020, 15, e0243830. [Google Scholar] [CrossRef] [PubMed]
- Gendelman, I.; Alibhai, A.Y.; Moult, E.M.; Levine, E.S.; Braun, P.X.; Mehta, N.; Zhao, Y.; Ishibazawa, A.; Sorour, O.A.; Baumal, C.R.; et al. Topographic analysis of macular choriocapillaris flow deficits in diabetic retinopathy using swept–source optical coherence tomography angiography. Int. J. Retin. Vitr. 2020, 6, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Parravano, M.; Ziccardi, L.; Borrelli, E.; Costanzo, E.; Frontoni, S.; Picconi, F.; Parisi, V.; Sacconi, R.; Di Renzo, A.; Varano, M.; et al. Outer retina dysfunction and choriocapillaris impairment in type 1 diabetes. Sci. Rep. 2021, 11, 15183. [Google Scholar] [CrossRef]
- de Carlo, T.E.; Chin, A.T.; Filho, M.A.B.; Adhi, M.; Branchini, L.; Salz, D.A.; Baumal, C.R.; Crawford, C.; Reichel, E.; Witkin, A.J.; et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina 2015, 35, 2364–2370. [Google Scholar] [CrossRef]
- Di, G.; Weihong, Y.; Xiao, Z.; Zhikun, Y.; Xuan, Z.; Yi, Q.; Fangtian, D. A morphological study of the foveal avascular zone in patients with diabetes mellitus using optical coherence tomography angiography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 873–879. [Google Scholar] [CrossRef]
- Takase, N.; Nozaki, M.; Kato, A.; Ozeki, H.; Yoshida, M.; Ogura, Y. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina 2015, 35, 2377–2383. [Google Scholar] [CrossRef]
- Bresnick, G.H.; Condit, R.; Syrjala, S.; Palta, M.; Groo, A.; Korth, K. Abnormalities of the foveal avascular zone in diabetic Retinopathy. Arch. Ophthalmol. 1984, 102, 1286–1293. [Google Scholar] [CrossRef]
- Conrath, J.; Giorgi, R.; Raccah, D.; Ridings, B. Foveal avascular zone in diabetic retinopathy: Quantitative vs qualitative assessment. Eye 2004, 19, 322–326. [Google Scholar] [CrossRef]
- Khadamy, J. Can Foveal Avascular Zone (FAZ) Assessment by OCT Angiography, Used for Grading of Diabetic Retinopathy? Adv. Ophthalmol. Vis. Syst. 2016, 5, 278–280. [Google Scholar] [CrossRef][Green Version]
- O’SHea, S.M.; O’dWyer, V.M.; Scanlon, G. Normative data on the foveal avascular zone in a young healthy Irish population using optical coherence tomography angiography. Eur. J. Ophthalmol. 2022, 32, 2824–2832. [Google Scholar] [CrossRef] [PubMed]
- Kannenkeril, D.; Nolde, J.M.; Kiuchi, M.G.; Carnagarin, R.; Lugo-Gavidia, L.M.; Chan, J.; Joyson, A.; Jose, A.; Robinson, S.; Matthews, V.B.; et al. Retinal Capillary Damage Is Already Evident in Patients with Hypertension and Prediabetes and Associated with HbA1c Levels in the Nondiabetic Range. Diabetes Care 2022, 45, 1472–1475. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Song, Y.Y.; Il-Jung; Na, Y.J.; Lee, Y.H.; Ki, J.Y.; Lee, M.W. The impairment of the deep vascular complex in prolonged type 2 diabetes patients without clinical diabetic retinopathy. PLoS ONE 2022, 17, e0269182. [Google Scholar] [CrossRef] [PubMed]

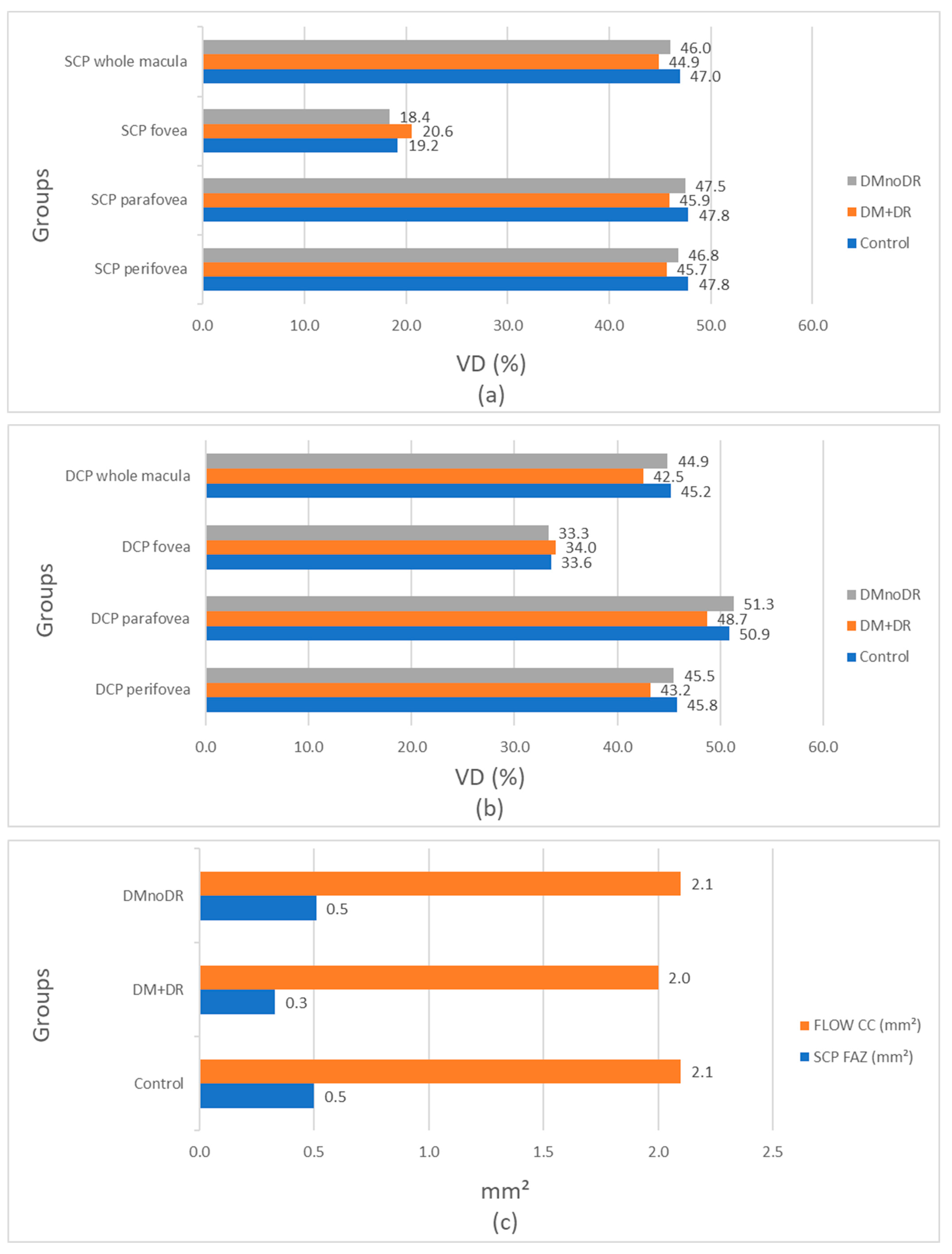
| DMnoDR * (N = 175) Mean ± SD (%) | DM + DR * (N = 84) Mean ± SD (%) | Control (N = 171) Mean ± SD (%) | DMnoDR vs. Control p Value ** | DM + DR vs. Control p Value ** | DMnoDR vs. DM + DR p Value ** | |
|---|---|---|---|---|---|---|
| SCP * macula | 46 ± 4.1 | 44.9 ± 4.2 | 47 ± 3.8 | 0.025 | 0.000 | 0.034 |
| SCP fovea | 18.4 ±7.8 | 20.6 ± 8 | 19.2 ± 7.7 | 0.308 | 0.177 | 0.029 |
| SCP parafovea | 47.5 ± 5.6 | 45.9 ± 4.9 | 47.8 ± 5.8 | 0.691 | 0.013 | 0.029 |
| SCP perifovea | 46.8 ± 4.2 | 45.7 ± 4.3 | 47.8 ± 3.8 | 0.022 | 0.000 | 0.051 |
| DMnoDR * (N = 175) Mean ± SD (%) | DM + DR * (N = 84) Mean ± SD (%) | Control (N = 171) Mean ± SD (%) | DMnoDR vs. Control p Value ** | DMnoDR vs. DM + DR p Value ** | DM + DR vs. Control p Value ** | |
|---|---|---|---|---|---|---|
| DCP * macula | 44.9 ± 5.4 | 42.5 ± 4.9 | 45.2 ± 4.8 | 0.574 | 0.001 | 0.000 |
| DCP fovea | 33.3 ± 8.7 | 34.0 ± 7.3 | 33.6 ± 8.2 | 0.522 | 0.730 | 0.719 |
| DCP parafovea | 51.3 ± 4.5 | 48.7 ± 4.3 | 50.9 ± 4.7 | 0.369 | 0.000 | 0.000 |
| DCP perifovea | 45.5 ± 6 | 43.2 ± 5.7 | 45.8 ± 5.5 | 0.612 | 0.002 | 0.001 |
| DMnoDR * (N = 175) Mean ± SD (mm2) | DM + DR * (N = 84) Mean ± SD (mm2) | Control (N = 171) Mean ± SD (mm2) | DMnoDR vs. Control p Value ** | DMnoDR vs. DM + DR p Value ** | DM + DR vs. Control p Value ** | |
|---|---|---|---|---|---|---|
| FLOW CC * | 2.1 ± 0.1 | 2.0 ± 0.1 | 2.1 ± 0.1 | 0.143 | 0.000 | 0.000 |
| FAZ SCP * | 0.51 ± 0.1 | 0.33 ± 0.08 | 0.50 ± 0.1 | 0.806 | 0.003 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuk, J.; Stanisavljevic, D.; Vasilijevic, J.; Jeremic Kaplarevic, M.; Micovic, M.; Risimic, A.; Risimic, D. Predictive Vascular Changes in OCTA in Diabetic Patients. Biomedicines 2025, 13, 1486. https://doi.org/10.3390/biomedicines13061486
Cuk J, Stanisavljevic D, Vasilijevic J, Jeremic Kaplarevic M, Micovic M, Risimic A, Risimic D. Predictive Vascular Changes in OCTA in Diabetic Patients. Biomedicines. 2025; 13(6):1486. https://doi.org/10.3390/biomedicines13061486
Chicago/Turabian StyleCuk, Jelena, Dejana Stanisavljevic, Jelena Vasilijevic, Milica Jeremic Kaplarevic, Milica Micovic, Aleksandar Risimic, and Dijana Risimic. 2025. "Predictive Vascular Changes in OCTA in Diabetic Patients" Biomedicines 13, no. 6: 1486. https://doi.org/10.3390/biomedicines13061486
APA StyleCuk, J., Stanisavljevic, D., Vasilijevic, J., Jeremic Kaplarevic, M., Micovic, M., Risimic, A., & Risimic, D. (2025). Predictive Vascular Changes in OCTA in Diabetic Patients. Biomedicines, 13(6), 1486. https://doi.org/10.3390/biomedicines13061486







