Pulmonary Involvement in Systemic Lupus Erythematosus: A Potentially Overlooked Condition
Abstract
1. Introduction
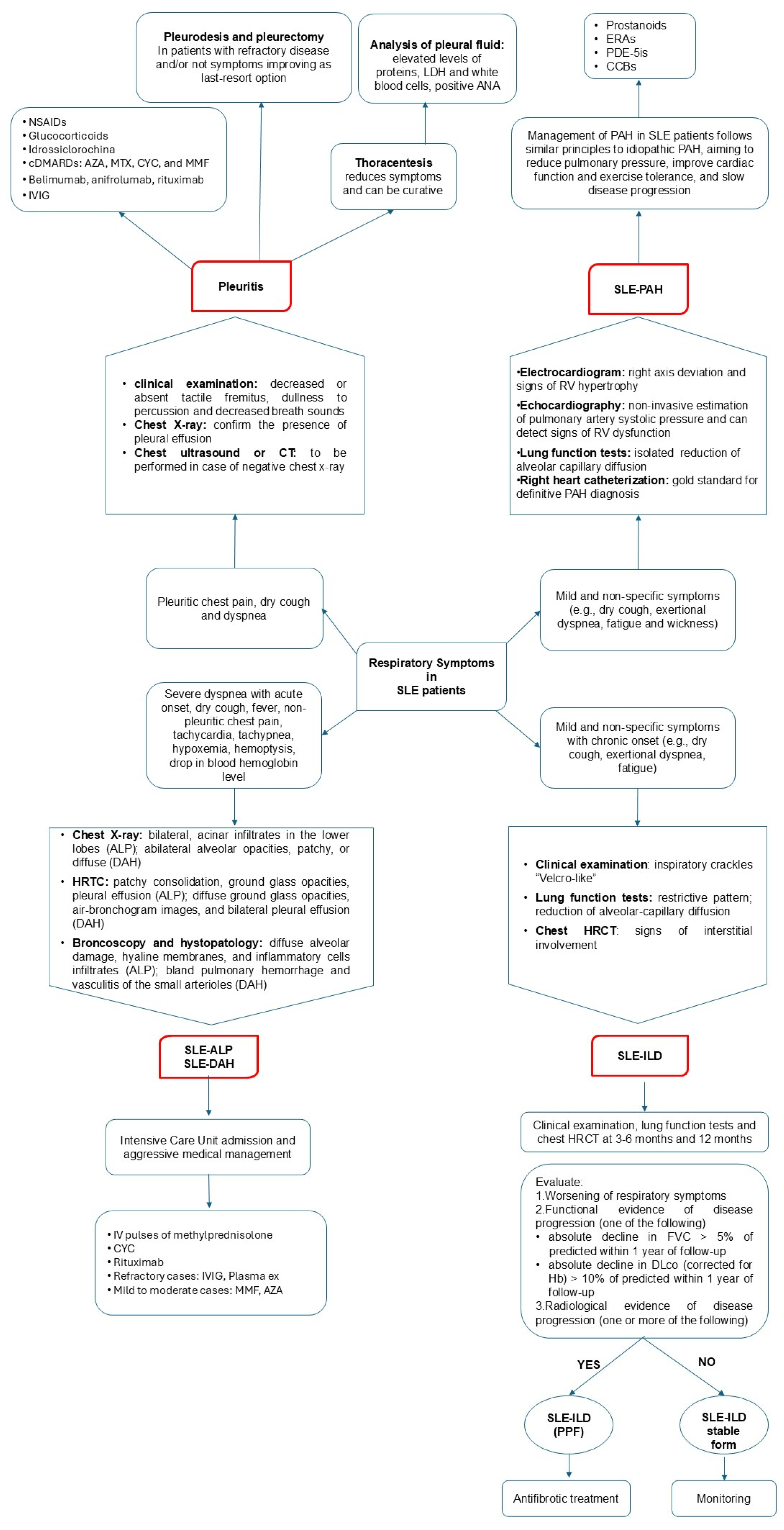
2. Clinical Patterns of Pulmonary Involvement in Systemic Erythematous Lupus
2.1. Pleuritis
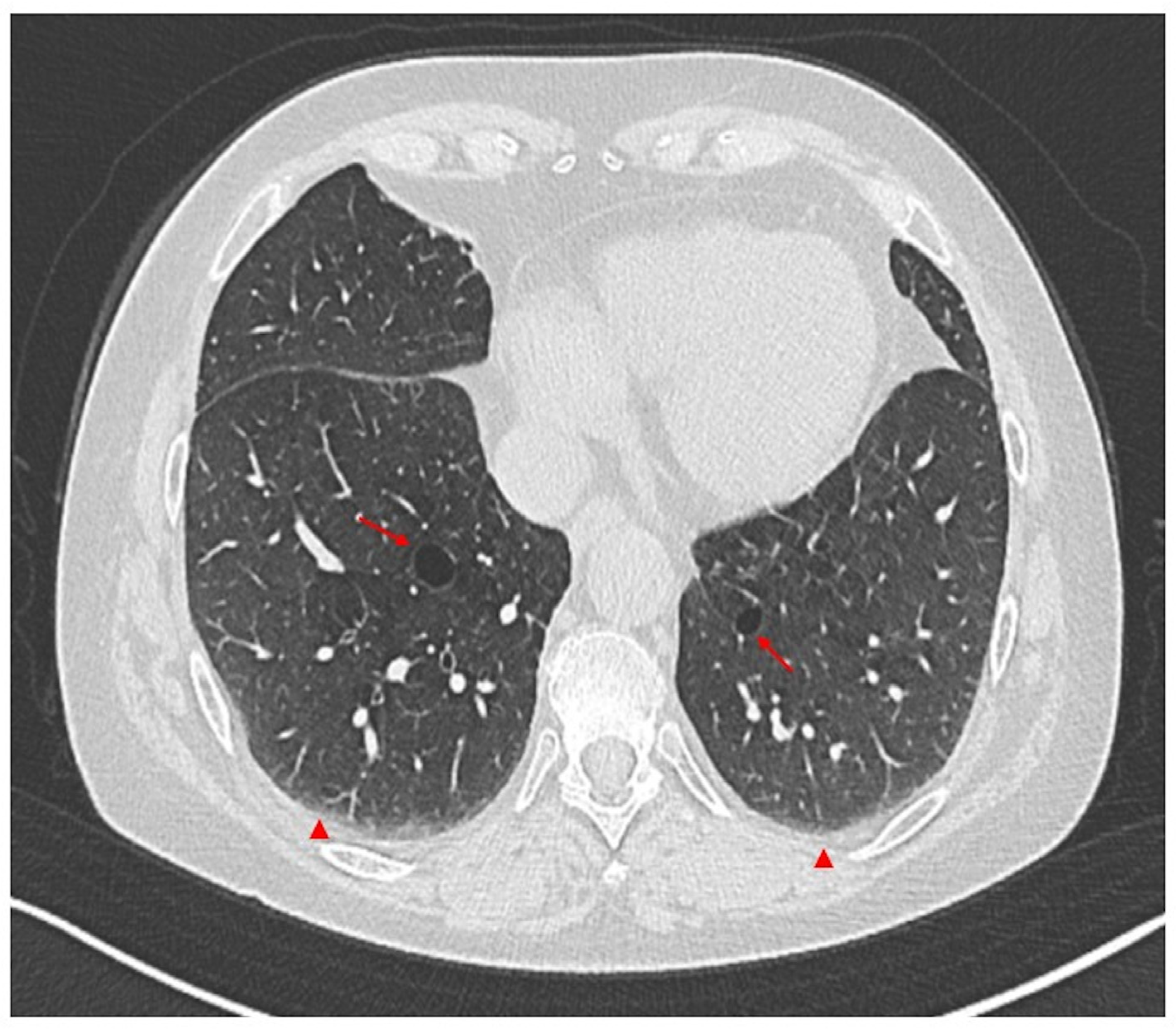
2.2. Lung Parenchymal Involvement
2.2.1. Acute Clinical Presentation
2.2.2. Chronic Clinical Presentation
2.3. Bronchiolitis Obliterans Organizing Pneumonia
2.4. Pulmonary Hypertension
2.5. Shrinking Lung Syndrome
2.6. Infections
2.7. Chronic Obstructive Pulmonary Disease
3. Predictive Factor of Lung Involvement in Systemic Lupus Erythematosus
4. Research Method
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lisnevskaia, L.; Murphy, G.; Isenberg, D. Systemic lupus erythematosus. Lancet 2014, 384, 1878–1888. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Kostopoulou, M.; Alunno, A.; Aringer, M.; Bajema, I.; Boletis, J.N.; Cervera, R.; Doria, A.; Gordon, C.; Govoni, M.; et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 2019, 78, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019, 71, 1400–1412. [Google Scholar] [CrossRef]
- Mormile, I.; Mosella, F.; Turco, P.; Napolitano, F.; de Paulis, A.; Rossi, F.W. Calcinosis Cutis and Calciphylaxis in Autoimmune Connective Tissue Diseases. Vaccines 2023, 11, 898. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, A.M.; Vila, L.M.; Apte, M.; Fessler, B.J.; Bastian, H.M.; Reveille, J.D.; Alarcon, G.S.; Group, L.S. Systemic lupus erythematosus in a multiethnic US Cohort LUMINA XLVIII: Factors predictive of pulmonary damage. Lupus 2007, 16, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.I.; Lee, K.H.; Park, S.; Yang, J.W.; Kim, H.J.; Song, K.; Lee, S.; Na, H.; Jang, Y.J.; Nam, J.Y.; et al. Systemic Lupus Erythematosus and Lung Involvement: A Comprehensive Review. J. Clin. Med. 2022, 11, 6714. [Google Scholar] [CrossRef]
- Kamen, D.L.; Strange, C. Pulmonary manifestations of systemic lupus erythematosus. Clin. Chest Med. 2010, 31, 479–488. [Google Scholar] [CrossRef]
- Memet, B.; Ginzler, E.M. Pulmonary manifestations of systemic lupus erythematosus. Semin. Respir. Crit. Care Med. 2007, 28, 441–450. [Google Scholar] [CrossRef]
- Jarrot, P.A.; Tellier, E.; Plantureux, L.; Crescence, L.; Robert, S.; Chareyre, C.; Daniel, L.; Secq, V.; Garcia, S.; Dignat-George, F.; et al. Neutrophil extracellular traps are associated with the pathogenesis of diffuse alveolar hemorrhage in murine lupus. J. Autoimmun. 2019, 100, 120–130. [Google Scholar] [CrossRef]
- Nielepkowicz-Gozdzinska, A.; Fendler, W.; Robak, E.; Kulczycka-Siennicka, L.; Gorski, P.; Pietras, T.; Brzezianska, E.; Antczak, A. Exhaled cytokines in systemic lupus erythematosus with lung involvement. Pol. Arch. Med. Wewn. 2013, 123, 141–148. [Google Scholar] [CrossRef]
- Herrada, A.A.; Escobedo, N.; Iruretagoyena, M.; Valenzuela, R.A.; Burgos, P.I.; Cuitino, L.; Llanos, C. Innate Immune Cells’ Contribution to Systemic Lupus Erythematosus. Front. Immunol. 2019, 10, 772. [Google Scholar] [CrossRef] [PubMed]
- Canny, S.P.; Jackson, S.W. B Cells in Systemic Lupus Erythematosus: From Disease Mechanisms to Targeted Therapies. Rheum. Dis. Clin. N. Am. 2021, 47, 395–413. [Google Scholar] [CrossRef]
- Tenbrock, K.; Rauen, T. T cell dysregulation in SLE. Clin. Immunol. 2022, 239, 109031. [Google Scholar] [CrossRef] [PubMed]
- Frangou, E.; Vassilopoulos, D.; Boletis, J.; Boumpas, D.T. An emerging role of neutrophils and NETosis in chronic inflammation and fibrosis in systemic lupus erythematosus (SLE) and ANCA-associated vasculitides (AAV): Implications for the pathogenesis and treatment. Autoimmun. Rev. 2019, 18, 751–760. [Google Scholar] [CrossRef]
- Cheng, P.; Li, S.; Chen, H. Macrophages in Lung Injury, Repair, and Fibrosis. Cells 2021, 10, 436. [Google Scholar] [CrossRef]
- Tseng, C.C.; Sung, Y.W.; Chen, K.Y.; Wang, P.Y.; Yen, C.Y.; Sung, W.Y.; Wu, C.C.; Ou, T.T.; Tsai, W.C.; Liao, W.T.; et al. The Role of Macrophages in Connective Tissue Disease-Associated Interstitial Lung Disease: Focusing on Molecular Mechanisms and Potential Treatment Strategies. Int. J. Mol. Sci. 2023, 24, 11995. [Google Scholar] [CrossRef]
- Depascale, R.; Del Frate, G.; Gasparotto, M.; Manfre, V.; Gatto, M.; Iaccarino, L.; Quartuccio, L.; De Vita, S.; Doria, A. Diagnosis and management of lung involvement in systemic lupus erythematosus and Sjogren’s syndrome: A literature review. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211040696. [Google Scholar] [CrossRef]
- Amarnani, R.; Yeoh, S.A.; Denneny, E.K.; Wincup, C. Lupus and the Lungs: The Assessment and Management of Pulmonary Manifestations of Systemic Lupus Erythematosus. Front. Med. 2020, 7, 610257. [Google Scholar] [CrossRef]
- Haye Salinas, M.J.; Caeiro, F.; Saurit, V.; Alvarellos, A.; Wojdyla, D.; Scherbarth, H.R.; de O e Silva, A.C.; Tavares Brenol, J.C.; Lavras Costallat, L.T.; Neira, O.J.; et al. Pleuropulmonary involvement in patients with systemic lupus erythematosus from a Latin American inception cohort (GLADEL). Lupus 2017, 26, 1368–1377. [Google Scholar] [CrossRef]
- Liang, Y.; Leng, R.X.; Pan, H.F.; Ye, D.Q. The prevalence and risk factors for serositis in patients with systemic lupus erythematosus: A cross-sectional study. Rheumatol. Int. 2017, 37, 305–311. [Google Scholar] [CrossRef] [PubMed]
- De Zorzi, E.; Spagnolo, P.; Cocconcelli, E.; Balestro, E.; Iaccarino, L.; Gatto, M.; Benvenuti, F.; Bernardinello, N.; Doria, A.; Maher, T.M.; et al. Thoracic Involvement in Systemic Autoimmune Rheumatic Diseases: Pathogenesis and Management. Clin. Rev. Allergy Immunol. 2022, 63, 472–489. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Brown, K.K. Interstitial lung disease associated with systemic sclerosis (SSc-ILD). Respir. Res. 2019, 20, 13. [Google Scholar] [CrossRef]
- Mormile, I.; Mormile, M.; Rossi, F.W.; Williams, M.; Valente, T.; Candia, C.; Granata, F.; Rega, R.; Orlandi, M.; Matucci-Cerinic, M.; et al. Radiological patterns and pulmonary function values of lung involvement in primary Sjogren’s syndrome: A pilot analysis. Front. Med. 2022, 9, 998028. [Google Scholar] [CrossRef]
- Romano, R.; Borrelli, M.; Cirillo, E.; Giardino, G.; Spadaro, G.; Crescenzi, L.; Mormile, I.; Venditto, L.; Pignata, C.; Santamaria, F. Respiratory Manifestations in Primary Immunodeficiencies: Findings From a Pediatric and Adult Cohort. Arch. Bronconeumol. 2021, 57, 712–714. [Google Scholar] [CrossRef]
- Deeb, M.; Tselios, K.; Gladman, D.D.; Su, J.; Urowitz, M.B. Shrinking lung syndrome in systemic lupus erythematosus: A single-centre experience. Lupus 2018, 27, 365–371. [Google Scholar] [CrossRef]
- Lv, T.T.; Wang, P.; Guan, S.Y.; Li, H.M.; Li, X.M.; Wang, B.; Pan, H.F. Prevalence of pulmonary hypertension in systemic lupus erythematosus: A meta-analysis. Ir. J. Med. Sci. 2018, 187, 723–730. [Google Scholar] [CrossRef]
- Borrell, H.; Narvaez, J.; Alegre, J.J.; Castellvi, I.; Mitjavila, F.; Aparicio, M.; Armengol, E.; Molina-Molina, M.; Nolla, J.M. Shrinking lung syndrome in systemic lupus erythematosus: A case series and review of the literature. Medicine 2016, 95, e4626. [Google Scholar] [CrossRef]
- Mageau, A.; Borie, R.; Crestani, B.; Timsit, J.F.; Papo, T.; Sacre, K. Epidemiology of interstitial lung disease in systemic lupus erythematosus in France: A nation-wide population-based study over 10 years. Respirology 2022, 27, 630–634. [Google Scholar] [CrossRef]
- Torre, O.; Harari, S. Pleural and pulmonary involvement in systemic lupus erythematosus. Presse Med. 2011, 40, e19–e29. [Google Scholar] [CrossRef]
- Pego-Reigosa, J.M.; Medeiros, D.A.; Isenberg, D.A. Respiratory manifestations of systemic lupus erythematosus: Old and new concepts. Best. Pract. Res. Clin. Rheumatol. 2009, 23, 469–480. [Google Scholar] [CrossRef]
- Wang, D.Y. Diagnosis and management of lupus pleuritis. Curr. Opin. Pulm. Med. 2002, 8, 312–316. [Google Scholar] [CrossRef]
- Good, J.T., Jr.; King, T.E.; Antony, V.B.; Sahn, S.A. Lupus pleuritis. Clinical features and pleural fluid characteristics with special reference to pleural fluid antinuclear antibodies. Chest 1983, 84, 714–718. [Google Scholar] [CrossRef]
- Hunninghake, G.W.; Fauci, A.S. Pulmonary involvement in the collagen vascular diseases. Am. Rev. Respir. Dis. 1979, 119, 471–503. Available online: https://www.atsjournals.org/doi/10.1164/arrd.1979.119.3.471?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 10 May 2025).
- Pines, A.; Kaplinsky, N.; Olchovsky, D.; Rozenman, J.; Frankl, O. Pleuro-pulmonary manifestations of systemic lupus erythematosus: Clinical features of its subgroups. Prognostic and therapeutic implications. Chest 1985, 88, 129–135. [Google Scholar] [CrossRef]
- Costa, M.F.; Said, N.R.; Zimmermann, B. Drug-induced lupus due to anti-tumor necrosis factor alpha agents. Semin. Arthritis Rheum. 2008, 37, 381–387. [Google Scholar] [CrossRef]
- Smith, P.R.; Nacht, R.I. Drug-induced lupus pleuritis mimicking pleural space infection. Chest 1992, 101, 268–269. [Google Scholar] [CrossRef]
- Crestani, B. The respiratory system in connective tissue disorders. Allergy 2005, 60, 715–734. [Google Scholar] [CrossRef] [PubMed]
- Cheema, G.S.; Quismorio, F.P., Jr. Interstitial lung disease in systemic lupus erythematosus. Curr. Opin. Pulm. Med. 2000, 6, 424–429. [Google Scholar] [CrossRef]
- Breuer, G.S.; Deeb, M.; Fisher, D.; Nesher, G. Therapeutic options for refractory massive pleural effusion in systemic lupus erythematosus: A case study and review of the literature. Semin. Arthritis Rheum. 2005, 34, 744–749. [Google Scholar] [CrossRef]
- Hunder, G.G.; McDuffie, F.C.; Hepper, N.G. Pleural fluid complement in systemic lupus erythematosus and rheumatoid arthritis. Ann. Intern. Med. 1972, 76, 357–363. [Google Scholar] [CrossRef]
- Winslow, W.A.; Ploss, L.N.; Loitman, B. Pleuritis in systemic lupus erythematosus: Its importance as an early manifestation in diagnosis. Ann. Intern. Med. 1958, 49, 70–88. [Google Scholar] [CrossRef]
- Karim, M.Y.; Alba, P.; Cuadrado, M.J.; Abbs, I.C.; D′Cruz, D.P.; Khamashta, M.A.; Hughes, G.R. Mycophenolate mofetil for systemic lupus erythematosus refractory to other immunosuppressive agents. Rheumatology 2002, 41, 876–882. [Google Scholar] [CrossRef]
- Altabas-Gonzalez, I.; Pego-Reigosa, J.M.; Mourino, C.; Jimenez, N.; Hernandez-Martin, A.; Casafont-Sole, I.; Font Urguelles, J.; Roman-Ivorra, J.A.; de la Rubia Navarro, M.; Galindo-Izquierdo, M.; et al. Thorough assessment of the effectiveness of belimumab in a large Spanish multicenter cohort of systemic lupus erythematosus patients. Rheumatology 2025, 64, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Carrion-Barbera, I.; Salman-Monte, T.C.; Castell, S.; Castro-Dominguez, F.; Ojeda, F.; Monfort, J. Successful treatment of systemic lupus erythematosus pleuropericarditis with belimumab. Eur. J. Rheumatol. 2019, 6, 150–152. [Google Scholar] [CrossRef]
- Ng, K.P.; Leandro, M.J.; Edwards, J.C.; Ehrenstein, M.R.; Cambridge, G.; Isenberg, D.A. Repeated B cell depletion in treatment of refractory systemic lupus erythematosus. Ann. Rheum. Dis. 2006, 65, 942–945. [Google Scholar] [CrossRef]
- Meissner, M.; Sherer, Y.; Levy, Y.; Chwalinska-Sadowska, H.; Langevitz, P.; Shoenfeld, Y. Intravenous immunoglobulin therapy in a patient with lupus serositis and nephritis. Rheumatol. Int. 2000, 19, 199–201. [Google Scholar] [CrossRef]
- Sherer, Y.; Langevitz, P.; Levy, Y.; Fabrizzi, F.; Shoenfeld, Y. Treatment of chronic bilateral pleural effusions with intravenous immunoglobulin and cyclosporin. Lupus 1999, 8, 324–327. [Google Scholar] [CrossRef] [PubMed]
- McKnight, K.M.; Adair, N.E.; Agudelo, C.A. Successful use of tetracycline pleurodesis to treat massive pleural effusion secondary to systemic lupus erythematosus. Arthritis Rheum. 1991, 34, 1483–1484. [Google Scholar] [CrossRef]
- Glazer, M.; Berkman, N.; Lafair, J.S.; Kramer, M.R. Successful talc slurry pleurodesis in patients with nonmalignant pleural effusion. Chest 2000, 117, 1404–1409. [Google Scholar] [CrossRef]
- Keane, M.P.; Lynch, J.P., 3rd. Pleuropulmonary manifestations of systemic lupus erythematosus. Thorax 2000, 55, 159–166. [Google Scholar] [CrossRef]
- Al-Adhoubi, N.K.; Bystrom, J. Systemic lupus erythematosus and diffuse alveolar hemorrhage, etiology and novel treatment strategies. Lupus 2020, 29, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Morales-Nebreda, L.; Alakija, O.; Ferguson, K.T.; Singer, B.D. Systemic lupus erythematosus-associated diffuse alveolar hemorrhage: A case report and review of the literature. Clin. Pulm. Med. 2018, 25, 166–169. [Google Scholar] [CrossRef]
- Matthay, R.A.; Schwarz, M.I.; Petty, T.L.; Stanford, R.E.; Gupta, R.C.; Sahn, S.A.; Steigerwald, J.C. Pulmonary manifestations of systemic lupus erythematosus: Review of twelve cases of acute lupus pneumonitis. Medicine 1975, 54, 397–409. [Google Scholar] [CrossRef]
- Tselios, K.; Urowitz, M.B. Cardiovascular and Pulmonary Manifestations of Systemic Lupus Erythematosus. Curr. Rheumatol. Rev. 2017, 13, 206–218. [Google Scholar] [CrossRef]
- Ahuja, J.; Arora, D.; Kanne, J.P.; Henry, T.S.; Godwin, J.D. Imaging of Pulmonary Manifestations of Connective Tissue Diseases. Radiol. Clin. N. Am. 2016, 54, 1015–1031. [Google Scholar] [CrossRef]
- Badsha, H.; Teh, C.L.; Kong, K.O.; Lian, T.Y.; Chng, H.H. Pulmonary hemorrhage in systemic lupus erythematosus. Semin. Arthritis Rheum. 2004, 33, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Zamora, M.R.; Warner, M.L.; Tuder, R.; Schwarz, M.I. Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine 1997, 76, 192–202. [Google Scholar] [CrossRef]
- Cucuzza, M.E.; Marino, S.D.; Schiavone, L.; Smilari, P.; Filosco, F.; Barone, P. Diffuse alveolar haemorrage as initial presentation of systemic lupus erythematosus: A case report. Lupus 2018, 27, 507–510. [Google Scholar] [CrossRef]
- Lara, A.R.; Schwarz, M.I. Diffuse alveolar hemorrhage. Chest 2010, 137, 1164–1171. [Google Scholar] [CrossRef]
- Hughson, M.D.; He, Z.; Henegar, J.; McMurray, R. Alveolar hemorrhage and renal microangiopathy in systemic lupus erythematosus. Arch. Pathol. Lab. Med. 2001, 125, 475–483. [Google Scholar] [CrossRef]
- Bajema, I.M.; Wilhelmus, S.; Alpers, C.E.; Bruijn, J.A.; Colvin, R.B.; Cook, H.T.; D’Agati, V.D.; Ferrario, F.; Haas, M.; Jennette, J.C.; et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: Clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018, 93, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, C.; Zhao, J.; Wang, Q.; Xu, D.; Zhang, S.; Shen, M.; Hou, Y.; Tian, X.; Li, M.; et al. Systemic lupus erythematosus-associated diffuse alveolar hemorrhage: A single-center, matched case-control study in China. Lupus 2020, 29, 795–803. [Google Scholar] [CrossRef]
- Ba-Shammakh, S.A.; Al-Zughali, E.A.; Kalaji, Z.H.; Al-Bourah, A.M.; Al-Shami, N.A. Clinical Dilemmas in Immune Thrombocytopenic Purpura with Diffuse Alveolar Hemorrhage: Diagnosis, Treatment, and Outcomes. Cureus 2023, 15, e47300. [Google Scholar] [CrossRef]
- Figueroa-Parra, G.; Meade-Aguilar, J.A.; Langenfeld, H.E.; Gonzalez-Trevino, M.; Hocaoglu, M.; Hanson, A.C.; Prokop, L.J.; Murad, M.H.; Cartin-Ceba, R.; Specks, U.; et al. Clinical features, risk factors, and outcomes of diffuse alveolar hemorrhage in antiphospholipid syndrome: A mixed-method approach combining a multicenter cohort with a systematic literature review. Clin. Immunol. 2023, 256, 109775. [Google Scholar] [CrossRef] [PubMed]
- Ta, R.; Celli, R.; West, A.B. Diffuse Alveolar Hemorrhage in Systemic Lupus Erythematosus: Histopathologic Features and Clinical Correlations. Case Rep. Pathol. 2017, 2017, 1936282. [Google Scholar] [CrossRef]
- Lee, C.T.; Strek, M.E. The other connective tissue disease-associated interstitial lung diseases: Sjogren’s syndrome, mixed connective tissue disease, and systemic lupus erythematosus. Curr. Opin. Pulm. Med. 2021, 27, 388–395. [Google Scholar] [CrossRef]
- Richter, P.; Cardoneanu, A.; Dima, N.; Bratoiu, I.; Rezus, C.; Burlui, A.M.; Costin, D.; Macovei, L.A.; Rezus, E. Interstitial Lung Disease in Systemic Lupus Erythematosus and Systemic Sclerosis: How Can We Manage the Challenge? Int. J. Mol. Sci. 2023, 24, 9388. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.H.; Chen, D.Y.; Lin, C.H.; Chao, W.C.; Chen, Y.M.; Chen, Y.H.; Huang, W.N.; Hsieh, T.Y.; Lai, K.L.; Tang, K.T.; et al. Risk of interstitial lung disease in patients with newly diagnosed systemic autoimmune rheumatic disease: A nationwide, population-based cohort study. Semin. Arthritis Rheum. 2020, 50, 840–845. [Google Scholar] [CrossRef]
- Narvaez, J.; Borrell, H.; Sanchez-Alonso, F.; Rua-Figueroa, I.; Lopez-Longo, F.J.; Galindo-Izquierdo, M.; Calvo-Alen, J.; Fernandez-Nebro, A.; Olive, A.; Andreu, J.L.; et al. Primary respiratory disease in patients with systemic lupus erythematosus: Data from the Spanish rheumatology society lupus registry (RELESSER) cohort. Arthritis Res. Ther. 2018, 20, 280. [Google Scholar] [CrossRef]
- Toyoda, Y.; Koyama, K.; Kawano, H.; Nishimura, H.; Kagawa, K.; Morizumi, S.; Naito, N.; Sato, S.; Yamashita, Y.; Takahashi, N.; et al. Clinical features of interstitial pneumonia associated with systemic lupus erythematosus. Respir. Investig. 2019, 57, 435–443. [Google Scholar] [CrossRef]
- Medlin, J.L.; Hansen, K.E.; McCoy, S.S.; Bartels, C.M. Pulmonary manifestations in late versus early systemic lupus erythematosus: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2018, 48, 198–204. [Google Scholar] [CrossRef]
- Jeleniewicz, R.; Suszek, D.; Majdan, M. Clinical picture of late-onset systemic lupus erythematosus in a group of Polish patients. Pol. Arch. Med. Wewn. 2015, 125, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, S.; Alunno, A.; Carubbi, F. Respiratory Manifestations in Systemic Lupus Erythematosus. Pharmaceuticals 2021, 14, 276. [Google Scholar] [CrossRef] [PubMed]
- Le Tallec, E.; Bourg, C.; Bouzille, G.; Belhomme, N.; Le Pabic, E.; Guillot, S.; Droitcourt, C.; Perlat, A.; Jouneau, S.; Donal, E.; et al. Prognostic value and predictors of the alteration of the diffusing capacity of the lungs for carbon monoxide in systemic lupus erythematosus. Rheumatology 2024, 63, 2178–2188. [Google Scholar] [CrossRef]
- Muangchan, C.; van Vollenhoven, R.F.; Bernatsky, S.R.; Smith, C.D.; Hudson, M.; Inanc, M.; Rothfield, N.F.; Nash, P.T.; Furie, R.A.; Senecal, J.L.; et al. Treatment Algorithms in Systemic Lupus Erythematosus. Arthritis Care Res. 2015, 67, 1237–1245. [Google Scholar] [CrossRef]
- Mwangi, J.; Litteken, C.; Gorthi, R.; Attoti, Y.; Atluri, R. Belimumab in the Treatment of Connective Tissue Disease-Associated Interstitial Lung Disease: Case Report and Literature Review. Cureus 2021, 13, e19218. [Google Scholar] [CrossRef]
- Vacchi, C.; Sebastiani, M.; Cassone, G.; Cerri, S.; Della Casa, G.; Salvarani, C.; Manfredi, A. Therapeutic Options for the Treatment of Interstitial Lung Disease Related to Connective Tissue Diseases. A Narrative Review. J. Clin. Med. 2020, 9, 407. [Google Scholar] [CrossRef]
- Keir, G.J.; Maher, T.M.; Ming, D.; Abdullah, R.; de Lauretis, A.; Wickremasinghe, M.; Nicholson, A.G.; Hansell, D.M.; Wells, A.U.; Renzoni, E.A. Rituximab in severe, treatment-refractory interstitial lung disease. Respirology 2014, 19, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.E.; Reyes-Caballero, K.S.; Samson, M.J.T.; Lichauco, J.J. Case of 54 Year-Old Patient with Systemic Lupus Erythematosus-Interstitial Lung Disease Managed with Intravenous Immunoglobulin. Am. J. Respir. Crit. Care Med. 2021, 203, A2059. [Google Scholar]
- Al-Ghanem, S.; Al-Jahdali, H.; Bamefleh, H.; Khan, A.N. Bronchiolitis obliterans organizing pneumonia: Pathogenesis, clinical features, imaging and therapy review. Ann. Thorac. Med. 2008, 3, 67–75. [Google Scholar] [CrossRef]
- Epler, G.R. Bronchiolitis obliterans organizing pneumonia: Definition and clinical features. Chest 1992, 102, 2S–6S. [Google Scholar] [CrossRef] [PubMed]
- Alasaly, K.; Muller, N.; Ostrow, D.N.; Champion, P.; FitzGerald, J.M. Cryptogenic organizing pneumonia. A report of 25 cases and a review of the literature. Medicine 1995, 74, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Imasaki, T.; Yoshii, A.; Tanaka, S.; Ogura, T.; Ishikawa, A.; Takahashi, T. Polymyositis and Sjogren’s syndrome associated with bronchiolitis obliterans organizing pneumonia. Intern. Med. 1996, 35, 231–235. [Google Scholar] [CrossRef]
- Otsuka, F.; Amano, T.; Hashimoto, N.; Takahashi, M.; Hayakawa, N.; Makino, H.; Ota, Z.; Ogura, T. Bronchiolitis obliterans organizing pneumonia associated with systemic lupus erythematosus with antiphospholipid antibody. Intern. Med. 1996, 35, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Takada, H.; Saito, Y.; Nomura, A.; Ohga, S.; Kuwano, K.; Nakashima, N.; Aishima, S.; Tsuru, N.; Hara, T. Bronchiolitis obliterans organizing pneumonia as an initial manifestation in systemic lupus erythematosus. Pediatr. Pulmonol. 2005, 40, 257–260. [Google Scholar] [CrossRef]
- Harvey, A.M.; Shulman, L.E.; Tumulty, P.A.; Conley, C.L.; Schoenrich, E.H. Systemic lupus erythematosus: Review of the literature and clinical analysis of 138 cases. Medicine 1954, 33, 291–437. [Google Scholar] [CrossRef]
- Mormile, I.; Osman, M.; Rossi, F.W. Editorial: Prognostic and predictive factors in autoimmune connective tissue disorders. Front. Immunol. 2024, 15, 1465572. [Google Scholar] [CrossRef]
- Cansu, D.U.; Korkmaz, C. Pulmonary hypertension in connective tissue diseases: Epidemiology, pathogenesis, and treatment. Clin. Rheumatol. 2023, 42, 2601–2610. [Google Scholar] [CrossRef]
- Parperis, K.; Velidakis, N.; Khattab, E.; Gkougkoudi, E.; Kadoglou, N.P.E. Systemic Lupus Erythematosus and Pulmonary Hypertension. Int. J. Mol. Sci. 2023, 24, 5085. [Google Scholar] [CrossRef]
- Nicolls, M.R.; Taraseviciene-Stewart, L.; Rai, P.R.; Badesch, D.B.; Voelkel, N.F. Autoimmunity and pulmonary hypertension: A perspective. Eur. Respir. J. 2005, 26, 1110–1118. [Google Scholar] [CrossRef]
- Thoreau, B.; Mouthon, L. Pulmonary arterial hypertension associated with connective tissue diseases (CTD-PAH): Recent and advanced data. Autoimmun. Rev. 2024, 23, 103506. [Google Scholar] [CrossRef] [PubMed]
- Cesoni Marcelli, A.; Loffredo, S.; Petraroli, A.; Carucci, L.; Mormile, I.; Ferrara, A.L.; Spadaro, G.; Genovese, A.; Bova, M. Nailfold Videocapillaroscopy Findings in Bradykinin-Mediated Angioedema. J. Investig. Allergol. Clin. Immunol. 2021, 31, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, T.; Bae, S.C.; Gu, H.; Huang, W.N.; Li, M.; Nikpour, M.; Okada, M.; Prior, D.; Atanasov, P.; Jiang, X.; et al. Risk Factors for Pulmonary Arterial Hypertension in Patients with Systemic Lupus Erythematosus: A Systematic Review and Expert Consensus. ACR Open Rheumatol. 2023, 5, 663–676. [Google Scholar] [CrossRef]
- Qu, J.; Li, M.; Zhang, X.; Zhang, M.; Zuo, X.; Zhu, P.; Ye, S.; Zhang, W.; Zheng, Y.; Qi, W.; et al. A prognostic model for systemic lupus erythematosus-associated pulmonary arterial hypertension: CSTAR-PAH cohort study. Respir. Res. 2023, 24, 220. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Dhala, A. Pulmonary arterial hypertension in systemic lupus erythematosus: Current status and future direction. Clin. Dev. Immunol. 2012, 2012, 854941. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Bogaard, H.J.; Condliffe, R.; Frantz, R.; Khanna, D.; Kurzyna, M.; Langleben, D.; Manes, A.; Satoh, T.; Torres, F.; et al. Definitions and diagnosis of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, D42–D50. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Mok, M.Y.; Tsang, P.L.; Lam, Y.M.; Lo, Y.; Wong, W.S.; Lau, C.S. Bosentan use in systemic lupus erythematosus patients with pulmonary arterial hypertension. Lupus 2007, 16, 279–285. [Google Scholar] [CrossRef]
- Kuzuya, K.; Tsuji, S.; Matsushita, M.; Ohshima, S.; Saeki, Y. Systemic Sclerosis and Systemic Lupus Erythematosus Overlap Syndrome with Pulmonary Arterial Hypertension Successfully Treated with Immunosuppressive Therapy and Riociguat. Cureus 2019, 11, e4327. [Google Scholar] [CrossRef]
- Gonzalez-Lopez, L.; Cardona-Munoz, E.G.; Celis, A.; Garcia-de la Torre, I.; Orozco-Barocio, G.; Salazar-Paramo, M.; Garcia-Gonzalez, C.; Garcia-Gonzalez, A.; Sanchez-Ortiz, A.; Trujillo-Hernandez, B.; et al. Therapy with intermittent pulse cyclophosphamide for pulmonary hypertension associated with systemic lupus erythematosus. Lupus 2004, 13, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Hennigan, S.; Channick, R.N.; Silverman, G.J. Rituximab treatment of pulmonary arterial hypertension associated with systemic lupus erythematosus: A case report. Lupus 2008, 17, 754–756. [Google Scholar] [CrossRef]
- Kommireddy, S.; Bhyravavajhala, S.; Kurimeti, K.; Chennareddy, S.; Kanchinadham, S.; Rajendra Vara Prasad, I.; Rajasekhar, L. Pulmonary arterial hypertension in systemic lupus erythematosus may benefit by addition of immunosuppression to vasodilator therapy: An observational study. Rheumatology 2015, 54, 1673–1679. [Google Scholar] [CrossRef]
- Duron, L.; Cohen-Aubart, F.; Diot, E.; Borie, R.; Abad, S.; Richez, C.; Banse, C.; Vittecoq, O.; Saadoun, D.; Haroche, J.; et al. Shrinking lung syndrome associated with systemic lupus erythematosus: A multicenter collaborative study of 15 new cases and a review of the 155 cases in the literature focusing on treatment response and long-term outcomes. Autoimmun. Rev. 2016, 15, 994–1000. [Google Scholar] [CrossRef]
- Laroche, C.M.; Mulvey, D.A.; Hawkins, P.N.; Walport, M.J.; Strickland, B.; Moxham, J.; Green, M. Diaphragm strength in the shrinking lung syndrome of systemic lupus erythematosus. Q. J. Med. 1989, 71, 429–439. [Google Scholar] [PubMed]
- Choudhury, S.; Ramos, M.; Anjum, H.; Ali, M.; Surani, S. Shrinking Lung Syndrome: A Rare Manifestation of Systemic Lupus Erythematosus. Cureus 2020, 12, e8216. [Google Scholar] [CrossRef]
- Hawkins, P.; Davison, A.G.; Dasgupta, B.; Moxham, J. Diaphragm strength in acute systemic lupus erythematosus in a patient with paradoxical abdominal motion and reduced lung volumes. Thorax 2001, 56, 329–330. [Google Scholar] [CrossRef]
- Hardy, K.; Herry, I.; Attali, V.; Cadranel, J.; Similowski, T. Bilateral phrenic paralysis in a patient with systemic lupus erythematosus. Chest 2001, 119, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.A.; Urowitz, M.B. Shrinking lung syndrome in SLE—A clinical pathologic study. J. Rheumatol. 1983, 10, 973–976. [Google Scholar]
- Krych, E.H.; Fischer, P.R.; Wylam, M.E. Pleural fibrosis mediates shrinking lungs syndrome in children. Pediatr. Pulmonol. 2009, 44, 90–92. [Google Scholar] [CrossRef]
- Gibson, C.J.; Edmonds, J.P.; Hughes, G.R. Diaphragm function and lung involvement in systemic lupus erythematosus. Am. J. Med. 1977, 63, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.A.; Loring, S.H.; Gill, R.R.; Liao, K.P.; Ishizawar, R.; Kim, S.; Perlmutter-Goldenson, R.; Rothman, D.; Son, M.B.; Stoll, M.L.; et al. Shrinking lung syndrome as a manifestation of pleuritis: A new model based on pulmonary physiological studies. J. Rheumatol. 2013, 40, 273–281. [Google Scholar] [CrossRef]
- Warrington, K.J.; Moder, K.G.; Brutinel, W.M. The shrinking lungs syndrome in systemic lupus erythematosus. Mayo Clin. Proc. 2000, 75, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Walz-Leblanc, B.A.; Urowitz, M.B.; Gladman, D.D.; Hanly, P.J. The “shrinking lungs syndrome” in systemic lupus erythematosus—Improvement with corticosteroid therapy. J. Rheumatol. 1992, 19, 1970–1972. [Google Scholar]
- Langenskiold, E.; Bonetti, A.; Fitting, J.W.; Heinzer, R.; Dudler, J.; Spertini, F.; Lazor, R. Shrinking lung syndrome successfully treated with rituximab and cyclophosphamide. Respiration 2012, 84, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Al-Karaja, L.; Alshayeb, F.O.; Amro, D.; Khdour, Y.F.; Alamlih, L. Shrinking Lung Syndrome in a Systemic Lupus Erythematous Patient Improved by Rituximab: A Case Report with Literature Review. Cureus 2023, 15, e50229. [Google Scholar] [CrossRef]
- DeCoste, C.; Mateos-Corral, D.; Lang, B. Shrinking lung syndrome treated with rituximab in pediatric systemic lupus erythematosus: A case report and review of the literature. Pediatr. Rheumatol. Online J. 2021, 19, 7. [Google Scholar] [CrossRef]
- Torres Jimenez, A.R.; Ruiz Vela, N.; Cespedes Cruz, A.I.; Velazquez Cruz, A.; Bernardino Gonzalez, A.K. Shrinking lung syndrome in pediatric systemic lupus erythematosus. Lupus 2021, 30, 1175–1179. [Google Scholar] [CrossRef]
- Shah, K.; Kondakindi, H.; Enabi, J.; Mukkera, S. Shrinking Lung Syndrome: A Rare Pulmonary Complication of Systemic Lupus Erythematosus. Cureus 2024, 16, e63990. [Google Scholar] [CrossRef]
- Fei, Y.; Shi, X.; Gan, F.; Li, X.; Zhang, W.; Li, M.; Hou, Y.; Zhang, X.; Zhao, Y.; Zeng, X.; et al. Death causes and pathogens analysis of systemic lupus erythematosus during the past 26 years. Clin. Rheumatol. 2014, 33, 57–63. [Google Scholar] [CrossRef]
- Lee, Y.H.; Choi, S.J.; Ji, J.D.; Song, G.G. Overall and cause-specific mortality in systemic lupus erythematosus: An updated meta-analysis. Lupus 2016, 25, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.R.W.; Clarke, A.E. Systemic lupus erythematosus and risk of infection. Expert. Rev. Clin. Immunol. 2020, 16, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Yang, M.; Xie, Y.S.; Xiao, W.G.; Lin, J.; Zhou, B.; Guan, X.; Luo, C.N.; Che, N.; Liu, X.Z.; et al. Causes of death in hospitalized patients with systemic lupus erythematosus: A 10-year multicenter nationwide Chinese cohort. Clin. Rheumatol. 2019, 38, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Hung, P.H.; Hu, H.Y.; Chen, Y.J.; Guo, H.R.; Hung, K.Y. Infection-related hospitalization and risk of end-stage renal disease in patients with systemic lupus erythematosus: A nationwide population-based study. Nephrol. Dial. Transplant. 2017, 32, 1683–1690. [Google Scholar] [CrossRef]
- Mormile, I.; Rossi, F.W.; Prevete, N.; Granata, F.; Pucino, V.; de Paulis, A. The N-Formyl Peptide Receptors and Rheumatoid Arthritis: A Dangerous Liaison or Confusing Relationship? Front. Immunol. 2021, 12, 685214. [Google Scholar] [CrossRef]
- Ramirez, G.A.; Cardamone, C.; Lettieri, S.; Fredi, M.; Mormile, I. Clinical and Pathophysiological Tangles Between Allergy and Autoimmunity: Deconstructing an Old Dichotomic Paradigm. Clin. Rev. Allergy Immunol. 2025, 68, 13. [Google Scholar] [CrossRef]
- Sawada, T.; Fujimori, D.; Yamamoto, Y. Systemic lupus erythematosus and immunodeficiency. Immunol. Med. 2019, 42, 1–9. [Google Scholar] [CrossRef]
- Mormile, I.; Punziano, A.; Riolo, C.A.; Granata, F.; Williams, M.; de Paulis, A.; Spadaro, G.; Rossi, F.W. Common Variable Immunodeficiency and Autoimmune Diseases: A Retrospective Study of 95 Adult Patients in a Single Tertiary Care Center. Front. Immunol. 2021, 12, 652487. [Google Scholar] [CrossRef]
- Garred, P.; Voss, A.; Madsen, H.O.; Junker, P. Association of mannose-binding lectin gene variation with disease severity and infections in a population-based cohort of systemic lupus erythematosus patients. Genes. Immun. 2001, 2, 442–450. [Google Scholar] [CrossRef]
- Doaty, S.; Agrawal, H.; Bauer, E.; Furst, D.E. Infection and Lupus: Which Causes Which? Curr. Rheumatol. Rep. 2016, 18, 13. [Google Scholar] [CrossRef]
- Teh, C.L.; Wan, S.A.; Ling, G.R. Severe infections in systemic lupus erythematosus: Disease pattern and predictors of infection-related mortality. Clin. Rheumatol. 2018, 37, 2081–2086. [Google Scholar] [CrossRef] [PubMed]
- Tektonidou, M.G.; Wang, Z.; Dasgupta, A.; Ward, M.M. Burden of Serious Infections in Adults with Systemic Lupus Erythematosus: A National Population-Based Study, 1996–2011. Arthritis Care Res. 2015, 67, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.R.; Barber, C.E.; Johnson, A.S.; Barnabe, C. Invasive fungal disease in systemic lupus erythematosus: A systematic review of disease characteristics, risk factors, and prognosis. Semin. Arthritis Rheum. 2014, 44, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Pope, J.; Jerome, D.; Fenlon, D.; Krizova, A.; Ouimet, J. Frequency of adverse drug reactions in patients with systemic lupus erythematosus. J. Rheumatol. 2003, 30, 480–484. [Google Scholar]
- Cheng, C.F.; Huang, Y.M.; Lu, C.H.; Hsieh, S.C.; Li, K.J. Prednisolone dose during treatment of tuberculosis might be a risk factor for mortality in patients with systemic lupus erythematosus: A hospital-based cohort study. Lupus 2019, 28, 1699–1704. [Google Scholar] [CrossRef]
- Pappa, M.; Panagiotopoulos, A.; Thomas, K.; Fanouriakis, A. Systemic Lupus Erythematosus and COVID-19. Curr. Rheumatol. Rep. 2023, 25, 192–203. [Google Scholar] [CrossRef]
- Conway, R.; Grimshaw, A.A.; Konig, M.F.; Putman, M.; Duarte-Garcia, A.; Tseng, L.Y.; Cabrera, D.M.; Chock, Y.P.E.; Degirmenci, H.B.; Duff, E.; et al. SARS-CoV-2 Infection and COVID-19 Outcomes in Rheumatic Diseases: A Systematic Literature Review and Meta-Analysis. Arthritis Rheumatol. 2022, 74, 766–775. [Google Scholar] [CrossRef]
- Tektonidou, M.G.; Lewandowski, L.B.; Hu, J.; Dasgupta, A.; Ward, M.M. Survival in adults and children with systemic lupus erythematosus: A systematic review and Bayesian meta-analysis of studies from 1950 to 2016. Ann. Rheum. Dis. 2017, 76, 2009–2016. [Google Scholar] [CrossRef]
- Bournia, V.K.; Fragoulis, G.E.; Mitrou, P.; Mathioudakis, K.; Tsolakidis, A.; Konstantonis, G.; Vourli, G.; Paraskevis, D.; Tektonidou, M.G.; Sfikakis, P.P. All-cause mortality in systemic rheumatic diseases under treatment compared with the general population, 2015–2019. RMD Open 2021, 7, e001694. [Google Scholar] [CrossRef]
- Pons-Estel, G.J.; Ugarte-Gil, M.F.; Alarcon, G.S. Epidemiology of systemic lupus erythematosus. Expert. Rev. Clin. Immunol. 2017, 13, 799–814. [Google Scholar] [CrossRef]
- Brusa, S.; Terracciano, D.; Bruzzese, D.; Fiorenza, M.; Stanziola, L.; Pinchera, B.; Valente, V.; Gentile, I.; Cittadini, A.; Mormile, I.; et al. Circulating tissue inhibitor of metalloproteinases 1 (TIMP-1) at COVID-19 onset predicts severity status. Front. Med. 2022, 9, 1034288. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Irastorza, G.; Ramos-Casals, M.; Brito-Zeron, P.; Khamashta, M.A. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: A systematic review. Ann. Rheum. Dis. 2010, 69, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Rolain, J.M.; Colson, P.; Raoult, D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int. J. Antimicrob. Agents 2007, 30, 297–308. [Google Scholar] [CrossRef]
- Mormile, I.; Della Casa, F.; Petraroli, A.; Furno, A.; Granata, F.; Portella, G.; Rossi, F.W.; de Paulis, A. Immunogenicity and Safety of mRNA Anti-SARS-CoV-2 Vaccines in Patients with Systemic Lupus Erythematosus. Vaccines 2022, 10, 1221. [Google Scholar] [CrossRef]
- Packard, T.A.; Li, Q.Z.; Cosgrove, G.P.; Bowler, R.P.; Cambier, J.C. COPD is associated with production of autoantibodies to a broad spectrum of self-antigens, correlative with disease phenotype. Immunol. Res. 2013, 55, 48–57. [Google Scholar] [CrossRef]
- Shen, T.C.; Lin, C.L.; Chen, C.H.; Tu, C.Y.; Hsia, T.C.; Shih, C.M.; Hsu, W.H.; Chang, Y.J. Increased risk of chronic obstructive pulmonary disease in patients with systemic lupus erythematosus: A population-based cohort study. PLoS ONE 2014, 9, e91821. [Google Scholar] [CrossRef] [PubMed]
- Katz, P.; Pedro, S.; Trupin, L.; Yelin, E.; Michaud, K. The Impact of Asthma and Chronic Obstructive Pulmonary Disease (COPD) on Patient-Reported Outcomes in Systemic Lupus Erythematosus (SLE). ACR Open Rheumatol. 2021, 3, 221–230. [Google Scholar] [CrossRef]
- Han, G.M.; Han, X.F. Comorbid Conditions are Associated with Emergency Department Visits, Hospitalizations, and Medical Charges of Patients with Systemic Lupus Erythematosus. J. Clin. Rheumatol. 2017, 23, 19–25. [Google Scholar] [CrossRef]
- Alamoudi, O.S.; Attar, S.M. Pulmonary manifestations in systemic lupus erythematosus: Association with disease activity. Respirology 2015, 20, 474–480. [Google Scholar] [CrossRef]
- El-Beheidy, R.; Domouky, A.M.; Zidan, H.; Amer, Y.A. Serum KL-6 as predictive and prognostic marker of interstitial lung disease in childhood connective tissue diseases: A pilot study. Reumatismo 2021, 73. [Google Scholar] [CrossRef]
- Oguz, E.O.; Kucuksahin, O.; Turgay, M.; Yildizgoren, M.T.; Ates, A.; Demir, N.; Kumbasar, O.O.; Kinikli, G.; Duzgun, N. Association of serum KL-6 levels with interstitial lung disease in patients with connective tissue disease: A cross-sectional study. Clin. Rheumatol. 2016, 35, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Lu, J.; Song, Y.; Wang, H.; Yin, S. The value of serum Krebs von den lungen-6 as a diagnostic marker in connective tissue disease associated with interstitial lung disease. BMC Pulm. Med. 2020, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Kilinc, A.A.; Arslan, A.; Yildiz, M.; Kucur, M.; Adrovic, A.; Barut, K.; Sahin, S.; Cokugras, H.; Kasapcopur, O. Serum KL-6 level as a biomarker of interstitial lung disease in childhood connective tissue diseases: A pilot study. Rheumatol. Int. 2020, 40, 1701–1706. [Google Scholar] [CrossRef]
- Winikajtis-Burzynska, A.; Brzosko, M.; Przepiera-Bedzak, H. Elevated Serum Levels of Soluble Transferrin Receptor Are Associated with an Increased Risk of Cardiovascular, Pulmonary, and Hematological Manifestations and a Decreased Risk of Neuropsychiatric Manifestations in Systemic Lupus Erythematosus Patients. Int. J. Mol. Sci. 2023, 24, 17340. [Google Scholar] [CrossRef]
- Santacruz, J.C.; Mantilla, M.J.; Rueda, I.; Pulido, S.; Rodriguez-Salas, G.; Londono, J. A Practical Perspective of the Hematologic Manifestations of Systemic Lupus Erythematosus. Cureus 2022, 14, e22938. [Google Scholar] [CrossRef]
- Jiang, Y.; Cheng, Y.; Ma, S.; Li, T.; Chen, Z.; Zuo, X.; Zhang, X. Systemic lupus erythematosus-complicating immune thrombocytopenia: From pathogenesis to treatment. J. Autoimmun. 2022, 132, 102887. [Google Scholar] [CrossRef]
- Granel, J.; Fernandes, H.; Bader-Meunier, B.; Guth, A.; Richer, O.; Pillet, P.; Leverger, G.; Ducassou, S.; Fahd, M.; Pasquet, M.; et al. Antinuclear antibody-associated autoimmune cytopenia in childhood is a risk factor for systemic lupus erythematosus. Blood 2024, 143, 1576–1585. [Google Scholar] [CrossRef]
- Thanou, A.; Jupe, E.; Purushothaman, M.; Niewold, T.B.; Munroe, M.E. Clinical disease activity and flare in SLE: Current concepts and novel biomarkers. J. Autoimmun. 2021, 119, 102615. [Google Scholar] [CrossRef] [PubMed]
- Lertchaisataporn, K.; Kasitanon, N.; Wangkaew, S.; Pantana, S.; Sukitawut, W.; Louthrenoo, W. An evaluation of the association of leukopenia and severe infection in patients with systemic lupus erythematosus. J. Clin. Rheumatol. 2013, 19, 115–120. [Google Scholar] [CrossRef]
- Pugh, D.; Farrah, T.E.; Gallacher, P.J.; Kluth, D.C.; Dhaun, N. Cyclophosphamide-Induced Lung Injury. Kidney Int. Rep. 2019, 4, 484–486. [Google Scholar] [CrossRef]
- Lateef, O.; Shakoor, N.; Balk, R.A. Methotrexate pulmonary toxicity. Expert. Opin. Drug Saf. 2005, 4, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Mormile, I.; Granata, F.; Punziano, A.; de Paulis, A.; Rossi, F.W. Immunosuppressive Treatment in Antiphospholipid Syndrome: Is It Worth It? Biomedicines 2021, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Deneuville, L.; Mageau, A.; Debray, M.P.; Sacre, K.; Costedoat-Chalumeau, N.; Hachulla, E.; Uzunhan, Y.; Le Tallec, E.; Cadranel, J.; Marchand Adam, S.; et al. Chronic interstitial lung disease associated with systemic lupus erythematosus: A multicentric study of 89 cases. Respirology 2024, 29, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Mathai, S.C.; Hassoun, P.M. Pulmonary arterial hypertension in connective tissue diseases. Heart Fail. Clin. 2012, 8, 413–425. [Google Scholar] [CrossRef]
- Lin, C.Y.; Ko, C.H.; Hsu, C.Y.; Chen, H.A. Epidemiology and mortality of connective tissue disease-associated pulmonary arterial hypertension: A national cohort study in taiwan. Semin. Arthritis Rheum. 2020, 50, 957–962. [Google Scholar] [CrossRef]
- Grygiel-Gorniak, B.; Lucki, M.; Daroszewski, P.; Lucka, E. Prevalence, molecular mechanisms and diagnostic approaches to pulmonary arterial hypertension in connective tissue diseases. Rheumatol. Int. 2025, 45, 87. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mormile, I.; Nazzaro, G.; Filippelli, M.; Della Casa, F.; Mormile, M.; de Paulis, A.; Rossi, F.W. Pulmonary Involvement in Systemic Lupus Erythematosus: A Potentially Overlooked Condition. Biomedicines 2025, 13, 1485. https://doi.org/10.3390/biomedicines13061485
Mormile I, Nazzaro G, Filippelli M, Della Casa F, Mormile M, de Paulis A, Rossi FW. Pulmonary Involvement in Systemic Lupus Erythematosus: A Potentially Overlooked Condition. Biomedicines. 2025; 13(6):1485. https://doi.org/10.3390/biomedicines13061485
Chicago/Turabian StyleMormile, Ilaria, Gerardo Nazzaro, Marco Filippelli, Francesca Della Casa, Mauro Mormile, Amato de Paulis, and Francesca Wanda Rossi. 2025. "Pulmonary Involvement in Systemic Lupus Erythematosus: A Potentially Overlooked Condition" Biomedicines 13, no. 6: 1485. https://doi.org/10.3390/biomedicines13061485
APA StyleMormile, I., Nazzaro, G., Filippelli, M., Della Casa, F., Mormile, M., de Paulis, A., & Rossi, F. W. (2025). Pulmonary Involvement in Systemic Lupus Erythematosus: A Potentially Overlooked Condition. Biomedicines, 13(6), 1485. https://doi.org/10.3390/biomedicines13061485






